Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

EXERCISE 22.1 What Exactly Is DNA?
You will very often read and hear the term molecules of DNA. Scientists use the
phrase routinely, and “DNA molecules” have become such a hot news story that
they are referred to regularly in general magazines and newspapers and on TV. But
when we closely examine the structure of DNA as we will do in this chapter,
we will see that the phrase a molecule of DNA is not strictly accurate. Why not?
Solution
Several factors introduce complications here. First of all, the negative charges on the
phosphate groups mean that DNA, in the form in which it exists in living things, is
a multi-charged ion, not a molecule at all. If hydrogen ions combined with all these
negative charges, it would become a molecule, but this is not the structure we ob-
serve in living things. Second, when people speak of “DNA molecules”they are often
referring to double-helical DNA, rather than to the single-stranded form. The DNA
double helix is held together by largely noncovalent forces from hydrogen bonds, so
it is really a complex formed from two intertwined molecules (or multi-charged
ions). It cannot properly be described as a molecule at all.
PRACTICE 22.1
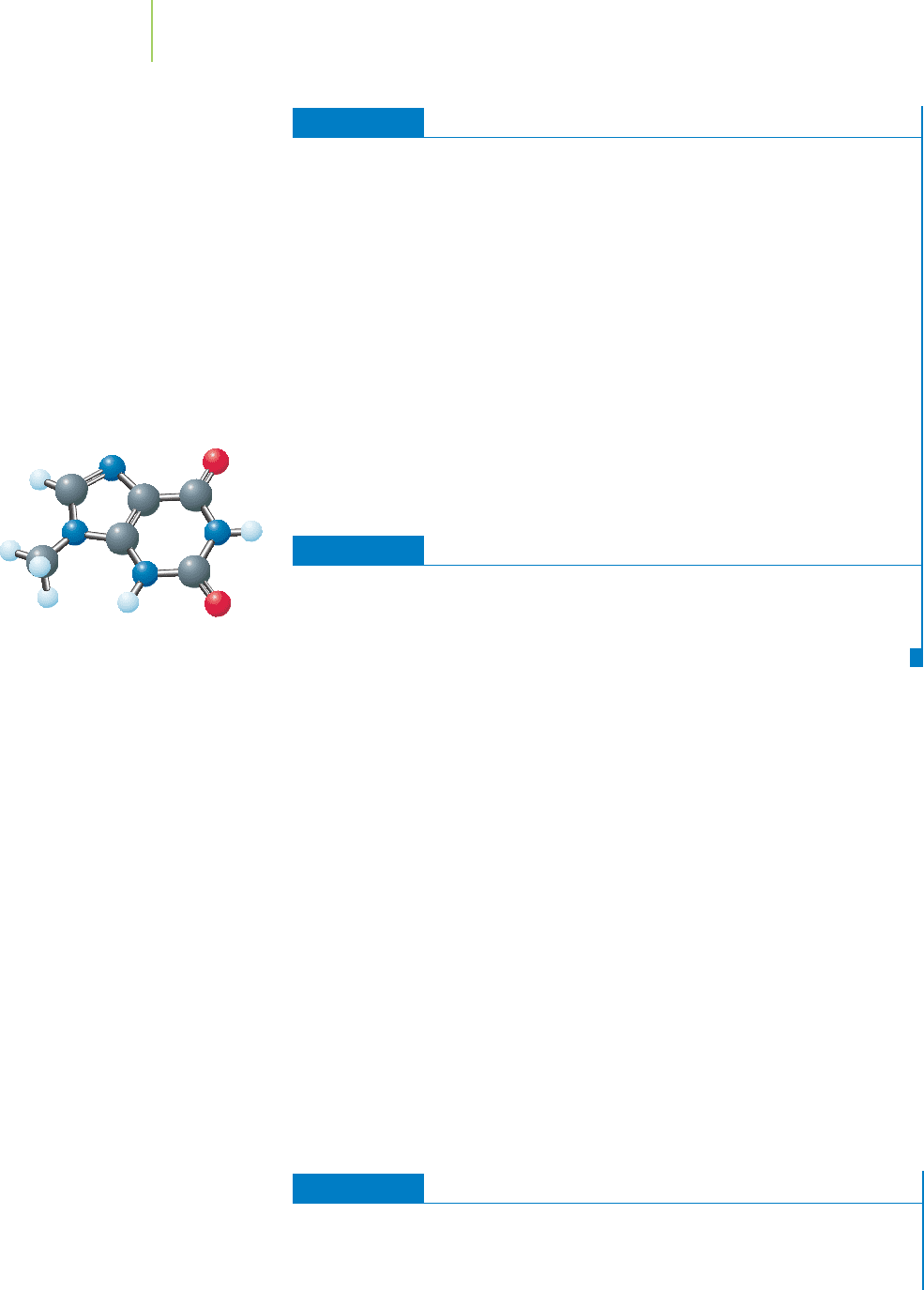
Suppose that a new base, shown in the margin, was discovered by scientists. To
which of the four nitrogenous organic bases would this new base be most likely
to form a stable base pair?
See Problems 3 and 4.
