Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

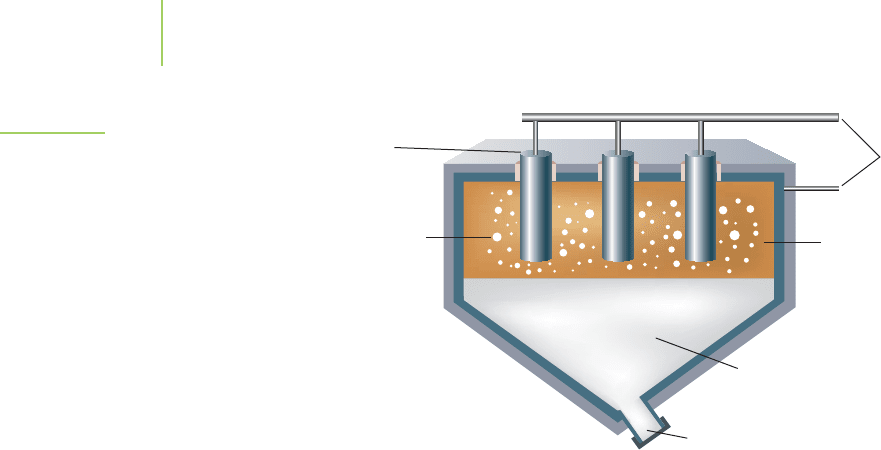
is the isolation of pure metals from a solution of metal ions, and it has been
known for quite some time. Humphrey Davey, an English chemist, used this
process to isolate metallic sodium from NaCl in 1807. However, the electrowin-
ning of aluminum wasn’t invented until 1886. Working independently, Charles
M. Hall (an American chemist) and Paul Heroult (a French chemist) discovered
that if bauxite ore was purified to alumina (Al
2
O
3
) and dissolved in molten cryo-
lite (Na
3
AlF
6
), metallic aluminum could be made. In the Hall–Heroult process,an
electrical current is passed into a molten mixture of alumina and cryolite to make
molten aluminum (Figure 19.19). The overall reaction used in the Hall–Heroult
process, shown below, is much simpler than the reactions that take place at the
electrodes. Why can’t an electric current be applied directly to an aqueous solu-
tion of Al
3+
in order to produce aluminum metal? Because the potential is so
large, instead of obtaining aluminum metal from the aqueous solution, the water
undergoes electrolysis to form hydrogen and oxygen gases.
2Al
2
O
3
(l) + 3C(s) n 4Al(l) + 3CO
2
(g) E° ≈−2.1 V
The negative potential tells us that this redox reaction is not spontaneous.
In fact, the reverse reaction—that is, 4Al(l) + 3CO
2
(g) n 2Al
2
O
3
(l) + 3C(s)—
is quite spontaneous (E
° ≈
+2.1 V). Modifying the concentrations and tempera-
tures helps a little to make the potential of the overall reaction less negative, via
the Nernst equation, but not enough to make the reaction spontaneous. If we
apply a positive potential to the reaction that is larger than the negative potential
expressed by the electrochemical cell, we can force the reaction to go forward.
Examination of a simpler redox reaction can be helpful here. For example, in
concept, if we wish to make the copper–iron redox reaction spontaneous, we
must supply a potential of just over +0.78 V to the reaction to make up for the
fact that E° is −0.78 V.
Cu(s) + Fe
2+
(aq) n Fe(s) + Cu
2+
(aq) E° =−0.78 V
When we do this, however, we note experimentally that the reaction still doesn’t
proceed toward products. If we supply a still greater positive potential, the reac-
tion does proceed. The extra voltage required is called the
overpotential of the
reaction. Overpotentials can be fairly high, especially when the products of
the reaction are gases. Unfortunately, we can’t easily predict what the overpo-
tential for a particular reaction will be. Instead we measure the overpotential
experimentally.
858 Chapter 19 Electrochemistry
CO
2
gas
by-product
Exit port
Molten
Al
2
O
3
/Na
3
AlF
6
mixture
Power source
Graphite
anodes
Molten
aluminum
FIGURE 19.19
The Hall–Heroult process. This process is still
used today to satisfy the world’s demand for
aluminum products. The product, molten alu-
minum, is relatively dense and settles to the
bottom of the electrochemical cell, where it is
drawn off and poured into castings.
