Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

++
more stable nuclei than their neighbors. Check the graph of
binding energy per
nucleon (proton or neutron in the nu-
cleus) versus the mass number shown in Figure 21.9 to see
that these elements sit at local maxima. The nuclides
16
8
O
and
40
20
Ca are “doubly magic”; they have magic numbers for
both protons and neutrons. In February 2000, a French re-
search team created another doubly magic nucleus, Ni-48,
which contains 28 protons and 20 neutrons.
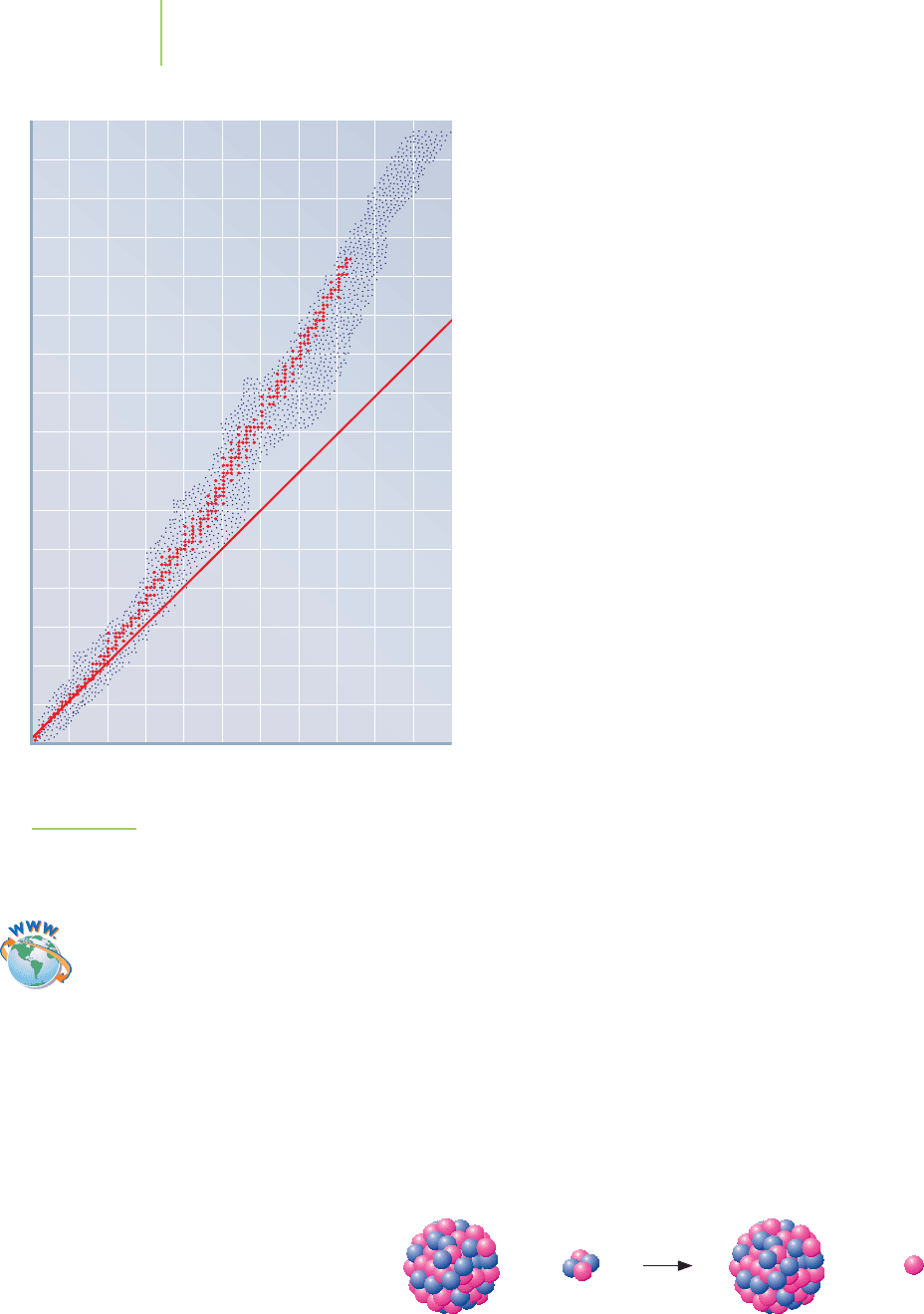
When you examine a larger set of nuclides, other trends
appear. There are 279 stable isotopes, a few naturally
occurring radioactive elements, and hundreds of synthetic
isotopes. Many of these find application in nuclear medi-
cine. Figure 21.10 sketches the band of stable isotopes for
each element with at least one stable isotope. Note the fol-
lowing points:
■
At higher atomic numbers, stable nuclei have increas-
ingly more neutrons than protons.
■
Some radioactive elements have too many neutrons
relative to the stable isotopes. These tend to decay by beta
emission, where a neutron changes into a proton and an
electron.
■
Some radioactive elements have too few neutrons relative
to the stable isotopes. These tend to decay by positron
emission, where a proton changes into a neutron and a
positron.
The plot ends at bismuth, Z
= 83, for no elements beyond
this have stable isotopes. Alpha emission is typical for heav-
ier elements that are unstable, simply because too many nu-
cleons are present, be they protons or neutrons. Table 21.7
lists some guidelines that are helpful in determining the
type of decay for a particular nucleus.
The radioactive decay can occur by the process of
positron emission, or
beta-plus emission,
0
+1
. This process
does not occur naturally on Earth and was not discovered
until scientists began creating new isotopes in the laboratory. Physically this is
done by slamming high-energy particles, ranging from protons and neutrons to
atomic nuclei, into a target nucleus. This can result in changing the nucleus’s
mass number and giving it additional energy. In general, the laboratory process is
Nucleus + small particle → bigger nucleus
The product nucleus that is formed may undergo radioactive decay.
For example, in 1930 phosphorus-30 was synthesized in the laboratory by the
bombardment of an aluminum target with alpha particles,
4
2
He:
27
13
Al +
4
2
He →
30
15
P +
1
0
n
More modern work includes the formation of tiny amounts of superheavy ele-
ments, which we will define as those beyond atomic number 106. Recent (1999
and 2000) reactions include
208
82
Pb +
86
36
Kr →
293
118
Uuo +
1
0
n
(t
1/2
< 1 msec)
249
97
Bk +
22
10
Ne →
267
107
Bh + 4
1
0
n
(t
1/2
= 17 seconds)
918 Chapter 21 Nuclear Chemistry
0
Number of
p
rotons
(
Z
)
20 40 60 80 100
20
40
60
80
100
120
140
160
Number of neutrons (A–Z)
0
1:1 neutron-to-proton ratio
Unstable region
(too many neutrons;
spontaneous beta
production)
Unstable region
(too many protons;
spontaneous positron
production)
Stable nuclides in the
zone of stability
FIGURE 21.10
The band of known isotopes for each element with at least
one stable isotope. n/p is the neutron-to-proton ratio. Note
that the n/p ratio is greater than 1 for most stable isotopes.
