Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


41. Which of these species would produce the greater number of
ions per mole when dissolved in water?
K
2
[Cr(C
2
O
4
)
2
(H
2
O)
2
]or
tetraamminediaquachromium(III) nitrate
42. Which of these species possesses the larger positive charge on
the complex ion?
tetraaquacopper(II) nitrate or
dichlorobis(ethylenediamine)iron(III) bromide
20.7 Color and Coordination Compounds
Skill Review
43. What is the electron configuration for each of these transi-
tion metal ions?
a. Fe
2+
b. Cr
2+
c. Zn
2+
44. What is the electron configuration for each of these transi-
tion metal ions?
a. Pd
4+
b. Ag
+
c. Mn
2+
45. Consider the following two transition metal ions as free
gaseous ions. Which would have the greater number of un-
paired d electrons, Fe
3+
or Cu
2+
?
46. Which free ion has the greater number of unpaired d elec-
trons, Ti
2+
or Co
2+
?
47. Draw the orbital diagram for the d orbitals in an octahedral
complex containing each of these metal centers. (Assume
that P < ∆
o
.)
a. Fe
3+
b. Co
2+
c. Ni
2+
48. Draw the orbital diagram for the d orbitals in an octahedral
complex containing each of these metal centers. (Assume
that P > ∆
o
.)
a. Mn
2+
b. Fe
2+
c. Cr
+2
49. Repeat Problem 47, but assume that the metal centers are in-
volved in tetrahedral complexes. Although tetrahedral com-
plexes typically have ∆
t
> P, what would you draw if the
tetrahedral complex existed with P > ∆
t
?
50. Repeat Problem 48, but assume that the metal centers are in-
volved in square planar complexes. Assume that P > ∆ in this
problem.
51. Draw the orbital diagram for the metal center in each of these
complexes. Use the information in the spectrochemical series
to assist you in placing the orbitals.
a. [FeCl
4
]
−
b. [Co(CN)
6
]
3−
c. [Mn(CO)
6
]
+
52. Draw the orbital diagram for the metal center in each of these
complexes. Use the information in the spectrochemical series
to assist you in placing the orbitals.
a. [CuF
6
]
4−
b. [Ni(OH)
6
]
4−
c. [Cr(NO
2
)
6
]
4−
53. Which of the complexes in Problems 47 and 48 is(are)
paramagnetic?
54. Calculate the magnetic moment for each of the complexes in
Problems 47 and 48.
Chemical Applications and Practices
55. Which one of these complexes would you predict to absorb
blue light: [M(CN)
6
2−
], [M(H
2
O)
6
4+
], [MCl
6
2−
], or
[M(NH
3
)
6
4+
]?
56. Which of these complexes would you predict to absorb
the longest wavelength of visible light: [M(CN)
6
2−
],
[M(H
2
O)
6
4+
], [MCl
6
2−
], or [M(NH
3
)
6
4+
]?
57. The colors of common gemstones are due to the presence of
transition metal ions. The color is produced when the metal
ion absorbs visible light. Would you predict the common
gemstones to have different “colors” under infrared light?
58. Coordination compounds with Zn
2+
ions typically are white
or colorless. Explain why this particular metal does not form
brightly colored compounds the way many other transition
metals do.
59. The crystal field theory provides an explanation of color in
various coordination complexes. For example, [Cr(H
2
O)
6
]
3+
can be detected as a violet color when dissolved.
a. What colors would the complex be absorbing?
b. How many unpaired electrons does the central chromium
ion have?
c. The compound [Cr(NH
3
)
6
]
3+
, when dissolved, appears
yellow. Would you expect it to absorb light at a higher or
lower frequency than Cr(H
2
O)
6
]
3+
? Explain.
d. Which ligand, NH
3
or H
2
O, is causing the greater value of
∆
o
?
60. Compare the two iron complexes [Fe(H
2
O)
6
]
3+
and
[Fe(CN)
6
]
3−
.
a. Which is more likely to be paramagnetic?
b. Which is more likely to absorb light of greater energy?
c. Which is more likely to be “high-spin”?
20.8 Chemical Reactions
Skill Review
61. Explain why the chelate effect typically provides for a very
favorable entropy change when a ligand exchange reaction
involves a complex going from a nonchelated complex to a
chelate complex.
62. If a ligand exchange reaction produced a large positive ∆G
value, would you expect the reaction to have a large or a small
equilibrium constant? Justify your choice.
63. A common chemical demonstration is to change a light blue
solution containing [Cu(H
2
O)
4
]
2+
quickly to a deep purple
solution by changing [Cu(H
2
O)
4
]
2+
into [Cu(NH
3
)
4
]
2+
with
the addition of ammonia to the solution. Would you con-
sider the first compound labile or inert? Is the exchange of
oxygen in hemoglobin considered to be representative of a
labile or an inert complex?
64. Except through loss of blood, the level of iron in humans is
fairly constant. One way that iron is moved throughout the
body, particularly from the liver, is within a molecule known
as ferritin. The iron(III) is held in a six-coordinate system
through bonds to oxygen and nitrogen that are part of several
amino acids. Would it be more logical for this molecule’s iron
site to be labile or inert with respect to other metals? Explain.
(Remember, the terms inert and labile refer to kinetic consid-
erations, not to equilibrium predictions.)
898 Chapter 20 Coordination Complexes

Focus Your Learning 899
Comprehensive Problems
65. In addition to the coordinationcomplexes of rhodium,cobalt,
and molybdenum described at the start of the chapter, use
other resources (the Internet or journals) to select another
transition metal that has a catalytic role in a chemical reaction.
66. Visit the pharmacy section of a grocery store and list the met-
als found in a mineral supplement. Use a reference (the In-
ternet or journals) to determine the primary biochemical
function of two of the metals from your list.
67. If a complex were assembled from a Co
3+
ion, four NH
3
, and
two Cl
−
, would you expect to see a neutral compound, an
anion, or a cation? Show a formula that justifies your answer.