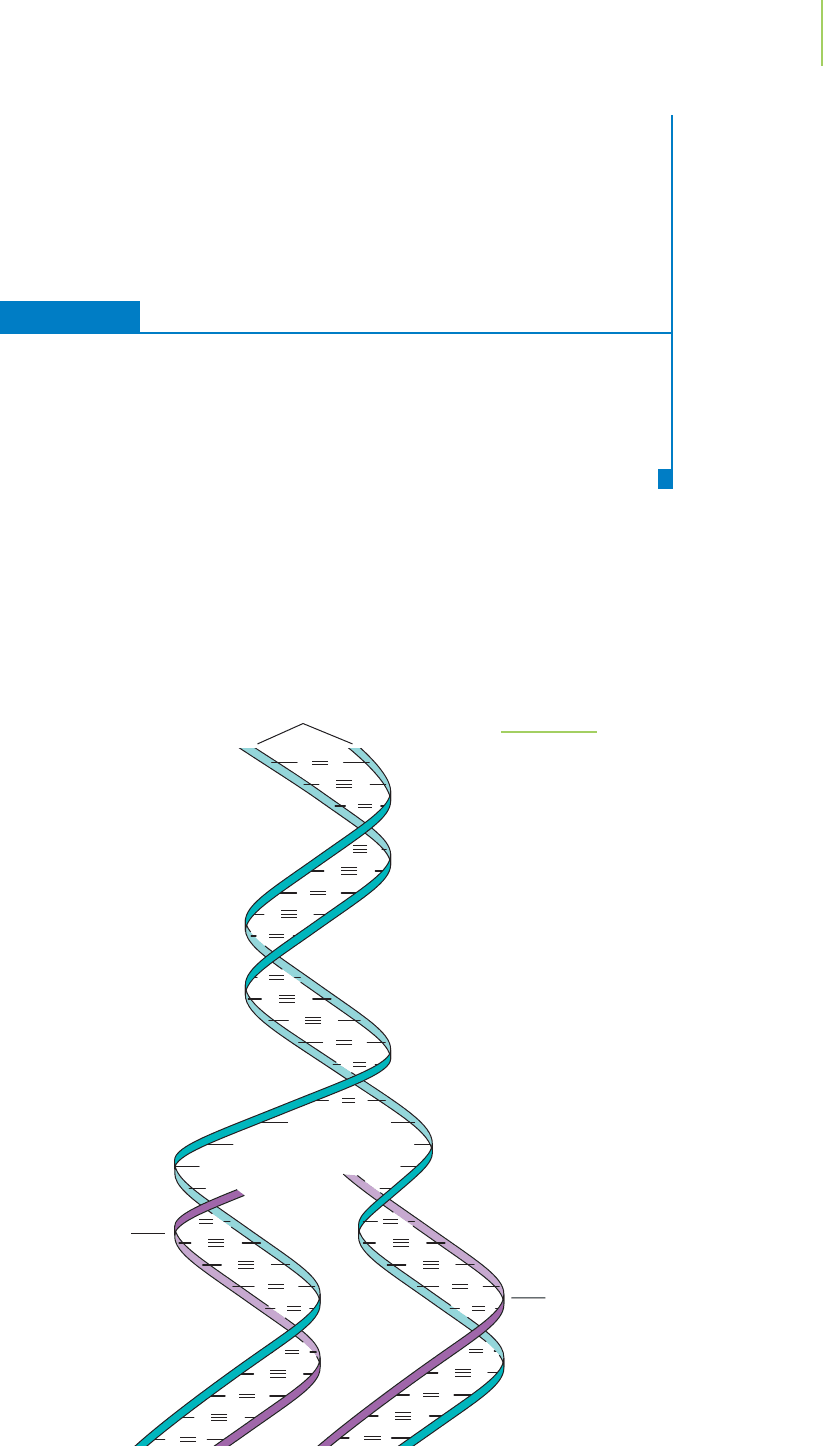
DNA Replication—The Secret of Reproduction
One of the most important characteristics of living creatures (animal or plant) is
that they make more of themselves. In other words, they “reproduce.” Reproduc-
tion in this sense depends on the ability of double-helical DNA to copy itself or
“replicate.”
Because the bases form complementary pairs in a double strand of DNA, the
sequence of nucleotide bases in one strand can be readily determined by examin-
ing the other strand of DNA. In other words, the A on one strand is paired with a
T on the other, each T with an A, each G with a C, and each C with a G. This
means that within a reproducing cell, the double helix can unravel and separate
into individual strands. Then, each strand can serve as the template on which a new
complementary strand assembles, as shown in Figure 22.9. The chemical reactions
involved in this DNA
replication are catalyzed by molecules within the cell known
as enzymes. However, the structure of the existing DNA strands determines the
sequence of the new DNA strands. Nucleotides carrying the appropriate bases are
the only ones that can bind to the existing strand in a manner that allows the en-
zymes to link them up into a new DNA strand. The result of this process is two
identical strands of DNA, one for the original cell and one for the new cell when
it is formed.
EXERCISE 22.2 Using the Template
If the DNA strand shown below serves as the template strand during DNA replica-
tion, what will be the sequence of the new double-helical DNA that results?
AATTGCGGGTCCGACC
938 Chapter 22 The Chemistry of Life
A new base for DNA.
GC
TA
A
A
T
T
C
AT
CG
TA
G
GC
TA
CG
A
A T
GC
CG
TA
A
AT
CG
AT
AT
CG
T
T
GC
CG
TA
A
T
CG
A
T
T
GC
CG
TA
A
A
T
GC
TA
A T
CG
A
T
Parent strands
Complementary
new strand
Complementary
new strand
FIGURE 22.9
Single-stranded DNA can serve as a tem-
plate for the creation of a new strand.
22.1 DNA—The Basic Structure
939
Solution
Remember the rules of base-pairing. Only two types of base pairs are allowed:
AT and GC (either way round), so the double-helical DNA will have the following
sequence:
AATTGCGGGTCCGACC
TTAACGCCCAGGCTGG
PRACTICE 22.2
A small piece of one of the strands of DNA is added to a single strand of DNA.
Indicate the correct alignment of the small piece with the long strand.
. . . AAAATGCTGGCATAGCGTTCCAGATACGGACTGACTGC . . .
CTATGC
See Problems 5 and 6.
DNA is located inside cells within a structure known as the nucleus. It is a
beautiful chemical structure with the ability to act as a template on which new
copies of itself are formed.
But what makes DNA so important to life? The answer
is that it carries coded information within its base sequence—information that
can be decoded to specify which molecules are made in a cell. These molecules,
known as
proteins, then perform most of the chemical activities that actually sus-
tain life.
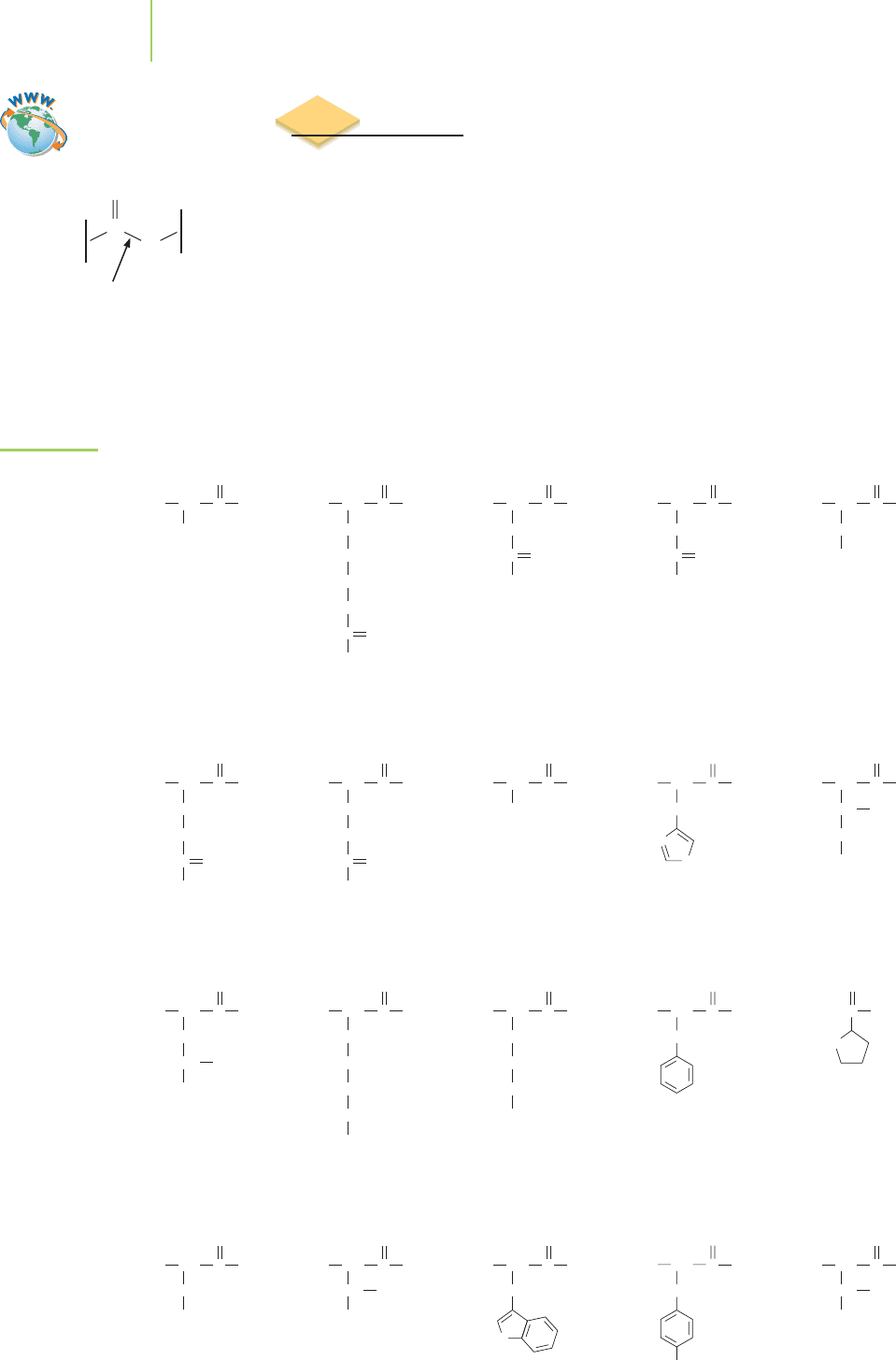
22.2 Proteins
Proteins are polymers that are formed when molecules called amino acids un-
dergo condensation reactions. The amine on one amino acid forms an
amide
bond
with the carboxylic acid on another amino acid. This process is repeated
many times as the protein is formed. Some of the important amino acids found
in living cells are shown in Figure 22.10. Note that all these amino acids contain
very similar arrangements of the carboxylic acid and amine functional groups.
