Salby M.L. Fundamentals of Atmospheric Physics
Подождите немного. Документ загружается.

1000
900
000
700
600
s
Y
E
500
Q
400
300
200
100
n
0
.2 .4
.6
.8
1.0
1.2 1.4
1.6
1.8
2.0
"
(L)
1000
900
800
700
600
5-
a
v
E
500
400
300
200
100
0
0
50
100
150 200 250
300
350
400
T
(K)
h
d
>
0
50
100
150 200 250
300
350
400
T
(K)
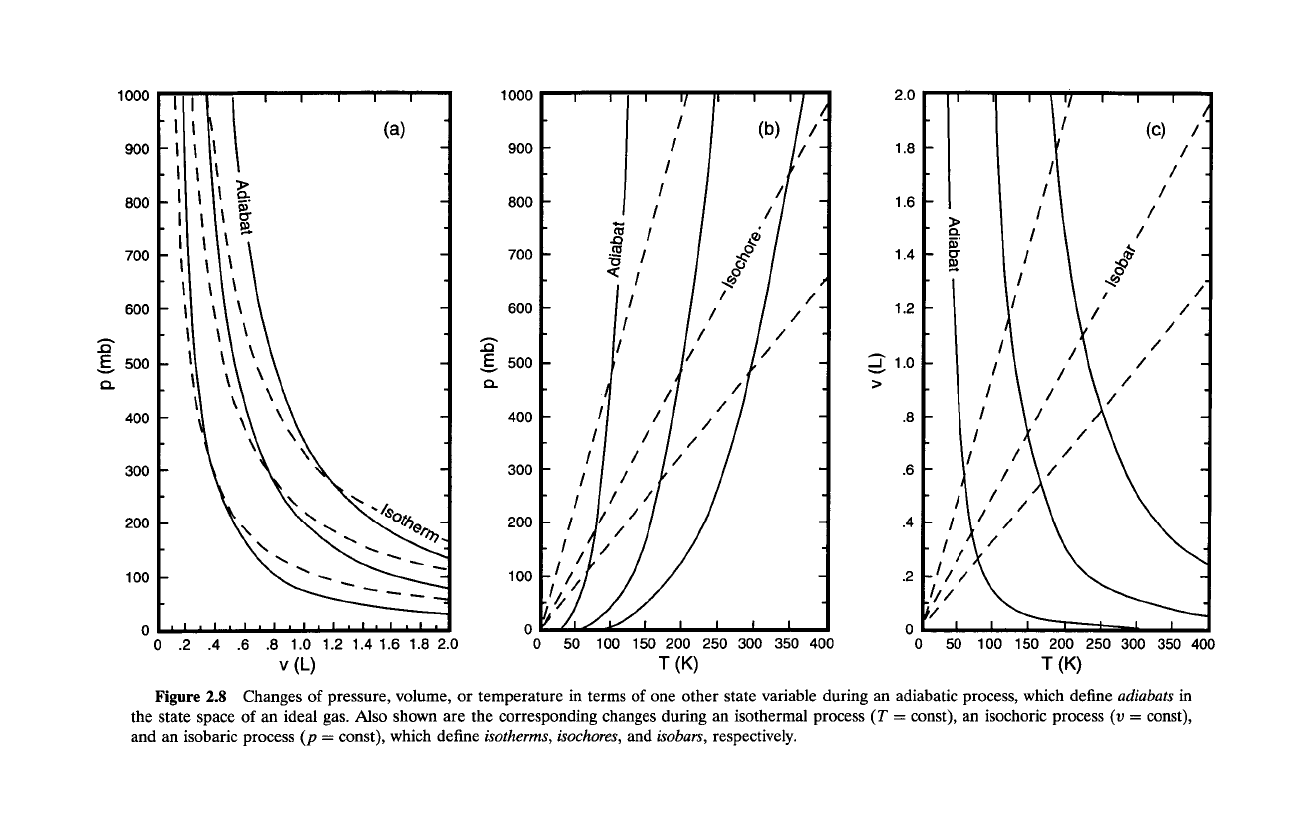
Figure
2.8
Changes
of
pressure, volume,
or
temperature in terms
of
one other state variable during an adiabatic process, which define
adiabats
in
the state space
of
an
ideal gas.
