Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

The Bottom Line
928 Chapter 21 Nuclear Chemistry
■
Each element is composed of atoms containing
the same number of protons. These may contain
isotopes with differing numbers of neutrons.
(Section 21.1)
■
Some nuclear configurations are unstable. They
decay in a stepwise progression toward stable nuclei.
(Section 21.2)
■
There are three main types of radioactive decay:
alpha-particle emission, beta-particle emission, and
gamma-ray emission. (Section 21.2)
■
Ionizing radiation can interact with living tissue and
cause damage to the DNA of a cell. This damage may
be repaired and cause no harm or, in some cases,
may lead to cancer. (Section 21.3)
■
Radioactive decay occurs via first-order kinetics.
(Section 21.4)
■
Energy is released in radioactive decay processes as
a consequence of the mass defect in nuclei.
(Section 21.5)
■
Nuclei with a “magic” number of protons and/or
neutrons (2, 8, 20, 28, 50, or 82) are stable. Nuclei
with even numbers of protons and/or neutrons are
also more likely to be stable. (Section 21.6)
■
Nuclear fission is the splitting of heavier nuclei into
lighter ones. Nuclear fusion results when smaller nu-
clei combine into heavier nuclei. (Section 21.7)
■
Radioisotopes can be used in medicine for imaging
the body and for treating and eliminating cancerous
tissues. (Section 21.8)
alpha decay A type of radioactive decay wherein an
alpha particle is emitted from the nucleus of a
radioactive nuclide. Common for elements whose
nuclei are larger than bismuth, alpha decay is often
accompanied by the release of a gamma ray. (p. 905)
alpha particles (α particles) Particles emitted from the
nucleus of a radioactive element during the process
of alpha decay. They are helium nuclei (2 protons,
2 neutrons), with a +2 charge. (p. 905)
antimatter Particles that have the same mass as, but
charges opposite to, corresponding matter.
Antimatter particles such as the positron and anti-
neutrino are similar to the electron and neutrino,
respectively, but have opposite characteristics.
(p. 905)
antineutrino A subatomic particle produced in beta-
minus decay that has no charge, has essentially no
mass, and interacts only rarely with matter. (p. 904)
becquerel (Bq) An SI unit of activity equivalent to one
nuclear disintegration per second. (p. 911)
beta particles (β particles) Particles emitted from the
nucleus of a radioactive atom during the process of
beta decay. These particles are high-speed electrons.
(p. 904)
beta-particle emission A naturally occurring type of
radioactive decay wherein an electron is ejected at
high speed from the nucleus, typical of nuclei that
have too many neutrons to be energetically stable.
An antineutrino accompanies this emission, and
sometimes one or more gamma rays as well. Also
known as beta emission. (p. 904)
beta-plus emission A type of radioactive decay wherein a
positron is ejected at high speed from the nucleus,
typical of nuclides that have too few neutrons. A
neutrino accompanies this emission, and usually one
or more gamma rays as well. Also known as positron
emission. (p. 918)
binding energy The energy released when a nucleus is
formed from protons and neutrons. Binding energies
are expressed as a positive number. (p. 915)
chain reaction In nuclear chemistry, a reaction that is
self-sustaining as one event in turn causes more
events. (p. 923)
critical mass The amount of fissionable fuel needed to
sustain a chain reaction. (p. 923)
curie (Ci) A larger unit of activity than the becquerel,
equivalent to 3.7 ×10
10
Bq. (p. 911)
daughter nuclide An isotope that is the product of a
nuclear reaction. (p. 904)
decay series A series of nuclear reactions that a large
nuclide undergoes as it changes from an unstable and
radioactive nucleus to a stable nucleus. (p. 907)
electron capture (EC) A type of radioactive decay that
occurs when an inner-core electron is captured by a
proton from the nucleus to form a neutron. The
process is usually accompanied by the emission of
X-rays. (p. 907)
fission (or nuclear fission) A type of nuclear reaction
wherein a large nucleus splits into two or three
smaller nuclei with the release of energy. (p. 917)
fusion (or nuclear fusion) A type of nuclear reaction
wherein small nuclei are joined to form a larger
Key Words
nucleus with the release of energy. Nuclear fusion
powers the stars. (p. 916)
gamma rays ( rays) A high-energy form of electro-
magnetic radiation that is emitted from the nucleus.
Gamma rays sometimes accompany alpha and beta
decays. (p. 904)
gray (Gy) A measure of absorbed radiation equal to
100 rad. (p. 911)
ionizing radiation Radiation such as alpha and beta
particles, gamma rays, or X-rays that is capable of
removing an electron from an atom or a bond when
it interacts with matter. (p. 910)
mass defect (Section 21.5) The loss in mass that occurs
when a nucleus is formed from its protons and
neutrons. (p. 915)
metastable state An energetically unstable arrangement
of protons and neutrons in a nucleus after a neutron
has become a proton. (p. 906)
neutrino A subatomic particle that is produced in beta-
plus decay and that has no charge, has essentially no
mass, and interacts only rarely with matter. (p. 905)
nuclear equation An equation showing a nuclear trans-
formation, where the atomic and mass numbers are
provided. (p. 904)
nuclear radiation The particles and/or energy emitted
during radioactive decay. (p. 901)
nucleon The name given to a particle (proton or
neutron) that is part of a nucleus. For example,
13
C
contains 13 nucleons, 6 protons, and 7 neutrons.
(p. 918)
positron The antimatter equivalent of an electron.
Positrons have a positive charge and the same mass as
an electron. (p. 907)
positron emission See beta-plus emission.(p. 907)
positron emission tomography A medical imaging tech-
nique that images metabolic processes within the
body. Also known as a PET scan. (p. 926)
rad A unit of energy absorbed by irradiated material
equal to 0.01 J/kg of exposed material. (p. 911)
radioactive decay The process by which an unstable
nucleus becomes more stable via the emission or
absorption of particles and energy. (p. 904)
radioactivity The emission of radioactive particles
and/or energy. (p. 901)
radiopharmaceuticals Compounds containing radioac-
tive nuclides that are used for imaging studies in
nuclear medicine. (p. 924)
rem A unit that measures the “equivalent dose” of
radiation; that is, it takes into account the interaction
of radiation with human tissue. The word stands for
“roentgen equivalent in man.” The rem is not an SI
unit but is related to the SI unit, the sievert. (p. 911)
roentgen A unit used to measure exposure to radiation.
