Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


888 Chapter 20 Coordination Complexes
Orbital Occupancy
The crystal field energy-level diagrams are the basis for understanding both
physical and chemical properties of transition metal coordination complexes.
However, as with all other chemicals, these properties are related to the electron
configuration of the species. This configuration still follows the Aufbau rule,
Pauli’s exclusion principle, and Hund’s rule of maximum multiplicity (Chap-
ter 6). However, the splitting between the energy levels of the d orbitals becomes
similar to the electrostatic repulsion that occurs when two electrons occupy the
same orbital. This repulsion is called the
pairing energy (P). Depending on the val-
ues of ∆ and P, it may be favorable to place an electron in a higher energy orbital
rather than pair it with another electron in one orbital where the negatively
charged electrons will repel each other.
Octahedral Complexes
An octahedral coordination complex containing a central metal atom with one
d electron, such as Ti
3+
or V
4+
, will have one electron occupying one of the t
2g
orbitals. For a central metal with a d
2
configuration, such as V
3+
, one electron will
occupy each of two t
2g
orbitals, as shown in Figure 20.28. This configuration can
be symbolized t
2g
2
, where the superscript represents the number of electrons in
the orbital set labeled t
2g
. An octahedral complex with three d electrons would
give rise to a t
2g
3
orbital occupancy. However, if another electron is added, it may
either pair up with one of the electrons already in a t
2g
orbital at an energy cost of
+P, or occupy one of the e
g
orbitals at an energy cost of +∆
o
. The electron will
tend to attain the lowest energy situation and hence will occupy the e
g
orbital if
∆
o
is less than the pairing energy, P. Therefore, depending on the values of ∆
o
and
P, the configuration could either be t
2g
4
with two unpaired electrons or t
2g
3
e
g
1
with four unpaired electrons, as shown in Figure 20.29.
These two configurations of electrons in the d orbitals are termed
low-spin,
(containing the minimum number of unpaired electrons, in this case two) or
high-spin (with the maximum number of unpaired electrons, in this case four),
respectively. For example, chromium complexes containing the chromium(II)
ion (four d electrons) may be either high-spin with four unpaired electrons, as in
[Cr(H
2
O)
6
]
2+
, or low-spin with two unpaired electrons, as in [Cr(CN)
6
]
4−
.
Continuing with our orbital occupancy evaluation, a d
5
configuration could
be either t
2g
5
(low-spin) or t
2g
3
e
g
2
(high-spin). At the d
6
configuration, electron
pairing must occur, but either low-spin (t
2g
6
) or high-spin (t
2g
4
e
g
2
) may exist. A
d
7
configuration could also be either low-spin (t
2g
6
e
g
1
) or high-spin (t
2g
5
e
g
2
). Like
the electron configurations with one, two, or three electrons, only one option is
available for occupancy of the t
2g
and e
g
orbital sets for the d
8
(t
2g
6
e
g
2
), d
9
(t
2g
6
e
g
3
),
and d
10
(t
2g
6
e
g
4
) configurations.
d
2
High-spin (t
2 g
2
) d
4
High-spin (t
2 g
3
e
g
1
) d
6
High-spin (t
2 g
4
e
g
2
) d
8
High-spin (t
2 g
6
e
g
2
)
d
4
Low-spin (t
2 g
4
) d
6
Low-spin (t
2 g
6
)
FIGURE 20.28
Electron distribution in d
2
,d
4
, d
6
, d
8
octahedral coordination complexes.
d
4
High-spin (t
2 g
3
e
g
1
)
d
4
Low-spin (t
2 g
4
)
P >
∆
P <
∆
FIGURE 20.29