68. The following complex contains an iron ion in the +3 state.
However, the resulting charge has been omitted from the
complex.Assign the charge for the complex, and indicate how
many counter ions (either Na
+
or Cl
−
) would have to be ion-
ically bonded to the complex to form a neutral compound.
[FeBr
4
(H
2
O)
2
]
69. The appearance of a visible color from a compound is associ-
ated with three fundamental phenomena. Describe a color-
producing compound’s properties associated with:
a. electron excitation
b. the energy of the photon of light being absorbed
c. the relative occupancy of lower and higher level orbitals
70. If 35.7 g of the complex Ca
3
[Fe(C
2
O
4
)
3
]
2
formed as a result of
using an oxalate-containing rust remover, how many grams
of iron would be removed?
71. Polydentate ligands have been very effective in applications
of soil chemistry. EDTA has been added to soil near citrus
trees to concentrate iron. Some polydentates have been used
to extract heavy metals from soil samples for further analysis.
Some internal digestive functions use polydentate ligands to
extract metals from foods we eat. Does this indicate that the
relative equilibrium constant for these reactions is larger or
smaller than one?
Thinking Beyond the Calculation
72. A new ligand is developed for use in a study to mimic the
electron transport reactions of copper metal.
a. Draw the octahedral complex that would result from the
use of this ligand and copper(II) ions.
b. Draw the d orbital diagram for an octahedral complex of
this ligand.
c. Compare the color of this octahedral complex to that of
[Cu(NH
3
)
6
]
2+
. Where does this ligand most likely fall in
the spectrochemical series?
d. Under certain conditions, 1.00 g of copper(II) produces
3.96 g of the copper–ligand complex. What is the coordi-
nation number of the complex under these conditions?
e. The equilibrium constant for the ligand exchange of this
new ligand and chloride ions is 1.45
×
10
−4
. What does
this indicate about the new ligand?
Ethanedithiol
HS SHCH
2
CH
2
Cu
+
–ligand [Cu(NH
3
)
6
]
+

Nuclear
Chemistry
Enrico Fermi used this very large cyclotron
at the University of Chicago in the 1950s.
The cyclotron can be used to prepare
radioactive isotopes for medical imaging.
The cyclotron generates fast-moving
subatomic particles that can be directed
at nonradioactive nuclei. The resulting
collision produces radioactive nuclei such
as fluorine-18, which can be used to help
doctors observe a particular biological
function within a patient’s body.
900
Contents and Selected Applications
21.1 Isotopes and More Isotopes
21.2 Types of Radioactive Decay
21.3 Interaction of Radiation with Matter
Chemical Encounters: Radiation and Cancer
21.4 The Kinetics of Radioactive Decay
21.5 Mass and Binding Energy
21.6 Nuclear Stability and Human-made Radioactive Nuclides
21.7 Splitting the Atom: Nuclear Fission
Chemical Encounters: Nuclear Weapons
Chemical Encounters: Nuclear Reactors as a Vital Source of Electricity
21.8 Medical Uses of Radioisotopes
Chemical Encounters: Tracer Isotopes for Diagnosis
Go to college.hmco.com/pic/kelterMEE for online learning resources.
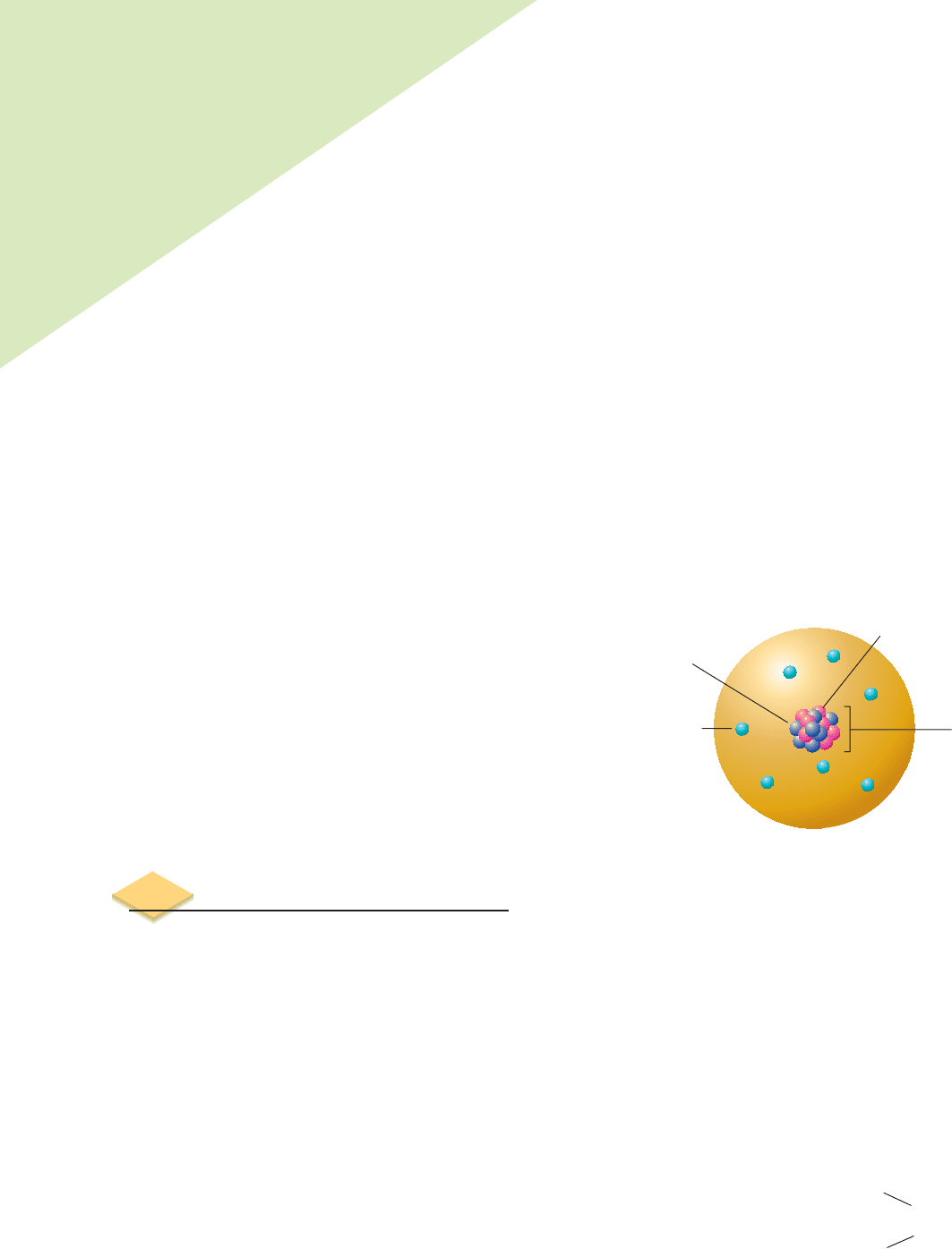
19
K
40
Mass
number
Atomic
number
The odds are that you know somebody who
has fought cancer. If you have talked with a friend
or relative who is a cancer patient, you may have heard
that radioactive substances are used in the process of pro-
ducing images of internal organs. You may know that radiation
can shrink a tumor or kill cancer cells. You may even be aware of the
dramatic procedure using full-body radiation that is given before a bone
marrow transplant. These applications illustrate how a health care team can
use
nuclear radiation for the benefit of a cancer patient.