Video Lesson: The Stability
of Atomic Nuclei
Some nuclides are expected to be quite stable, others not so. The nature of the
nucleus becomes clearer when we study the half-lives of these nuclides.
EXERCISE 21.6 Ten Tin Isotopes
Tin has more stable isotopes than any other element. Explain why you might expect
this to be the case. Then examine the radioisotopes of tin and discuss their decay
modes.
Stable isotopes
112
Sn,
114
Sn,
115
Sn,
116
Sn,
117
Sn,
118
Sn,
119
Sn,
122
Sn,
124
Sn
(
120
Sn,
118
Sn, and
116
Sn are the most abundant.)
Radioisotopes
121
Sn,
123
Sn,
125
Sn,
126
Sn,
127
Sn
that decay by
−
(plus others with higher mass and short half-life)
Radioisotopes that
110
Sn,
111
Sn,
113
Sn
decay by
+
or by (plus others with lower mass and short half-life)
electron capture
First Thoughts
Data like these exist for every element; in general, they are most conveniently ac-
cessed on the Web or in a chemistry handbook. You cannot reason out which iso-
topes actually exist. Although tin’s location provides some guidance, you must still
look them up.
Solution
For several reasons, tin would be expected to have a number of stable isotopes. First,
tin is in the middle of the periodic table where nuclei are more stable and where a
few extra neutrons do little to upset the balance of nuclear forces. Second, it has
an atomic number of 50, one of the “magic numbers.” Finally, 8 of the 10 isotopes
have even numbers of protons and neutrons, and the even isotopes are the most
abundant—another indication of their stability.
Further Insights
Isotopic stabilities hold some surprises. For example, a radioisotope may fall be-
tween a pair of stable isotopes. We noted this above for tin. It also happens for chlo-
rine, where Cl-35 and Cl-37 are stable, but Cl-36, which has an odd number of both
protons (17) and neutrons (19), is radioactive. Again, nuclear stability (or instabil-
ity) arises from a combination of several factors and is therefore difficult to predict.
PRACTICE 21.6
Predict whether each of the following isotopes might be stable or radioactive.
a.
79
Br b.
101
Ru c.
136
Ba d.
180
Ta
See Problems 59–62.
21.6 Nuclear Stability and Human-made Radioactive Nuclides 919
Predicting Nuclear Decay
Type of Decay Reason for Instability Change in n/p Ratio
Alpha emission Nucleus too heavy Increase (small for heavy nuclides)
Beta emission n/p too high (*) Decrease
Positron emission n/p too low (*) Increase
Gamma Too much energy None
Nucleus energetically excited
Electron capture n/p too low (*) Increase
*n/p represents the neutron-to-proton ratio.
TABLE 21.7
n
0
1
n
0
1
n
0
1
n
0
1
n
0
1
S
r
38
90
C
s
55
144
0
1
n
n
0
1
n
0
1
n
0
1
n
0
1
U
92
235
U
92
235
U
92
23
5
Kr
36
9
4
Ba
56
13
9
Xe
54
14
2
n
0
1
n
0
1
n
0
1
U
92
235
R
b
37
90
Ba
56
139
K
r
36
9
4
Application
C
HEMICAL
ENCOUNTERS:
Nuclear Weapons
920 Chapter 21 Nuclear Chemistry
21.7
Splitting the Atom: Nuclear Fission
With the discovery of fission came the birth of new radioisotopes and of a new
consciousness that the nuclear age was upon us. Nuclear fission was discovered in
the 1930s through the work of scientists such as Enrico Fermi, Fritz Strassman,
Otto Hahn, and Lise Meitner. Work on fission continued in the early 1940s in
both Germany and the United States, culminating in the deployment by the
United States of the first atomic bomb used in war, fueled by uranium-235, on the
town of Hiroshima, Japan, on August 6, 1945, and of a second combat-based
atomic bomb, fueled by plutonium-239, on Nagasaki, Japan, on August 9, 1945.
The war ended shortly after the second bomb was dropped, but the nuclear age
had just begun.
Why do
235
U and
239
Pu split and release energy? The answer lies in part in the
thermodynamics of nuclear stability. Refer to Figure 21.9. Uranium nuclei are not
so thermodynamically stable as iron, bromine, and other elements in the region
of greatest stability near the top of the curve. The answer also lies in considering
the precarious balance that exists in large nuclei such as uranium and plutonium.
These nuclei are very heavy and are held together by the strong force between nu-
cleons. However, opposing the strong force are the many proton–proton repul-
sions in an atom of this size. For some atoms, the injection of extra mass into the