The difference between them can be found in the group of atoms attached to the
same carbon as the amine. The amino acids are often referred to as
residues be-
cause they are what remains after the protein is broken up into its individual
amino acids. Twenty different residues are common within your body, and each
has been given a specific name.
940 Chapter 22 The Chemistry of Life
The amide bond
N
H
C
O
FIGURE 22.10
Twenty common
amino acids. The
names of the
amino acids are
also shown with
their three-letter
abbreviation and
one-letter code.
Alanine
(Ala, A)
H
2
N CH
CH
3
OHC
O
Arginine
(Arg, R)
H
2
N CH
CH
2
CH
2
CH
2
NH
2
NH
2
NH
+
C
OHC
O
Lysine
(Lys, K)
H
2
N CH
CH
2
CH
2
CH
2
NH
2
CH
2
OHC
O
Methionine
(Met, M)
H
2
N CH
CH
2
CH
2
S
CH
3
OHC
O
Isoleucine
(Ile, I)
H
2
N CH
CH CH
3
CH
2
CH
3
OHC
O
Leucine
(Leu, L)
H
2
N CH
CH
2
CH
3
CH
CH
3
OHC
O
Serine
(Ser, S)
H
2
N CH
CH
2
OH
OHC
O
Threonine
(Thr, T)
H
2
N CH
CH
CH
3
OH
OH
C
O
Valine
(Val, V)
H
2
N CH
CH
CH
3
OH
CH
3
C
O
Tryptophan
(Trp, W)
H
2
N CH
CH
2
HN
OHC
O
Asparagine
(Asn, N)
H
2
N CH
CH
2
NH
2
OC
OHC
O
Aspartic acid
(Asp, D)
H
2
N CH
CH
2
OH
OC
OHC
O
Glutamic acid
(Glu, E)
H
2
N CH
CH
2
CH
2
OH
OC
OHC
O
Glutamine
(Gln, Q)
H
2
N CH
CH
2
CH
2
NH
2
OC
OHC
O
Glycine
(Gly, G)
H
2
N CH
H
OHC
O
Histidine
(His, H)
H
2
N CH
CH
2
OHC
N
NH
O
Cysteine
(Cys, C)
H
2
N CH
CH
2
SH
OHC
O
HN
Phenylalanine
(Phe, F)
H
2
N CH
CH
2
OHC
O
Proline
(Pro, P)
OHC
O
Tyrosine
(Tyr, Y)
H
2
N CH
CH
2
OH
OHC
O
Video Lesson: Proteins
Proteins are made in living cells by the sequential addition of amino acids to
a lengthening
polypeptide chain. This sequence of the protein constitutes the first
level of structure of the protein. As indicated in Figure 22.11, it is often called the
primary structure of the protein. Just like every individual person, each type of
protein within a living cell is unique thanks to its amino acid sequence. The se-
quence of the amino acids determines the resulting shape of the protein and the
resulting function of the protein.
We can now appreciate that both DNA and proteins are chemicals whose
structure is determined by the sequence in which monomer units are bonded into
polymers. Each DNA polymer has a unique base sequence (also know as its nu-
cleotide sequence). Each protein has a unique amino acid sequence. The key to
understanding how DNA can specify which proteins a living thing contains is to
understand how the base sequence of DNA can specify the amino acid sequence of a
protein. This is achieved by the operation of a simple chemical code.
22.3 How Genes Code for Proteins
A gene is a section of DNA that encodes a specific protein molecule.
This means that the base sequence of the gene determines the amino
acid sequence of the resulting protein. The whole process of convert-
ing the genetic code into a particular protein is known as gene expres-
sion, which occurs in two distinct phases:
transcription, in which an
RNA copy of the gene is made, and
translation, in which the RNA copy
directs production of the protein.
What is RNA?
Ribonucleic acid (RNA) is a substance that bears a
close resemblance to DNA. In fact, there are only two main differences
between DNA and RNA (see Figure 22.12). The sugar in RNA is ribose,
which carries one more oxygen atom than the deoxyribose found in
22.3 How Genes Code for Proteins 941
FIGURE 22.11
Primary structure of a protein. The
primary structure is the order of the
amino acid monomers in the strand of
the protein.
H
3
N CCN
H
Amino-terminal
residue
Primary structure of protein
Carboxyl-terminal
residue
O
R
1
CC
H
O
R
2
H
NCC
H
O
R
3
H
NCC
H
O
R
4
H
NCC
H
O
O
R
5
H
+
–
C
N O
NH
Phosphate
Ribose sugar
Nitrogenous organic base
Nucleoside
Nucleotide
O
P
O
–
O
O
O
H
H
CC
OH
H
OH
O
–
H
CC
CH
2
HC
HC C
The basic components of the monomers that
make up RNA.
DNA
RNA
FIGURE 22.12
The difference between DNA and RNA
lies in the ribose sugar.