Although radiation can save lives, it can also damage and kill. Back in the early
1900s, early radiologists held film plates in an X-ray beam to get pictures of their pa-
tients. These radiologists often developed sores on their hands that would not heal,
and some eventually lost parts of their fingers. In August 1945, people in the Japanese
cities of Hiroshima and Nagasaki were exposed to huge bursts of radiation from the
only use of atomic bombs in warfare. Some died immediately, and others succumbed
a few weeks later from a then-unknown disease that we now call radiation sickness.
In the United States, those who worked in the nuclear weapons industry are now dis-
proportionately contracting lung, stomach, lymphatic, and other cancers.
How can nuclear radiation both cure and cause cancer? The answer to this seeming
paradox rests on our understanding of
radioactivity (the release of particles and en-
ergy accompanying a nuclear change) and how it is pro-
duced from nuclear processes. In this chapter we will exam-
ine types of radioactive decay, touching on the mathematics
of half-lives, the relationship between mass and energy, and
the interactions of ionizing radiation with matter. We also
will examine nuclear fission, a process that helps produce a
whole host of substances useful in nuclear medicine. The
story of radioactivity begins, however, at the tiny center of
the atom—its nucleus.
901
Nucleus
–
–
–
–
–
–
–
+
+
+
+
+
+
+
Proton
Neutron
Electron
21.1 Isotopes and More Isotopes
Nuclei that occur naturally on Earth can be as small as the nucleus of a hydrogen
atom (a single proton) or as large as uranium (92 protons plus 146 neutrons). All
of these naturally occurring atoms, and all those that are human-made, are rep-
resented by writing their element symbol in the manner described in Chapter 2.
The symbol is placed next to a superscripted mass number and a subscripted
atomic number. Some of these nuclei are stable—that is, they do not sponta-
neously decompose—and others are radioactive, decomposing to other nuclei.
As we will soon see, the size and makeup of the nucleus determine whether it is
stable or radioactive.
Recall from Chapter 2 that we can use nuclide notation, in which we list the
symbol for the element, accompanied by its atomic number and the mass num-
ber, to indicate the isotope of the element that we wish to describe. For example,
consider the element potassium, whose atomic number (Z) is 19. As we know
from the periodic table, this element has 19 protons. If the nucleus of a potassium
atom has 21 neutrons, we represent this in nuclide notation as
40
19
K
. The num-
ber 40, the mass number, is the sum of 19 protons and 21 neutrons. Because “19
protons” is always potassium, we can more simply represent
40
19
K
as
40
K, K-40 or
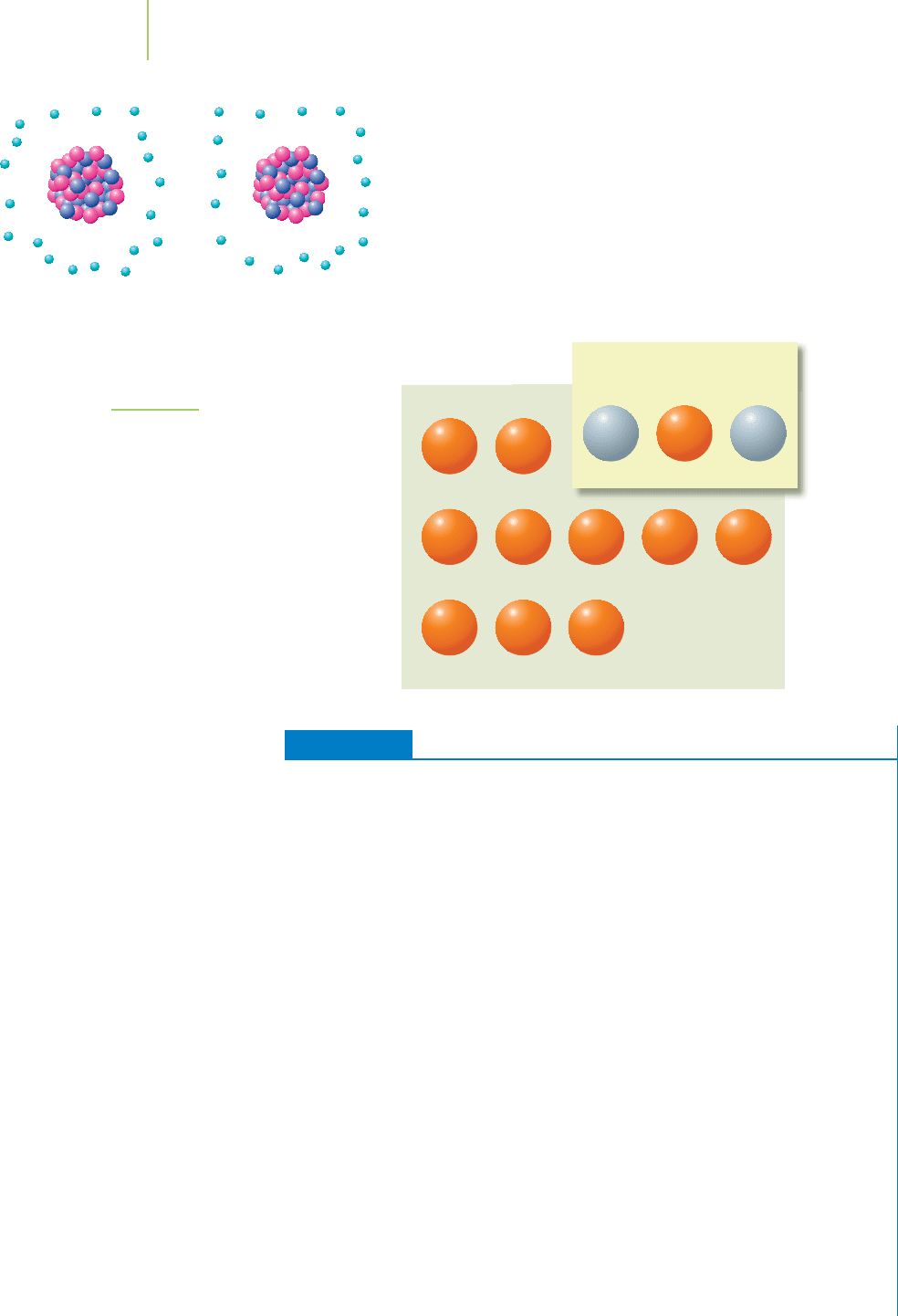
37
K
38
K
39
K
40
K
41
K
42
K
43
K
44
K
45
K
46
K
47
K
48
K
49
K
Human-made isotopes
Naturally occurring
isotopes of potassium
93.258% 0.012% 6.730%
potassium-40. In nuclear chemistry, we are often not concerned
with the number of electrons around a particular nucleus. We are
interested only in the nucleus of the atom, so we often omit
charges (even though they may exist). This means that in our
chemist’s shorthand for the discussion of changes in the nucleus,
we’ll consider
40
K
+
as
40
K. The nucleus is our only focus here.
Potassium atoms come in several varieties,shown in Figure 21.1,
some with fewer than 21 neutrons and some with more. These
varieties are known as isotopes, and each isotope is known as a