One roentgen is equal to 2.58 ×10
−4
C/kg of dry air
at STP. (p. 911)
sievert (Sv) A unit that measures the “equivalent dose”
of radiation; that is, it takes into account the inter-
action of radiation with human tissue. One sievert
equals 100 rem. (p. 911)
subcritical mass A mass of a radioactive isotope that is
too small to sustain a chain reaction. (p. 923)
supercritical mass A mass of a radioactive isotope that
not only supports a chain reaction but causes the
majority of the nuclei to undergo unfettered radio-
active decay within a very short period of time,
releasing huge amounts of energy. (p. 923)
Focus Your Learning 929
The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
Section 21.1 Isotopes and More Isotopes
Skill Review
1. What is the atom with smallest atomic number? The smallest
mass number?
2. Can different elements both have the same number of pro-
tons? The same number of neutrons? Explain.
3. Can an atom have no neutrons? Explain.
4. Can an atom have no protons? No electrons? Explain.
5. Can a helium nuclide have a smaller mass number than a hy-
drogen nuclide? Explain.
6. Can a carbon nuclide have a smaller mass number than a ni-
trogen nuclide? Explain.
7. Identify the number of protons, neutrons, and electrons in
each of the following isotopes.
a.
12
Nb.
124
Sb c.
152
Eu d.
9
Be
8. Identify the number of protons, neutrons, and electrons in
each of the following isotopes.
a.
7
Li b.
122
Cs c.
17
Od.
18
F
Chemical Applications and Practices
9. A person who weighs 60 kg (132 lb) has about 120 g of potas-
sium in his or her body. How many grams of K-40 does this
include? K-40 has a natural abundance of 0.0118%.
10. No isotopes of potassium are chemically stable in the pres-
ence of water and/or oxygen.Potassium-39 and potassium-41
are stable isotopes. Explain these different meanings of the
word stable.
11. Fallout from a nuclear weapon includes the radioactive
nuclide Sr-90.
a. In the biosphere, which chemical form for Sr-90 is more
likely, Sr
2+
or Sr?
b. Once Sr-90 lands downwind, it is extremely difficult to
separate from the plants and soils. Suggest reasons why.
12. Strontium-90 from fallout gets into the food chain and even-
tually can end up in cows’ milk. Can you remove the radioac-
tivity from cows’ milk by boiling it? Why or why not?
Focus Your Learning

Section 21.2 Types of Radioactive Decay
Skill Review
13. Both gamma rays and infrared radiation are forms of electro-
magnetic radiation. How do they differ?
14. Beta decay involves the emission of a high-speed electron
from an atom, yet the overall charge on the atom does not
become less negative. Explain why.
15. Write nuclear equations for the following processes:
a. An alpha particle (along with a gamma ray) is emitted
from plutonium-239.
b. Carbon-14 undergoes beta decay.
c. Cesium-137 emits a beta particle with an accompanying
gamma ray.
16. Write nuclear equations for the following processes:
a. Plutonium-238 emits an alpha particle with an accompa-
nying gamma ray.
b. Radon-222 is produced from the decay of a radium
isotope with the emission of a gamma ray.
c. Radium-225 emits a gamma ray followed by an alpha
particle.
17. Write nuclear equations for the following processes:
a. Polonium-215 (with a gamma ray) is produced by an
alpha emission.
b. Strontium-90 decays by beta emission. Little or no gamma
radiation is released.
c. Tc-99 decays by beta-minus emission.
18. Write nuclear equations for the following processes:
a. Nitrogen-14 is formed from a radioisotope of carbon.
b. Cadmium-110 is formed from a radioisotope of silver.
c. Technetium-99 is formed from Technetium-99m.
Chemical Applications and Practices
19. Samarium-146 is the lightest element found naturally on our
planet to undergo alpha emission. Write the equation for this
alpha decay. There is no accompanying gamma ray.
20. Iodine-131, used in medical imaging, undergoes beta decay.
Write the nuclear equation for this reaction.
21. Darlene Hoffman, an award-winning nuclear chemist, pos-
tulated the existence of Pu-244 before it was discovered. Into
which of the four natural decay series does it fit?
22. A chemistry source states that radon-219 is produced in the
actinium-227 radioactive decay series. Into which of the four
decay series mentioned in Section 21.2 does actinium-227 fit?
Section 21.3 Interaction of Radiation with Matter
Skill Review
23. Classify the following as ionizing or nonionizing radiation:
cosmic rays, infrared radiation, gamma rays, visible light,
microwaves, X-rays.
24. You can cook food using microwaves, and you can sterilize
food using gamma rays. Why do these two types of radiation
produce such different results?
25. Suppose that you had administered a gamma emitter to a pa-
tient in order to diagnose how well his or her heart was func-
tioning. Name three things you could do to minimize your
exposure to the radiation.
930 Chapter 21 Nuclear Chemistry
26. Which cells in your body are most susceptible to radiation?
Why?
27. Explain the similarities and differences between:
a. a curie and a becquerel
b. a rad and a rem
28. Explain the similarities and differences between:
a. a rem and a sievert
b. a curie and a rem
29. Smoke detectors use only a small quantity of americium, less
than 35 kBq. How many disintegrations per second is this?
30. Using the information in Problem 29, show that the result is
comparable to 1 microcurie.
Chemical Applications and Practices
31. In the mid-1990s, a watch was advertised that glowed in the
dark. The source of the glow was the radioisotope tritium in-
teracting with a luminous paint. The annual dose for a per-
son wearing the watch as estimated at 4.0 microsieverts.
a. How many rem is this?
b. Do you think this amount of radiation warrants concern?
Note the radiation symbol
between the hour and
minute hands and the H3
(
3
H, or tritium) on the face
of this watch.
32. In the previous problem, we noted that tritium, a radioiso-
tope of hydrogen, was used in some watches.
a. What mode of decay would you predict for tritium?
b. Given that radiation escapes from the watch case, does this
evidence support your prediction?
33. Three metals—aluminum, iron, and cadmium—are pro-
posed as materials that could be used to shield nuclear
radiation.
a. What property of these materials would you need to look
up to determine which one would have to be used in the
greatest thickness?
b. What else might you need to know about a substance
before you use it in shielding?
34. If radon-222 gas decays in your lungs to produce solid polo-
nium, which is then trapped there, how many decays does the
polonium progress through before reaching the stable iso-
tope of lead-206? How many alpha and beta particles are
emitted in the process?