The relative magnitude of the pair-
ing energy and the crystal field
energy determine the electron
configuration.
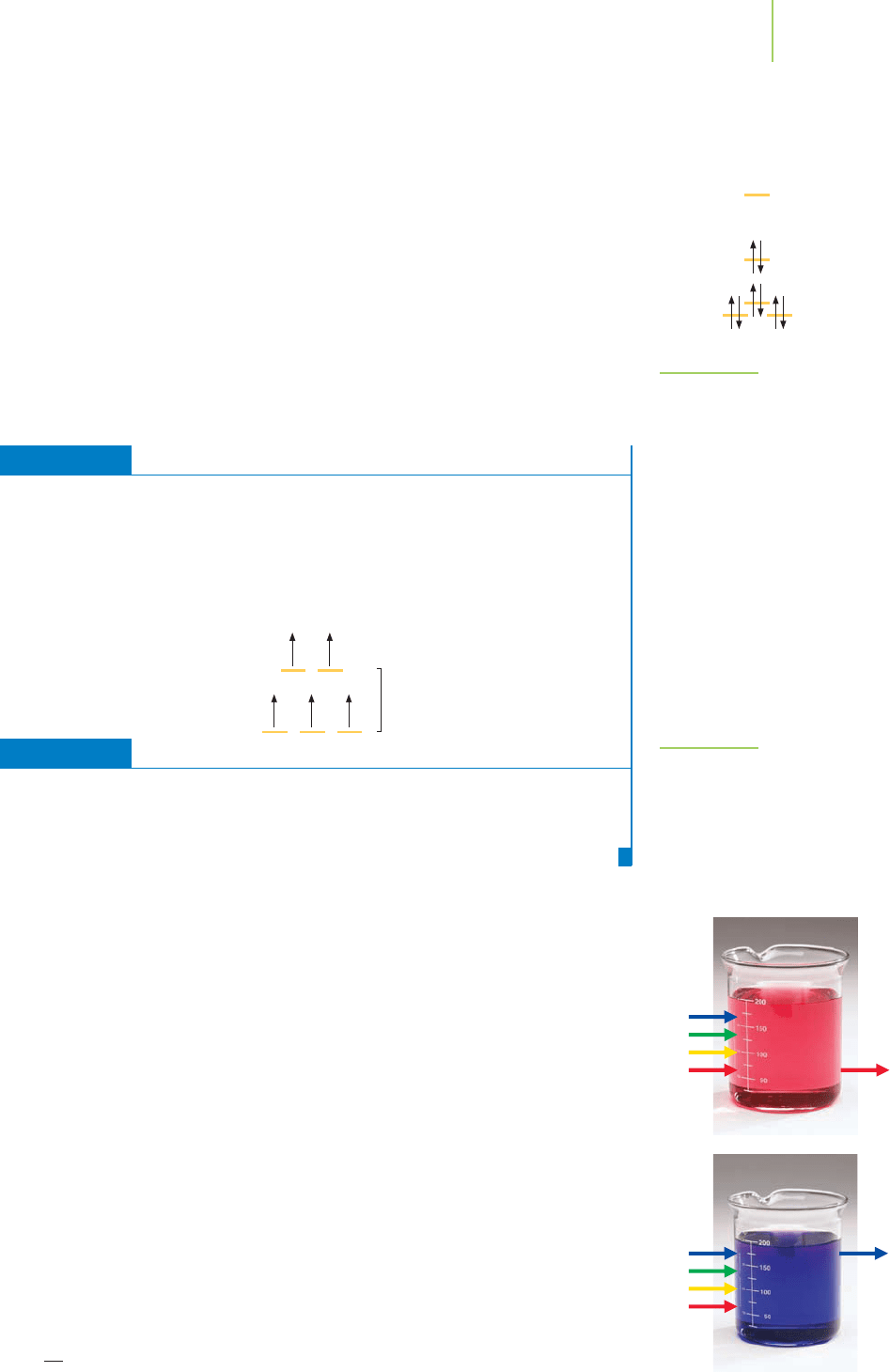
FIGURE 20.31
Absorption of light (arrow) by cobalt
complexes. The red solution is a solution
of [Co(H
2
O
6
)
2+
]; the blue solution is the
coordination of chloride with cobalt(II)
in [CoCl
4
]
2−
. Note that different wave-
lengths of light are absorbed by these
complexes.
20.7 Color and Coordination Compounds
889
Tetrahedral Complexes
Although it would seem that similar high-spin and low-spin electron configura-
tions would be possible for the d
3
− d
6
metals in tetrahedral complexes, such
situations are not observed. The magnitude of ∆
t
is usually less than the pairing
energy P. Recall that ∆
t
for a tetrahedral complex is only about
4
⁄9 that of ∆
o
for an
octahedral complex. Because P is always greater than ∆
t
, high-spin complexes
are nearly always the only possibility for tetrahedral complexes.
Square Planar Complexes
Similar analyses could be done with square planar complexes. However, it turns
out that most square planar complexes occur for metal centers and ligands that
generate a low-spin configuration. For example, cisplatin (cis-[PtCl
2
(NH
3
)
2
]) has
the orbital occupancy shown in Figure 20.30.
EXERCISE 20.4 Drawing Crystal Field Diagrams
Draw crystal field splitting diagrams with electron occupancy for [Mn(H
2
O)
6
]
2+
(high-spin).
Solution
Manganese(II) has a d
5
configuration. With a coordination number of 6, an octa-
hedral crystal field is expected. A high-spin d
5
(t
2g
3
e
g
2
) configuration will result.
PRACTICE 20.4
How many unpaired electrons would you predict for the low-spin complex
[Fe(CN)
6
]
4−
? Show the basis of your prediction using the crystal field splitting dia-
gram for the iron metal ion.
See Problems 47–50.
The Result of d Orbital Splitting
A really dramatic example of color differences occurs in the two complexes of
Co
2+
shown in Figure 20.31. When water is coordinated to Co
2+
in the
[Co(H
2
O)
6
]
2+
ion, the color is red. When chloride ligands coordinate to Co
2+
in [CoCl
4
]
2−
, the color is blue. How does this fit into our understanding of color?
The valence electron configurations of all of these species are shown in Fig-
ure 20.32 on page 890. For [Co(H
2
O)
6
]
2+
with an octahedral ligand field, the red
color arises when an electron is excited from a t
2g
orbital to an e
g
orbital by ab-
sorption of a photon of energy ∆
o
. For [CoCl
4
]
2−
with a tetrahedral ligand field,
the blue color arises when an electron is excited from an e orbital to a t orbital by
absorption of a photon of energy ∆
t
. Because, in general, ∆
t
is smaller than ∆
o
, the
photon absorbed by [CoCl
4
]
2−
must be lower in energy than the photon ab-
sorbed by [Co(H
2
O)
6
]
2+
. In this case, [Co(H
2
O)
6
]
2+
absorbs higher-energy blue
light and appears red, and [CoCl
4
]
2−
absorbs lower-energy red light and appears
blue.
The absorption spectra in Figure 20.33, which show how much light of vari-
ous wavelengths is absorbed, exhibit a maximum for [CoCl
4
]
2−
at 660 nm
(equivalent to 181 kJ/mol) and a maximum for [Co(H
2
O)
6
]
2+
at 510 nm (equiv-
alent to 221 kJ/mol). Recall that longer wavelength corresponds to lower energy,
E =
hc
λ
, so the [CoCl
4
]
2−
complex absorbs lower-energy photons. The key point
∆
d
xy
d
z
2
d
yz
d
xz
d
x
2
–y
2
FIGURE 20.30
Electron distribution in square
planar cis-[PtCl
2
(NH
3
)
2
].