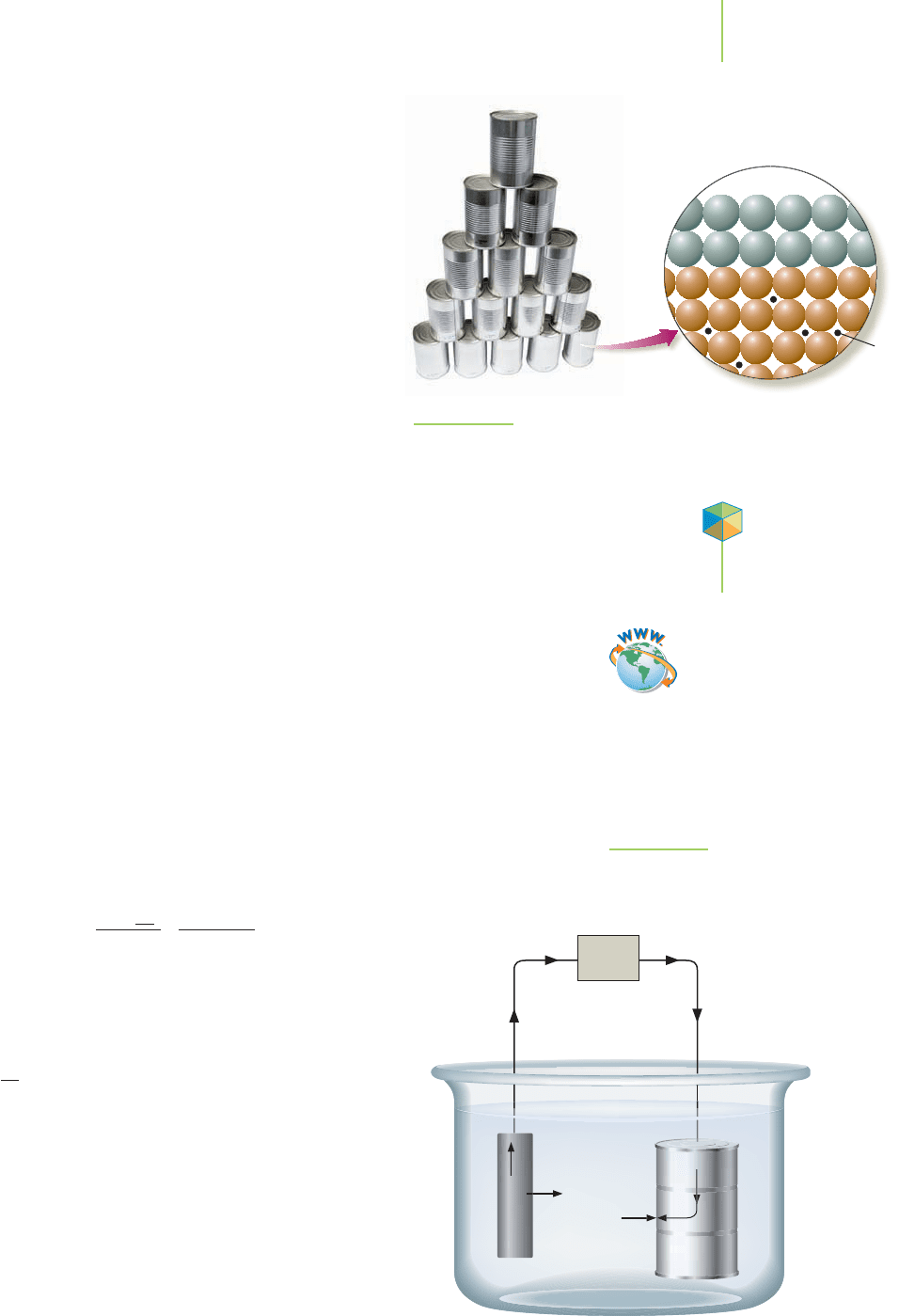
The Applications of Electrolysis
The average American uses 142 tin cans each year. From
what are tin cans made? That might sound like a silly
question, but “tin” cans are actually made of steel
coated with a very thin layer of tin (Figure 19.20). Ap-
proximately 0.25% of the mass of a tin can is actually
tin, and chromium is becoming more common as a
coating on the steel. Without the coating, the steel
would rust and the contents would spoil. The tin coat-
ing on a steel can isn’t applied like paint on a house.
How is the tin applied to the steel?
The most common use for electrolysis is
electroplat-
ing
, or depositing metals onto a conducting surface.
The result is a coating that is very tightly integrated into
the surface of the metal. Because of this tight integra-
tion into the metal surface, the coating resists flaking
and peeling. This coating makes the item more attrac-
tive and imparts some corrosion resistance or chemical
resistance to the surface. Electroplating is used in the manufacture of inexpensive
jewelry and chrome bumpers for your car, but most common electroplating
today occurs in the manufacture of tin cans.
Calculations Involving Electrolysis
The process used to coat a steel can with tin is an example of electrolysis. How is
it done? The steel can is hooked up to a power supply and dipped into a solution
of tin ions (Sn
2+
). A block of tin metal is also placed in the solution and con-
nected to the power supply. Then a current is applied to the can (Figure 19.21). In
the terminology of the electrolytic cell, the block of tin metal becomes the anode
and the can becomes the site of reduction (the cathode) for tin ions.
Michael Faraday (remember him from our discussion on potentials and
spontaneity) noted that the amount of current applied to a cell is directly
proportional to the amount of metal that can be deposited in an electrolytic re-
action. We can represent this mathematically as
g =
A·s·(M
)
F
mol metal
mol e
−
where A is the number of amperes applied to the can
(1 ampere =1 coulomb of charge per second)
s is the number of seconds that the current is
applied
M
is the molar mass of the metal
F is Faraday’s constant (96,485 coulombs/mol
electrons)
the ratio (mol metal/mol e
−
) is the mole ratio
of the reduction half-reaction
It is helpful to look at this calculation as an extended
unit conversion problem. Exercise 19.12 shows how this
is done. Note that the unit conversion problem is the
same as the equation shown above.
19.7 Electrolytic Reactions 859
Fe
C
Sn
FIGURE 19.20
Tin cans are actually steel cans electroplated with a thin coat of tin. The
tin resists corrosion and helps keep the contents fresh.
Application
C
HEMICAL
ENCOUNTERS:
Electroplating
Power
source
Anode Cathode
e
–
e
–
e
–
e
–
e
–
Sn Sn
2+
Sn
2+
e
–
FIGURE 19.21
Electroplating a tin can. The tin can
acts as the cathode in the electrolysis
experiment.
Video Lesson: The Stoichiometry
of Electrolysis

Fe
C
Au
EXERCISE 19.12 Golden Forks
To save money and still have a beautiful set of dinnerware, a chemist decides to elec-
troplate the metal with gold. How many grams of gold will be electroplated on a
fork if 2.5 A is applied to the fork for 20 s?
Solution
The number of grams can be calculated by starting with the current and perform-
ing a unit conversion. Note that the unit amperes (amps, A) can be written as
coulombs/second (C/s). We also need to examine the reduction half-reaction from
Table 19.3 to determine the number of electrons involved in the process.
Au
3+
(aq) + 3e
−
n Au(s)
2.5C
s
×
20 s
×
1 mol e
−
96,485 C
×
1 mol Au
3 mol e
−
×
196.97 g Au
1 mol Au
= 0.034 g Au
Amperes × time ×
1
faraday
× mole ratio × M
= grams
This means that our chemist would need to make sure to buy at least 0.034 g of gold
per fork.
PRACTICE 19.12
How many grams of tin will be deposited from a solution of tin(II) nitrate on a steel
can if 0.45 A are applied to the can for 1.5 h?
See Problems 73–78.