Section 21.4 The Kinetics of Radioactive Decay
Skill Review
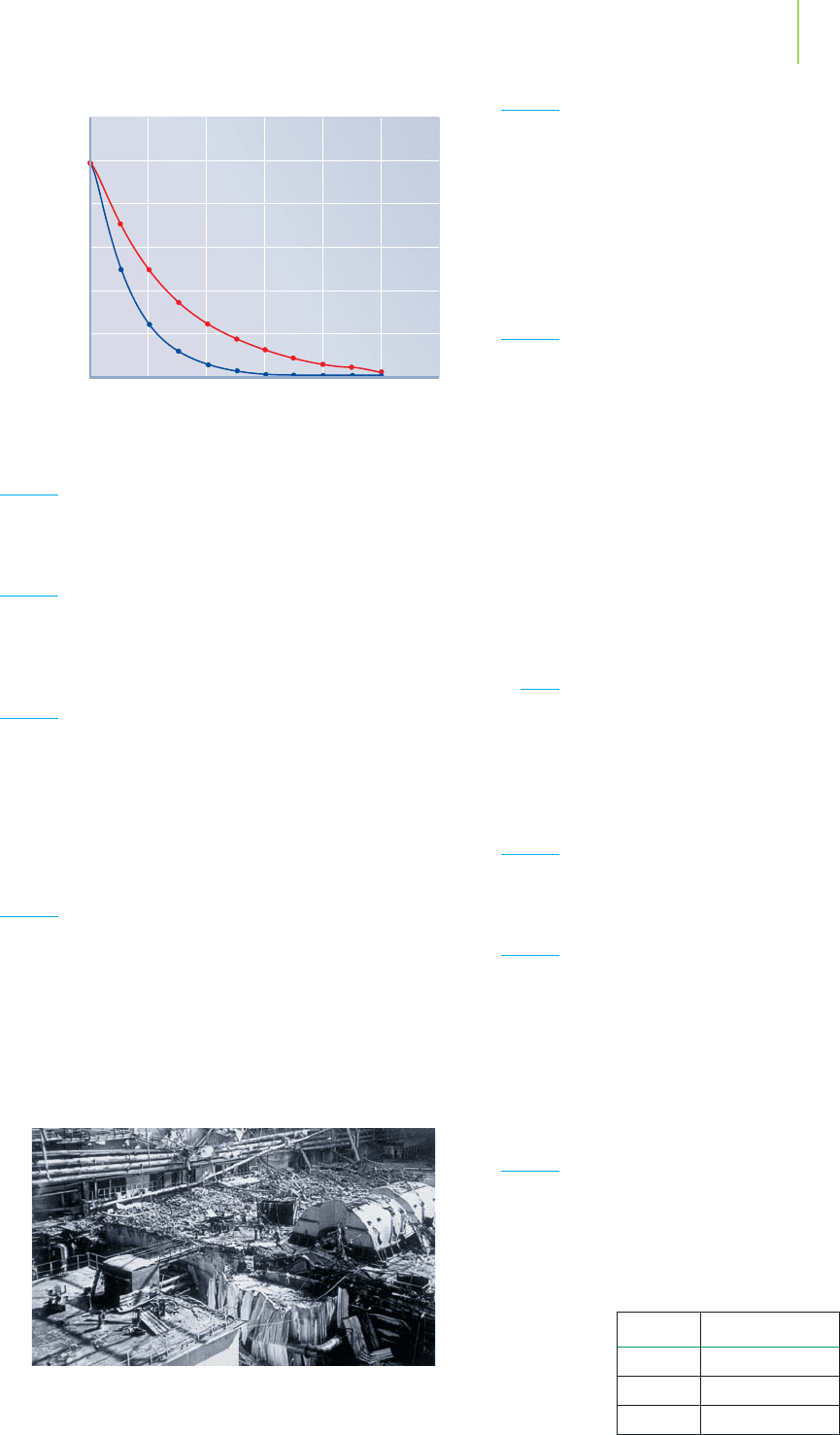
35. Here are the decay plots for two different hypothetical radio-
active nuclei. The plot denoted by the red line is A; that de-
noted by the blue line is B. From these graphs, determine:
a. Which nuclide has the longer half-life?
b. What is the half-life of the nuclide indicated by the red
line?
c. Which one has the higher activity?
d. Which one would be more dangerous if swallowed?
45. A 0.1-microcurie sample of polonium-210 is used to demon-
strate alpha decay in the lecture hall, because there is little ac-
companying gamma radiation. The half-life of this isotope is
138 days. About how often does an instructor need to buy a
new source? State any assumptions you made in arriving at
your answer.
46. The world uranium reserves are currently estimated at
3.4 million tonnes, where a tonne is a metric ton, or 1000 kg.
Current knowledge places Earth at 4.5 billion years old. How
much uranium was present at the time our world formed?
47. The half-life of plutonium-239 is about 24,000 years. After
approximately how much time will over 99% of a sample of
plutonium-239 have decayed? How can this element exist on
our planet if its half-life is so short?
48. Plutonium oxide, used to power the Cassini space-probe to
Saturn, was stored in corrosion-resistant materials designed
to contain the fuel for 10 half-lives, or 870 years.
a. What is the half-life of Pu-238?
b. Why do you think the figure 10 half-lives was selected?
c. The Cassini batteries contained 72 lb (33 kg) of Pu-238 in
the form of plutonium dioxide. How much Pu-238 (in
kilograms and in grams) will remain after 870 years?
Section 21.5 Mass and Binding Energy
Skill Review
49. a. Why don’t the masses of the neutrons and protons that
make up an oxygen-16 atom add up to the mass of the
oxygen nucleus?
b. Is this true for all isotopes of oxygen?
50. a. Is an atom of carbon likely ever to fall apart into its com-
ponent protons, electrons, and neutrons?
b. Explain why or why not.
51. Explain how the mass defect and the binding energy are
related.
52. Is mass conserved in nuclear reactions, such as alpha decay,
that proceed spontaneously? Why or why not?
53. Calculate the mass defect and the resulting energy for the
following nuclear reaction:
(
1
0
n = 1.008665 g/mol;
4
He = 4.002603 g/mol)
170
Ir (169.974970 g/mol) decays to
166
Re (165.965740 g/mol)
54. Calculate the mass defect and the resulting energy for the fol-
lowing nuclear reaction:
40
19
K (39.963999 g/mol) n
40
20
Ca (39.962591 g/mol) +
0
−
1
Chemical Applications and Practices
55. The masses of the neutral atoms involved for an alpha emis-
sion from U-238 are shown below. These values include the
masses of the electrons.
a. Would you expect the U-238 or its decay products to have
more mass?
b. What is the source/result of any difference in these masses?
4
2
He 4.002603 u
238
92
U 238.050784 u
234
90
Th 234.040945 u
e
–
0.005485799 u
Focus Your Learning 931
0
20,000
40,000
60,000
80,000
100,000
120,000
0 102030405060
Time
(
minutes
)
Number of counts
36. Using the plot in Problem 35, estimate the half-life of each
nucleus. What does a shorter half-life imply?