nucleus can tip the balance in favor of the proton repulsions and send the nucleus
flying apart into two or more pieces. This is what happens with
235
U and
239
Pu. A
neutron, with no charge, is an ideal particle to shoot into a nucleus. Once it slips
into the nucleus, a heavier nuclide is formed that fragments in a matter of
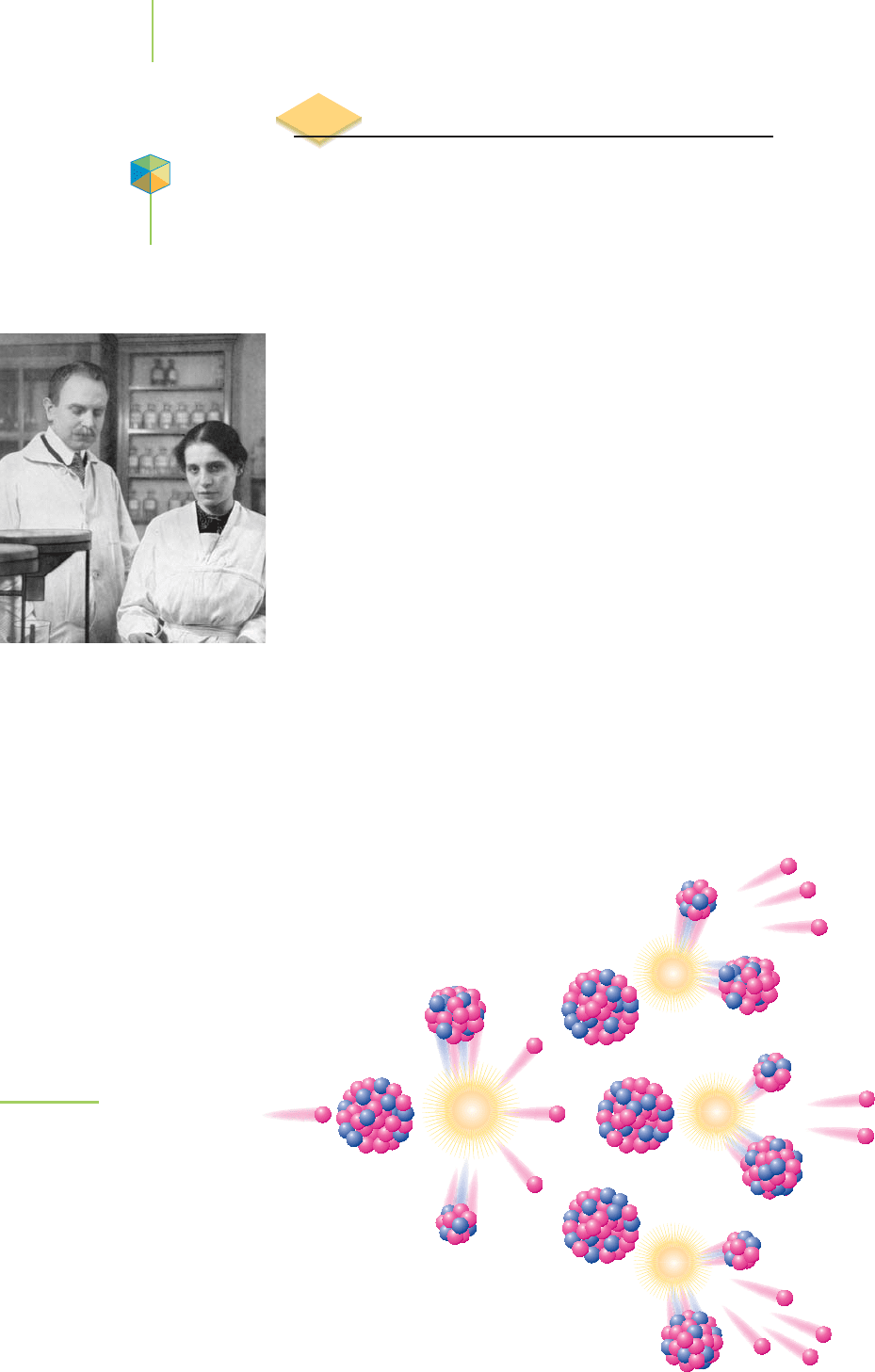
nanoseconds. Figure 21.11 illustrates the process for the fission of uranium-235.
239
94
Pu +
1
0
n → [
240
Pu] →
70
30
Zn +
167
64
Gd +3
1
0
n +energy
235
92
U +
1
0
n → [
236
U] →
139
56
Ba +
94
36
Kr + 3
1
0
n +energy
Nuclear reactions such as these were initially of interest because the tremen-
dous energy they released could be unleashed in a weapon. Since the violent birth
of fission in 1945,these reactions have found a variety of other,morehumanitarian
Lise Meitner (1878–1968) and Otto Hahn
(1879–1968). As a woman in the male-
dominated world of the early 1900s,
Meitner worked as a physicist with Otto
Hahn. She was responsible for the dis-
covery of protactinium and was the first
to explain nuclear fission correctly. De-
spite her contributions, Otto Hahn did
not acknowledge her work when he re-
ceived the 1944 Nobel Prize. In belated
recognition of her work on radioisotopes,
element 109 is named meitnerium.
FIGURE 21.11
A nucleus of
235
U undergoing nuclear
fission.

uses. Today, using non-weapons-grade fissionable fuel, they provide the energy
in nuclear power plants worldwide, they power spacecraft and submarines, and
they are the source of many isotopes used in nuclear medicine.
Nuclear Reactors as a Vital Source of Electricity
The 442 nuclear reactors currently in operation worldwide are alternatives to the
pollution and greenhouse gas emissions caused by coal-burning and oil-burning
power generation. The world’s first nuclear power plant went on line in 1954 in
the Russian city of Obninsk. This was immediately followed by the construction
of a nuclear facility in Sellafield, England. It wasn’t until 1957 that the first full-
scale power plant in the United States began operation in Shippingport, Pennsyl-
vania. Municipal power generation by nuclear fission is not new. However, the
use of nuclear fission has been controversial because of safety concerns, the two
most important being the possible accidental release of radiation, and the dis-
posal and long-term (thousands, and perhaps millions, of years!) safeguarding of
radioactive wastes. Accidental releases of radiation occurred on a relatively small
scale at the Three Mile Island nuclear facility in Pennsylvania in 1979 and on a
much larger scale just outside the Ukrainian town of Chernobyl, in 1986. Still,
much of the world uses nuclear power to meet its energy needs, as shown in
Figure 21.12.
The goal of any large power plant is to generate electricity by turning a tur-
bine, which converts the mechanical energy into electricity. Steam, resulting from
21.7 Splitting the Atom: Nuclear Fission 921
Application
C
HEMICAL
ENCOUNTERS:
Nuclear Reactors
as a Vital Source
of Electricity
0 10,000 20,000 30,000 40,000 50,000 60,000 70,000 80,000 90,000 100,000
United States
France
Japan
Russian Federation
Germany
South Korea
Canada
Ukraine
United Kingdom
Sweden
Spain
China
Belgium
Czech Republic
Switzerland
India
Bulgaria
Finland
Slovak Republic
Brazil
South Africa
Hungary
Mexico
Lithuania
Argentina
Slovenia
Romania
Netherlands
Pakistan
Armenia
376 MW
425 MW
449 MW
655 MW
656 MW
935 MW
1185 MW
1310 MW
1755 MW
1800 MW
1901 MW
2442 MW
2676 MW
2722 MW
3040 MW
3220 MW
3528 MW
5801 MW
6602 MW
7585 MW
8869 MW
11,852 MW
13,107 MW
12,599 MW
16,810 MW
20,339 MW
21,743 MW
46,772 MW
63,363 MW
99,210 MW
Megawatts (MW) of electricity
FIGURE 21.12
Nuclear power plants are located in many countries of the world. The United States, France, Japan,
and the Russian Federation possess the majority of the plants, with the capability to produce over
230,000 MW of energy annually.
the heating of water, supplies the energy to turn the turbine. The essential differ-
ence among the different types of power plants is the fuel source that creates the
steam from water. In conventional nuclear reactors, this energy is supplied by the
controlled fission of
235
U (we say that the fission “goes critical”).