nuclide of that element, as we learned in Chapter 2.
902 Chapter 21 Nuclear Chemistry
KK
+
FIGURE 21.1
Potassium has many isotopes, most of
which are listed here. Those colored
orange are radioactive. Natural abun-
dances are provided for those isotopes
that occur in nature. Note that 99.988%
of the potassium atoms on Earth are
not radioactive.
EXERCISE 21.1 Decoding Isotopes
Describe the differences and similarities in nuclear structure among the members of
each of these sets:
a.
12
6
C
,
13
6
C
, and
14
6
C
b.
40
Ar,
40
K, and
40
Ca
c.
40
Ca
2+
and
40
Ca
First Thoughts
The numbers at the bottom of each representation are simply a restatement of the
element symbol. The mass number is the sum of the number of neutrons and
protons. To find the number of neutrons in a particular nuclide, just subtract the
atomic number from the mass number. Remember from Chapter 2 that numbers
written on the upper right-hand side of the nuclide notation indicate a deviation in
the number of electrons.
Solution
a.
12
6
C
,
13
6
C
, and
14
6
C
are isotopes of carbon, each containing six protons. But each
has a different mass number and hence a different number of neutrons: 6, 7,
and 8, respectively.
b.
40
Ar,
40
K, and
40
Ca have the same mass number but different numbers of
protons. Ar has 18p and 22n, K has 19p and 21n, and Ca has 20n and 20p.
c.
40
Ca
2+
and
40
Ca differ only in the number of electrons, so there is no differ-
ence in nuclear structure. The
40
Ca atom loses two electrons to form the
40
Ca
2+
ion.
Further Insights
This exercise, while conceptually important, involves just notation and bookkeep-
ing. The nuclide notations, by themselves, say little about the stability or radio-
activity of an isotope. Far more useful and interesting are the topics of natural
abundance, radioactivity, and half-life, which we discuss below.
PRACTICE 21.1
Indicate the number of protons and neutrons in each of the following nuclides.
a.
32
S c. radon-222
b.
23
11
Na
d. Tc-98
See Problems 7 and 8.
Potassium is considered an essential nutrient. Deficiencies in potassium can
result in muscle pain, angina, and even heart problems. Eating bananas is one way
in which we can ensure a healthy supply of potassium in our bodies. From Fig-
ure 21.1 we can see that three isotopes of potassium occur naturally:
39
19
K
,
40
19
K
, and
41
19
K
. However, whereas potassium-39 and potassium-41 possess stable nuclei,
40
19
K
is radioactive. This means that when we consume a banana, we get a measurable
amount of radioactive potassium-40. How much? The natural abundance of
potassium-40 is only 0.012%, or approximately 1 atom in 10,000. A typical ba-
nana has approximately 300 mg of potassium. Therefore, with each banana we
eat, we ingest approximately 0.036 mg of radioactive potassium-40.
Although potassium has 18 known isotopes, most do not occur in nature and
must be produced in a laboratory. Each of these isotopes behaves essentially the
same in chemical reactions, which involve the interaction of electrons with other
atoms. For example, on exposure to oxygen or moisture, potassium metal ionizes
to form K
+
. All isotopes of potassium behave in this manner. Because our planet
is wet and blanketed in oxygen, all naturally occurring potassium isotopes are
found in nature as K
+
. Remember, however, that we often omit the charge when
writing nuclides, and you are likely to see
40
K rather than
40
K
+
. The key idea here
is that we differentiate between chemical reactions (electron interactions between
atoms) and nuclear processes (changes within the nucleus of an individual atom).