Also
shown are the corresponding changes during an isothermal process
(T
=
const), an isochoric process
(v
=
const),
and an isobaric process
(p
=
const), which define
isotherms, isochores,
and
isobars,
respectively.

2.4 Adiabatic Processes 71
and isochoric processes describe a family of
isochores
(v = const), which are
superposed in Fig. 2.8. Just as each of the foregoing paths is characterized by
invariance of a certain state variable, so too are adiabats.
2.4.1 Potential Temperature
Poisson's relation between pressure and temperature motivates the introduc-
tion of a new state variable that is preserved during an adiabatic process. The
potential temperature 0
is defined as that temperature assumed by the system
when compressed or expanded adiabatically to a reference pressure of P0 =
1000 mb. According to (2.30.2), an adiabatic process from the state (p, T) to
the reference state (P0, 0) satisfies
Opo~= rp-~.
Hence, the potential temperature is described by
0
~= P . (2.31)
A function of pressure and temperature, 0 is a state variable. According to
(2.31) and Poisson's relation (2.30.2), 0 is invariant along an adiabatic path in
state space.
Adiabatic behavior of individual air parcels is a good approximation for
many atmospheric applications. Above the boundary layer and outside of
clouds, the timescale of heat transfer is of order two weeks and thus long
compared to the characteristic timescale of displacements, which influence an
air parcel through changes of pressure and expansion work. For instance, ver-
tical displacements of air and accompanying changes of pressure and volume
occur in cumulus convection on a timescale of minutes to hours. Even in mo-
tions of large horizontal dimension (e.g., in sloping convection associated with
synoptic disturbances), air displacements occur on a characteristic timescale
of order one day. Thus, over a fairly wide range of motions, the timescale for
an air parcel to adjust to changes of pressure and to perform expansion work
is short compared to the characteristic timescale of heat transfer.
Under these circumstances, the potential temperature of individual air
parcels is approximately conserved. An air parcel descending to greater pres-