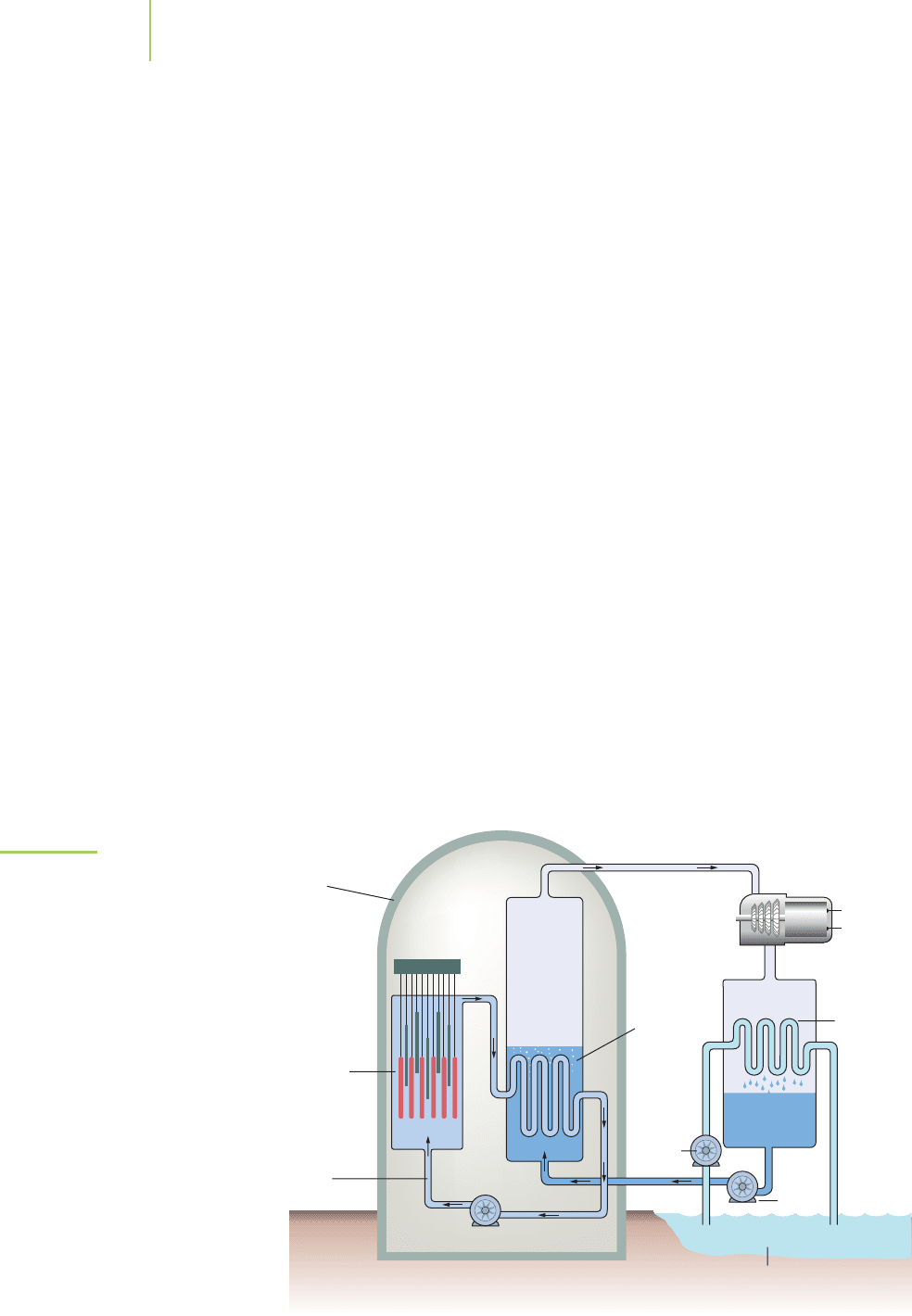
Conventional nuclear reactors have three essential parts, as shown in Fig-
ure 21.13. The nuclear reactor part comprises 100 to 200 fuel rods that contain
the fissionable uranium. A series of movable control rods, typically made of
boron or cadmium, absorb neutrons as a way of controlling the rate of fission.
The rods are located at the bottom of a pool of water, which acts as a moderator
to slow these neutrons so that they can be captured by the uranium in the fuel
rods. The other two parts of the system, the steam generator and the turbine and
condenser, are common to many types of conventional power plants. Other types
of nuclear reactors also exist. Breeder reactors, for example, are so named because
they produce more fissionable fuel than they consume. In these reactors, rela-
tively abundant
238
U is bombarded with neutrons to “breed”
239
Pu, along with the
emission of -particles.
238
92
U +
1
0
n →
239
92
U
−→
239
93
Np
−→
239
94
Pu
Prototype large breeder reactors either have been built or are being built in
China, France, Scotland, the United States, India, Japan, and the former Soviet
Union. Breeder reactors are not currently used in the commercial production of
energy because of the exceptionally long (24,400 years) half-life of
239
Pu. In these
reactors, sodium metal is used as a coolant because it can transfer heat away from
the reactor core much better than water and has a much higher boiling point,
allowing it to remain liquid without being pressurized. Because of the high oper-
ating temperature of the liquid sodium, along with sodium’s capacity to absorb
neutrons (becoming radioactive after it travels by the core), sodium is viewed
as particularly hazardous. The future of breeder reactors is, at the very least,
uncertain.
922 Chapter 21 Nuclear Chemistry
High-
pressure
water
Pump
Pump
Pump
Steam
turbine
Electrical
output
Cooling pond
27°C38°C
Steam
generator
Reactor
Containment
shell
Steam
Control
rods
Condenser
FIGURE 21.13
The essential parts of a nuclear power
plant. The nuclear reactor, submersed in
a pool of water, contains the uranium-
based fuel rods and a series of control
rods to slow the fission process. The
high-pressure water, heated by the fis-
sion, travels to a steam generator where
the heat vaporizes water and creates
high-pressure steam. The steam is used
to turn a turbine and generate electricity.
The steam is then condensed by passing
large amounts of water in a cooling pond
through the condenser.