The calculations can also be done in reverse. If we want to coat our steel can
with a certain number of grams of tin, we can use either the equation or the unit
conversion method to calculate the number of amps that need to be applied.
As we’ve seen in this chapter, electrochemistry is a very useful topic, especially
in today’s society. Learning how muscles in our body begin a contraction can help
us understand how the heart works and why it is so important to maintain suffi-
cient levels of electrolytes during physical exercise. And as we become increas-
ingly mobile, the demand for longer-lasting batteries will only increase. Batteries
power our cell phones, our portable CD players, and even our cars. Electroplating
the surfaces of many everyday items makes them appear expensive and confers
resistance to corrosion. In fact, everywhere we look, there’s electrochemistry!
860 Chapter 19 Electrochemistry
The Bottom Line
■
Redox reactions involve both a reduction and an oxi-
dation half-reaction. (Sections 19.1 and 19.3)
■
Redox reactions can be identified by determining the
oxidation state of the atoms involved in a reaction.
(Section 19.2)
■
Redox reactions can be balanced by summation of
balanced half-reactions. (Section 19.3)
■
Positive cell potentials indicate a spontaneous reac-
tion and are related to the free energy change by
∆G° =−nFE°. (Section 19.3)
■
Electrochemical cells require both a path for the
electrons and a path for other ions. (Section 19.4)
■
The oxidation reaction takes place at the anode. The
reduction reaction takes place at the cathode.
(Section 19.4)
■
The Nernst equation relates the actual potential of a
redox reaction to conditions other than the standard
conditions. (Section 19.6)
■
Half-reaction potentials can be used to determine
the relative reactivity of metals. Organization of the
metals in this fashion is known as a chemical reac-
tivity series. (Section 19.5)
■
Cell potentials enable us to calculate equilibrium
constants. (Section 19.6)
■
Electrowinning and electroplating are examples
of electrolysis reactions. In electrolysis, a positive
potential that includes the overpotential is applied
to force the reaction to run in reverse. (Section 19.7)

anode The electrode at which oxidation takes place.
(p. 826)
battery Two or more voltaic cells joined in series.
(p. 826)
cardiologist A heart specialist. (p. 824)
cathode The electrode at which reduction takes place.
(p. 826)
cell notation The chemist’s shorthand used to describe
electrochemical cells. (p. 847)
concentration cell A cell in which different concentra-
tions of identical ions on both sides of the cell
provide the driving force for the reaction. (p. 853)
corrosion The deterioration of a metal as a consequence
of oxidation. (p. 850)
disproportionation A reaction in which a single reactant
is both the oxidizing and the reducing agent.
(p. 830)
electrochemical cell A device that allows the exchange
between chemical and electrical energy. (p. 825)
electrochemistry The study of the reduction and oxida-
tion processes that occur at the meeting point of
different phases of a system. (p. 825)
electrochemists Scientists who create or analyze systems
that allow exchanges between chemical and electrical
energy. (p. 824)
electrode A metal surface that acts as a collector or
distributor for electrons. (p. 826)
electrodics The study of the interactions that occur
between a solution of electrolytes and an electrical
conductor, often a metal. (p. 825)
electrolytic cell A cell that requires the addition of
electrical energy to drive the chemical reaction under
study. (p. 826)
electromotive force (emf) A measure of how strongly a
species pulls electrons toward itself in a redox process.
Also known as voltage. (p. 834)
electroplating The process of depositing metals onto a
conducting surface. (p. 859)
electrowinning The isolation of pure metals from a
solution of metal ions. (p. 857)
Faraday’s constant A unit of electric charge equal to the
magnitude of charge on a mole of electrons. (p. 836)
fuel cell An electrochemical cell that utilizes continually
replaced oxidizing and reducing reagents to produce
electricity. (p. 826)
galvanic cell A cell that produces electricity from a
chemical reaction. Also known as a voltaic cell.
(p. 825)
gel electrophoresis The use of electric fields to separate
ions. (p. 825)
Key Words 861
Key Words
half-reaction An equation that describes the reduction
or oxidation part of a redox reaction. (p. 826)
Hall–Heroult process The most widely used process for
the preparation of aluminum from bauxite. (p. 858)
ionics The study of the behavior of ions dissolved in
liquids. (p. 825)
Nernst equation The equation used to determine the cell
potential at nonstandard conditions. (p. 852)
nitrogen cycle The path that nitrogen follows through its
different oxidation states on Earth. (p. 831)
overpotential The extra potential needed, above that
which is calculated, in order to make an electrochem-
ical process proceed. (p. 858)
oxidation The loss of electrons. (p. 826)
oxidation reaction In a redox reaction, the half-reaction
that supplies electrons. (p. 826)
oxidation state A bookkeeping tool that gives us insight
into the distribution of electrons in a compound.
Also known as oxidation number. (p. 827)
oxidizing agent A substance that causes the oxidation of
another substance. (p. 830)
potential The driving force (to perform a reaction) that
results from a difference in electrical charge between
two points. (p. 824)
reactivity series A ranking of the electrochemical reac-
tivity of some elements. (p. 849)
redox reactions Chemical reactions in which reduction
and oxidation occur. (p. 824)
reducing agent A substance that causes the reduction of
another substance. (p. 830)
reduction The gain of electrons. (p. 827)
reduction reaction In a redox reaction, the half-reaction
that acquires electrons. (p. 826)
sacrificial anode A material that will oxidize more easily
than the one we seek to protect from oxidation.
(p. 850)
salt bridge A device containing a strong electrolyte
that allows ions to pass from beaker to beaker. (p. 845)
standard hydrogen electrode reaction (SHE) A reference
half-reaction of the reduction of hydrogen ion to
hydrogen gas, against which to compare our reduc-
tion. (p. 835)
standard potential (E°) The measure of the potential of a
reaction at standard conditions. (p. 834)
volt The SI unit of potential. (p. 834)
voltage A measure of how strongly a species pulls elec-
trons toward itself. Also known as electromotive force
(emf). (p. 834)
voltaic cell A cell that produces electricity from a chem-
ical reaction. Also known as a galvanic cell. (p. 825)
The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
19.1 What Is Electrochemistry?
Skill Review
1. Explain why the descriptive term battery is often used, but is
technically not correct, to describe these most commonly
purchased sources of electricity.