∆
o
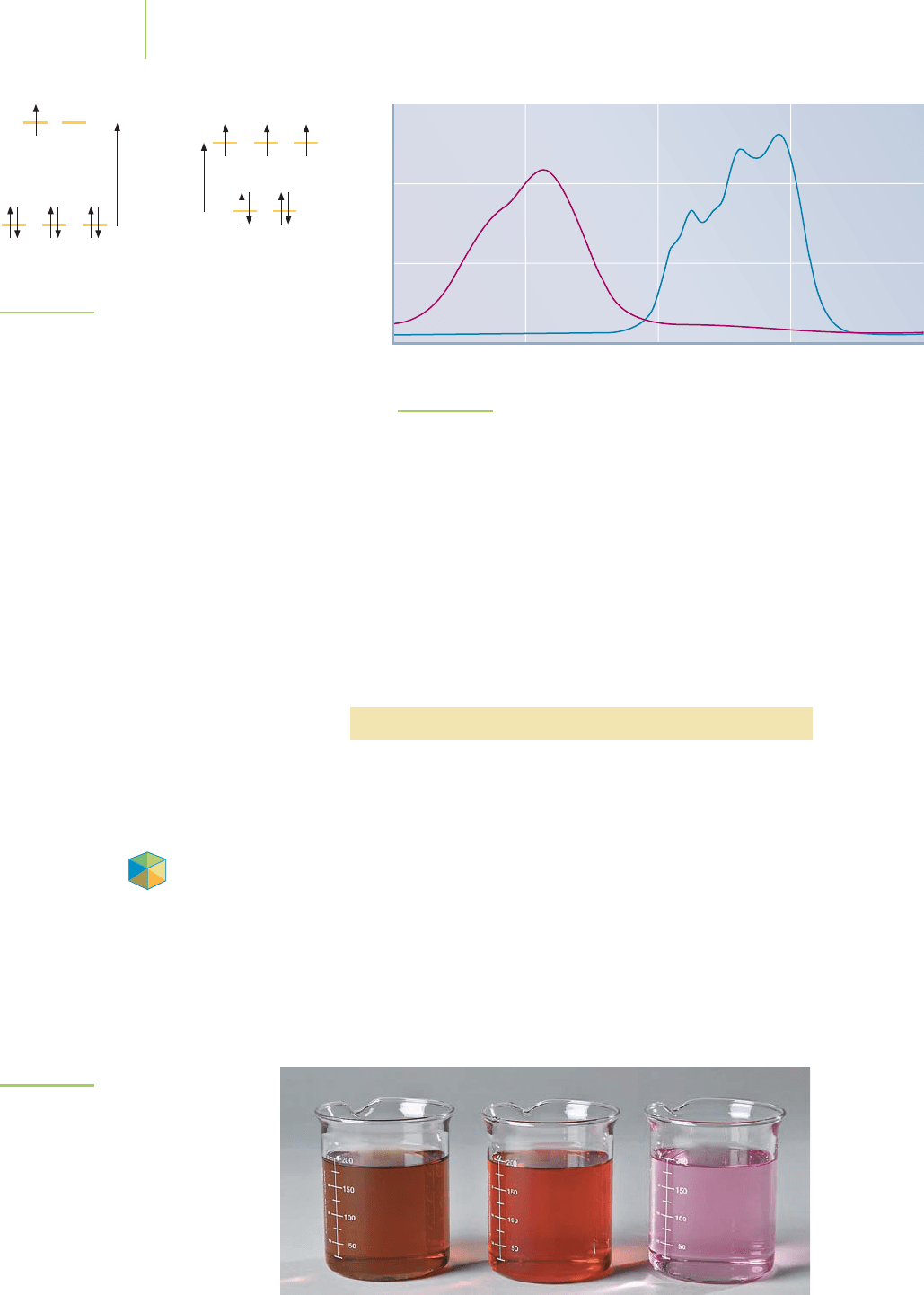
890 Chapter 20 Coordination Complexes
is that based on the model and with experimental evidence to support it, the
geometry of the ligand field around a metal center influences the size of ∆.
The nature of the ligands around the metal center also influences the magni-
tude of ∆. Figure 20.34 shows solutions of three cobalt(III) complexes with dif-
ferent ligands around the cobalt center. The wavelength of light that is absorbed
decreases, as shown in Figure 20.35, as the unique ligand varies from Cl
−
to H
2
O
to NH
3
. As the ligand varies, the value of ∆
o
increases and the wavelength of max-
imum absorbance decreases. Using this type of information from a wide variety
of complexes, a
spectrochemical series was developed that illustrates the effect of
a particular ligand on the value of ∆. The series is
Cl
−
< F
−
< OH
−
< H
2
O < NH
3
< NO
2
−
< CN
−
< CO
Conveniently, this series is generally the same for all metals. Knowing this in-
formation, we can predict that the wavelength of light absorbed by [FeF
6
]
3−
would be longer (lower in energy) than the wavelength of light absorbed by
[Fe(H
2
O)
6
]
3+
because a water ligand produces a larger value of ∆ than the fluo-
ride ligand.
Let’s use this information to answer a practical question: Why is the blood in
your veins dark red, whereas the blood in arteries is bright red? When blood in a
vein is exposed to air, its color changes to bright red. Although the exact origin of
this color change is fairly complex, it turns out that the crystal field splitting in-
creases when oxygen binds to the iron center. When oxygen binds to the Fe
2+
cen-
ter, higher-energy light is absorbed, longer-wavelength light is transmitted by the
hemoglobin, and the blood changes color. Oxyhemoglobin (with a larger crystal
field splitting) absorbs higher-energy blue light and is red in the arteries;
Co
2+
(d
7
)
∆
o
> ∆
t
FIGURE 20.32
The electron configurations of two cobalt com-
plexes, [Co(H
2
O)
6
]
2+
and [CoCl
4
]
2−
. The difference
in the magnitude of the crystal field energy deter-
mines what wavelengths of light they absorb.
0.00
0.25
0.50
0.75
400 500 600 700 800
Wavelength (nm)
Absorbance
Red [Co(H
2
O)
6
]
2+
Blue [CoCl
4
]
2–
FIGURE 20.33
Absorption spectra of [Co(H
2
O)
6
]
2+
and [CoCl
4
]
2−
.