sure experiences an increase of temperature according to (2.30.2) due to com-
pression work performed on it, but in such proportion to its increase of pres-
sure as to preserve the parcel's potential temperature through (2.31). Similar
considerations apply to an air parcel that is ascending. It follows that, un-
der adiabatic conditions, 0 is a conserved quantity and therefore behaves as
a tracer of air motion. On timescales for which individual parcels can be re-
garded as adiabatic, particular values of 0 track the movement of those bodies
of air. Conversely, a collection of air parcels that has a particular value of 0
72
2
Thermodynamics of Gases
Adiabatic
O 7 ~ ...................
Diabatic
._ "
0=01
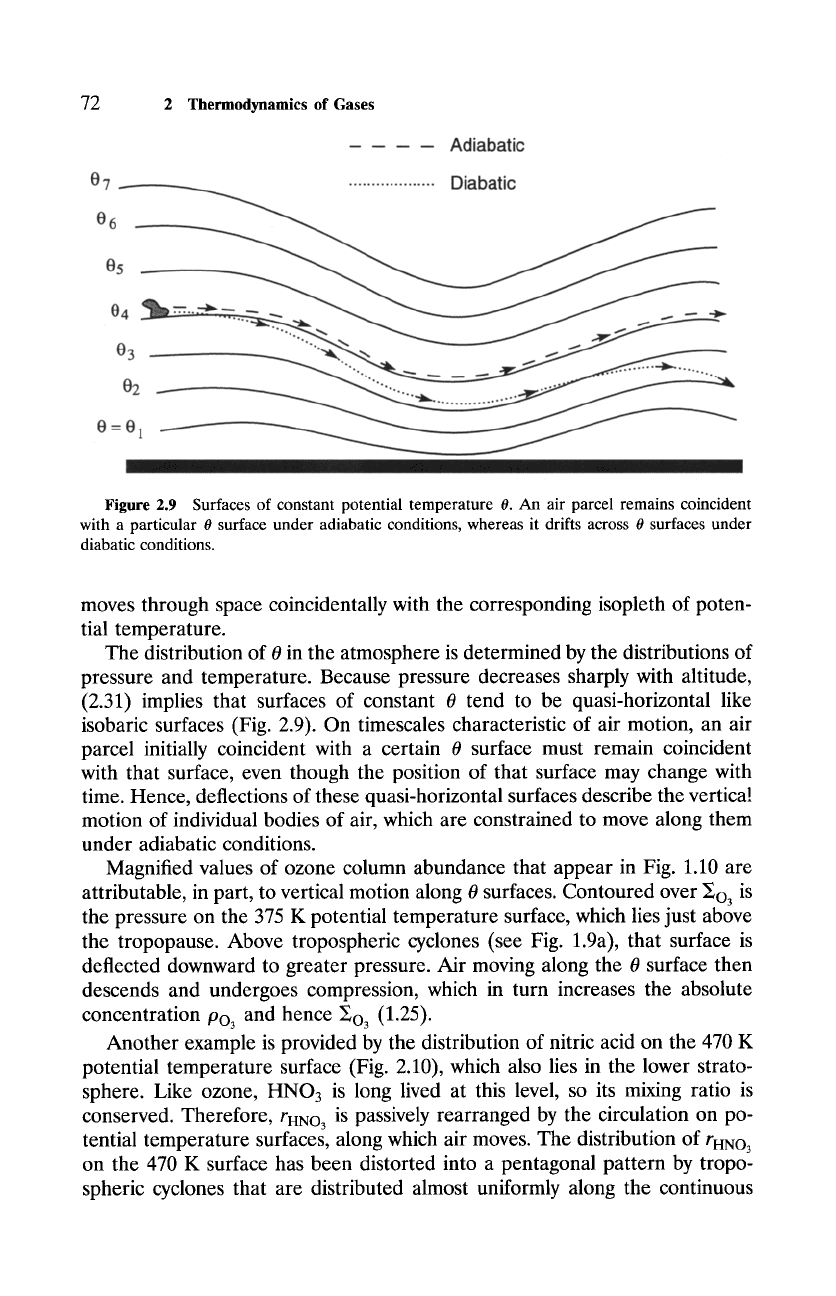
Figure
2.9 Surfaces of constant potential temperature 0. An air parcel remains coincident
with a particular 0 surface under adiabatic conditions, whereas it drifts across 0 surfaces under
diabatic conditions.
moves through space coincidentally with the corresponding isopleth of poten-
tial temperature.
The distribution of 0 in the atmosphere is determined by the distributions of
pressure and temperature. Because pressure decreases sharply with altitude,
(2.31) implies that surfaces of constant 0 tend to be quasi-horizontal like
isobaric surfaces (Fig. 2.9). On timescales characteristic of air motion, an air
parcel initially coincident with a certain 0 surface must remain coincident
with that surface, even though the position of that surface may change with
time. Hence, deflections of these quasi-horizontal surfaces describe the vertical
motion of individual bodies of air, which are constrained to move along them
under adiabatic conditions.
Magnified values of ozone column abundance that appear in Fig. 1.10 are
attributable, in part, to vertical motion along 0 surfaces. Contoured over Eo3 is
the pressure on the 375 K potential temperature surface, which lies just above
the tropopause. Above tropospheric cyclones (see Fig. 1.9a), that surface is
deflected downward to greater pressure. Air moving along the 0 surface then
descends and undergoes compression, which in turn increases the absolute
concentration Po3 and hence Eo3 (1.25).