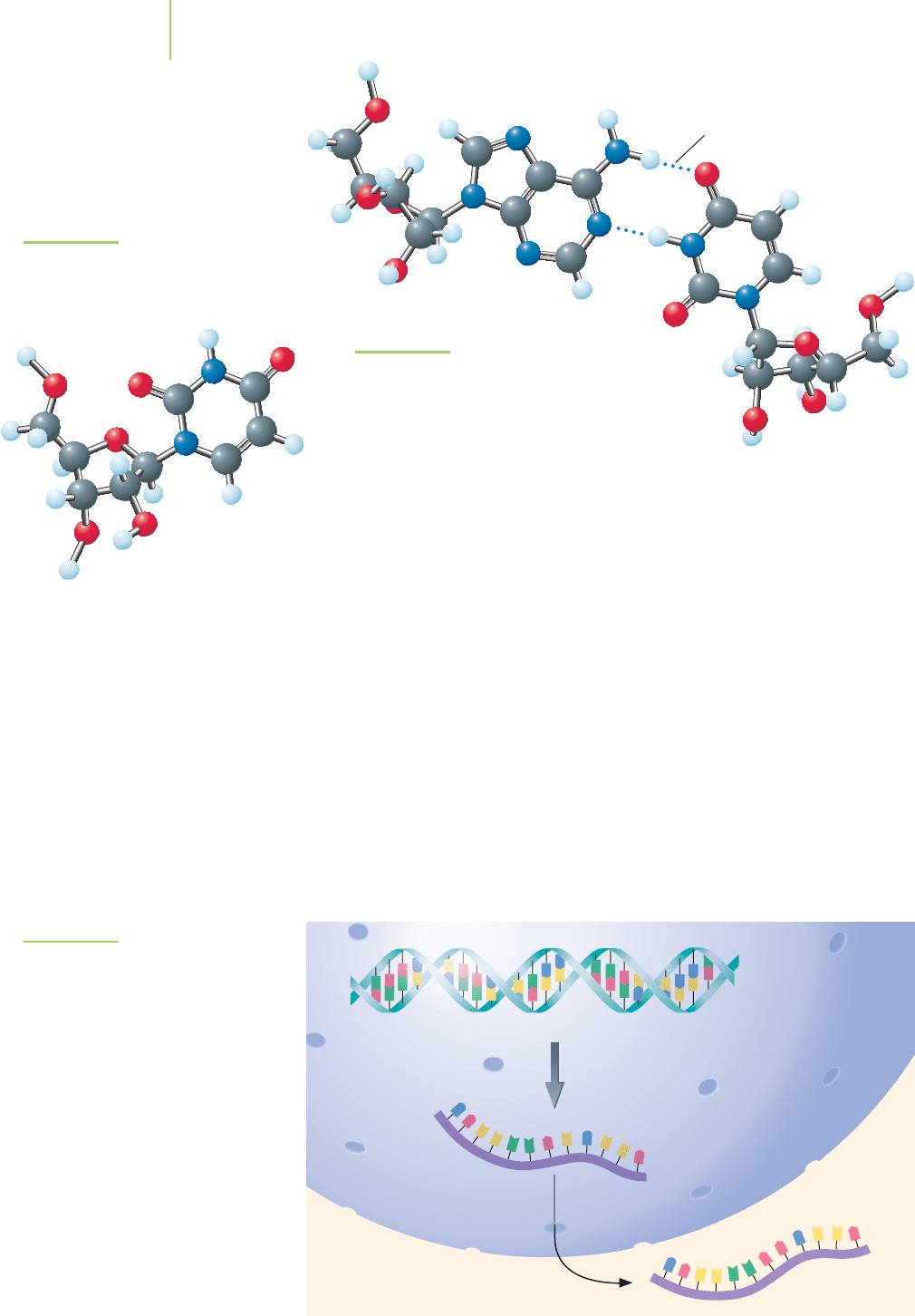
Hydrogen bonds
Adenosine
942 Chapter 22 The Chemistry of Life
DNA. Also, in RNA the nitrogenous base uracil, shown in Figure 22.13, is found
in place of the thymine of DNA. Uracil can participate in an A–U base pair as
shown in Figure 22.14, in the same way that thymine can participate in an A–T
base pair. The result is that RNA can form base pairs with a complementary
strand of DNA.
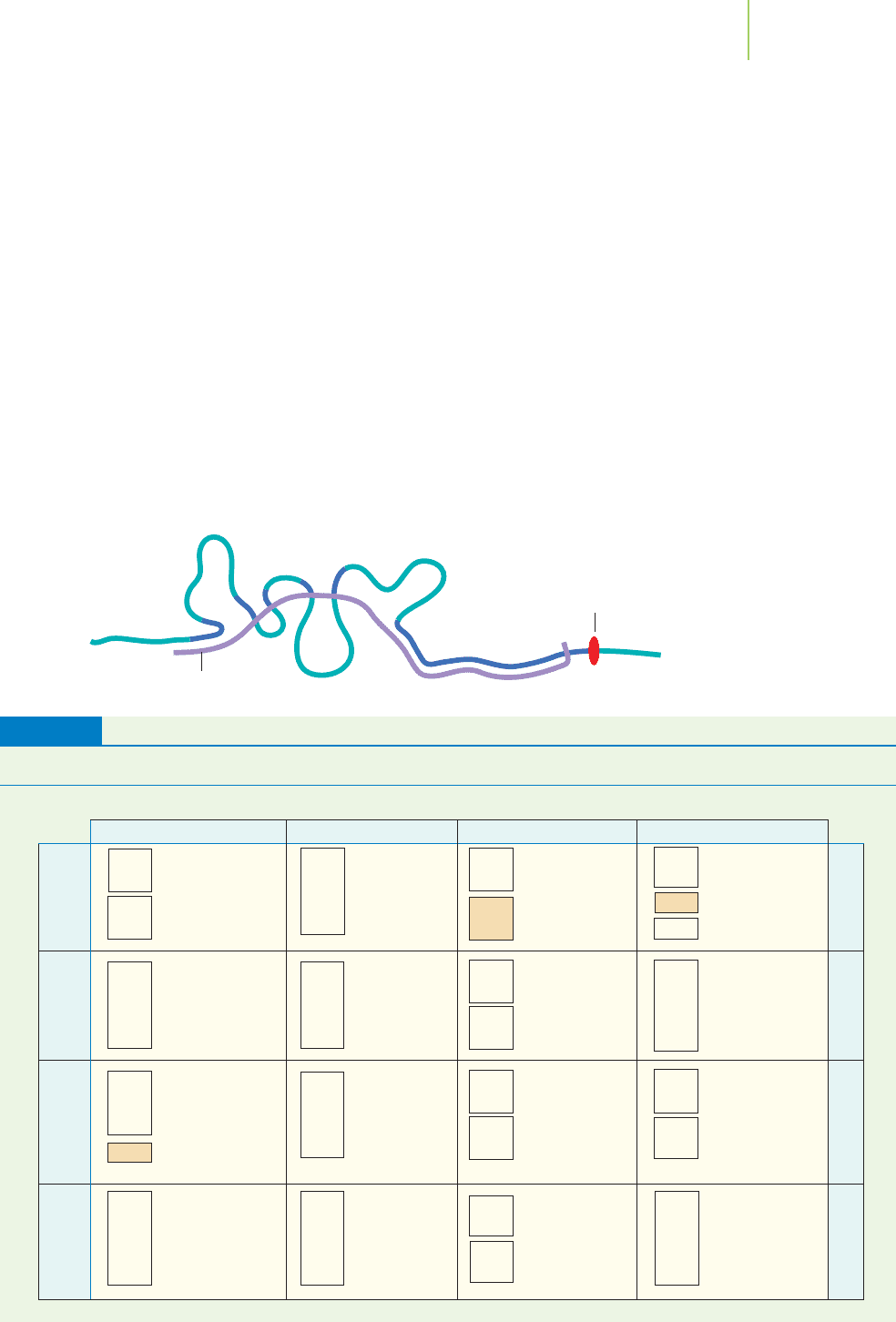
Transcription—Making the Message
The process of gene transcription occurs in a very similar way to DNA replica-
tion. Inside the nucleus of the cell, the DNA unwinds into the individual strands
of DNA, and a new, complementary strand of RNA is made. The complementary
strand is manufactured using enzymes via condensation reactions in much the
same manner as in the manufacture of DNA. The single-stranded RNA copy of a
gene that is produced in transcription is known as
messenger RNA (mRNA). This
copy carries the genetic “message” from the nucleus to the ribosomes (see below)
in the cytoplasm, where the message is used (decoded) to make protein. mRNA
gets its name from the fact that it takes the message held within the genes to the
site of protein synthesis, as illustrated in Figure 22.15.