FIGURE 20.34
The influence of coordinated ligands on
color of coordination complexes. Each
complex of cobalt(III) contains five
NH
3
ligands and one other ligand.
From left to right: [Co(NH
3
)
6
]
3+
,
[Co(NH
3
)
5
(H
2
O)]
3+
, and [Co(NH
3
)
5
Cl]
2+
.
Application
Mn(OH
2
)
6
2
+
d
5
High-spin Mn(CN)
6
4
–
d
5
Low-spin
e
g
e
g
t
2g
t
2g
deoxyhemoglobin (with a smaller crystal field splitting) absorbs lower energies
and appears bluish-red in the veins.
Magnetism
When magnetism is mentioned, it is common to think of bar magnets and their
attraction for metal objects. This common form of magnetism shown by iron,
nickel, and cobalt is known as
ferromagnetism. However, there is a more common
but also more subtle form of magnetism called
paramagnetism (Chapter 9). This
property exists in any substance that contains unpaired electrons. A substance
that is paramagnetic is attracted to a magnetic field, though less strongly than in
a ferromagnetic substance. A material that has all of its electrons paired exhibits
diamagnetism, and these materials are very weakly repelled by a magnetic field.
The origin of paramagnetism is in the spin of the unpaired electrons in a sub-
stance. In the absence of a magnetic field, the spins of the unpaired electrons are
randomly oriented. When these unpaired electrons are placed in a magnetic field,
their spins align with the field and result in a net attraction. The experimental
characterization of transition metal coordination complexes was greatly aided by
measurement of the paramagnetism of various species. Measurement of the
strength of the paramagnetism provides an experimental quantity called the
mag-
netic moment (µ)
. In many cases, µ is related to the number of unpaired electrons,
n, and is nearly equal to or slightly greater than the theoretical value given by the
formula
µ = [n(n + 2)]
1/2
where n = number of unpaired electrons.
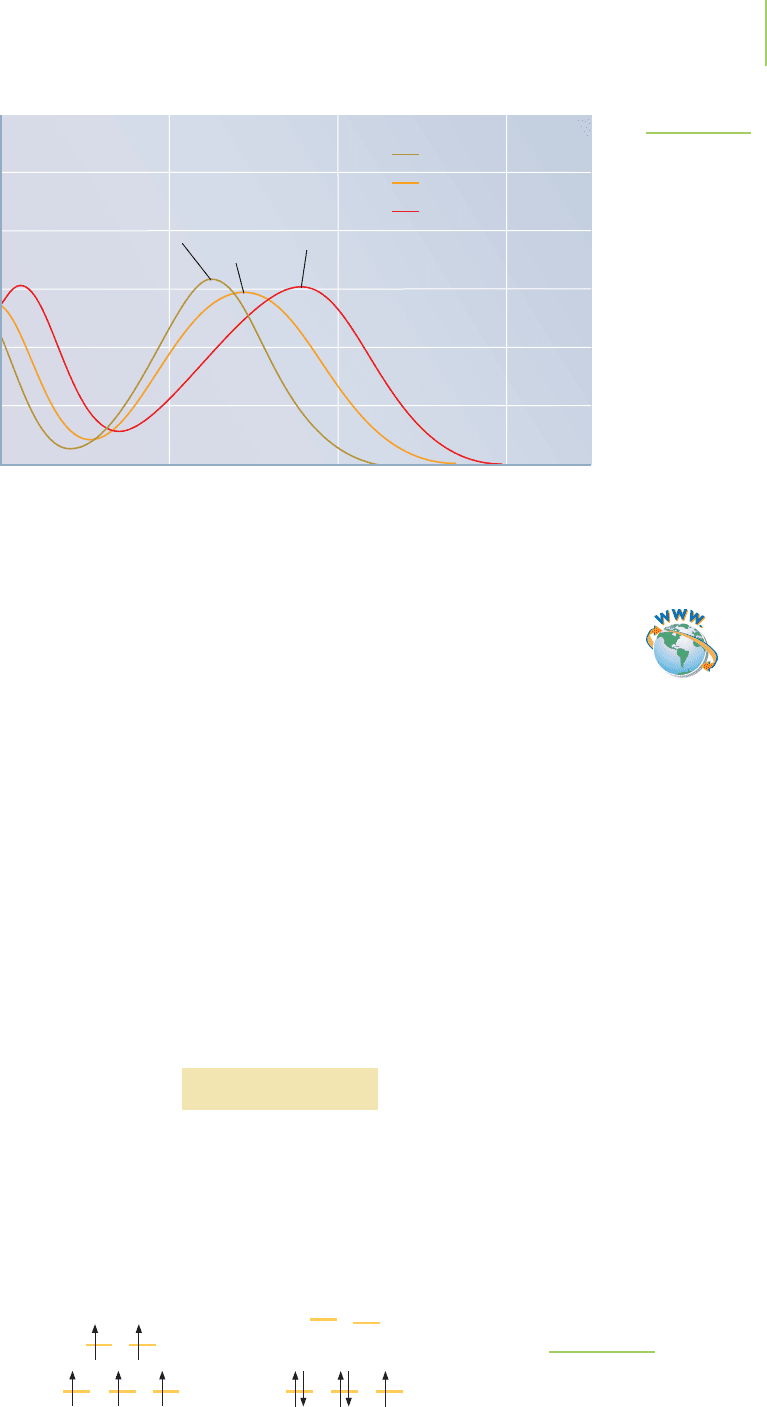
The complex [Mn(H
2
O)
6
]
2+
has a magnetic moment of 5.9, whereas the
magnetic moment of [Mn(CN)
6
]
4−
is 2.2. Both have five d electrons. Why should
they have such different magnetic moments? Using the relationship between
magnetic moment and the number of unpaired electrons, µ = [n(n + 2)]
1/2
,we
find that if n = 5, then µ should be 5.92 for the aqua complex. Thus the
[Mn(H
2
O)
6
]
2+
complex is a high-spin d
5
complex, as shown in Figure 20.36.