2. Provide two other names for an electrochemical cell.
Chemical Applications and Practices
3. Every electrochemical cell is developed around two types of
chemical reactions. Name and describe both of these con-
nected reactions.
4. List three properties of electrochemical cells that are consid-
ered when designing an appropriate power source.
Section 19.2 Oxidation States—Electronic Bookkeeping
Skill Review
5. Determine the oxidation number of each atom in the struc-
ture of dimethylsulfoxide (DMSO).
10. Put the following compounds in order from the lowest to the
highest oxidation number for carbon.
C
6
H
12
O
6
CO
2
CH
3
OH CH
4
C
6
H
6
11. Determine the oxidation state for each atom in the following
compounds:
a. KMnO
4
b. LiMnO
2
c. NH
4
ClO
4
12. Determine the oxidation state for each atom in the following
compounds:
a. K
2
MnCl
4
b. Cr
2
O
3
c. C
12
H
22
O
11
13. Use the following four terms or expressions to identify each
of the chemical situations indicated: (is oxidized; is reduced; is
an oxidizing agent; is a reducing agent). You may use as many
terms as apply.
a. An atom has gained an electron.
b. An atom increases its oxidation number.
c. The oxidation number of an atom changes from −2 to –3.
14. Use the following four terms or expressions to identify each
of the chemical situations indicated: (is oxidized; is reduced; is
an oxidizing agent; is a reducing agent). You may use as many
terms as apply.
a. An atom decreases its oxidation number.
b. An atom loses two electrons.
c. The oxidation number of an atom changes from +3 to +5.
15. Supply the oxidation number of each atom, on both sides of
the reaction arrow, in the equation
3Mg(s) + 2H
3
PO
4
(aq) n Mg
3
(PO
4
)
2
(aq) + 3H
2
(g)
16. Supply the oxidation number of each atom, on both sides of
the reaction arrow, in the equation
2AgNO
3
(aq) + Cu(s) n Cu(NO
3
)
2
(aq) + 2Ag(s)
Chemical Applications and Practices
17. The main active ingredient in commercial household bleach
is the hypochlorite ion, ClO
−
.
a. What is the oxidation number of the Cl atom in the ion?
b. Hypochlorite is known as a good oxidizing agent. Would
that property indicate that the Cl tends to gain or lose
electrons as it reacts? Explain the logic of your response.
18. The combustion of propane in a portable burner is a com-
mon redox reaction. Examine each component of the equa-
tion. Assign an oxidation number to each atom, and deter-
mine which component is acting as the reducing agent.
C
3
H
8
(g) + O
2
(g) n CO
2
(g) + H
2
O(g)
19. The brilliant red color of many fireworks is due to the pres-
ence of strontium. However, strontium metal is not typically
found in its pure state. The following redox reaction depicts
the isolation of strontium from molten strontium chloride.
SrCl
2
(l) n Sr(s) + Cl
2
(g)
a. Assign an oxidation number to each of the atoms in the
reaction.
b. What has been reduced in the reaction?
862 Chapter 19 Electrochemistry
Focus Your Learning
6. Determine the oxidation number of each atom in the struc-
ture of periodic acid.
7. In which of the following compounds would the chlorine
atom have the most positive oxidation number? In which
would chlorine have the most negative oxidation number?
Cl
2
;ClO
2
;NaClO
4
;HCl
8. Which species in the following list shows the nitrogen atom
in its most reduced form? Which depicts nitrogen in its most
oxidized form? N
2
; HNO
3
;NH
3
9. Put the following compounds in order from the lowest to the
highest oxidation number for nitrogen.
NO N
2
ONO
2
N
2
H
4
NH
3

20. Sodium thiosulfate is familiar to photographers as “hypo.” It
helps dissolve some of the silver salts used in developing pho-
tographs. Aqueous solutions of sodium thiosulfate can un-
dergo disproportionation reactions, as shown here:
S
2
O
3
2−
(aq) + H
+
(aq) n H
2
O(l) + S(s) + SO
2
(g)
a. What is the change in the oxidation number of S in S
2
O
3
2−
in the oxidation portion of the reaction?
b. What is the change in the oxidation number of S in S
2
O
3
2−
in the reduction portion of the reaction?
21. Four equations can be written to describe the rusting of iron:
O
2
(aq) + 2H
2
O(l) + 4e
−
n 4OH
−
(aq)
Fe(s) n Fe
2+
(aq) + 2e
−
Fe
2+
(aq) + 2OH
−
(aq) n Fe(OH)
2
(s)
Fe(OH)
2
(s) + OH
−
(aq) n FeO(OH)(s) + H
2
O(l) + e
−
a. Which of these are reduction or oxidation half-reactions?
b. What other kind of equation is present here?
c. Combine the equations to give the overall equation that
describes the formation of rust, FeO(OH)(s), from iron,
Fe(s).
22. In the chapter, we discussed the reaction of hydrogen and
oxygen to give water. How is it possible for this reaction to
proceed either as an explosion (as in the space shuttle main
engines) or as a gentle, readily managed source of electricity?
Section 19.3 Redox Equations
Skill Review
23. The superscript on the symbol E°
refers to standard condi-
tions for electrochemical reactions. What specifically does
that indicate for the concentrations and pressure of the react-
ing system?
24. The voltage values (emf) given on a standard reduction table
are referenced to a standard called SHE.
a. To what do the letters refer?
b. What is the voltage of the reference?
25. Complete each of these statements using the word positive,
negative, spontaneous,or nonspontaneous.
a. When E
° is negative, the electrochemical reaction is
___________.
b. When E° is positive, the value for
G° is ___________.
c. When a reaction is spontaneous, the values for
G° will
be __________, and the values for E° will be__________.
d. Nonspontaneous redox reactions have a ___________
value for E°.