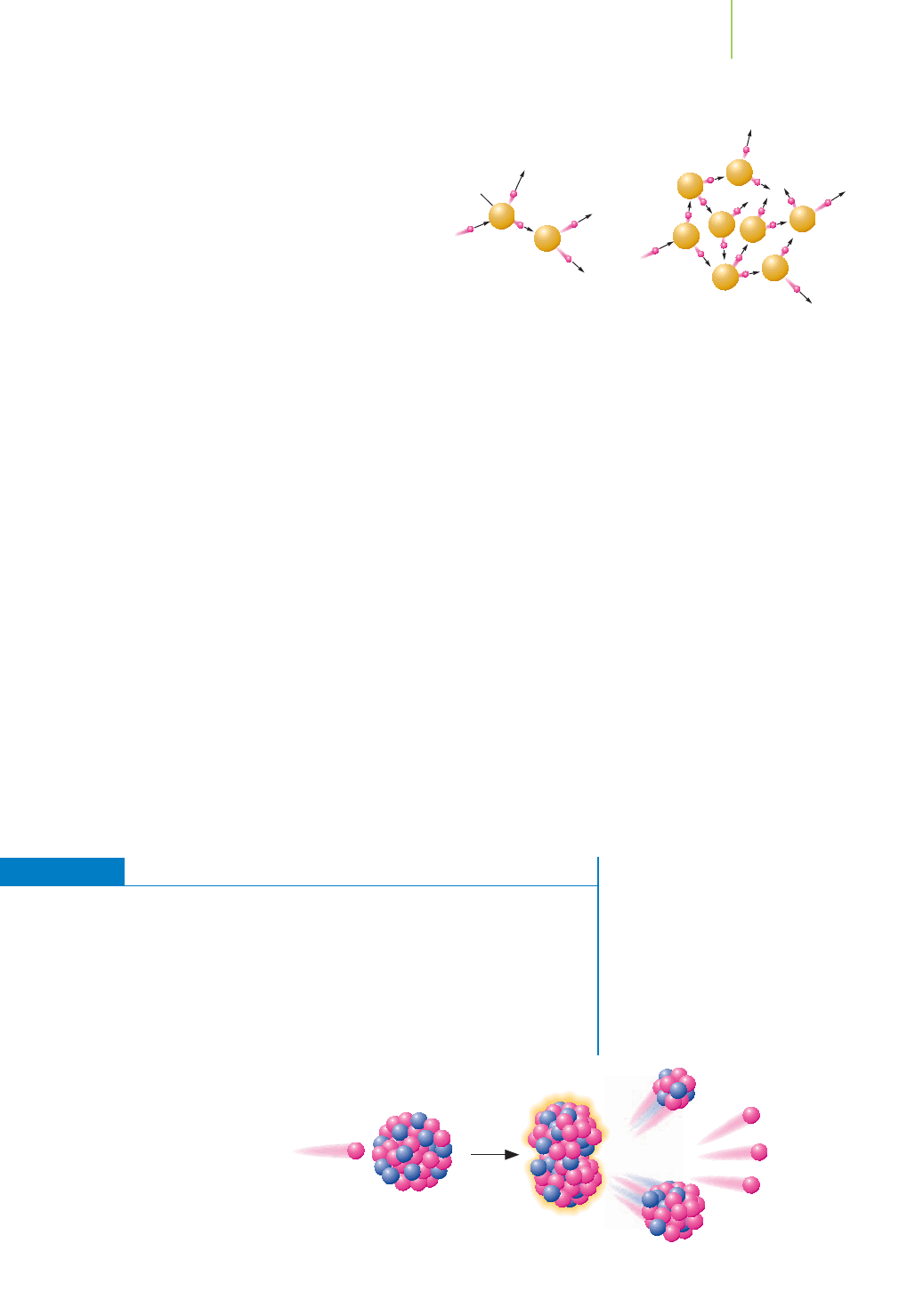
Here are the details of fission as we understand
it today:
■
Fission reactions release energy. The masses of the
product nuclei are less than those of the starting
nuclei, and the source of the energy is this mass dif-
ference. The energy released is orders of magnitude
higher than that of “ordinary” chemical reactions.
■
With rare exception, fission is not a naturally
occurring process. We initiate it in some nuclei
by brute force—that is, by smashing them with a
high-energy neutron. The impact simply drives
the nucleus apart. A few nuclei can be induced to
undergo fission after they capture lower-energy
neutrons. Furthermore, the only naturally occurring fissionable nucleus is
235
U, which is present in only 0.72% of all uranium atoms. The low availabil-
ity of fissionable fuels slowed the development of fission. It also spurred the
breeding of human-made fissionable fuels such as
233
U and
239
Pu.
■
Once induced, fission releases more neutrons, typically 2 to 3 neutrons, per
event. These daughter neutrons usually are traveling fast and may escape with-
out further interaction with a fissionable nucleus. In this instance, we have a
condition known as a
subcritical mass. But if enough fissionable nuclei are
nearby (a
critical mass) or if the neutrons are slowed, these neutrons can con-
tinue the fission process in the absence of a neutron source. A self-sustaining
chain reaction is possible. If too much fissionable material (a supercritical mass)
is present, the reaction goes out of control.
■
Nuclei can split in more than one way, forming a whole host of fission prod-
ucts. The split is usually into two or sometimes three fragments. This means
that fission is “messy” because of the many products.
■
Fission is also messy because the products are usually radioactive. They tend
to be neutron-rich and beta emitters. In the case of nuclear reactors, the ra-
dioactive products end up in high-level and low-level nuclear waste—in other
words, storage. In the case of weapons testing above ground, they result in
nuclear fallout.
EXERCISE 21.7 Fission: A Chain Reaction
Draw a sketch to show why the fission of
235
U can be called a chain reaction. What
factors do you think will influence whether the fission chain reaction will merely
sustain itself or will be explosive?
First Thoughts
Fission of
235
U is initiated by neutrons and produces neutrons. This can result in a
chain reaction if enough of the neutrons released are able to interact with more
235
U.
Solution
As shown here, the fission events
quickly multiply, and the reaction
becomes explosive. But if each fission
event simply produced one more
fission (instead of three), the chain
reaction would simply sustain itself.
21.7 Splitting the Atom: Nuclear Fission 923
n
0
1
n
0
1
n
0
1
n
0
1
U
Fissionable
nucleus
92
235
U
Unstable
intermediate
Fission
products
Energ
y
+
92
236
Ba
56
139
Kr
36
94
Fission of
235
U.
Nucleus
Supercritical massSubcritical mass
Most radioactive
particles escape
the reaction.
Few radioactive
particles escape
the reaction.
The conditions needed to sustain a chain reaction.