20.7 Color and Coordination Compounds 891
0
0.1
0.2
0.3
0.4
0.5
0.6
350 450 550 650
Wavelen
g
th (nm)
Absorbance
[Co(NH
3
)
6
]
3+
[Co(NH
3
)
5
(H
2
O)]
3+
[Co(NH
3
)
5
Cl]
2+
528 nm
493 nm
475 nm
FIGURE 20.35
Absorption spectra of [Co(NH
3
)
6
]
3+
,
[Co(NH
3
)
5
(H
2
O)]
3+
, and [Co(NH
3
)
5
Cl]
2+
,
showing higher-energy, shorter-
wavelength absorption in the order
[Co(NH
3
)
6
]
3+
< [Co(NH
3
)
5
(H
2
O)]
3+
<
[Co(NH
3
)
5
Cl]
2+
. (Spectra provided by Jerry
Walsh.)
FIGURE 20.36
High-spin and low-spin manganese complexes. In the
high-spin case, the crystal field energy is less than the
pairing energy. In the low-spin case, the magnitude of
is greater than the pairing energy.
Video Lesson: Magnetic
Properties and Spin
Using the same relationship, if n = 1 (as it would be in the low-spin case), we ex-
pect µ to be 1.73, which is close to the value observed in the cyano complex.
Therefore, the [Mn(CN)
6
]
4−
complex must be a low-spin complex. This makes
sense when we note that CN
−
imparts a strong ligand field (high in the spectro-
chemical series) and H
2
O imparts a weak ligand field. For CN
−
, the magnitude of
∆ is large enough that the electrons prefer to be paired up in the t
2g
orbitals rather
than unpaired and occupying the higher energy e
g
orbitals (Figure 20.37).
A fascinating change related to the paramagnetism of the iron ion in hemo-
globin occurs upon binding of oxygen. Deoxyhemoglobin contains a high-spin,
paramagnetic Fe
2+
ion with four unpaired electrons. Upon binding with oxygen,
the increased ligand field causes an increase in ∆, and the Fe
2+
becomes low-spin
and diamagnetic. This change in spin state, which can be followed by measure-
ment of its magnetism, is critical to the ability of the iron to bind oxygen and
release it under physiological conditions, as we will discuss later.
20.8 Chemical Reactions
Aside from possessing interesting color and magnetism properties, transition
metal coordination complexes also undergo useful chemical reactions. Of partic-
ular biological importance is a class of reactions known as ligand exchange reac-
tions. This class of reactions includes the coordination of oxygen and its release
from an iron ion in hemoglobin. Another class of reactions, known as electron
transfer reactions, are also important in biological processes. This class of reac-
tions is quite common in photosynthesis and respiration.
Ligand Exchange Reactions
Why do coordination complexes form? All chemical reactions proceed when the
total free energy of the system, ∆G, decreases. Because the free energy change de-
pends on two factors, entropy and enthalpy, these must be considered in a reac-
tion involving the association of a ligand with a metal. Consider the formation of
hexaamminenickel(II) from the aqua complex:
[Ni(H
2
O)
6
]
2+
+ 6NH
3
→ [Ni(NH
3
)
6
]
2+
+ 6H
2
O K = 4 × 10
8
Because the two different ligands, NH
3
and H
2
O, are similar in size and the same
number of each are involved in the reaction, the change in entropy for the reac-
tion is small. The driving force for this reaction must then rely on the stability of the
resulting coordinate covalent bonds. In this example, the Ni
2+
ON bond is stronger
than the Ni
2+
OO bond, so ∆G is relatively large and negative because of the
favorable enthalpy change in nickelOnitrogen bond formation. This translates
into a large equilibrium constant for the formation of [Ni(NH
3
)
6
]
2+
via the equa-
tion ∆G =−RT lnK.
Consider the following reaction of our nickel complex. Each mole of this
complex can react with three moles of ethylenediamine to form [Ni(en)
3
]
3+
.
Both complexes contain Ni
2+
ON bonds. Does it make sense that this reaction
should also have a very large equilibrium constant?
[Ni(NH
3
)
6
]
2+
+ 3en n [Ni(en)
3
]
2+
+ 6NH
3
K = 5 × 10
9
There doesn’t appear to be much change in the enthalpy of this reaction (the
change in enthalpy is expected to be small because the bonds created in the prod-
uct are similar to the bonds broken in the reactant), so we must focus on changes
in entropy for this reaction. A very favorable entropy change is observed; the
reaction produces three more moles of product than moles of reactant. This
892 Chapter 20 Coordination Complexes
[Mn(CN)
6
]
4–
FIGURE 20.37
Visualization: Nickel(II)
Complexes

exceptionally high favorability for forming complexes with polydentate ligands is
known as the
chelate effect.
Although we can predict the direction of a reaction by using thermodynam-
ics, only an examination of the kinetics of the reaction will determine the rate of
the reaction. Some coordination complexes exchange their ligands very rapidly
and are referred to as
labile; others do so more slowly and are referred to as inert.
For example, the blue [CoCl
4
]
−
complex rapidly exchanges chloro ligands with
water to produce the red [Co(H
2
O)
6
]
2+
complex, both of which are shown in Fig-
ure 20.38. In contrast, the chromium complexes shown in Figure 20.39, green
[CrCl
2
(H
2
O)
4
]
+
and purple [Cr(H
2
O)
6
]
3+
, take at least a day to exchange ligands.
In the interaction between cisplatin and DNA molecules, it is important for the
platinum complex to bond to the DNA and remain bonded long enough for
the complex to be toxic to the system. The platinum–DNA complex is inert—and
must be inert to exhibit the kind of anticancer activity that it does.