26. The typical battery used in a standard flashlight produces ap-
proximately 1.5 V. If the value for
G° of the reaction were
−289,500 J, what would you calculate as the moles of elec-
trons exchanged in the balanced redox reaction?
27. Combine these half-reactions in such a way that a galvanic
cell results, and calculate the cell potential.
Fe
3+
+ e
−
n Fe
2+
E° = 0.77 V
Fe
2+
+ 2e
−
n Fe E° =−0.44 V
28. Combine these half-reactions in such a way that a galvanic
cell results, and calculate the cell potential.
Sn
2+
+ 2e
−
n Sn E° =−0.14 V
Sn
4+
+ 2e
−
n Sn
2+
E° =+0.15 V
29. Calculate the free energy change for the cell in Problem 27.
30. Calculate the free energy change for the cell in Problem 28.
31. What is the free energy change for the following reaction at
standard conditions?
PbO
2
+4H
+
+2Hg +2Cl
−
n Pb
2+
+2H
2
O +Hg
2
Cl
2
E°
cell
=1.12 V
32. What is the free energy change for the following reaction at
standard conditions?
O
2
+ 4H
+
+ 2Ni n 2H
2
O + 2Ni
2+
E°
cell
= 1.46 V
33. What are three considerations or aspects that must be
“balanced” in a balanced redox reaction?
34. In this balanced redox reaction, chlorine is shown to replace
bromide ions from a solution.
Cl
2
(aq) + 2Br
−
(aq) n 2Cl
−
(aq) + Br
2
(aq)
What is the value of n in the overall reaction?
35. Balance these half-reactions in acidic solution. Is each a
reduction or an oxidation? Which substance is oxidized and
which is reduced?
a. CO
2
n H
2
C
2
O
4
b. Np
4+
n NpO
2
+
36. Balance these half-reactions in acidic solution. Is each a re-
duction or an oxidation? Which substance is oxidized and
which is reduced?
a. I
2
n IO
3
−
b. NO
3
−
n NO
37. Balance these redox equations in acidic solution:
a. Sn
2+
+ Cu
2+
n Sn
4+
+ Cu
+
b. S
2
O
3
2−
+ I
3
−
n S
4
O
6
2−
+ 3I
−
c. SO
3
−
+ Fe
3+
n SO
4
2−
+ Fe
2+
38. Balance these redox equations in acidic solution:
a. Al + Cu
2+
n Al
3+
+ Cu
b. UO
2
2+
+Ag + Cl
−
n U
4+
+AgCl
c. H
2
SO
4
+ HBr n SO
2
+ Br
2
39. Balance the redox equation that illustrates the reaction of
solid copper and dichromate, first in acidic and then in basic
solution.
Cu(s) + Cr
2
O
7
2−
(aq) n Cu
2+
(aq) + Cr
3+
(aq)
40. Balance the redox equation that illustrates the reaction of
permanganate and methanol, first in acidic and then in basic
solution.
MnO
4
−
+ CH
3
OH n CO
3
2−
+ MnO
4
2−
41. Balance this redox reaction, first in acidic and then in basic
solution.
ClO
4
−
+ I
−
n ClO
−
+ IO
3
−
42. Balance this redox reaction, first in acidic and then in basic
solution.
Zn + NO
3
−
n Zn
2+
+ NH
3
Focus Your Learning 863
Chemical Applications and Practices
43. Using the standard reduction potentials found in the appen-
dix, locate the half-cell reaction for zinc. Zinc is often used in
the production of dry cell batteries. It is also used to protect
other metals from oxidation.
a. What is the E
° value for this reaction?
b. What would be the value of
G
° for the half-reaction?
c. What would be the E
° value if the reaction stoichiometry
were doubled?
44. Splitting water into hydrogen gas and oxygen gas is one tech-
nique being investigated as a way to produce hydrogen gas for
use in fuel cells. The reaction is shown here as
2H
2
O n 2H
2
+ O
2
If the E° value for this nonspontaneous reaction were ap-
proximately −2.00 V, what would you calculate as the value
for
G
°?
45. Use the Standard Table of Reduction Potentials to answer the
following questions:
a. Which of the three alkali metal ions Na, K, and Li has the
least potential to attract electrons?
b. Of the three halogens which would be the strongest oxi-
dizing agent, F
2
,Cl
2
,or Br
2
?
c. If you prepared a battery by connecting two of the follow-
ing three half-cells, which combination could produce the
highest potential? Zn, Cu, Ni
46. Suppose you attempted to build a battery using lead (and
Pb
2+
) with chromium (and Cr
3+
).
a. Write out the half-cell reduction reactions and their E
°
values for each.
b. Which of the two would provide the reduction reaction,
and which would provide the oxidation reaction?
c. What would be the total E
°
of the overall redox reaction?
d. How many moles of electrons would be exchanged in the
overall reaction?
47. The oxidation of copper metal gives rise to a beautiful green
patina. An equation that illustrates the oxidation of copper is
shown here. Balance this redox reaction.
Cu(s) + CO
2
(g) n CuO(s) + C
2
O
4
2−
(aq)
48. One technique used for the detection of ethanol involves the
following redox reaction with dichromate ions. Balance this
redox reaction.
C
2
H
5
OH(aq) + Cr
2
O
7
2−
(aq) n CH
3
CO
2
H(aq) + Cr
3+
(aq)
49. As with acid–base reactions, careful addition of a solution
containing an oxidizing agent to a solution containing a re-
ducing agent can be used for titration analysis. Standardizing
a solution of potassium permanganate to be used later in a
redox titration often involves reaction with a known amount
of sodium oxalate. Balance the redox reaction used in the
standardization process.
C
2
O
4
2−
(aq) + MnO
4
−
(aq) n CO
2
(g) + Mn
2+
(aq) + H
2
O(l)
50. Once a standard solution of potassium permanganate is pre-
pared, it can be used to determine the concentration of iron
in an unknown sample. For example, the iron content of a
small steel sample could be obtained through a titration re-
action with a standard permanganate solution. The redox
reaction that would take place in the analysis is shown here
(unbalanced, and after the iron has been prepared as +2 ion).