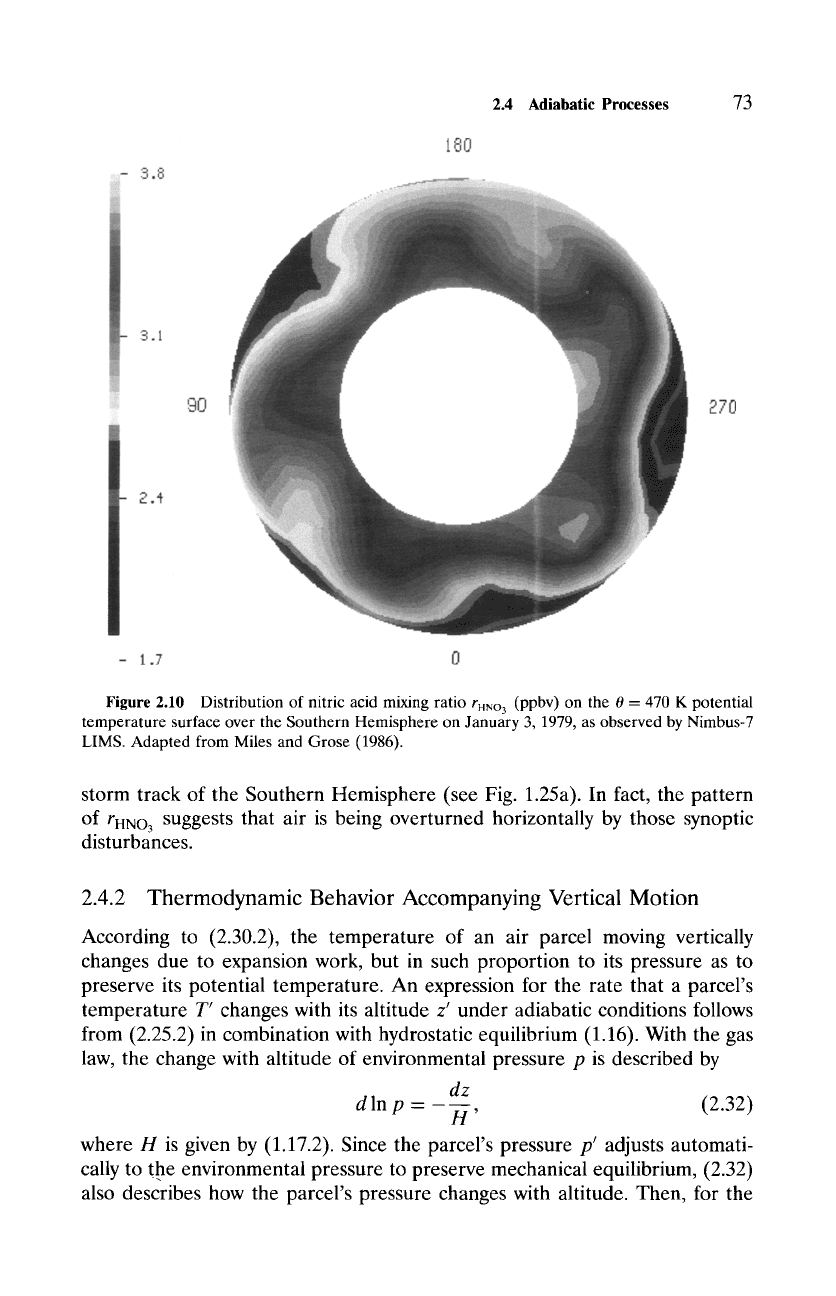
Another example is provided by the distribution of nitric acid on the 470 K
potential temperature surface (Fig. 2.10), which also lies in the lower strato-
sphere. Like ozone, HNO3 is long lived at this level, so its mixing ratio is
conserved. Therefore, rHNO3 is passively rearranged by the circulation on po-
tential temperature surfaces, along which air moves. The distribution of rHNO3
on the 470 K surface has been distorted into a pentagonal pattern by tropo-
spheric cyclones that are distributed almost uniformly along the continuous
.... :3.8
:~iiiiiiiiil
~iiiiiiiiii~
............
ij{iiiiiii~ii
>;. 1
!ii!ii!
2.4
90
.... 4
180
2.4
Adiabatic Processes
73
70
, .g
-
1.7 0
Figure 2.10 Distribution of nitric acid mixing ratio
rHNO3
(ppbv) on the 0 = 470 K potential
temperature surface over the Southern Hemisphere on January 3, 1979, as observed by Nimbus-7
LIMS. Adapted from Miles and Grose (1986).
storm track of the Southern Hemisphere (see Fig. 1.25a). In fact, the pattern
of rHNO3 suggests that air is being overturned horizontally by those synoptic
disturbances.