Potassium-39 is a stable nuclide, but potassium metal certainly is not a stable
chemical when in the presence of water. On the other hand, argon is chemically
inert, but it has isotopes that are radioactive.
Natural isotopic abundances for potassium or
for any element can be found in many of the chem-
istry handbooks in the library. Table 21.1 offers a
brief look at what you’ll find there. As we just saw,
the nuclei of elements present in nature are not
necessarily stable. In fact, some elements, such as
uranium and radon, exist naturally only in ra-
dioactive forms. Other elements, such as potas-
sium and carbon, have both stable and radioactive
isotopes. Still others, such as aluminum, have only
one stable naturally occurring form.
21.1 Isotopes and More Isotopes 903
Sources of potassium.
Application
Natural Isotopic Abundances for Selected Elements
Carbon
12
C 98.90%
13
C 1.10
Oxygen
16
O 99.762%
17
O 0.038
18
O 0.200
Magnesium
24
Mg 78.99%
25
Mg 10.00
26
Mg 11.01
Aluminum
27
Al 100%
TABLE 21.1
Potassium
39
K 93.2581%
40
K 0.0117
41
K 6.7302
Iron
54
Fe 5.8%
56
Fe 91.72
57
Fe 2.2
58
Fe 0.28
Silver
107
Ag 51.84%
109
Ag 48.16
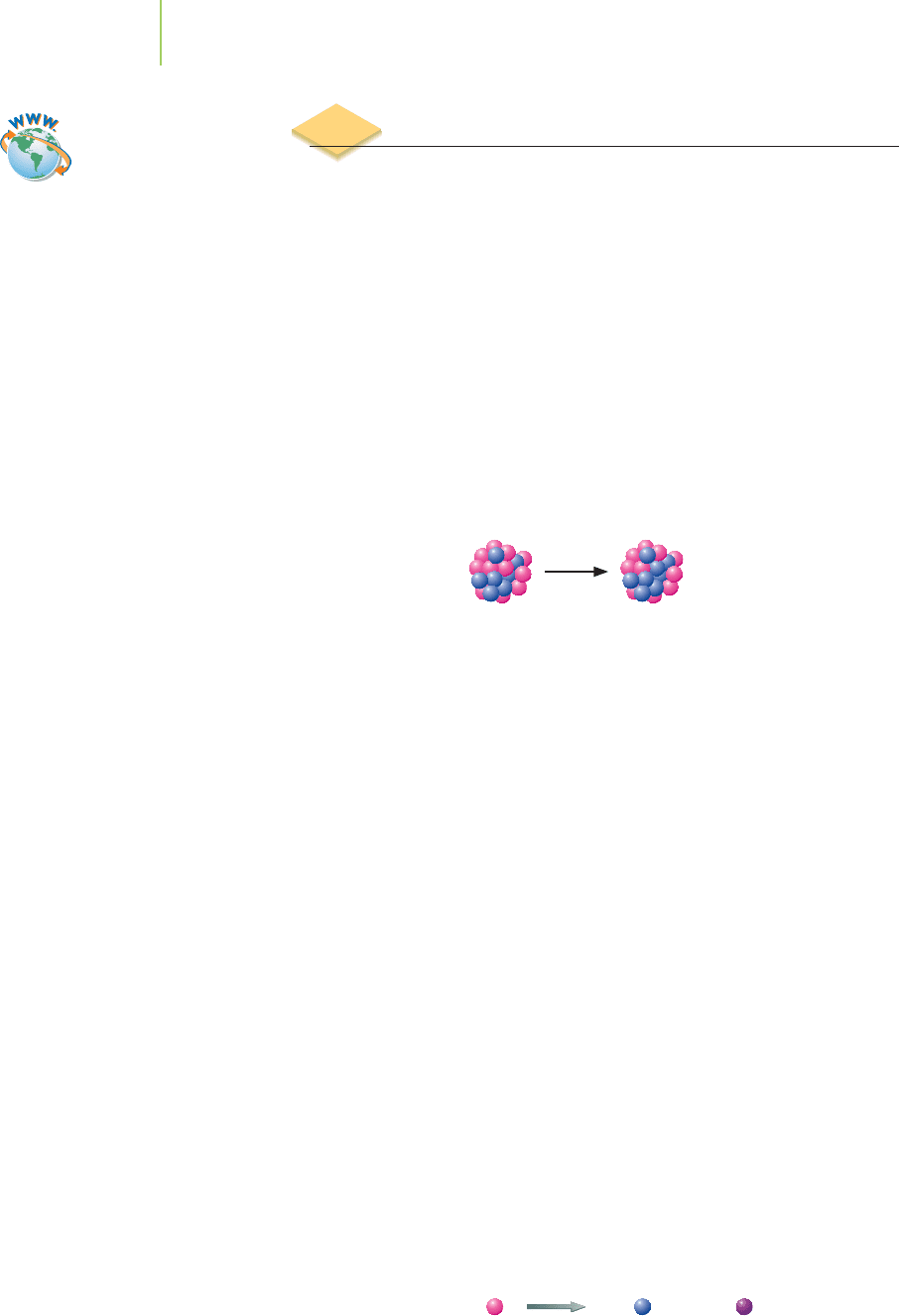
21.2 Types of Radioactive Decay
There is no way for you hold a potassium atom in your hand and peer into its nu-
cleus. But if you could keep an eye on a few
40
K atoms for a period of time (per-
haps over a billion years), you would observe that some of the potassium atoms
had been replaced by atoms of calcium.
How do we account for this nuclear sleight-
of-hand?