Balance the redox titration and identify the reducing agent.
MnO
4
−
(aq) + Fe
2+
(aq) n Mn
2+
(aq) + Fe
3+
(aq) + H
2
O(l)
51. One step in a common method for the analysis of vitamin C
in juice drinks is to oxidize the vitamin C (ascorbic acid) with
aqueous I
2
. Balance this redox reaction.
C
6
H
8
O
6
(aq) + I
2
(aq) n I
−
(aq) + C
6
H
6
O
6
(aq)
52. There are many half-cell reaction combinations that could be
used to make batteries. One such reaction would be to use
system consisting of silver(II) oxide and zinc. The reaction
shown below would have to occur in a basic environment.
Balance this redox reaction.
Zn(s) +AgO(s) n Zn(OH)
2
(s) +Ag(s)
Section 19.4 Electrochemical Cells
Skill Review
53. Define the following terms in your own words: cell, half-
reaction, galvanic cell, voltaic cell, electromotive force.
54. Compare and contrast an electrolytic cell with a galvanic cell
on the basis of sign on the E° value, ability to do work, chem-
ical process taking place at the anode, and spontaneity.
55. The following notation refers to a specific voltaic cell. Iden-
tify the species that would serve as the anode and the species
that is the oxidizing agent.
Zn(s) | Zn
2+
(aq) || Cu
2+
(aq) | Cu(s)
56. The following notation refers to a specific voltaic cell. Iden-
tify the species that would serve as the anode and the species
that is the oxidizing agent.
Mg(s) | Mg
2+
(aq) || Co
2+
(aq) | Co(s)
57. The following schematic diagram represents two metals and
their cations in separate beakers joined by a NaNO
3
salt
bridge. If the metals were lead and silver (with their respec-
tive cations Pb
2+
and Ag
+
), which beaker would require the
silver and which the lead if the nitrate ions were moving from
left to right through the salt bridge? Justify your answer.
864 Chapter 19 Electrochemistry
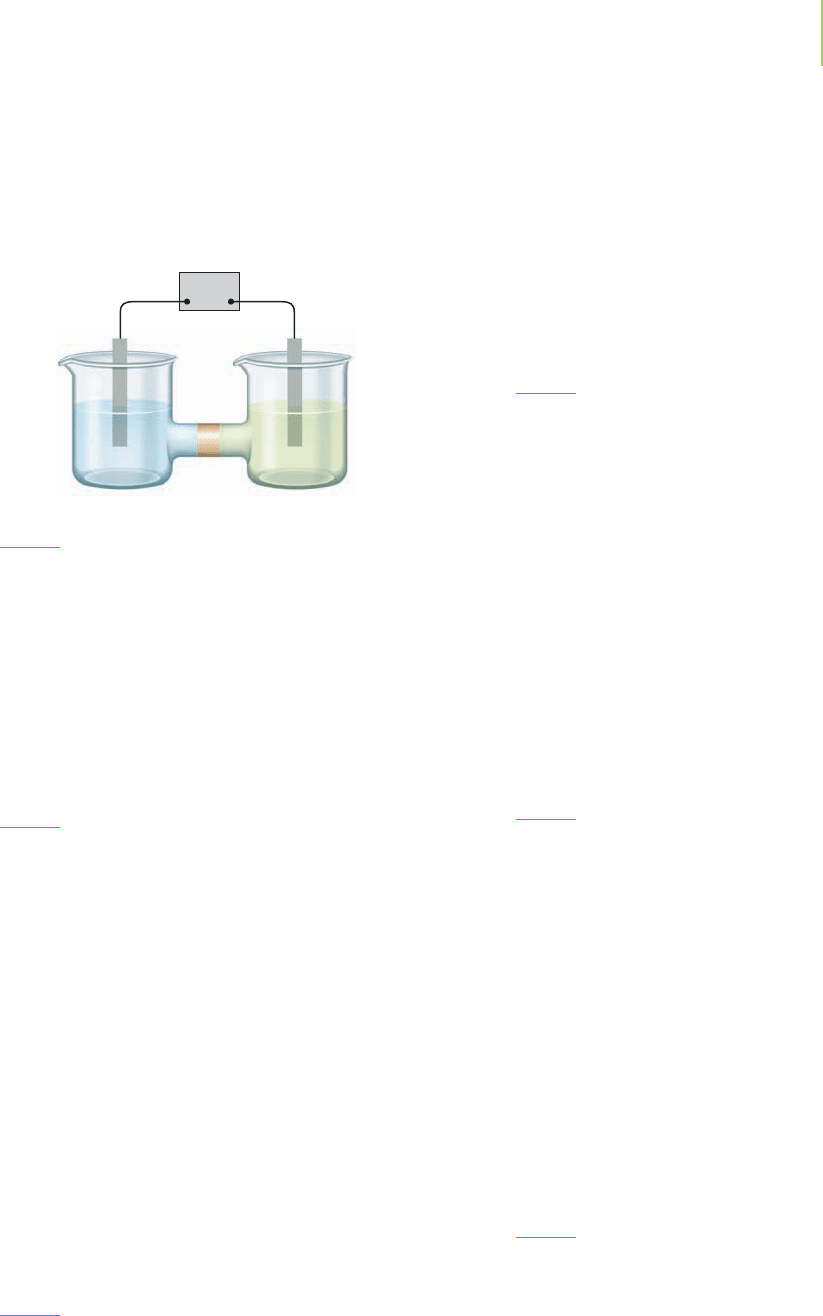
58. The following schematic diagram represents two metals and
their cations in separate beakers joined by a NaNO
3
salt
bridge. If the metals were copper and iron (with their respec-
tive cations Cu
2+
and Fe
3+
), which beaker would require the
copper and which the iron if the nitrate ions were moving
from left to right through the salt bridge? Justify your answer.
c. In which direction, to the left or to the right, would the
following reaction take place spontaneously? (Assume
standard conditions.)