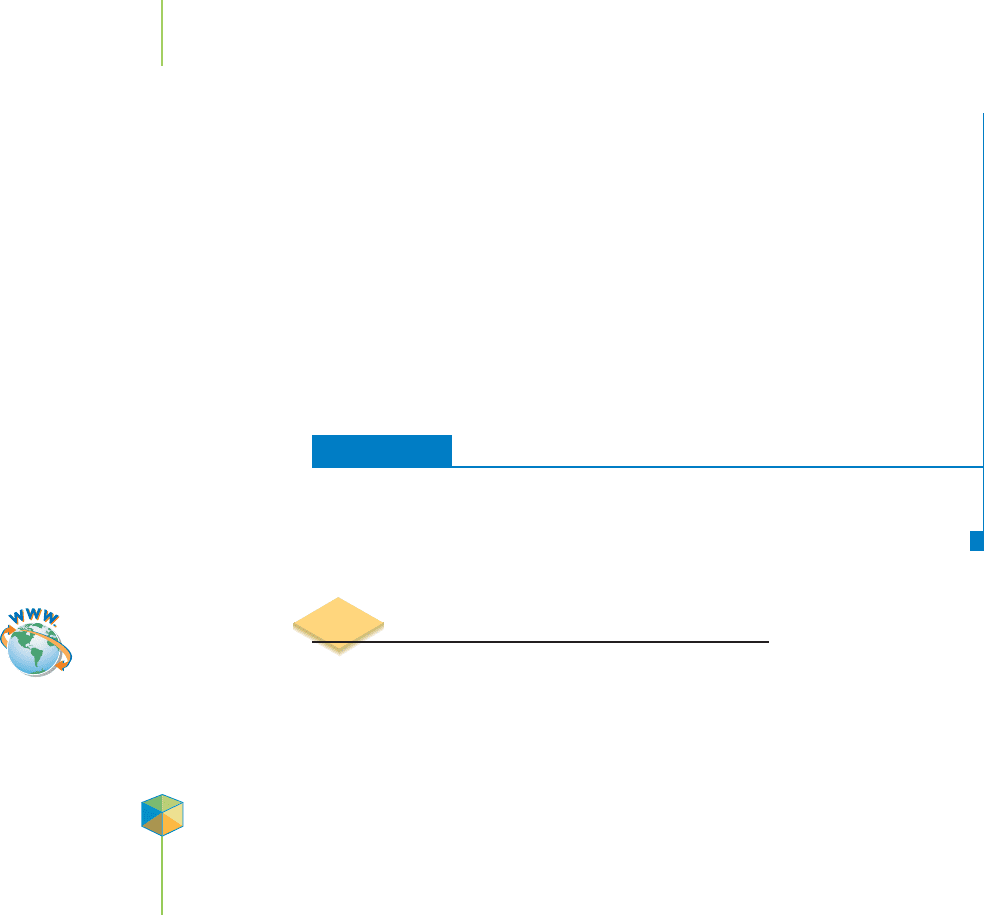
Further Insights
For a chain reaction to be explosive, two conditions must be met: (1) The fission re-
action must produce more than one neutron, and (2) these neutrons must effi-
ciently produce more fission events.
235
U produces 2 to 3 neutrons per event, so the
first condition is met. The second is a bit tricky. First, you need a critical mass of
235
U if you want each fission event to induce one additional event. For
235
U, this is a
fairly large (on the order of kilograms) amount. To be explosive, you need to have
a mass of
235
U that is even greater. Second, you need to minimize the presence of
substances such as
238
U that absorb neutrons and stop the reaction. Finally, you
need to keep the
235
U in one place, which is difficult because the energy released
tends to blow it apart. What are the implications? Although the principles of build-
ing a nuclear weapon are fairly simple, engineering one can be very difficult.
PRACTICE 21.7
Draw a sketch that would illustrate the fission reaction of
235
U that is just sustain-
able. (Hint: A sustainable reaction is not a supercritical chain reaction.)
See Problems 67 and 68.
21.8 Medical Uses of Radioisotopes
Now used almost routinely for diagnosis, radioisotopes enable us to image inter-
nal organs, bone, and tissue structures. We can watch biological processes such as
oxygen uptake and brain activity. Radioisotopes also make possible the treatment
of tumors without anesthesia or invasive surgery. There are more than 40,000
such procedures are carried out in the United States each day.
Tracer Isotopes for Diagnosis
We have already mentioned that you can image certain internal structures such as
bones (or bullets or swallowed coins) using X-rays. The bones, by absorbing
the incoming radiation, show up as shadows on the X-ray films or detectors.
However, the heart, liver, and thyroid gland do not show up very well on X-ray
films because they absorb so little of the X-rays.
Imaging these organs is the job of radioactive tracers, more technically
called diagnostic
radiopharmaceuticals. Radiopharmaceuticals are used in small
amounts, so they release only low amounts of radiation into the body. They are
considered safe to use for diagnostic purposes. Through introduction into the
body by mouth, inhalation, or injection, the radiopharmaceutical can be used to
outline the organs of interest. This imaging occurs by measuring the emitted ra-
diation outside the patient. Either a “hot spot” (a tumor that preferentially ab-
sorbs the radioisotope) or a “cold spot” (a place where the surrounding tissues
preferentially absorb the radioisotope) will produce the desired result. Using an
alpha or beta emitter as a radioactive source would not work, because these parti-
cles would be absorbed by the tissues inside the body before they could be detected
outside the body.
To understand imaging, we no longer can ignore the chemical form of the nu-
clide, as we have done throughout most of this chapter. The art of getting a good
image lies in understanding the chemical behavior of the nuclide in the body. For
example, although you can make elemental iodine,
131
I
2
, from radioactive
131
I,
you cannot feed this chemical to a patient to image the thyroid gland, because io-
dine is chemically reactive and would damage the mouth and stomach. Similarly,
an organic fat-soluble compound containing iodine would tend to concentrate in
924 Chapter 21 Nuclear Chemistry
Application
C
HEMICAL
ENCOUNTERS:
Tracer Isotopes
for Diagnosis
Video Lesson: Applications
of Nuclear Chemistry
the lymph system rather than travel via the bloodstream to the thyroid. The
chemical form of choice for thyroid studies is the iodide ion,
131
I
–
, in the water-
soluble form of sodium iodide,Na
131
I,which can be swallowed in a salty“cocktail.”
Each radioactive substance must be prepared in a carefully tailored chemical
form, because each organ in the body has a different chemical profile.
EXERCISE 21.8
123
I and
131
I : Cousins But Not Twins
Radioactive
131
I is used for both treatment and diagnostic scans, whereas radioac-
tive
123
I is used only for diagnosis. Account for this difference after looking up the
decay mode for each nuclide.
Solution
Both of these isotopes of iodine behave the same chemically in the body (they both
are taken up by a normal thyroid gland), so the difference must lie in their nuclear
properties. A table of radioactive decay shows that both
131
I and
123
I release gamma
rays. Because gamma rays easily penetrate matter, they can be detected outside the
body. Therefore, both can be used for diagnosis. For destruction of a tumor, you
need alpha or beta particles that damage tissue at short distances.
131
I is a beta emit-
ter;
123
I is not.
PRACTICE 21.8
Understanding the biological action of salicylic acid can be enhanced by imaging a
patient fed with a radiopharmaceutical. Which of the following salicylic acid deriv-
atives would not be a suitable choice for such a study?
See Problems 73–76.
In many cases, technetium-99m is the nuclide of choice. The existence of
technetium was predicted by Mendeleev as “eka-manganese,” and it is the only
non-rare-Earth element he foresaw that was not discovered in his lifetime.
Although it was first produced in 1939 by Glen Seaborg and Emilio Segrè, it was
not used in nuclear medicine until the 1960s. Technetium has a versatile chem-
istry and is dispensed in dozens of chemical compounds for imaging different
parts of the body. Furthermore, technetium-99m emits only gamma rays, which
are little absorbed by the body and therefore expose the patient to only a small ra-
diation dose. Because it has such a short half-life, Tc-99m cannot be stored in a
flask on a shelf. Rather, it usually is generated in the hospital from a source of
molybdenum called a “molybdenum (or sometimes technetium) cow”:
99
Mo n
99m
Tc +
0
−1
+
0
0
99m
Tc n
99
Tc +
0
0
The molybdenum-99 isotope used in the cow is not naturally occurring on Earth.