37. If 75% of a radioactive sample is gone after 30 days, what is
the half-life of the nuclide?
38. If 50% of a radioactive sample remains after 30 days, what is
the half-life of the nuclide?
39. Tritium has a half-life of 12.3 years. What fraction of a sam-
ple of tritium will remain after 12.3 years? After 24.6 years?
40. What percent of the original sample of strontium-90 (half-
life = 28.9 years) will remain
a. after 14 years? b. after 49.6 years? c. after 1000 years?
41. The half-life of strontium-90 is 28.9 years. How long would it
take for the activity of a Sr-90 sample to diminish by 87.5%?
42. The half-life of strontium-85 (about 64 days) is considerably
shorter than that of strontium-90. How long would it take for
the activity of a sample of Sr-85 to diminish by 93.75%? Why
do you think that Sr-85 is used diagnostically for bone scans,
but Sr-90 is not?
Chemical Applications and Practices
43. A 10-mCi sample of a tracer isotope is used to diagnose
blood flow from the heart. If it is desirable that nearly all of
the radioactivity (99%) be gone after 3 days, approximately
what half-life should the isotope have?
44. It is estimated that the nuclear accident at Chernobyl released
1.85 × 10
18
becquerels, a large amount of radiation. How
many curies is this? Why is it not easy to translate either of
these values into the number of radioactive atoms present?
Chernobyl nuclear power plant.
56. Use the data in Problem 55 to calculate the mass defect for
the process. What is the source/result of any difference in
these masses?
57. Describe the similarities and differences between beta-minus
and beta-plus emission.
58. The stable isotopes of carbon are
12
C and
13
C. What decay
mode would you predict for
14
C?
Section 21.6 Nuclear Stability
and Human-made Radioactive Nuclides
Skill Review
59. a. What does “doubly magic” mean, in reference to nuclides?
b. Give two examples of nuclei that are doubly magic and
two examples of nuclides that have no magic numbers at
all. How would you expect these nuclei to differ?
60. Suggest a reason why the heavier elements have proportion-
ately more neutrons than the lighter ones.
Chemical Applications and Practices
61. Element 114 was recently discovered. Why was this element
sought, but not the neighboring elements 115 and 113?
62. Some claims to the discovery of element 118 have been made.
Give reasons why this element may be considered to have
both nuclear and chemical stability.
Section 21.7 Splitting the Atom: Nuclear Fission
Skill Review
63. Why is alpha emission a better prediction for the mode of ra-
dioactive decay for uranium or plutonium than it is for iron,
carbon, or hydrogen?
64. Answer the following questions for the fission of plutonium:
239
94
Pu +
1
0
n n [
240
94
Pu] n
136
51
Sb +
100
43
Tc + 4
1
0
n + energy
a. What is the significance of the fact that neutrons are
produced?
b. What is the source of the energy in this equation?
65. Iron and cobalt are not expected to undergo nuclear fission.
Why?
66. Would you expect helium to undergo nuclear fission? Will
hydrogen-1 undergo fission?
67. Write nuclear reactions for the fission of
235
U to form:
a.
94
Kr and
139
Ba and neutrons
b.
80
Sr and
153
Xe and neutrons
68. An isotope of the element technetium can be produced by
bombarding molybdenum-97 with deuterium nuclei. Two
neutrons are also formed. Write the nuclear equation.
Chemical Applications and Practices
69. On our planet, both U-235 and U-238 occur naturally.
a. How do these isotopes differ?
b. What is the natural abundance of each?
c. Propose a reason why it is very difficult to separate these
two isotopes.
932 Chapter 21 Nuclear Chemistry
70. Using “conventional” explosives such as TNT, you can make
tiny explosive devices as well as huge ones. Is it possible to
make a similarly tiny nuclear bomb? Why or why not?
71. When fission of U-235 or Pu-239 occurs, elements such as
americium, californium, and berkelium are not found in the
fallout. Explain why.
72. One particularly nasty component of nuclear fallout is stron-
tium-90. Explore the reactivity of this particular isotope and
explain why it may be harmful to living creatures.
Section 21.8 Medical Uses of Radioisotopes
Skill Review
73. What questions should you ask about a radionuclide to be in-
jected for diagnostic purposes?
74. Why aren’t alpha emitters useful for diagnostic scans, such as
a scan of the heart or of the thyroid gland?
75. Technetium-99m samples should not be stored overnight
but, rather, should be freshly prepared each day for diagnos-
tic scans. Why?
76. Why is molybdenum-99 not used directly as a component of
a radiopharmaceutical?
Chemical Applications and Practices
77. A patient was injected with 10 mg of fluorine-18–labeled glu-
cose for a PET scan. Fluorine-18 has a half-life of 110 min-
utes and disintegrates by positron emission. Write the nu-
clear equation for the decay.
78. Using the information in Problem 77, determine the amount
of time needed to reduce the radioactivity of fluorine-18 to
1/16 of its original activity.
79. In the chapter, it was mentioned that it takes about two and a
half weeks for Tc-99 to be eliminated from the body. The
Department of Energy reports that it takes approximately
60 hours for the body to eliminate half of the technetium. Are
these two figures consistent with each other?
80. Gallium-67 citrate is used as a radiopharmaceutical for diag-
nosing tumors and infections.
a. What type of radioactive decay would you predict for this
nuclide?
b. A typical activity of a radionuclide used in the treatment
of an adult lymphoma is on the order of 10 mCi. After how
many days would radiation levels drop to less than a
millicurie?
Comprehensive Problems
81. Use the Internet to research the connection between smoking
and exposure to the nuclides polonium-210 and lead-210.
82. For the same dose of radiation, which has a higher dose
equivalent, strontium-90 or radon-222?
83. How do you know whether or not a gamma ray accompanies
an alpha or a beta emission?
84. Write nuclear equations for the following processes:
a. A positron is emitted by oxygen-15.
b. Boron-11 is formed by positron emission.
c. A positron is emitted by chlorine-35.
d. Oxygen-18 is formed by positron emission.

85. One of the radioactive decay series is shown below. Identify
the mode of radioactive decay at each step.
232
90
Th n
228
88
Ra n
228
89
Ac n
228
90
Th n
224
88
Ra n
220
86
Rn n
216
84
Po n
212
82
Pb n
212
83
Bi n
212
84
Po n
208
82
Pb
86. In the radioactive decay series in the previous problem, lead-
212 was formed. Why didn’t the decay series stop at lead?
87. In the radioactive decay series given in Problem 85, which el-
ements are represented by the symbols Rn and Ra? Which
one is a gas? Which one is a metal? Which one is chemically
inert?