2.4.2 Thermodynamic Behavior Accompanying Vertical Motion
According to (2.30.2), the temperature of an air parcel moving vertically
changes due to expansion work, but in such proportion to its pressure as to
preserve its potential temperature. An expression for the rate that a parcel's
temperature T' changes with its altitude z' under adiabatic conditions follows
from (2.25.2) in combination with hydrostatic equilibrium (1.16). With the gas
law, the change with altitude of environmental pressure p is described by
dz
dlnp- H' (2.32)
where H is given by (1.17.2). Since the parcel's pressure p' adjusts automati-
cally to the environmental pressure to preserve mechanical equilibrium, (2.32)
also describes how the parcel's pressure changes with altitude. Then, for the
74
2 Thermodynamics of Gases
b
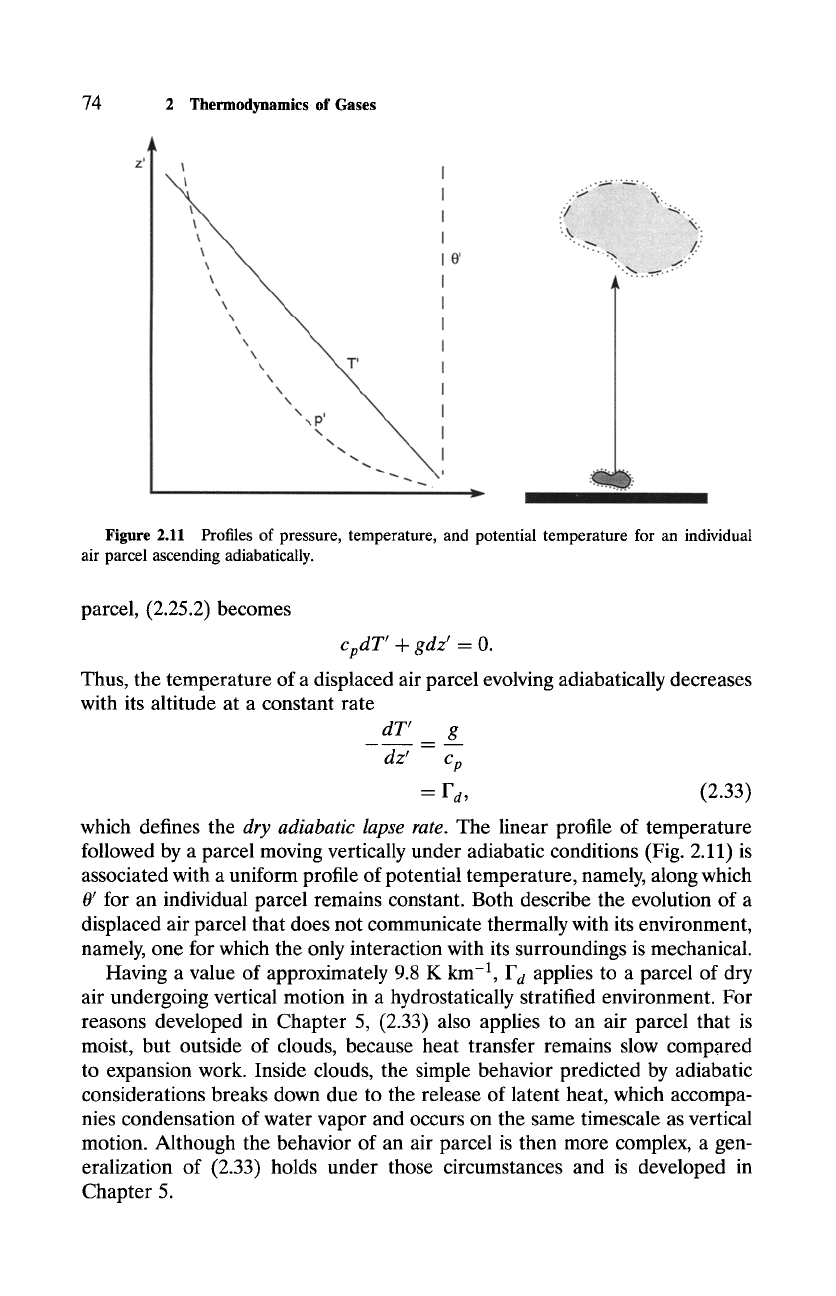
Figure 2.11 Profiles of pressure, temperature, and potential temperature for an individual
air parcel ascending adiabatically.
parcel, (2.25.2) becomes
cpdT' + gdz' = O.