It is generated as a fission product of uranium in nuclear reactors and shipped to
hospitals worldwide. The
99m
Tc nuclide is separated from the molybdenum as it
is needed in a process called “milking the cow.” Figure 21.14 shows the earliest
chromatographic column used to do a hands-on separation of
99m
Tc from
99
Mo.
O
OH
O
3
H
O
131
I
OH
OH
14
C
O
OH
OH
21.8 Medical Uses of Radioisotopes 925
FIGURE 21.14
This photograph shows the early column
chromatography apparatus used to sepa-
rate Tc-99m from the parent isotope
(Mo-99). The molybdenum remains on
the column, and the technetium
passes through. The early work on
technetium-99m was done at
Brookhaven National Lab.
926 Chapter 21 Nuclear Chemistry
Why does nuclear medicine employ such
exotic nuclei as technetium and palla-
dium yet seemingly ignore the elements that make up
most of our body, including hydrogen, oxygen, carbon,
nitrogen, sulfur, and potassium? All of these elements
play biochemical roles in the development of cells and
tissues, yet none has been mentioned as a diagnostic or
therapeutic tool. Why the omission?
Think back on what is needed to do imaging. First is
a source of radiation that penetrates well enough to be
detected outside the body. Gamma emitters usually are
the nuclides of choice. Second, you need availability of
the nuclide in sufficient quantity to do a study. Third,
you need a half-life that is reasonably short, and if it is
very short, the nuclide must be generated on site. Finally,
you need to create a chemical form of the nuclide that
will give either a “hot spot” or a “cold spot” in the area of
medical interest.
Carbon-14, though biologically active, has too long
a half-life and is a pure beta-minus emitter (see Fig-
ure 21.15). However,
11
C, with a n/p that is lower than
those of the stable isotopes, is a positron emitter. Other
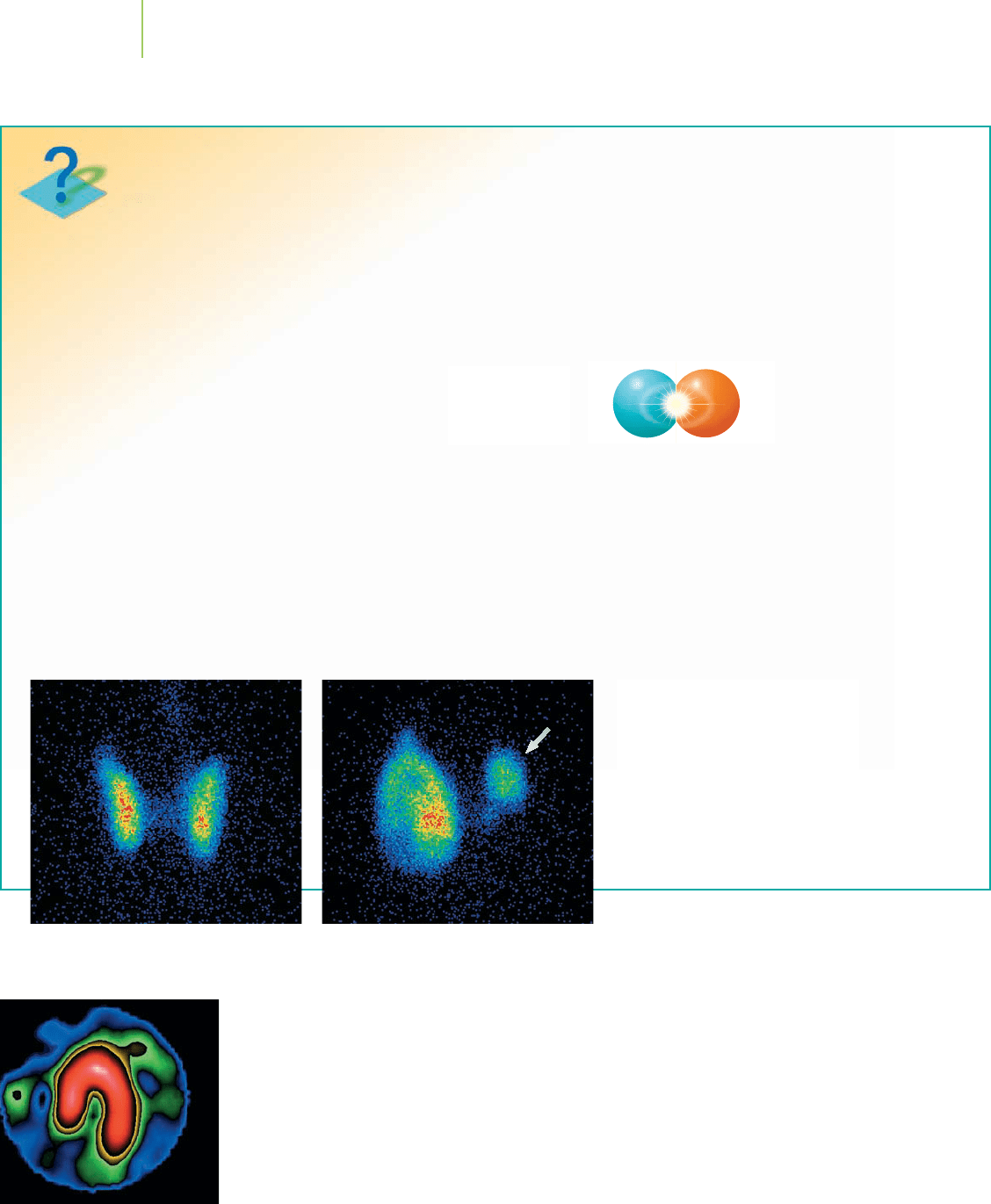
How do we know?
Imaging with Positron Emission Tomography (PET)
These thyroid scans were taken using
radioactive iodine (I-123). The normal
scan on the left shows uniform iodine
uptake; the two thyroid lobes are simi-
lar in size. The lobe marked with an
arrow in the photo on the right is not
functioning properly, as is typical in thy-
roid cancer. A biopsy would follow to
confirm the presence of cancer.
positron emitters include
15
O,
13
N, and
30
S. Positrons
themselves do not penetrate very far. But when a positron
encounters an electron, which happens almost imme-
diately, an annihilation occurs whereby the particle
(positron) and antiparticle (electron) are converted into
energy:
0
+
1
+ e
−
n 2
0
0
The photons from the two gamma rays are emitted in
exactly opposite directions. When a gamma detector is
positioned both above and below the patient, if each one
simultaneously records an event, then a positron was
annihilated. By feeding the data from the detectors into a
computer, it is possible to reconstruct an image of where
the positron emission took place.