Beta-Particle Emission
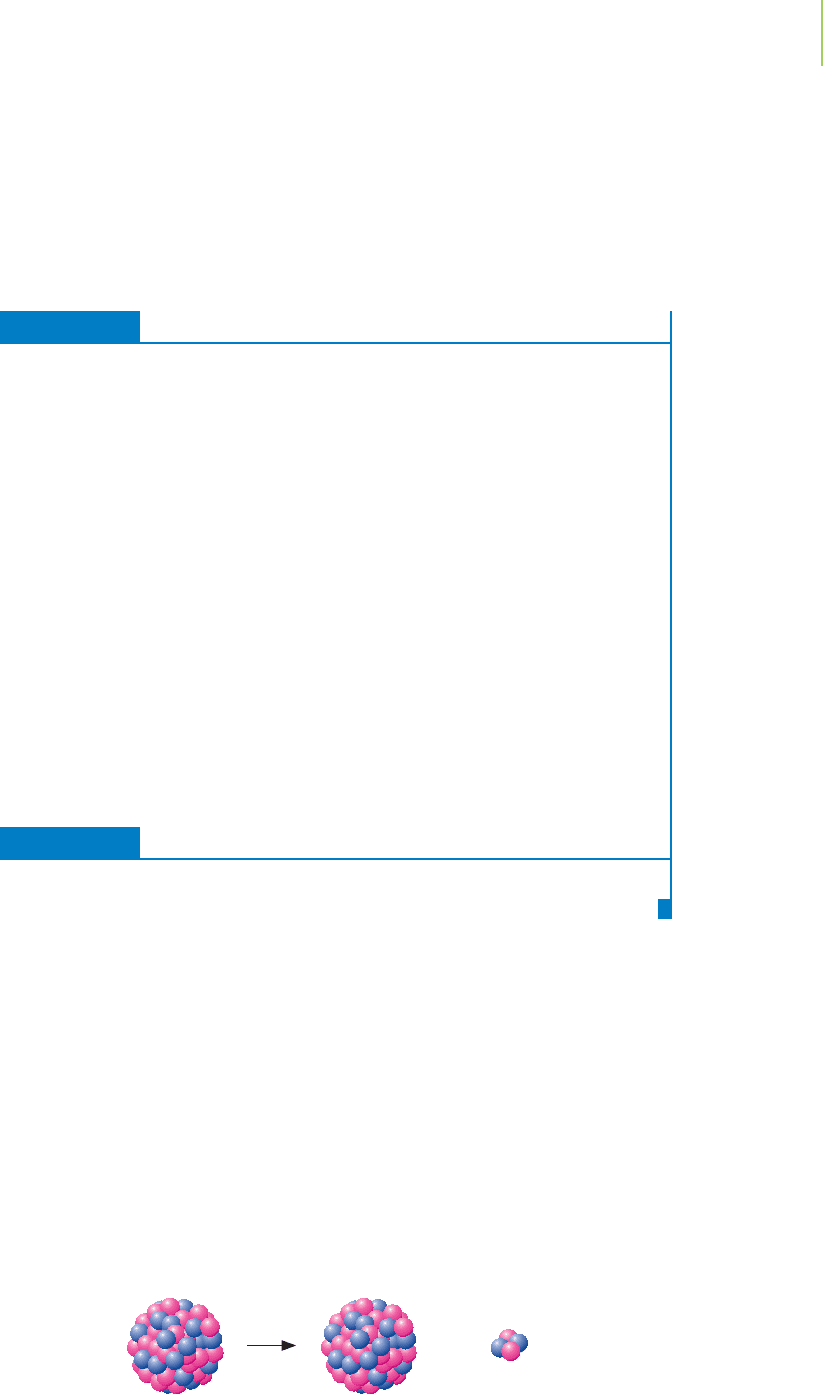
The answer in this case is beta-particle emission, a type of radioactive decay. In
beta emission, the nucleus of
40
19
K
ejects a
beta particle,
0
−1
, that travels at 90% the
speed of light. A new nucleus,
40
20
Ca
, is formed that has one more proton. Because
calcium-40 has an energetically stable nucleus (you can verify this in a table of
radioisotopes), no further nuclear reactions take place. For now, let’s write the
nuclear equation for the beta-minus emission, or “beta emission” for short, as
40
19
K
n
40
20
Ca
+
0
−
1
We note that the sums of the atomic masses, as well as the sums of the atomic
numbers, are the same on both sides of our equation. The nuclide that results
from the beta emission,
40
20
Ca
, is comparable in size to potassium. In addition, a
tiny product is ejected with mass number zero and a negative charge—an elec-
tron. In the context of nuclear decay, this electron is called a beta emission parti-
cle. The subscript –1 in
0
−
1
may look strange as an atomic number. Interpret it as
a “negative one charge” rather than a “minus one proton.”
Beta emission, like many other of the nuclear reactions we will study, has the
following features:
■
A nuclide of one element, through the process of radioactive decay, is trans-
formed into a nuclide of another. The resulting
daughter nuclide may be stable
or radioactive.
■
The sum of the atomic numbers on one side of the nuclear equation is equal
to the sum on the other. For the beta emission of
40
K, the sum of the mass
numbers on each side of the equation is 40, and the sum of the atomic num-
bers on each side of the nuclear equation is 19.
■
Energy is released in radioactive decay reactions. Gamma rays of varying en-
ergy nearly always accompany the nuclear reaction.
How can an electron be emitted from a nucleus when there are no electrons in
the nucleus?
To see how this could happen, let’s venture down from the
macroworld into the nanoworld to look at the beta emission of a single neutron.
Neutrons aren’t something you can keep in a bottle in your chemistry lab. But if
you had the proper specialized equipment, you could demonstrate that a neutron
decays to form a proton, an electron, and an
antineutrino,
0
0
´ν
.
1
0
n
n
1
1
p
+
0
−
1
+
0
0
´ν
The net effect of beta decay is the transformation of a neutron into a proton with
the release of an electron, an antineutrino, and energy. This is consistent with the
beta decay we wrote for
40
19
K
, where the product nuclide has one more proton.
–1
0
+
904 Chapter 21 Nuclear Chemistry
–1
0
0
0
0
n
1
1
p
In nucleus In nucleus expelled Expelled
1
++
Video Lesson: The Nature
of Radioactivity
The antineutrino,
0
0
´ν
, is an example of antimatter. Each antimatter particle has
a mate in our world of “real” matter. For the antineutrino, this mate is the
neutrino, or “little neutron”—a particle similarly hidden within the neutron. With
no charge and probably very little mass, the neutrino required a sophisticated
piece of scientific detective work to prove its existence. However, because this
ghostly particle and its antimatter mate interact little if at all with matter, neutri-
nos typically are omitted from nuclear equations.