88. Radon is produced in three of the naturally occurring decay
series. Which three? Which isotopes of radon are formed?
How would you expect these isotopes to differ? How would
you expect them to be the same?
89. Of the three radon isotopes mentioned in Problem 88, only
radon-222 is a health hazard. Propose a reason why, and then
research your answer to see whether you are correct.
90. Many tropical islands are volcanic in origin and contain ura-
nium in the rocks and minerals beneath the soils. However,
radon is less likely to be a problem in homes built in the trop-
ics. Propose two reasons why (and more if you can).
91. How does the nucleus of a carbon atom compare in density
with that of elemental lead (d = 11.3 g/cm
3
)? To answer this
question, calculate the volume of a
12
C nucleus, assuming
that the nucleus is spherical and that the radius is 1.2 ×
10
−13
cm. The mass of the nucleus in
12
C is 11.96709 u, or
1.98718 × 10
−23
g.
92. The transformation of elements into other elements also
takes place in stars. Write the nuclear equation for the forma-
tion of oxygen-16 when carbon-12 is hit with an alpha parti-
cle. A gamma ray is also released in this reaction.
93. Does food irradiation make the food radioactive? Find an an-
swer to this question using the resources of the World Wide
Web. Cite your sources.
94. Look up on the Internet the current maximum allowed ex-
posure of workers in the nuclear industry. The Department
of Energy (DOE) sets this standard. How does this standard
vary for some individuals?
95. Irene Curie and her mother Marie are not the only scientists
to have won the Nobel Prize for their pioneering work in
nuclear chemistry and physics. Others include Ernest
Rutherford, Ernest O. Lawrence, and Emilio G. Segrè. Use the
Internet to find out why these and/or other prizes for nuclear
work were awarded.
96. We pointed out in the text that palladium-103 (half-life =
16.97 days) is used in the treatment of prostate cancer, re-
placing iodine-125 (half-life = 59.4 days), though both are
widely used. Recently, Cs-131 (half-life = 9.7 days) has been
used. Why are these different products being used? That is,
what are the advantages and disadvantages of each?
97. Palladium-103 has a half-life of 16.97 days.
a. What is the half-life in years?
b. How many protons, neutrons, and electrons are in an atom
of palladium-103 in PdCl
2
?
c. A researcher develops cisplatin (Pd(NH
3
)
2
Cl
2
) using the
palladium-103 isotope. Assume that the process involves
the direct conversion of palladium(II) chloride to cisplatin
in one step, but the process itself requires 24 hours to
complete. How many grams of radioactive cisplatin would
remain if the researcher started with 100.0 g of pure
palladium-130(II) chloride?
Thinking Beyond the Calculation
98. Americium oxide is typically used in household smoke detec-
tors. A document reports that a gram of americium oxide
provides enough active material for “more than 5000 house-
hold smoke detectors.” The particular isotope used in this ap-
plication is americium-241.
a. How much americium is present in a typical smoke
detector?
b. Give two reasons why Am-240 and Am-242 would not be
appropriate isotopes to use.
c. Americium-241 decays by alpha emission. Write the
nuclear reaction for this process.
d. The half-life of americium-241 is 432.2 years. How long
will it take the reactivity of a sample of this nuclide to drop
to 1% of its original activity?
e. Beta-particle emission by americium-242 is found in 83%
of the sample. The rest of an americium-242 sample
decays by electron capture. Write nuclear reactions for
these processes.
Focus Your Learning
933
Smoke detectors contain about 150 mil-
lionths of a gram of americium-241,
which is extracted from spent fuel rods.
The
Chemistry
of Life
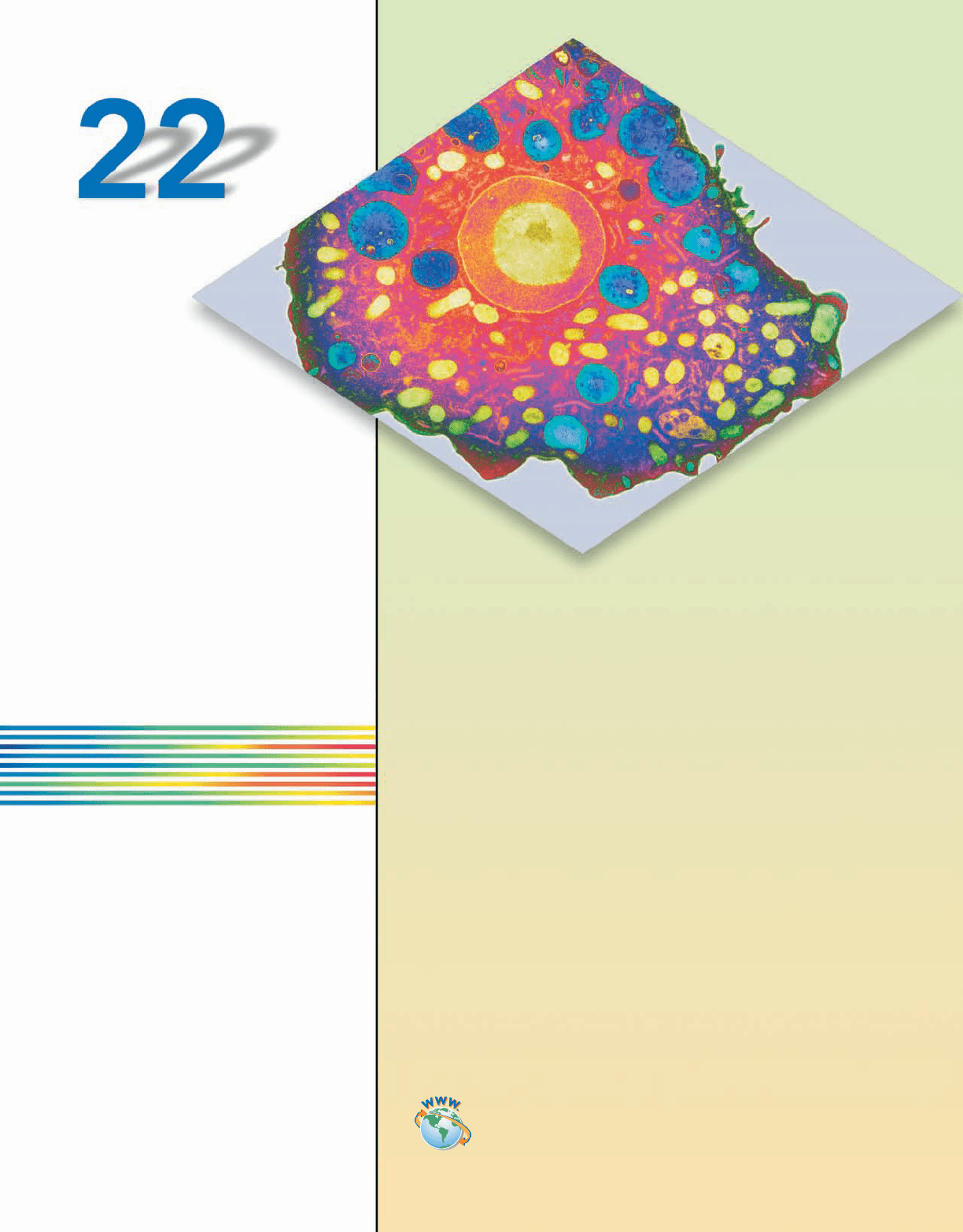
The cell is an intricate ballet of huge poly-
mers, proteins, hormones, and lipids.