FIGURE 22.15
mRNA is made in the nucleus and then
transferred to the cytoplasm for protein
synthesis.
Transcription
Nucleus
DNA
mRNA
Cytoplasm
Uridine
FIGURE 22.14
An A–U base pair. Note that the hydrogen bond-
ing is similar to that found in an A–T base pair.
Uracil and adenine attached to a ribose sugar
unit (without a phosphate) are known as uri-
dine and adenosine, respectively.
FIGURE 22.13
The different monomer used in RNA,
shown here without the attached phos-
phate group, is similar in structure to
thymidine.
22.3 How Genes Code for Proteins 943
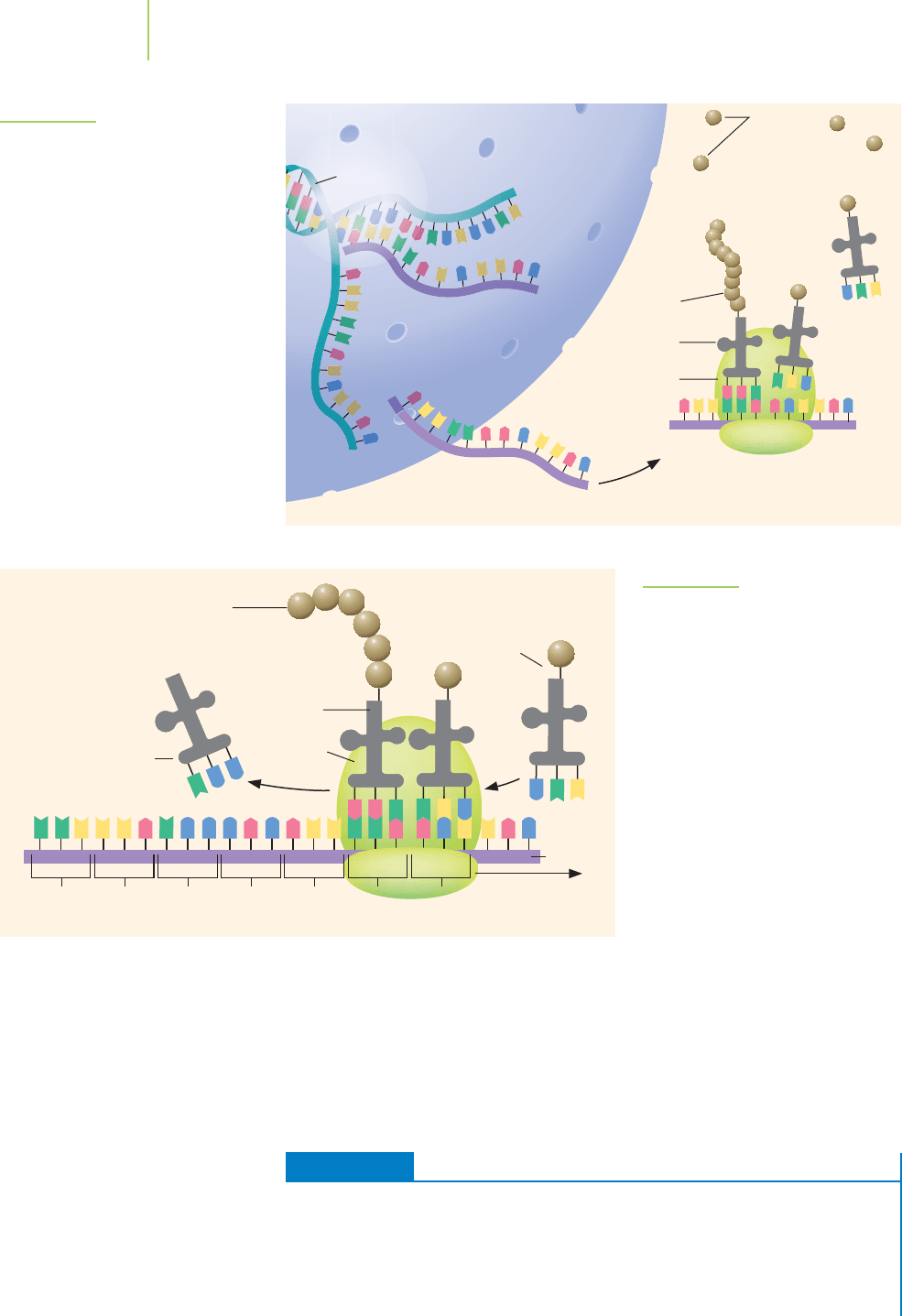
Translation—Making Proteins
Once at the site of protein synthesis, the mRNA is read by another class of RNA
molecules known as
transfer RNA (tRNA). Each tRNA molecule includes a set of
three nitrogenous bases that can form base pairs with three sequential bases of
mRNA. Each set of three sequential bases within the mRNA is known as a
codon.
The codon binds to the complemetary
anticodon on the tRNA. What is the pur-
pose of the tRNA? Each tRNA also carries a specific amino acid at its amino acyl
binding site. When the tRNA anticodon binds to the codon on the mRNA, the
amino acid on that tRNA becomes incorporated into the growing protein chain.
If the anticodon and codon are not complementary, the tRNA leaves without
donating its amino acid to the protein. The code, indicating the relationship
between each specific codon and amino acid is called the genetic code and is
summarized in Table 22.1. The entire process of translation is outlined in
Figure 22.16.
The mRNA contains a special“start” sequence that identifies the beginning of
a protein. In fact, every protein that is made begins with the amino acid methion-
ine. From that point onward, the amino acids are identified by each codon until
the final “stop” codon is reached. Sometimes all of the mRNA is translated into a
protein.Some genes make an mRNA that contains a lot of extraneous nucleotides.
The Genetic Code
The three-letter codes listed are mRNA codons. For example, AAA is the mRNA codon for the amino acid lysine.