Thus, the temperature of a displaced air parcel evolving adiabatically decreases
with its altitude at a constant rate
dT' g
dz ~ Cp
= F d, (2.33)
which defines the
dry adiabatic lapse rate. The linear profile of temperature
followed by a parcel moving vertically under adiabatic conditions (Fig. 2.11) is
associated with a uniform profile of potential temperature, namely, along which
0' for an individual parcel remains constant. Both describe the evolution of a
displaced air parcel that does not communicate thermally with its environment,
namely, one for which the only interaction with its surroundings is mechanical.
Having a value of approximately 9.8 K
km -1,
IF" d applies to a parcel of dry
air undergoing vertical motion in a hydrostatically stratified environment. For
reasons developed in Chapter 5, (2.33) also applies to an air parcel that is
moist, but outside of clouds, because heat transfer remains slow compared
to expansion work. Inside clouds, the simple behavior predicted by adiabatic
considerations breaks down due to the release of latent heat, which accompa-
nies condensation of water vapor and occurs on the same timescale as vertical
motion. Although the behavior of an air parcel is then more complex, a gen-
eralization of (2.33) holds under those circumstances and is developed in
Chapter 5.
2.5 Diabatic Processes
75
2.5 Diabatic Processes
Whereas for many purposes the behavior of an air parcel can be regarded
as adiabatic, that condition is violated in certain locations and over long
timescales. Near the surface, thermal conduction and turbulent mixing be-
come important on timescales of order one day. A similar conclusion holds
inside clouds, where the release of latent heat operates on the same timescale
as vertical motion. Over timescales longer than a week, radiative transfer be-
comes important.
Under diabatic conditions, the system interacts with its environment ther-
mally as well as mechanically. The potential temperature of an air parcel is
then no longer conserved. Instead, 0 changes in proportion to heat transferred
into the system, where 0 and other unprimed variables are hereafter under-
stood to refer to an individual air parcel. Taking the logarithm of (2.31) gives
a differential relation among the variables 0, p, and T
dln 0 - dln T -- -Kdln p.
(2.34)
Likewise, dividing the first law (2.22.2) by T and incorporating the gas law
leads to a similar relation
6q
d In T - Kd In p = ~. (2.35)
cpT
Combining (2.34) and (2.35) then yields
6q
d In 0
=
cpT"
(2.36)
Thus, the increase of potential temperature is a direct measure of the heat
transferred into the system. For this reason, an air parcel will drift across
potential temperature surfaces in proportion to the net heat exchanged with
its environment (Fig. 2.9). Equation (2.36) can be regarded as an alternate
and more compact expression of the first law, one in which expansion work
has been absorbed into the state variable 0. In this light, 0 for a compressible
fluid like air is analogous to T for an incompressible fluid like water. Each
increases in direct proportion to the heat absorbed by the system.
2.5.1 Polytropic Processes
Most of the energy exchanged between the earth's surface and the atmosphere
and between one atmospheric layer and another is accomplished through ra-
diative transfer (refer to Fig. 1.27). In fact, radiative transfer is the primary
diabatic influence outside of the boundary layer and clouds. It is sometimes
convenient to model radiative transfer as a polytropic process
3q = cdT, (2.37)
76
2
Thermodynamics of Gases
wherein the heat transferred into the system is proportional to the system's
change of temperature. The constant of proportionality c is the
polytropic spe-
cific heat capacity. Newtonian cooling,
in which heat transfer is proportional to
the departure from an equilibrium temperature, can be regarded as a poly-
tropic process. For deep temperature anomalies, as is characteristic of plan-
etary waves, Newtonian cooling is a useful approximation to IR cooling to
space.