EXERCISE 21.2 Beta Emissions In and Around Us
A look at the world around us reveals a lot of naturally occurring radiation.
Elements responsible for this radiation include carbon-14, potassium-40, and
hydrogen-3 (tritium).
a. Write the nuclear equation for a beta emission by carbon-14.
b. If
3
2
He is formed via a beta emission, what radioisotope produced it?
First Thoughts
Beta emissions follow a set pattern: an increase of +1 in Z and no change in A.
Solution
a.
14
6
C n
0
−
1
+
14
7
N
b.
3
1
H n
0
−
1
+
3
2
He
Further Insights
We discussed the radioactivity of carbon-14 in Chapter 2. Knowing the rate of this
nuclear reaction is part of a process that enables us to predict the age of an archae-
ological artifact.
PRACTICE 21.2
Write the nuclear equation for the beta emission of
60
Co.
See Problems 14, 15b, 15c, and 20.
Alpha-Particle Emission
There are two other common forms of radioactivity: alpha-particle emission and
gamma-ray emission. To appreciate how these differ, let’s revisit potassium-40.
Imagine again that you were watching several individual atoms of the nuclide. No
matter how long you waited, you would not observe an
alpha decay, the emission
of a helium nucleus (
4
2
He
) from a larger nucleus. Why not? Potassium-40, like
many radioisotopes, does not exhibit this form of radioactivity. Why not? The
simple answer is that the nucleus is made more energetically stable by beta emis-
sion than by alpha decay.Why? The answer to this is a little more complex and will
be the focus of Sections 21.5 and 21.6.
Which elements decay by alpha emission? Radon is one example. The nuclear
reaction for the alpha decay of radon-222 is
222
86
Rn →
218
84
Po +
4
2
He
21.2 Types of Radioactive Decay 905
+
Energy is given off, along with an alpha particle (
4
2
He
), which is energetic but trav-
els more slowly than a beta particle, at only about 5–10% of the speed of light.
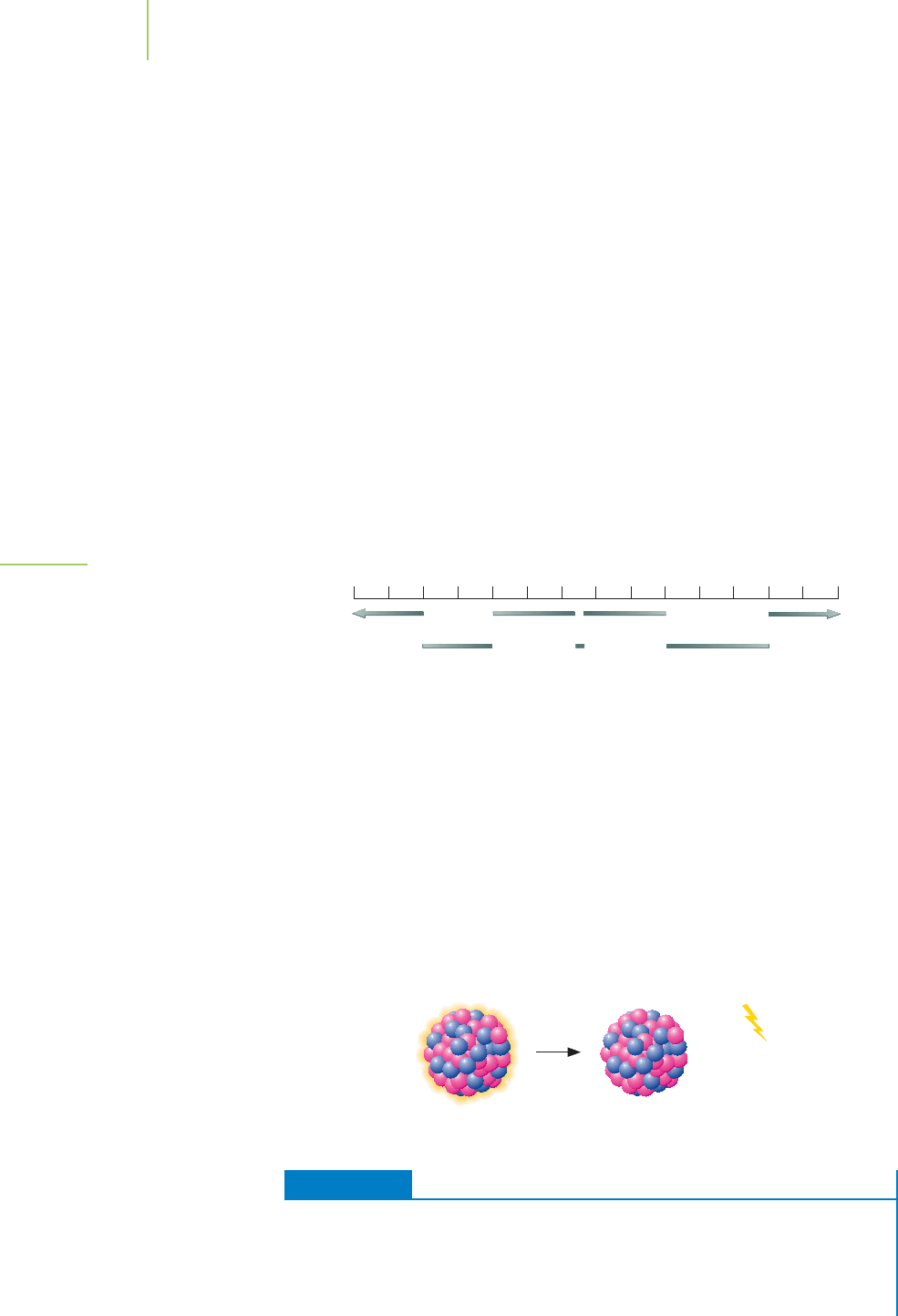
Technetium-99m is generated on demand in nuclear medicine imaging.