Positron emission imaging is better known as a PET
scan, short for positron emission tomography. It is a
Today such processes are automated. The half-life of
99
Mo is a brief 67 hours, so
it is shipped to medical suppliers for immediate distribution to hospitals.
One of the more widely used technetium compounds is sodium pertechne-
tate, NaTcO
4
. The pertechnetate ion, TcO
4
–
, has properties similar to those of the
chloride ion, Cl
–
, and concentrates in brain tumors, in the thyroid and salivary
glands, and in areas of the body where blood is pooling (as happens in internal
bleeding). Similarly, technetium pyrophosphate, TcP
2
O
7
, can be used to image
the heart to see the extent of damage to heart muscle after a heart attack.
Although it is well developed, nuclear medicine is still a relatively young
field; radioisotope tracers were developed in the 1930s and put into widespread
Tc image of heart muscle.
e
–
e
+
A burst of energy is
released as a
positron and an
electron collide.
21.8 Medical Uses of Radioisotopes 927
FIGURE 21.15
The isotopes of carbon.
9
C
10
C
11
C
12
C
13
C
14
C
15
C
16
C
17
C
Type of decay
Half-life
n/p ratio too low
EC
0.127 s 19.3 s 2.45 s 0.75 s 0.19 s
20.3
min
5715
years
EC
+
or EC
Stable Stable –– – –
n/p ratio too high
use in the 1950s and 1960s. In the 1980s, the ready availability of computers
to help process images led to explosive growth in the field that continues
today. There are announcements of new techniques, new types of images, or
methods that require lower amounts of radiation almost daily. (There are also
cautions that costly scans are being used too routinely to warrant either the
risks or the costs involved.) The odds are that you know somebody who has
waged battle with cancer. Nuclear medicine is undoubtedly a part of that per-
son’s health history.
much trickier procedure than other types of nuclear
imaging, largely because positron emitters tend to have
half-lives on the order of minutes. To have enough
radioactive material present, they must be produced
nearby or on site at a hospital. In either case, tech-
nicians are needed to maintain the production
equipment. PET scans also require the injection of
radioactive material, which is not the case for MRI
or CT scans.
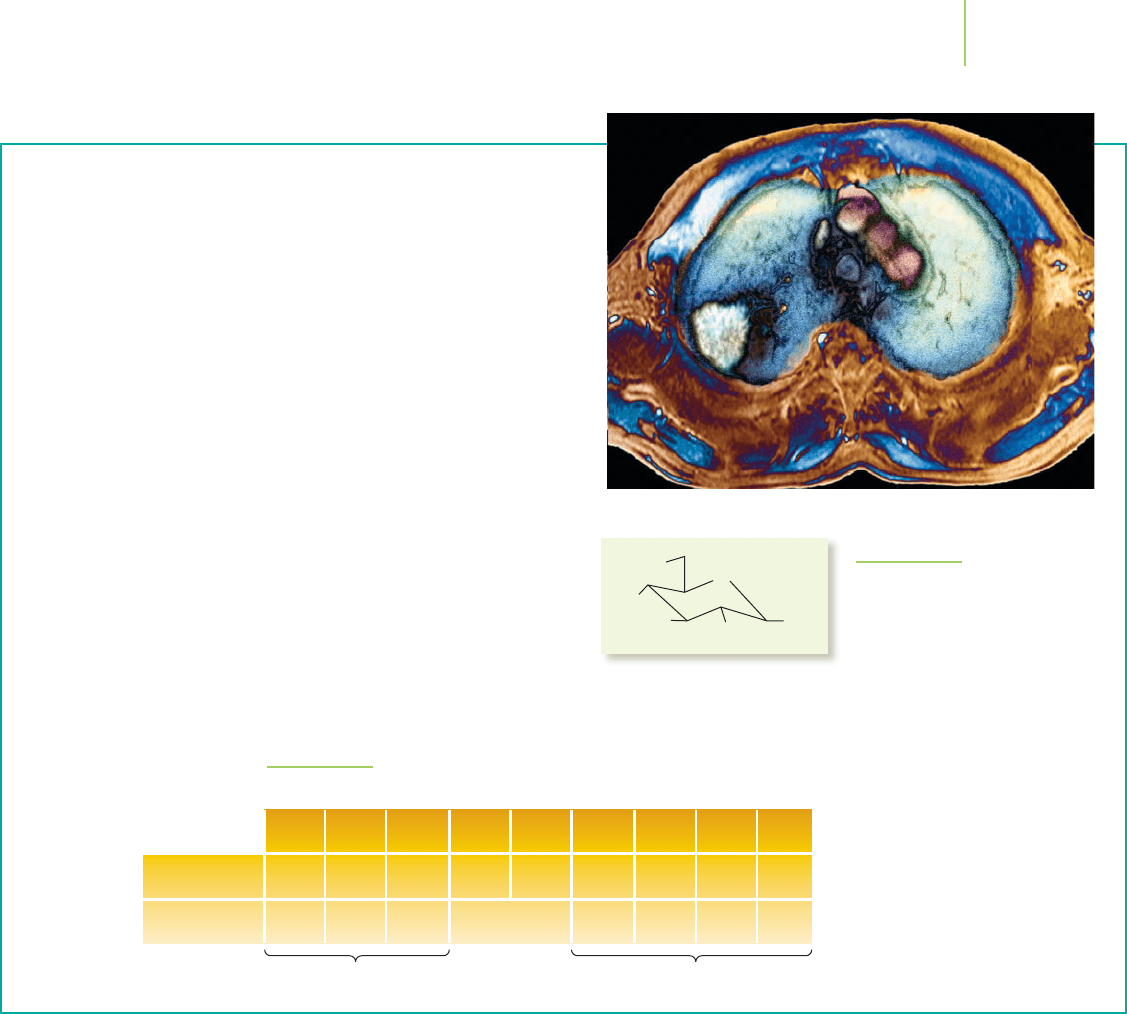
The payoff with PET, though, is impressive. It
produces “functional imaging”—that is, images of
chemical processes in action. For example, it can
record the brain in action during a seizure or when
the patient is hearing music, thus decoding the
neural pathways. Using glucose labeled with
11
C or
with
18
F (see Figure 21.16), PET can reveal brain
metabolism. Similarly,
18
F-labeled estrogen can be
used within a patient to show how tumors grow.
The color-enhanced real-time images are far more
dramatic than the black-and-white slices produced
by other means.
HO
HO
HO
O
OH
18
F
FIGURE 21.16
18
F-labeled 2-deoxyglucose, or FDG,
is used to study glucose metabolism
in the body. It can be used to differ-
entiate benign tumors from cancer-
ous ones, because the latter use glu-
cose at a higher rate. After a patient
consumes this radiopharmaceutical,
tumors within her or his body show
up as white spots where glucose
metabolism is higher.