For a polytropic process, the two expressions of the first law (2.22) reduce
to
(c v - c)dT + pdv
= 0,
(2.38.1)
(Cp - c)dT - vdp
= 0. (2.38.2)
Equations (2.38) resemble the first law for an adiabatic process, but with
modified specific heats. Consequently, previous formulas valid for an adiabatic
process hold for a polytropic process with the transformation
Cp --> (Cp - C),
(2.39)
Then (2.39) may be used in (2.31) to define a
polytropic potential temperature
0, which is conserved during a polytropic process (Problem 2.9). Analogous to
potential temperature, O is the temperature assumed by an air parcel if com-
pressed polytropically to 1000 mb, and it characterizes a family of polytropes
in state space.
In terms of potential temperature, the first law becomes
dln O = (~p)dln T.
(2.40)
If c is positive, the effective specific heats
(Cp- c)
and (c o -c) are reduced
from their adiabatic values. Therefore, a given change of pressure or volume
in (2.38) leads to a greater change of temperature. For an increase of temper-
ature, the additional warming implies heat absorption from the environment
and an increase of 0 by (2.40). This would be the situation for an air parcel
that is cooler than its surroundings. For a decrease of temperature, the ad-
ditional cooling implies heat rejection to the environment and a decrease of
0, as would occur for an air parcel that is warmer than its environment. The
reverse behavior follows if c is negative.
Suggested Reading
A thorough discussion of thermodynamic properties of gases is given in
Atmo-
spheric Thermodynamics
(1981) by Iribarne and Godson.
Statistical Thermodynamics
(1973) by Lee, Sears, and Turcotte provides a clear
treatment of specific heats and other gas properties from the perspective of
statistical mechanics.

Problems 77
Problems
2.1.
2.2.
2.3.
2.4.
2.5.
2.6.
2.7.
2.8.
2.9.
2.10.
2.11.
A plume of heated air leaves the cooling tower of a power plant at 1000
mb with a temperature of 30~ If the air may be treated as dry, to
what level will the plume ascend if the ambient temperature varies with
altitude as: (a)
T(z)
= 20- 8z(C) and (b)
T(z)
= 20 + z(C), with z in
kilometers.
Suppose that the air in Problem 2.1 contains moisture, but its effect is
negligible beneath a cumulus cloud, which forms overhead between 900
and 700 mb. (a) What is the potential temperature inside the plume at
950 mb? (b) How is the potential temperature inside the plume at 800 mb
related to that at 950 mb? (c) How is the buoyancy of air at 800 mb relat-
ed to that if the air were perfectly dry?
One mole of water is vaporized at 100~ and 1 atm of pressure (1.013 x
105 Pa), with an observed increase in volume of 3.02 x 10 -2 m 3. (a) How
much work is performed by the water? (b) How much work would be
performed if the water behaved as an ideal gas and accomplished the
same change of volume isobarically? (c) The enthalpy of vaporization
for water is 4.06 x 10
4
J mol -~. What heat input is necessary to accom-
plish the above change of state? Why does the heat input differ from the
work performed?
Demonstrate (a) that h =
h(T)
and (b) relation (2.19).
Demonstrate that 0 is conserved during an adiabatic process.
A large helium-filled balloon carries an instrumented payload from sea
level into the stratosphere. If the balloon ascends rapidly enough for
heat transfer across its surface to be negligible, what relative increase in
volume must be accommodated for the balloon to reach 10 mb?
(Note:
The balloon exerts negligible surface tension~it serves merely to contain
the helium.)
Derive (2.27) for an ideal gas.
Describe physical circumstances under which polytropic heat transfer
would be a useful approximation to actual heat transfer.
Use transformation (2.29) to define a
polytropic potential temperature O,
which is conserved during a polytropic process. How is 0 related to 0?
Through sloping convection, dry air initially at 20~ ascends from sea
level to 700 mb. Calculate (a) its initial and final specific volumes, (b) its
final temperature, (c) the specific work performed, and (d) changes in
its specific energy and enthalpy.
A commercial aircraft is en route to New York City from Miami, where
the surface pressure was 1013 mb and its altimeter was adjusted at
takeoff to indicate 0 ft above sea level. If the surface pressure at New

78
2
Thermodynamics of Gases
2.12.