Second letter
TABLE 22.1
First letter
Third letter
U
C
A
G
U
Phenyl-
alanine
Leucine
Leucine
Isoleucine
Methionine,
initiation
codon
Valine
UCU
UCC
UCA
UCG
CCU
CCC
CCA
CCG
ACU
ACC
ACA
ACG
GCU
GCC
GCA
GCG
C
Serine
Proline
Threonine
Alanine
A
Tyrosine
Stop codon
Stop codon
Histidine
Glutamine
Asparagine
Lysine
Aspartic acid
Glutamic
acid
G
Cysteine
Stop codon
Tryptophan
Arginine
Serine
Arginine
Glycine
U
C
A
G
U
C
A
G
U
C
A
G
U
C
A
G
Not all of the mRNA is used to
create a protein.
Mature mRNA
“Stop”
signal
UGU
UGC
UGA
UGG
CGU
CGC
CGA
CGG
AGU
AGC
AGA
AGG
GGU
GGC
GGA
GGG
UAU
UAC
UAA
UAG
CAU
CAC
CAA
CAG
AAU
AAC
AAA
AAG
GAU
GAC
GAA
GAG
UUU
UUC
UUA
UUG
CUU
CUC
CUA
CUG
AUU
AUC
AUA
AUG
GUU
GUC
GUA
GUG
944 Chapter 22 The Chemistry of Life
FIGURE 22.16
The overall process of translation.
Nucleus
DNA
mRNA
Ribosome aligns tRNA
with mRNA and constructs
the protein
Ribosome
Protein
tRNA brings
amino acids
to ribosome
Amino
acids
tRNA
Cytoplasm
To hold the molecules close together and to assist in catalyzing the process of
adding amino acids into the protein, the process of translation takes place on
giant assemblies of protein and RNA known as
ribosomes (see Figure 22.17). As
the ribosome moves along an mRNA molecule, the appropriate tRNAs are able to
bind to special sites on the ribosome, allowing enzymes to link the amino acids
they carry into a new protein chain. The end result is the translation of the base
sequence of the gene (via the mRNA) into the amino acid sequence of the protein.
EXERCISE 22.3 Examining the Genetic Code
Examine the genetic code shown in Table 22.1. Do all codons encode amino acids?
Does each codon specify a unique amino acid?
First Thoughts
Because there are four different bases and three positions, we can figure out the pos-
sible number of arrangements of these bases into the three positions.
FIGURE 22.17
The ribosome slides along the mRNA.
tRNAs then bring specific amino acids to
the ribosome and allow the protein chain
to be constructed. In this figure, the
amino acids (aa) and the tRNAs are num-
bered to indicate their position.
Movement
of ribosome
aa
6
aa
7
aa
8
aa
5
aa
4
aa
3
aa
2
aa
1
Growing
polypeptide chain
tRNA
Ribosome
tRNA
5
leaving
aa
8
– tRNA
8
arriving
Codon
aa
1
Codon
aa
2
Codon
aa
3
Codon
aa
4
Codon
aa
5
Codon
aa
6
Codon
aa
7
mRNA
CCG GUA
GGGGGCCCCCCAAAAAAUUUUUUU
G
A
A
GUA
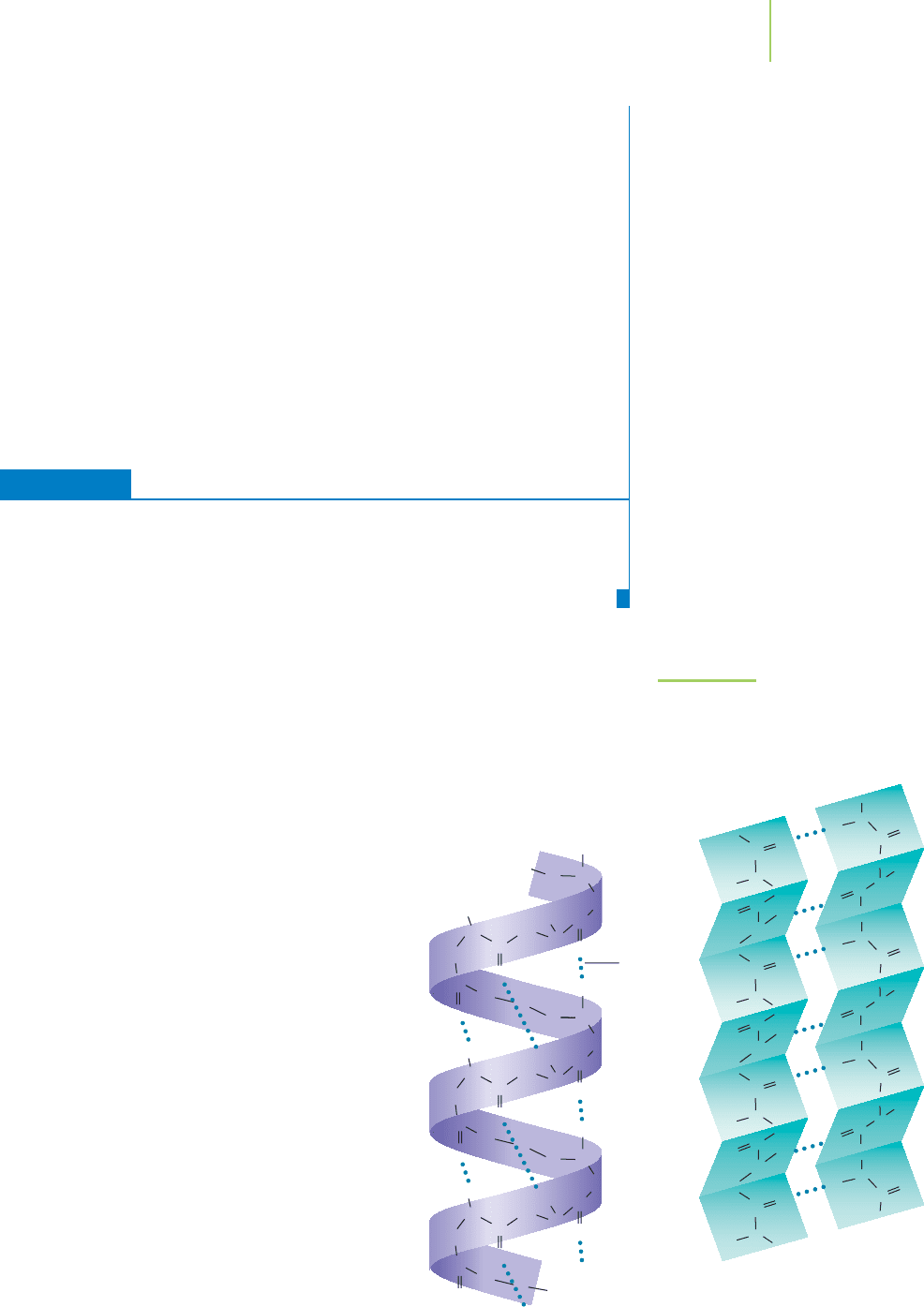
Solution
There are 64 possible codons—different ways to arrange the four bases into sets of
three. There are only about 20 amino acids found in proteins, so there must be more
codons available than amino acids. Examining the genetic code reveals that each
amino acid can be encoded by several alternative codons. It also reveals that three
codons act as “stop” signals, indicating the point at which the synthesis of a protein
chain should end.