The kinetic and thermodynamic factors exhibited in the reactivity of coordi-
nation complexes are intriguingly complementary in hemoglobin. The Fe
2+
metal center is kinetically labile, which is important for the rapid exchange of
oxygen ligands:
HbFe
2+
+ O
2
HbFeO
2
2+
rapid
However, in spite of the fact that Fe
2+
can exchange its ligands rapidly, the chelate
effect of the tetradentate porphyrin ligand maintains stability of the iron–
porphyrin portion of the complex.
HbFe
2+
+ 6H
2
O
Hb + [Fe(H
2
O)
6
]
2+
equilibrium lies to the left
The balance of chemical characteristics for molecules in living systems is exquis-
ite. This is why the scientific challenge of generating a chemical substitute for he-
moglobin to transport oxygen in the bloodstream has been formidable.
20.8 Chemical Reactions 893
FIGURE 20.38
Rates of ligand exchange.
[CoCl
4
]
2−
exchanges chloro
ligands immediately on
mixing.
FIGURE 20.39
[CrCl
2
(H
2
O)
4
]
+
exchanges
ligands slowly, taking a day
or more to fully exchange
its ligands.
t = 3min
−−−−−−−→
t = 1day
−−−−−−−→
t = 3 min
−−−−−−−→
t = 1day
−−−−−−−→
894 Chapter 20 Coordination Complexes
Fe
N
Protein
Protein
N
N
N
N
S
FIGURE 20.40
Iron coordination in
cytochrome electron
transfer protein.
Application
C
HEMICAL
ENCOUNTERS:
Cytochromes
Electron Transfer Reactions
Transition metals typically exhibit several stable oxidation states, and the +2 and
+3 states are fairly common. Because many transition metals exhibit stability in
two or more oxidation states, transition metal complexes can play important
roles in electron transfer processes. For example, cytochromes are electron
transfer agents in biological systems. Within a cytochrome pro-
tein, an iron ion coordinates to a porphyrin ring. The other sites
on the octahedral complex are occupied by ligands that are part of
the protein structure, as shown in Figure 20.40. During respiration
and photosynthesis, the iron changes oxidation state from Fe
2+
to
Fe
3+
to Fe
2+
as the cytochrome shuttles electrons between two
biological reaction sites (Figure 20.41).
Chlorophyll a
II
Receptor
II
Light
absorbed
Chlorophyll a
I
Receptor
I
Light
absorbed
Carbohydrates
Cytochrome(Fe
+2/+3
)
Cytochrome(Fe
+3/+2
)
Plastocyanin
H
2
O
O
2
CO
2
FIGURE 20.41
The role of iron (cytochrome) and copper (plastocyanin) oxidation states in electron
transfer reactions in photosynthesis.
The Bottom Line
■
Coordination complexes are present in simple metal
ions in solution and as the reaction center in many
biological molecules. (Section 20.1)
■
A Lewis base that donates a lone pair of electrons to
a metal to form a coordinate covalent bond acts as a
ligand in producing a coordination complex.
(Section 20.2)
■
The coordination numbers of various metal
centers are commonly observed to be 2, 4, or 6.
(Section 20.3)
■
Common coordination geometries are linear, tetra-
hedral, square planar, and octahedral. (Section 20.4)
■
A variety of isomer classes are observed for metal
complexes. (Section 20.5)
■
The proper names and formulas for coordination
complexes are specified by IUPAC rules.
(Section 20.6)
■
In transition metal complexes, the d orbitals are no
longer degenerate but split into two or more energy
levels, depending on coordination geometry.
(Section 20.7)
■
The electron configuration for octahedral complexes
gives rise to high-spin and low-spin complexes for d
4
to d
7
metal centers. (Section 20.7)
■
The color of many transition metal compounds
arises when a photon of visible light is absorbed and
an electron is excited to a higher energy d orbital.
(Section 20.7)
Key Words 895
bidentate Capable of forming two coordinate covalent
bonds to the same metal center. (p. 874)
chelate A polydentate ligand that forms strong
metal–ligand bonds. (p. 874)
chelate effect An unusually large formation constant
due to a favorable entropy change for the formation
of a complex between a metal center and a polyden-
tate ligand. (p. 893)
cis isomer An isomer containing two similar groups on
the same side of the compound. (p. 880)
coordinate covalent bond A covalent bond that results
when one atom donates both electrons to the bond.
(p. 870)
coordinates Forms a coordinate covalent bond. (p. 871)
coordination complex A metal bonded to two or more
ligands via coordinate covalent bonds. (p. 870)
coordination number The number of coordinate covalent
bonds that form in a complex. (p. 875)
coordination sphere isomers Substances that contain dif-
ferent ligands in the coordination spheres of complex
cations and complex anions. (p. 879)
crystal field splitting energy The difference in energy be-
tween the d orbital sets that arises because of the
presence of ligands around a metal; symbolized by ∆.
(p. 887)
diamagnetism The ability of a substance to be repelled
from a magnetic field. This property arises because all
of the electrons in the molecule are paired. (p. 891)
ferromagnetism Occurs when paramagnetic atoms are
close enough to each other (such as in iron) that they
reinforce their attraction to the magnetic field, so the
whole is, in effect, greater than the sum of its parts.