Their dance keeps the cell alive. Shown
here is a colored transmission electron mi-
crograph of the protozoa responsible for
causing meningoencephalitis (inflamma-
tion of the brain and its membranes). The
nucleus (red) contains a large body known
as a nucleolus (yellow), where RNA is
made.
934
Contents and Selected Applications
22.1 DNA—The Basic Structure
Chemical Encounters: The Human Genome Project
22.2 Proteins
22.3 How Genes Code for Proteins
22.4 Enzymes
22.5 The Diversity of Protein Functions
22.6 Carbohydrates
22.7 Lipids
22.8 The Maelstrom of Metabolism
22.9 Biochemistry and Chirality
22.10 A Look to the Future
Go to college.hmco.com/pic/kelterMEE for online learning resources.
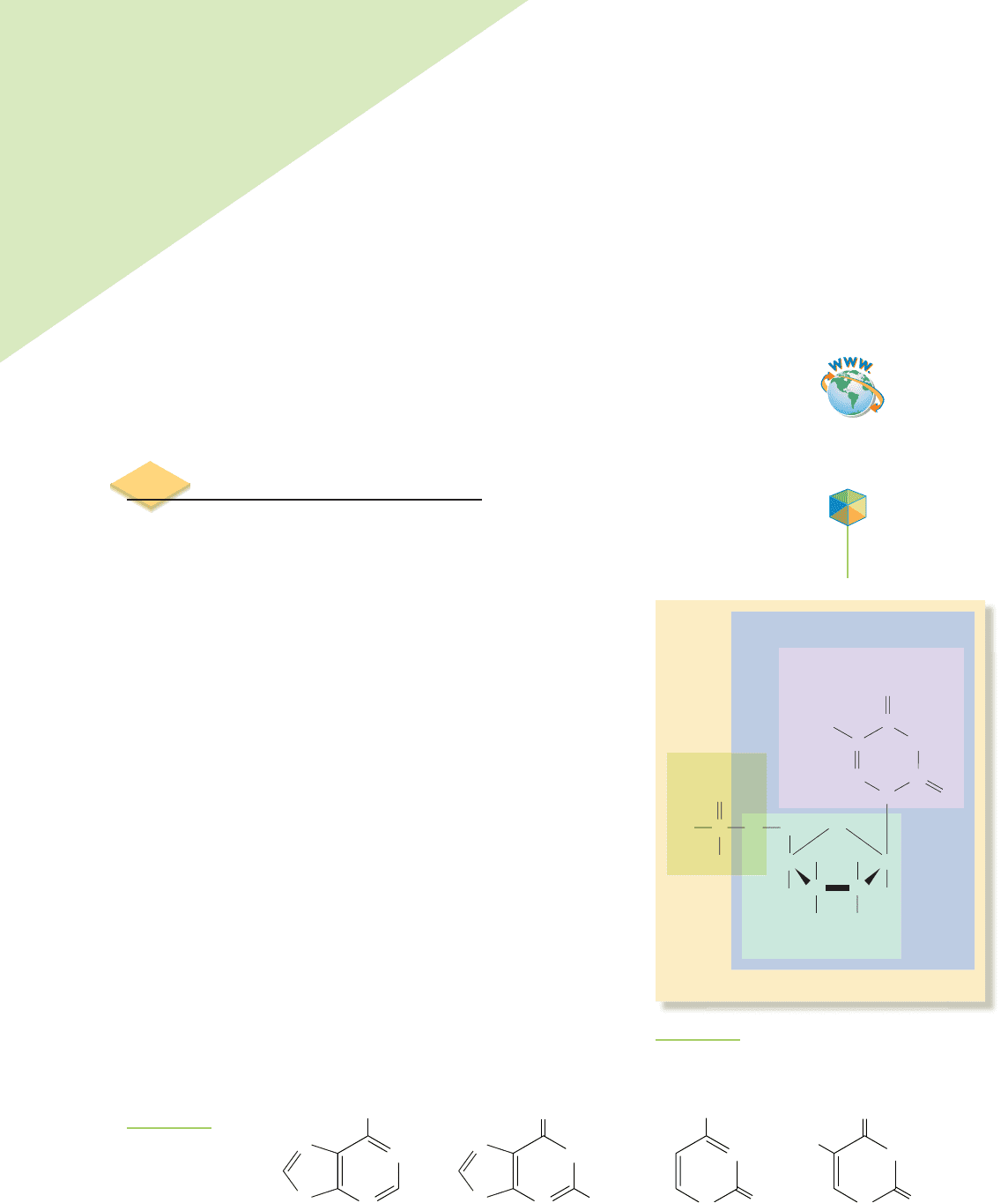
Guanine
O
N
NH
NH
2
N
N
H
Adenine
NH
2
N
N
N
N
H
NH
2
N
H
N
O
Cytosine
O
N
H
NH
O
Thymine
FIGURE 22.2
The four nitrogenous
organic bases found
in DNA.
Life is an adventure of changes. From the mo-
ment of conception, through birth, growth, adoles-
cence, maturity, aging, and death, we are sustained by an
endless process of change. The chemistry of life is the chem-
istry of these changes, and as you might expect, it is exceptionally
complex. The human body is one of the most intricate chemical sys-
tems we know, and yet there is a wonderful simplicity at the root of it all.
Our introduction to chemistry has given us the basic tools with which to
examine the chemistry of life in some detail. At the root of this detail is a chemical
we call DNA. This substance serves as the set of instructions that operate the chemi-
cal processes in living things. To understand the chemical reactions that take place in
the body, we must understand the nature of DNA because the chemistry of life is
based on DNA and the chemical species it produces.