Further Insights
Damage to DNA can result in changes to the genetic code. For example, a specific
base within a codon could be changed as a result of the damage. Some of these
changes have no effect on the protein encoded by the DNA, because they change a
codon into one of the other codons specifying the same amino acid. Other such
changes cause a different amino acid to appear in the encoded protein. This can
cause problems with the resulting protein that is made using the mutant codon.
PRACTICE 22.3
What is the primary structure of a protein made using the following mRNA
sequence?
AUGUGGCCAAAAUUGGACAUGUUCGACUAG
See Problems 15 and 16.
The human genome contains between about 20,000 and 30,000 genes. These
genes are able to encode an even greater number of proteins, because the RNAs
that are originally made from the genes can be edited to make many different
proteins in a kind of enzymic “cut-and-paste” process. The way in which genes
encode proteins is an astonishing demonstration of the power of chemistry to
sustain the complex processes that underpin all life. To understand more fully
just how powerfully genes influence the chemistry of life, we need to look at the
proteins encoded by our genes and investigate the things these proteins can do.
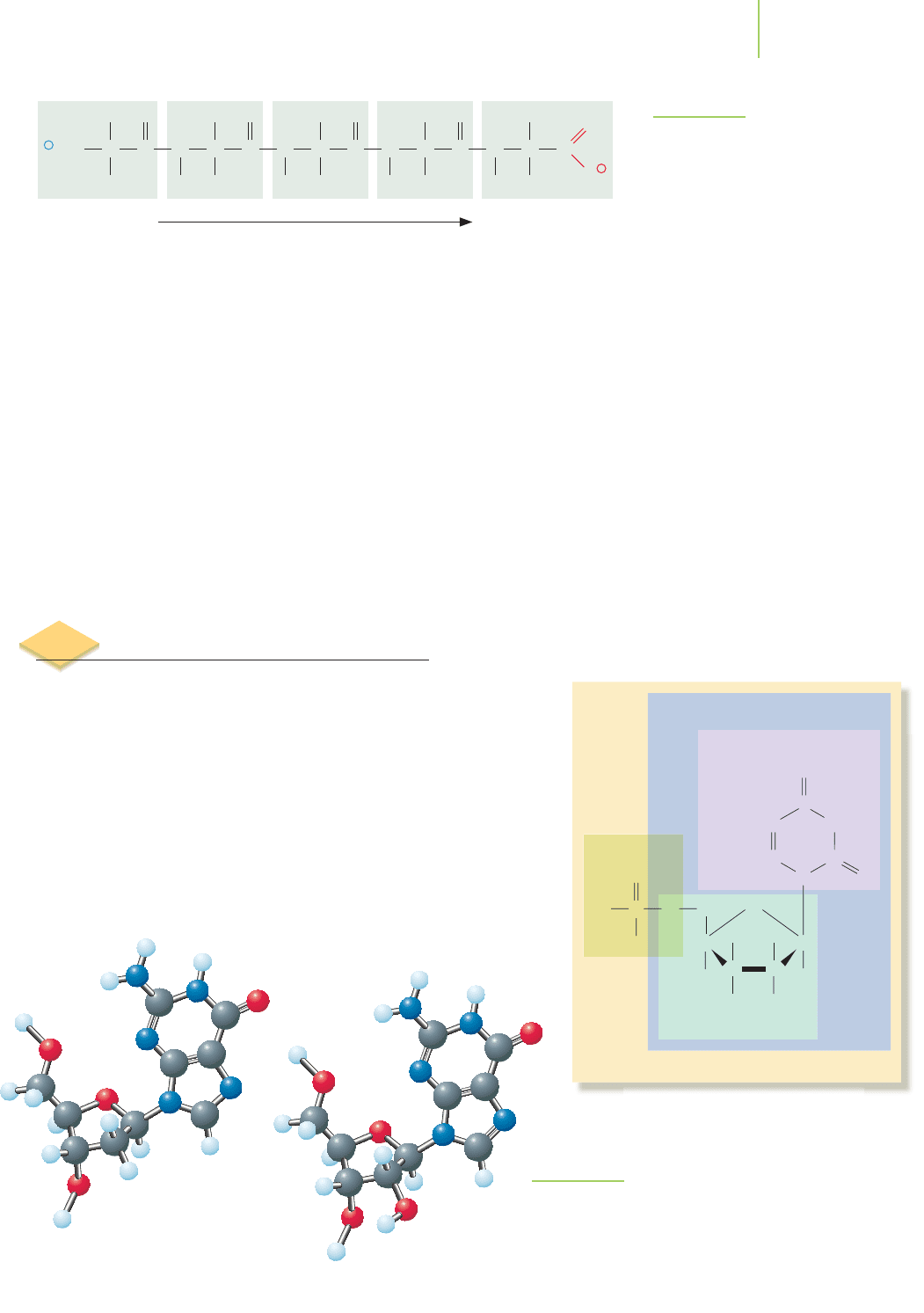
Protein Folding
As soon as a protein chain begins to be formed, it
starts to fold into a specific three-dimensional con-
formation. The folding process is governed princi-
pally by noncovalent interactions among the amino
acids themselves, and also among the amino acids
and the water molecules surrounding the protein.
Localized regions within a polypeptide chain that
fold in a particular way are examples of a protein’s
secondary structure. The most significant secondary
structures are the alpha helix ( helix) and the beta
pleated sheet ( sheet), shown in Figure 22.18. The
helix forms as a consequence of hydrogen bonds
between the NOH and CPO groups of the
polypeptide chain, holding the chain in the form of
a helix. The sheet is also held together by hydro-
gen bonds between NOH and CPO groups, but
with the hydrogen bonding occurring between
neighboring portions of a polypeptide chain.
Regions of specific secondary structure are linked
by turns in the polypeptide chain, and by less ordered
structures, to form the overall three-dimensional
22.3 How Genes Code for Proteins 945
FIGURE 22.18
Two common types of secondary struc-
tures: the alpha helix and the beta sheet.
These structures are held together by hy-
drogen bonding (shown as dotted lines).
C
C
N
N
N
N
H
H
H
H
O
O
O
C
C
C
C
O
C
C
C
O
C
C
N
N
N
N
H
Hydrogen
bonds
Alpha helix
H
H
H
O
O
O
C
C
C
C
O
C
C
C
C
N
N
N
N
H
H
H
H
O
O
O
C
C
C
C
C
N
N
N
O
O
O
H
H
H
H
N
C
C
C
C
C
C
O
C
C
Beta sheet
N
N
N
O
O
O
H
H
H
H
N
C
C
C
C
C
C
O
C
C
N
N
N
O
O
O
H
H
H
H
N
C
C
C
C
C
C
O
C
C
N
N
O
O
H
H
C
C
C
C
C
C

conformation of a protein. The folding of the secondary structures
into a three-dimensional conformation is known as the protein’s
ter-
tiary structure
. Just as is true of helices and sheets, there are many
common tertiary structures. Some of these are shown in Figure 22.19.
However, many proteins have what can loosely be described as a
“globular” tertiary structure; see the example shown in Figure 22.20.