(p. 891)
geometric isomers Substances in which all of the atoms
are attached with the same connectivity, or bonds,
but the geometric orientation differs. (p. 880)
high-spin A coordination complex with the maximum
number of unpaired electrons. (p. 888)
inert The opposite of labile; said of a compound that is
very slow to exchange ligands. (p. 893)
ionization isomers Isomers that differ in the placement
of counter ions and ligands. (p. 879)
labile The opposite of inert; said of a compound that
exchanges ligands rapidly. (p. 893)
ligand A Lewis base that donates a lone pair of elec-
trons to a metal center to form a coordinate covalent
bond. (p. 870)
linkage isomers Isomers that differ in the point of
attachment of a ligand to a metal. (p. 879)
low-spin A coordination complex with the minimum
number of unpaired electrons. (p. 888)
magnetic moment (µ) The strength of the paramagnet-
ism of a compound. Can be used to determine the
number of unpaired electrons. (p. 891)
octahedral The geometry indicated by six electron
groups (lone pairs and/or bonds) positioned sym-
metrically around a central atom such that the bond
angles are 90°. (p. 877)
pairing energy (P) The energy required to spin-pair two
electrons within a given orbital. (p. 888)
paramagnetism The ability of a substance to be attracted
into a magnetic field. This attraction arises because of
the presence of unpaired electrons within the mole-
cule. (p. 891)
polydentate A ligand that contains two or more lone
pairs on different atoms, each forming a coordinate
covalent bond to a metal center. (p. 874)
spectrochemical series The series of ligands organized
with respect to their effect on the value of the crystal
field splitting energy. (p. 890)
square planar The geometry indicated by four electron
groups (lone pairs and/or bonds) positioned sym-
metrically in a plane around a central atom such that
the bond angles are 90°. (p. 877)
tetradentate Capable of forming four coordinate cova-
lent bonds to the same metal center. (p. 874)
tetrahedral The geometry indicated by four electron
groups (lone pairs and/or bonds) positioned sym-
metrically around a central atom such that the bond
angles are 109°. (p. 877)
trans isomer An isomer containing two similar groups
on opposite sides of the compound. (p. 880)
tridentate Capable of forming three coordinate covalent
bonds to the same metal center. (p. 874)
Key Words
■
The order of ligands, in terms of their influence on
the magnitude of the d orbital splitting and the en-
ergy of the photon of light absorbed, is defined as
the spectrochemical series. (Section 20.7)
■
The number of unpaired electrons determines the
magnetic moment of the complex. (Section 20.7)
■
Metal centers that exchange ligands rapidly are called
labile; those that exchange ligands slowly are
called inert. (Section 20.8)
■
Chelate complexes have high formation constants
because of an entropy effect. (Section 20.8)
■
Because transition metal complexes often exhibit
several oxidation states, transition metal complexes
are good electron transfer (redox) agents.
(Section 20.8)
896 Chapter 20 Coordination Complexes
Focus Your Learning
The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
20.1 Bonding in Coordination Complexes
Skill Review
1. Define each of these terms in your own words:
a. ligand b. coordinate covalent bond c. Lewis acid
2. Define each of these terms in your own words:
a. Lewis base b. complex c. metal center
3. For each of these coordination complexes, state the number
of ligands, the oxidation number of the coordinated metal,
and the number of coordinate covalent bonds that are
formed within the complex.
a. [Fe(H
2
O)
6
]
2+
b. [Co(NH
3
)
6
]
2+
c. [Zn(NH
3
)
4
]
2+
d. [Pt(CO)
4
]
4. For each of these coordination complexes, state the number
of ligands, the oxidation number of the coordinated metal,
and the number of coordinate covalent bonds that are
formed within the complex.
a. [Fe(CN)
6
]
3−
b. [CuCl(NH
3
)
3
]
+
c. [Ni(H
2
O)
6
]
2+
d. [Au(NH
3
)
2
]
2+
5. Diagram a structure that depicts a metal ion (M) surrounded
by four symmetrically arranged ligands (L).
6. Diagram a structure that depicts a metal ion (M) surrounded
by six symmetrically arranged ligands (L).Why are coordina-
tion complexes rarely found with more than six ligands?
20.2 Ligands
Skill Review
7. Diagram the Lewis dot structure for ammonia (NH
3
). Ex-
plain why this molecule is classified as a Lewis base.
8. Diagram the Lewis dot structure for methylamine
(CH
3
NH
2
). Is this molecule a Lewis base?
9. Would you predict the following molecule to be a ligand? If
so, indicate whether it would be monodentate, bidentate, tri-
dentate, or tetradentate.
11. Which of these are not likely act as ligands in forming coor-
dination complexes?
a. H
2
Ob.CN
−
c. Ca
2+
d. O
2
e. C
2
H
6
12. Which of these can act as a ligand in forming coordination
complexes?
a. CH
4
b. Br
2
c. O
2−
d. H
2
O
2
e. Li
+
Chemical Applications and Practices
13. Explain why the structure of ethylenediammine (en) enables
it to act as a bidentate ligand, whereas nitrogen gas, which
also contains two nitrogen atoms, cannot act as a bidentate
ligand.
14. EDTA
4−
can coordinate with metal ions at six different sites.
EDTA
4−
is often used as a food preservative in such food
products as mayonnaise. Explain how EDTA
4−
functions in
such an effective manner.
15. The formula Co(A
2
B
2
) represents a coordination complex
with two ligands, A and B. The Co ion is coordinated through
six sites. If two A ligands occupy two of those sites, what must
be true about the bonding for ligand B?
16. Amino acids can act as bidentate ligands. This is due to the
presence of both oxygen and nitrogen. For example, alanine
(Ala) could form the metal complex [Fe(ala)
3
]
3+
. Use the ala-
nine structure to draw a structure of the metal complex.
10. Would you predict the following molecule to be a ligand? If
so, indicate whether it would be monodentate, bidentate, tri-
dentate, or tetradentate.
20.3 Coordination Number
Skill Review
17. Determine the oxidation state and the coordination number
of the central metal in each of these coordination complexes:
a. [Cr(NH
3
)
5
Br]
3+
b. [Mn(NH
2
CH
2
CH
2
NH
2
)
3
]
2+
c. [Cd(NH
2
CH
3
)
4
]
2+
18. Determine the oxidation state and the coordination number
of the central metal in each of these coordination complexes:
a. [Co(NH
3
)
6
]
3+
b. [Pd(en)Cl
2
] c. [Mo(ox)
3
]
3−
19. Explain how it is possible that the central metals in these two
complexes can have the same coordination number even
though the number of ligands differs in the complexes.