935
22.1 DNA—The Basic Structure
In the summer of the year 2000, scientists participating in the International
Human Genome Project announced that they had reached the first major mile-
stone in working out the structure of all the genetic material that is needed to
make a human. They had deciphered a rough “first draft” of a com-
plete set of human genes. Work will continue for many years to
complete the draft and then to look at the different versions of genes
that underlie many of the differences among us. The information
gained about the structure of our genes will be used to develop new
medicines and biotechnologies and to understand much more
about how life works. What exactly are genes?
Why are they the focus
of such intense scrutiny?
To best uncover the meaning and significance of the gene, we
will begin with DNA.
Deoxyribonucleic acid (DNA) is the name for a
series of polymers composed of chemicals called
nucleotides.In
DNA, each nucleotide is itself composed of a phosphate group
bonded to a
deoxyribose sugar group, bonded to one of four ni-
trogenous organic bases
(schematically shown in Figure 22.1). The
four nitrogenous organic bases, shown in Figure 22.2, are adenine,
guanine, thymine, and cytosine.
DNA is a polymer of the four nucleotides bonded together in the
manner shown in Figure 22.3. The phosphate groups on the nu-
cleotides participate in condensation reactions to form phosphodi-
ester bonds that bind the individual nucleotides into the DNA chain.
Recall that condensation reactions result in the coupling of two
molecules with the elimination of a molecule of water (Chapter 12).
C
N O
NH
Phosphate
Deoxyribose sugar
Nitrogenous organic base
Deoxynucleoside
Deoxynucleotide
O
P
O
–
O
O
O
H
H
CC
H
H
OH
O
–
H
CC
CH
3
CH
2
C
HC C
FIGURE 22.1
A nucleotide contains a nitrogenous organic base, a
deoxyribose sugar, and a phosphate.
Application
C
HEMICAL ENCOUNTERS:
The Human Genome
Project
Video Lesson:
Nucleic Acids
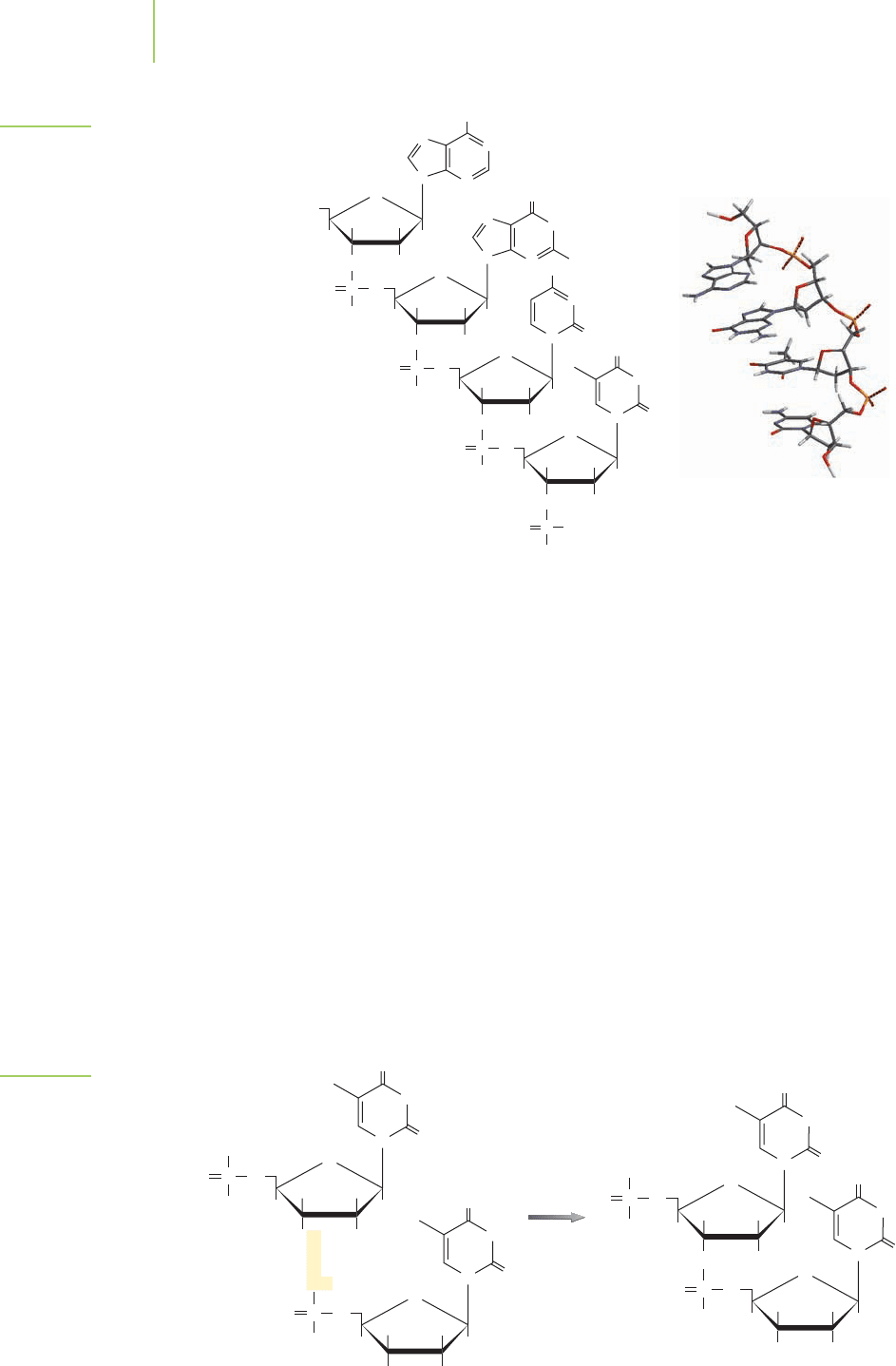
936 Chapter 22 The Chemistry of Life
FIGURE 22.3
The structure of DNA. Note how a strand
of DNA naturally twists when the nitro-
genous bases are on the same side of
the molecule.
NH
2
NH
2
N
N
N
N
O
H
H
O
PO
O
–
H H
H
HO
O
N
N
NH
N
O
H
H
O
O
H H
H
O
PO
O
–
O
NH
2
O
N
N
O
H
H
O
N
NH
O
PO
O
–
H H
H
O
H
H
O
H H
H
O
PO
O
–
O
–
FIGURE 22.4
The condensation of two
nucleotides and the elim-
ination of water form the
backbone of the DNA
molecule.
O
O
–
PO
O
–
O
O
H
H
O
N
NH
O
O
N
NH
O
PO
O
–
H H
H
O
H
H
OH
H H
H
O
O
O
–
PO
O
–
O
O
H
H
O
N
NH
O
O
N
NH
OH
OH
PO
O
–
H H
H
O
H
H
OH
H H
H
O
+ H
2
O
In DNA, the phosphate’s —OH group is lost as the sugar’s oxygen adds to the
phosphorus atom (Figure 22.4).