This is the general three-dimensional shape found for most of the
proteins that act as enzymes.Other proteins have linear tertiary struc-
tures, largely composed of one or more extended helices or sheets.
Many such proteins form strong fibers that contain several protein
molecules intertwined. For example, the protein
collagen, shown in
Figure 22.21, gives strength to connective tissues such as tendons, lig-
aments, and bones. The structural strength of collagen is a result of
three helical proteins wound around one another to form a triple
helix.
When several polypeptide chains are held together by forces of
attraction, as in collagen, we say that a protein with
quaternary structure has been
formed. Many globular proteins also possess quaternary structure. For example,
hemoglobin, the protein that carries oxygen to your muscles, is made of four
polypeptide chains that have folded around each other as shown in Figure 22.22.
Not all proteins have quaternary structure. For example, myoglobin, the oxygen
storage protein that resides in your muscles, consists of only a single polypeptide
chain. The precisely folded structure of proteins can be disrupted, or
denatured,
by heat or by variations in the chemical surroundings, including changes in pH
and in the concentration of salt ions. Denaturation reduces or destroys a protein’s
biochemical activity, depending on how severe it is. This explains, for example,
why the protein albumin in an egg comes out of solution and turns white upon
heating.
946 Chapter 22 The Chemistry of Life
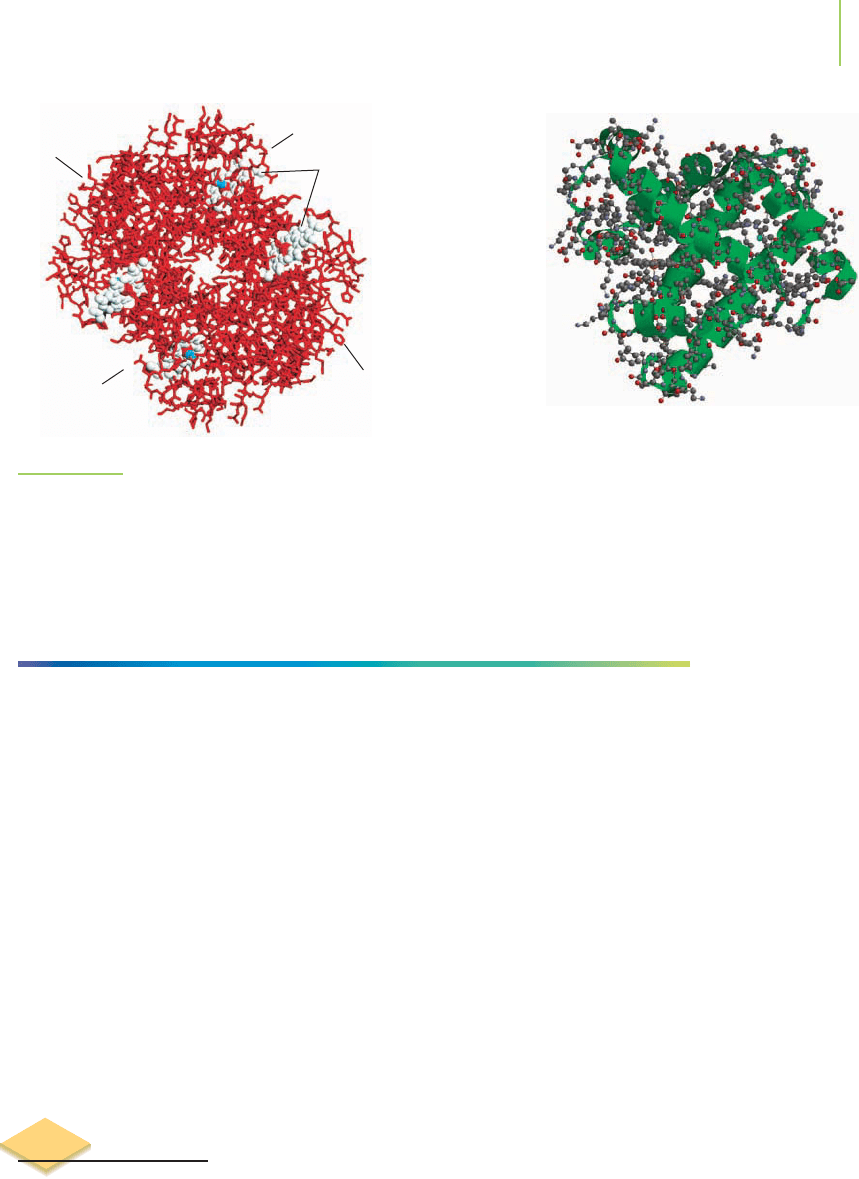
FIGURE 22.19
Some common tertiary structures. These structures are held in place by intermolecular forces of
attraction such as London forces, dipole–dipole interactions, and hydrogen bonding.
Helix-turn-helix Beta-alpha-beta Hairpin Greek key
Collagen
FIGURE 22.21
Collagen is a fibrous protein.
FIGURE 22.20
Lactate dehydrogenase is a globular enzyme
involved in the biochemical process of harvesting
energy from sugars.
22.4 Enzymes 947
FIGURE 22.22
Hemoglobin. There are four subunits, each with its own
iron-containing heme, that come together to form hemo-
globin. The subunits are held together to form the quater-
nary structure of the protein.
Subunit B
Heme groups
Subunit A
Subunit D
Subunit C
Myoglobin. The green ribbons represent the
backbone of the chain of amino acids. Note
the curled structures in the ribbons. They
illustrate the alpha helix secondary structure
in this protein.
HERE’S WHAT WE KNOW SO FAR
■
DNA is a polymer of nucleotides made from two complementary single
strands wrapped around each other to form a double helix.
■
Proteins are polymers of amino acids linked through an amide bond. They
contain primary, secondary, tertiary, and sometimes quaternary structure.
■
Proteins are made by translating the genetic code from mRNA. tRNA supplies
the specific amino acids to the growing polypeptide chain. The construction
occurs at the ribosomes within the cytoplasm of a cell.
■
Primary structure is the sequence of amino acids that make up the protein.
■
Secondary structures are the specific regions of a polypeptide chain that fold
into an helix or a sheet.
■
Tertiary structures result from folding of the secondary structures within a
polypeptide chain into a three-dimensional shape. They can be globular or
linear in arrangement.
■
Quaternary structure results when two or more polypeptide chains are folded
into a specific shape. Not all proteins have quaternary structure.
22.4 Enzymes
The covalent bond that links glucose and fructose together in a molecule of su-
crose can be hydrolyzed by water, although the reaction is quite slow. In the lab-
oratory, we can speed the reaction by adding a little acid to an aqueous solution
of sucrose. The reaction can be monitored and the rate of the catalyzed reaction
measured.
Why is this reaction important? In the body, much of the “fuel” that we
ingest is sucrose. This sugar is broken down into its components, glucose and
fructose. As we noted in Chapter 14, both of the sugars are metabolized to pro-
vide energy to operate other biological processes and sustain life. Because these
molecules are vital to our existence, any reaction that makes them, or metabolizes
them, needs to be very rapid. Therefore, almost every chemical reaction within