Ag + Fe
3+
n Fe
2+
+Ag
+
64. Under standard conditions one of the following two reac-
tions will not occur spontaneously. Use the Standard Table of
Reduction Potentials to explain how you could correctly pre-
dict which one will not take place.
Fe + Sn
2+
n Fe
3+
+ Sn
Cu
2+
+ Fe n Fe
3+
+ Cu
Chemical Applications and Practices
65. Suppose you have a shiny piece of metal that is unlabeled. It
is known to be either aluminum or tin. You also have a solu-
tion of 1.0 M nickel(II) nitrate. Suggest a chemical test that
you could use to determine the identity of the metal using the
solution. Explain your expected results and the basis of your
conclusion.
66. One technique to protect against oxidation of structures such
as ship hulls and underground iron pipes is to place the
structure in contact with a metal that will oxidize more easily
than the iron in the structure. The more active metal is then
referred to as a sacrificial anode. Neglecting such issues as
cost and availability, would copper or zinc make the better
sacrificial anode in an attempt to protect an iron-based struc-
ture? Explain the basis of your choice.
Section 19.6 Not-So-Standard Conditions:
The Nernst Equation
Skill Review
67. The constant used in the Nernst equation, 0.0257, is derived
from the combination of three other constants. Obtain the
three values, assuming standard temperature, and derive the
constant used in the equation. Some texts refer to the Nernst
equation using the base 10 logarithmic scale. What would be
the value of the constant in that scale?
68. The simplified equation presented here represents the redox
reaction taking place inside the typical, nonalkaline flashlight
battery. Use the principles described in the Nernst equation
to answer the questions that follow.
Zn(s) + 2MnO
2
(s) + 2NH
4
+
(aq) n
Zn
2+
(aq) + Mn
2
O
3
(s) + 2NH
3
(g) + H
2
O(l)
a. What would be the effect on the spontaneity of the re-
action if the concentration of NH
4
+
(aq) were decreased?
b. What would be the effect on the voltage of the cell if the
concentration of NH
4
+
(aq) were decreased?
c. What is the value of n in the equation?
d. At equilibrium what is the value of E?
69. Calculate the value of E
cell
for the reaction of iron and cop-
per(II), given the specific concentrations listed.
a. Fe(s) | Fe
2+
(0.10 M) || Cu
2+
(0.10 M) | Cu(s)
b. Fe(s) | Fe
2+
(1.5 M) || Cu
2+
(0.10 M) | Cu(s)
c. Fe(s) | Fe
2+
(0.10 M) || Cu
2+
(1.5 M) | Cu(s)
70. Calculate the value of E
cell
for the reaction of cobalt(II) chlo-
ride and zinc metal, given the specific concentrations listed.
a. Zn(s) | Zn
2+
(0.10 M) || Co
2+
(0.10 M) | Co(s)
b. Zn(s) | Zn
2+
(2.5 M) || Co
2+
(0.050 M) | Co(s)
c. Zn(s) | Zn
2+
(0.010 M) || Co
2+
(0.10 M) | Co(s)
Focus Your Learning
865
Chemical Applications and Practices
59. Diagram a battery consisting of two beakers and a salt bridge
that makes use of the two half-reactions silver and gold (sort
of expensive, but you’re worth it).
a. Predict the E
° value for the battery.
b. What is the value of n for the balanced equation?
c. Label the cathode.
d. Exactly what species is acting as the oxidizing agent?
e. Represent the battery in the ABC notation.
60. Search Internet references using the keywords “hydrogen fuel
cell” and report on the basic operation of such a cell. Give
particular emphasis to the electrolyte used in such a cell. Be
sure to report the necessary URL for your references.
61. Small button-sized batteries can be made using mercury and
zinc. If the two half-reactions are as follows, what would you
report as the ABC notation for the battery? (Mercury in the
batteries is considered a toxic substance and should be care-
fully recycled.)
Zn(s) n ZnO(s)
HgO(s) n Hg(l)
Note that the reactions are not balanced. The reaction takes
place in a basic medium.
62. Write out the standard reduction half-cell reactions and E
°
values for Zn, Ni, Pb, and H
2
(g).
a. Select the two half-reactions that, when combined, would
produce the battery with the greatest theoretical overall E
°
value, and report that value.
b. Which, if any, of those represented would have electrons
move along an external wire, when connected with the
hydrogen half-cell, toward the hydrogen electrode?
Section 19.5 Chemical Reactivity Series
Skill Review
63. Use the Standard Table of Reduction Potentials to answer the
following questions.
a. Which is the better reducing agent, Ba or Ca?
b. Which is the better oxidizing agent, Pb
2+
or Ni
2+
?
Chemical Applications and Practices
71. Suppose that at night on a campout your flashlight dims and
finally stops working. Your friend remarks, “I guess the bat-
tery is dead.” In what three other chemical ways, using Gibbs
free energy, equilibrium, and potential, could you state the
same conclusion as your friend?
72. A quick source of hydrogen gas in the lab is to carefully place
some solid magnesium in hydrochloric acid.
Mg(s) + 2HCl(aq) n MgCl
2
(aq) + H
2
(g)
If the Mg
2+
concentration were 1.0 M and the HCl concen-
tration were 0.10 M (assume completely dissociated HCl),
what would you calculate as the E value for the reaction at
25°C?
Mg(s) | Mg
2+
(aq) || H
+
(0.1 M) | H
2
(1 atm)
Section 19.7 Electrolytic Reactions
Skill Review
73. Calculate the number of grams of gold electroplated
onto a surface given these conditions. (Assume that the solu-
tion of Au
3+
ions is concentrated enough to complete the
electrolysis.)
a. 1.25 A for 60 s b. 2.11 A for 2.33 h c. 0.75 A for 1 d
74. Calculate the time required to electroplate 1.0 g of tin metal
onto a surface given these conditions. (Assume that the solu-
tion of Sn
2+
ions is concentrated enough to complete the
electrolysis.)
a. 2.25 A b. 0.11 A c. 1.38 A
75. Alessandro Volta is given historical precedence in the discov-
ery of the first battery in the sense that we think of batteries
today. The “Voltaic pile” consisted of dissimilar metals joined
by salt-moistened paper strips. Davy and his assistant
Michael Faraday later refined this. At that time, early electro-
plating businesses began developing in England. If such a
business silver-plated a teaspoon, would the teaspoon be the
cathode or the anode in such a process? If the spoon received
0.33 g of silver after being plated for 1.0 h, what amperage
was used?