The resulting condensation product is known as single-stranded DNA. How-
ever, the DNA that makes up genes is more complex in a very beautiful and sig-
nificant way. This occurs when a second strand of DNA (upside down in relation
to the first strand) interacts with the first strand.
Why does DNA occur as pairs of
strands?
Double-stranded DNA is much more stable than a single strand, because
the nitrogenous bases are able to participate in hydrogen bonds with each other.
The hydrogen bonding results in a
base pair. As a consequence of their relative
size, the amount of space available between the two DNA strands, and the types
of hydrogen bonds in the bases, adenine always base-pairs with thymine, and cy-
tosine always base-pairs with guanine (Figure 22.5). We say that these pairs of
bases are complementary; that is, they pair in a reciprocal fashion with one
another.
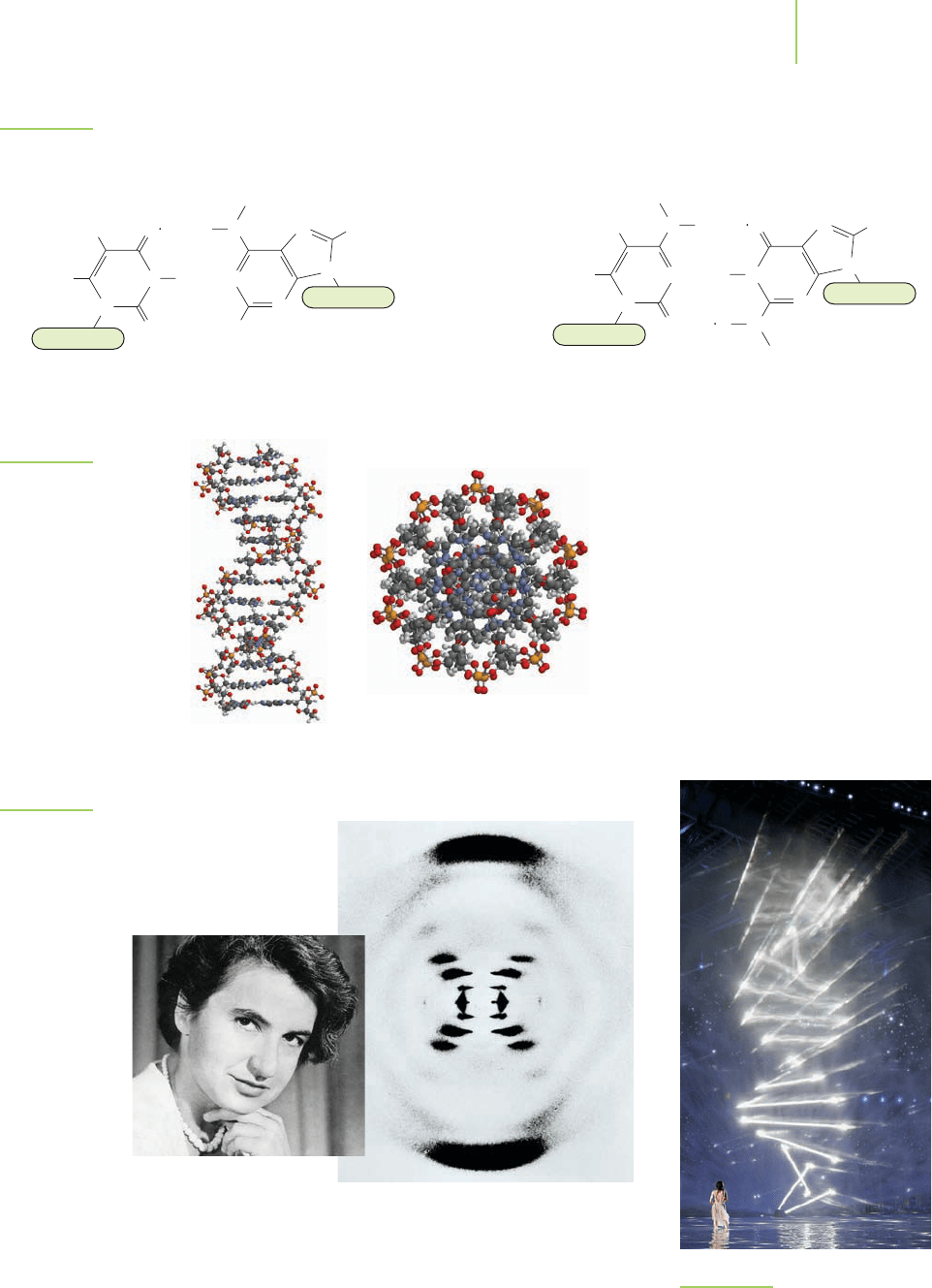
To improve the hydrogen bonding, the two strands of DNA wind around each
other to form a base-paired double helix, as shown in Figure 22.6. This structure
had eluded scientists for quite some time until James Watson and Francis Crick
22.1 DNA—The Basic Structure 937
FIGURE 22.5
Base pairs in DNA, looking down the axis of the DNA molecule. Note the proximity of the oxygen
atom to the amine group on the opposite base, and that of the nitrogen to the opposite amine.
N
N
H
N
H
H
N
NH
HO
N
N
CH
3
Adenine
Thymine
O
N
N
N
H
H
N
N
Guanine
Cytosine
O
H
H
H
H
N
H
O
H
Deoxyribose
Deoxyribose
Deoxyribose
Deoxyribose
N
N
H
TA
T–A
CG
C–G
obtained the 1953 X-ray diffraction photograph shown in Figure 22.7. The image
of this diffraction pattern was made by the chemist Rosalind Franklin and given
to Watson and Crick without her knowledge. It turned out to be the key piece of
data used to determine the structure of the double helix. In fact, the structure of
the DNA double helix has become an icon of science. It was even the centerpiece
of the 2004 Olympic Summer Games (Figure 22.8). Why is this structure so
important? Within the double helix, we find the secret of how life is able to
reproduce—and also the secret of how mere chemicals can contain the coded
information that controls the structure and activities of all living things.
FIGURE 22.6
The double helix of DNA.
Side view View down the axis
FIGURE 22.7
Rosalind Franklin (1920–1958) and the diffrac-
tion pattern she made that solved the double-
helical structure of DNA. Her contributions to
this discovery were never acknowledged during
her life. She died of cancer at a very early age.
FIGURE 22.8
The opening ceremony of the 2004 Sum-
mer Olympic Games in Athens, Greece.