2.13.
2.14.
2.15.
2.16.
York is 990 mb and the temperature is -3~ estimate the altimeter
reading when the aircraft lands if no subsequent adjustments are made.
Commercial aircraft normally cruise near 200 mb, where T = -60~
(a) Calculate the temperature of air if compressed adiabatically to the
cabin pressure of 800 mb. (b) How much specific heat must then be
added/removed to maintain a cabin temperature of 25~
A system comprised of pure water is maintained at 0~ and 6.1 mb,
at which the specific volumes of ice, water, and vapor are
vi
= 1.09 x
10 -3 m3kg -1,
v w
-- 1.00 x 10 -3 m 3 kg -1,
v v
= 206 m 3 kg -1. (a) How
much work is performed by the water substance if 1 kg of ice melts?
(b) How much work is performed by the water substance if 1 kg of
water evaporates? (c) Contrast the values in (a) and (b) in light of the
heat transfers required to accomplish the preceding transformations of
phase:
afusion-
0.334
• 10 6 J
and
Qvaporization ~-2.50 x 10 6 J.
Surface winds blow down a mountain range during a
Chinook,
which is
an Indian term for "snow eater." If the temperature at 14,000 ft along
the continental divide is -10~ and if the sky is cloud-free leeward
of the divide, what is the surface temperature at Denver, which lies at
approximately 5000 ft?
A scuba diver releases a bubble at 30~ at a depth of 32 ft, where the
absolute pressure is 2 atm. Presume that the bubble remains intact, is
large enough for heat transfer across its surface to be negligible, and
condensational heating in its interior can be ignored. Beginning from
hydrostatic equilibrium and the first law, (a) derive an expression for
the bubble's temperature as a function of its elevation z above its initial
elevation and (b) determine the bubble's temperature upon reaching
the surface under the foregoing conditions. (c) How would these results
be modified under fully realistic conditions?
(Note:
Normal respiration
processes only about 30% of the oxygen available, so the bubble can be
treated as air.)
An air parcel descends from 10 to 100 mb. (a) How much specific work
is performed on the parcel if its change of state is accomplished adiabat-
ically? (b) If its change of state is accomplished isothermally at 220 K?
(c) Under the conditions of (b), what heat transfer must occur to achieve
the change of state? (d) What is the parcel's change of potential temper-
ature under the same conditions?

Chapter 3
The Second Law and Its Implications
The first law of thermodynamics describes how the state of a system changes
in response to work it performs and heat absorbed by it. The second law of
thermodynamics deals with the direction of thermodynamic processes and the
efficiency with which they occur. Since these characteristics control how a
system evolves out of a given state, the second law also underlies the stability
of thermodynamic equilibrium.
3.1 Natural and Reversible Processes
A process for which the system can be restored to its initial state, without
leaving a net influence on the system or on its environment, is said to be
reversible.
A reversible process is actually an idealization: one that is devoid of
friction and for which changes of state occur slowly enough for the system to
remain in thermodynamic equilibrium. By contrast, a
naturalprocess
is one that
proceeds freely, for example, a spontaneous process which can be stimulated
by a small perturbation to the system. Because the system is then out of
thermodynamic equilibrium with its surroundings, a natural process cannot be
reversed entirely, that is, without leaving a net influence on either the system
or its environment. Thus, a natural process is inherently "irreversible,"
Irreversibility is introduced by any process that drives the system out of
thermodynamic equilibrium, as occurs during rapid changes of state. Perform-
ing work across a finite pressure difference, wherein the system is out of
mechanical equilibrium, and transferring heat across a finite temperature dif-
ference, wherein the system is out of thermal equilibrium, are both irreversible.
A process involving friction (e.g., turbulent mixing, which is accompanied by
frictional dissipation) is also irreversible, as are transformations of phase when
different phases are not at equilibrium with one another (e.g., in states away
from saturation).
An example of irreversible work follows from a gas that is acted on by a
piston and is maintained at a constant temperature through contact with a
heat reservoir (Fig. 3.1). Suppose the gas is expanded isothermally at temper-
ature T~2 from state 1 to state 2 and subsequently restored to state 1 through
79