EXERCISE 21.3 Alpha Emissions In and Around Us
Radon, discussed earlier, is present in the atmosphere at a concentration of about
1 part in 10
21
. In some caves and basements, however, test kits like those shown in
Figure 21.3 can detect it at much higher concentrations. Write nuclear equations for
the alpha decay of radon-220, radon-222, and radon-219. A gamma ray accompa-
nies each of these processes.
You also may see
4
2
He
2+
,
4
2
He or simply used to denote the alpha particle. Is
alpha emission possible for a hydrogen or helium atom? No, because these atoms
are too small to emit an alpha particle and still have any protons left for the re-
maining nucleus. In fact, alpha emission typically does not occur for small nuclei.
It is far more common for elements above bismuth (Z = 83).
Gamma-Ray Emission
For both alpha and beta emissions, the nuclear equations we have written so far
are not complete, because a gamma ray is usually produced as well. When we
include this gamma ray (
0
0
) in the alpha decay of radon, the nuclear equation
becomes
222
86
Rn →
218
84
Po +
4
2
He +
0
0
A gamma ray, as indicated in Figure 21.2, is a high-energy photon—a form of
electromagnetic radiation with a short wavelength (typically less than a picome-
ter) traveling at the speed of light. Loss of energy from the system, in the form of
a gamma ray, is favorable because the products of the reaction would then have
less energy than the starting material. In essence, the free energy of the system is
lowered.
906 Chapter 21 Nuclear Chemistry
10
–11
10
–9
10
–7
10
–5
10
–3
10
–1
10 10
3
Gamma
Radio
Ultraviolet Infrared
X-ray Microwave
Wavelength (cm)
Visible
FIGURE 21.2
Gamma rays are at the high-energy end
of the electromagnetic spectrum. They
have very short wavelengths on the
order of ten-trillionths of a meter or less.
The energy of cosmic rays is comparable
to that of gamma rays, but cosmic rays
are particles and are not part of the
electromagnetic spectrum.
+
γ
0
0
Gamma rays carry off excess energy in an amount depending on the particu-
lar nuclide. In some cases, lower-energy gamma rays are identical to X-rays;
however, we mentally distinguish between the two by noting that the former orig-
inated inside the nucleus and the latter outside of it. For medical purposes, as we
will see in the final section of this chapter, both X-rays and gamma rays can ac-
complish the same diagnostic and therapeutic tasks.
Few nuclides are pure or almost pure gamma emitters. A notable example is
technetium-99m, where the m indicates a
metastable state. It represents a mis-
arrangement of protons and neutrons in a nucleus after a neutron has become a
proton. In beta emitters, this arrangement occurs so rapidly that it appears to be
simultaneous with the original beta emission. In some cases, however, it is slow
enough to be obvious:
99m
Tc n
99
Tc +
0
0

21.2 Types of Radioactive Decay 907
Solution
222
86
Rn →
218
84
Po +
4
2
He +
0
0
220
86
Rn →
216
84
Po +
4
2
He +
0
0
219
86
Rn →
215
84
Po +
4
2
He +
0
0
PRACTICE 21.3
Write nuclear equations for the alpha decay of
218
Po and
230
Th.
See Problems 15a, 16a, 16c, 17a, and 19.
Other Types of Radioactive Decay
The three types of radioactive decay that we have discussed are the most com-
mon, but two other modes of nuclear decay are also important to a comprehen-
sive picture of radioactive processes.
Electron capture (EC) is the combination of
an inner-orbital electron and a proton from the nucleus to form a neutron. The
mass of the nuclide doesn’t change during the process because a proton and a
neutron are similar in mass, but the atomic number decreases by one as the pro-
ton is changed to a neutron. Typically, this process is accompanied by the emis-
sion of X-rays from the nuclide. The radioactive decay of iodine-125, which is
used to diagnose problems with the pancreas and intestines, occurs by the process
of electron capture:
125
53
I +
0
−1
→
125
52
Te
In positron emission (
0
+1
), a proton decays into
a neutron and a positron. A neutrino accompanies
this emission, and usually one or more gamma rays
as well. A
positron, or positive electron, is a particle
that has the same mass as an electron but carries a
charge of +1. Interestingly, the positron typically
doesn’t have a very long life, because when it comes
into contact with an electron, the two particles
combine to form two gamma rays. This type of ra-
dioactive decay is important in the lighter elements
such as aluminum-26.
26
13
Al →
0
1
+
26
12
Mg
Table 21.2 lists the five types of radioactive
decay that are important in understanding nuclear
decay processes.
Decay Series
Radon, discussed in Exercise 21.3, is one example in which the products of the
nuclear reaction are still radioactive. All isotopes of all elements past bismuth
(Z = 83) are radioactive. So far we have described nuclear decay as though it were
a one-step process. However, a nuclide may decay to form a second radioactive
nuclide, which in turn may decay more than a dozen times in a stepwise progres-
sion toward stability that is called a
decay series.
Consider the element uranium. Two natural isotopes exist for this element,
238
U and
235
U, with natural abundances of 99.28% and 0.72%, respectively. These
isotopes exhibit a fairly extensive decay series. Both series consist of alpha and
beta emissions, as shown in Figure 21.4. The accompanying gamma rays are not
shown in these series. Why do the lines in the decay series zig-zag? Alpha decays
FIGURE 21.3
Radon test kits can be purchased and
used to measure the concentration of
radon in a basement.
Radioactive Decay Processes
Change in Change in
Type of Decay Emission Atomic Number Mass Number
Alpha-particle
4
2
He
−2 −4
emission
Beta-particle
0
−1
+10
emission
Gamma-ray
0
0
00
emission
Positron
0
+1
−10
emission
Electron capture X-ray −10
TABLE 21.2
Uranium ore (known as yellow cake) can
be refined into pellets of uranium metal.