76. In addition to isolating sodium, Sir Humphrey Davy isolated
potassium, calcium, and magnesium from impure natural
ore samples.
a. If he used a current of 1.00 A for 1.00 h, how many grams
of magnesium metal could he obtain from molten MgCl
2
?
b. Using the same electrical set up, how many grams of
potassium metal could Sir Humphrey isolate from molten
KCl?
Chemical Applications and Practices
77. In the typical lead storage battery found in most automo-
biles, lead is oxidized to Pb
2+
(in the form of PbSO
4
). In
recharging, the reaction is reversed. If a battery were
recharged for 30.0 min at a current of 8.00 A, how many
grams of lead would be reduced, from PbSO
4
during the
process?
78. Germanium has become a valuable metal in semiconductor
fields. If a 1.00 A current were used for 1.00 h to plate out
0.677 g of germanium, what would you calculate as the
oxidation number of the germanium ions in the plating
solution?
Comprehensive Problems
79. Use the illustration below as the starting point to draw the
electrolytic cell that would be used to plate copper metal onto
a steel saucepan. Be sure to indicate the location of the cath-
ode and the anode, the location of the copper electrode, and
the steel saucepan.
866 Chapter 19 Electrochemistry
Power
source
Anode Cathode
80. The Nernst equation can be used just as well with half-cell re-
actions as with balanced redox reactions. An application of
this is the use of potential differences in the acid level of so-
lutions compared to a standard when producing the probes
for pH meters. Using the half-reaction 2H
+
(aq) + 2e
−
n
H
2
(g) at 1 atm, what would you calculate, using the Nernst
equation, as the value for E in these situations?
a. Pure water, pH = 7.00
b. An acid solution with pH = 2.00
c. A 0.10 M solution of nitric acid
d. A 0.10 M solution of acetic acid, K
a
= 1.8
×
10
−5
81. Potassium ferrate (K
2
FeO
4
) is a powerful oxidizing agent
(E
° = 2.20 V relative to SHE in acidic solution) in which the
iron(VI) ion is reduced to the iron(III) ion. It oxidizes water
to oxygen gas.
a. Determine the cell potential and equilibrium constant for
the reaction at standard conditions for the oxidation of
water by ferrate ion in acidic solution.
b. Balance the reaction in basic solution.
c. Calculate the cell potential, given the following initial
concentrations:
[FeO
4
2−
] = 1.5
×
10
−3
M;[Fe
3+
] = 1.1
×
10
−3
M;
P
O
2
= 8.3
×
10
−5
atm; pH = 2.8
d. The reaction results in the production of a yellow solid
that is especially apparent at high pH. Can you suggest
what this might be? Further, can you suggest how this solid
might help if the ferrate ion were used to treat wastewater
contamination?

82. Potassium permanganate (KMnO
4
) is a useful analytical
reagent for determining the percentage of iron in an iron ore.
The procedure includes dissolving the iron with HCl and
then converting all of the iron in the ore to Fe
2+
using several
reagents. The titration of the resulting Fe
2+
with MnO
4
−
is
Fe
2+
+ MnO
4
−
n Fe
3+
+ Mn
2+
A sample of the original iron ore weighing 3.852 g was
processed for titration in a total volume of 300.0 mL of solu-
tion. A 100.0 mL aliquot (accurately measured portion) was
removed and titrated with a 0.1025 M KMnO
4
solution. A
total of 23.14 mL was required to the light pink endpoint.
What is the percent of iron in the ore sample?
83. We noted in Chapter 14, Thermodynamics, that we can have
“standard conditions” at a given pressure, but the tempera-
ture can vary and must be stated as part of specifying your
standard values. If we define our standard values at 1 bar
pressure and 3000°C, how would that affect the values for our
“new” standard reduction potentials compared to the ones
we commonly use at 25°C?
84. We discuss rechargeable batteries in Section 19.4. You know
from your own experience that these batteries can be
recharged hundreds of times but eventually wear down and
must be replaced. Why can’t they be recharged an infinite
number of times? In particular, investigate the way in which
metals plate back onto the cathode when the recharging takes
place.
85. Chrome plating is the process by which Cr
6+
is electroplated
on automobile surfaces to give them a nice luster. The
chromium layer can be as little as 10.0 µm thick. How many
grams of CrO
3
would be necessary to plate the back surface
of a car mirror that has a coverable area of 150 cm
2
?
(d = 7.2 g/cm
3
.)
86. We discuss fuel cells at several places in the chapter, but never
actually calculate the electrochemical data for it. Calculate
the E°, G° and K at 298K for the hydrogen/oxygen fuel cell
described in Section 19.2. Once you have done so, can you
propose a fuel cell that might be more effective for use in au-
tomobiles? What are the criteria that you use to make that
judgment?
Thinking Beyond the Calculation
87. A battery designer wishes to prepare a solution-based battery
for use in a new automobile. The designer chooses iron and
zinc as the two metals for study.
a. Draw the setup (using beakers and a salt bridge) that
indicates the location of the iron and zinc electrodes, the
iron(II) and zinc(II) solutions, and the flow of electrons.
b. Which half-reaction is the oxidation reaction?
c. What is the cell potential for the reaction if the initial
concentrations of iron(II) and zinc(II) are 0.25 M at 25°C?
d. What would be the cell potential if the concentrations of
both solutions in part c were increased to 1.0 M? ...to
2.0 M?
e. If the battery is cooled to 10°C, is there a change in the cell
potential? If so, what is the new potential?
f. Describe at least one advantage to using a battery made
from these metals.
g. Describe at least one disadvantage to the use of iron in a
battery.
Focus Your Learning
867