[Fe(ox)
3
]
3−
[Co(SCN)
2
(H
2
O)
4
]
+
20. Explain how it is possible that the central metal in these two
complexes can have the same oxidation state even though the
coordination number differs in the complexes.
[Fe(en)
3
]
3+
[ScI
4
]
−
Focus Your Learning 897
Chemical Applications and Practices
21. Many heavy-metal-containing salts are
not very water soluble. One way to in-
crease the solubility is to form a complex.
Examine the structure of the bidentate
oxinate ion shown here. If two of these
complexed with a lead ion, what would be
the coordination number of the lead?
22. One method used to determine the “hardness” of water sam-
ples is to titrate the sample with EDTA. This reaction forms a
complex with the calcium ions in the water. Using the struc-
ture of EDTA depicted in Figure 20.9, determine what the
coordination number of the calcium ion would be if it com-
bined with one disodium EDTA ion. Diagram the structure
that justifies your answer.
20.4 Structure
Skill Review
23. Diagram the structures of the complexes listed in Problem 3,
and identify the geometry (bond angles and overall shape) of
each structure.
24. Diagram the structures of the complexes listed in Problem 4,
and identify the geometry (bond angles and overall shape) of
each structure.
25. Indicate the oxidation number, coordination number, and
geometry for the cobalt ion in the following compound.
[CoCl(NO
2
)(en)
2
]Cl
26. Indicate the oxidation number, coordination number, and
geometry for the silver ion in the following compound.
[Ag(NH
3
)
4
]Br
2
Chemical Applications and Practices
27. When nickel ore is refined, it must be removed from other
metals. This can be done by forming a coordination complex
between nickel and carbon monoxide. The highly poisonous
nickel–carbon monoxide complex Ni(CO)
4
evaporates easily
and can therefore be used to separate nickel from its impuri-
ties. What is the coordination number for nickel, and what
two possible geometries could you predict for this structure?
28. The readily availableelectron pairs found in the oxygen,nitro-
gen, and some sulfur atoms of amino acids (Chapter 22) pro-
vide bonding sitesformetals.The metal–aminoacid combina-
tions serve as the basis for many important biochemical
processes. For example, a certain copper-containing enzyme
utilizes an octahedral structure around a Cu
2+
ion to assist in
the transport of electrons within cells. Draw the structure of a
copper(II) complex that would form if three glycine amino
acids (shown below) formed coordinate covalent bonds with
the copper. (Assume that each glycine is bidentate.)
HC
H
NH
2
COH
O
Glycine
Oxinate ligand
N
O
20.5 Isomers
Skill Review
29. Define the type of isomer present, if isomerization exists, in
each of these pairs of complexes.
a. trans-[Pt(NH
3
)
2
Cl
2
] and cis-[Pt(NH
3
)
2
Cl
2
]
b. [Pt(CN)
2
(NH
3
)
4
]Cl
2
and [PtCl
2
(NH
3
)
4
](CN)
2
c. [Fe(H
2
O)
6
][CuBr
4
] and [Cu(H
2
O)
6
][FeBr
4
]
30. Define the type of isomer present, if isomerization exists, in
each of these pairs of complexes.
a. [Cu(NH
3
)
3
(ONO)] and [Cu (NH
3
)
3
(NO
2
)]
b. [Mn(H
2
O)
4
Cl
2
]Br
2
and [Mn(H
2
O)
4
Br
2
]Cl
2
c. cis-[PdCl
2
(NH
3
)
2
] and trans-[PdCl
2
(NH
3
)
2
]
31. Diagram two square planar geometric isomers with the for-
mula [PtI
2
(NH
3
)
2
]. Label the cis isomer. It is not possible to
diagram two tetrahedral geometric isomers of [Pt(NH
3
)
2
I
2
].
Explain why.
32. Diagram all of the possible isomers of [Co(NH
3
)
2
(SCN)
2
].
33. How many different isomers of [NiCl
3
F
3
]
4−
can be drawn?
Show the structure of each.
34. Show the structures of the coordination sphere isomers for
[Co(NH
3
)
6
][Cr(NO
2
)
6
].
Chemical Applications and Practices
35. Ethylenediammine (en) is a bidentate ligand, so it forms two
attachments to metal ions in coordination complexes. How-
ever, the square planar complex [Pt I
2
(en)] exhibits only one
type of geometric isomer. Draw possible structures for
[Pt I
2
(en)] and for a complex between iron(III) and en.
36. Diagram the Lewis dot structure for the cyanate ion (OCN
−
).
Showhowthis ionwould makeit possible tohavetwodifferent
forms of the following complex: [Co(OCN)(NH
3
)
5
]
2+
. What
type of isomerism does this example illustrate?
20.6 Formulas and Names
Skill Review
37. Provide names for these complex ions:
a. [Co(CN)(en)
2
(NH
3
)]
2+
b. [Cr(C
2
O
4
)
2
(NH
3
)
2
]
−
c. [Fe(NO
2
)
6
]
3−
d. [CoCl
3
(H
2
O)
3
]
38. Provide names for these complex ions:
a. [Mn(en)
3
]
2+
b. [Ni(H
2
O)
4
(NH
3
)
2
]
2+
c. [Cr(NO
2
)
6
]
3−
d. [V(SCN)
2
(H
2
O)
4
]
39. Write the chemical formula for each of these compounds and
complex ions:
a. tetraammineaquachlorocobalt(III)
b. trans-diaquabis(ethylenediamine)copper(II) chloride
c. sodium tetrachlorocobaltate(II)
d. pentacarbonylchloromanganese(I)
40. Write the chemical formula for each of these compounds and
complex ions:
a. tetraaaquadichlorocopper(II)
b. potassium cis-dibromooxalatoplatinate(II)
c. tetraamminenickel(II) sulfate
d. tetraaquathiosulfatoiron(III) nitrite
