Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

828 Chapter 19 Electrochemistry
IIIB IVB VB VIB VIIB IB IIB
IA
IIA IIIA IVA VA VIA VIIA
VIIIA
VIIIB
Li
+
Na
+
K
+
Rb
+
Cs
+
Mg
2+
Ca
2+
Sr
2+
Ba
2+
Sc
3+
Ti
2+
Ti
3+
Ti
4+
V
2+
V
3+
V
4+
V
5+
Cr
2+
Cr
3+
Cr
6+
Mn
2+
Mn
3+
Mn
4+
Mn
7+
Fe
2+
Fe
3+
Co
2+
Co
3+
Ni
2+
Cu
+
Cu
2+
Ag
+
Al
3+
Sn
2+
Sn
4+
Pb
2+
Pb
4+
Zn
2+
Cd
2+
Hg
2
2+
Hg
2+
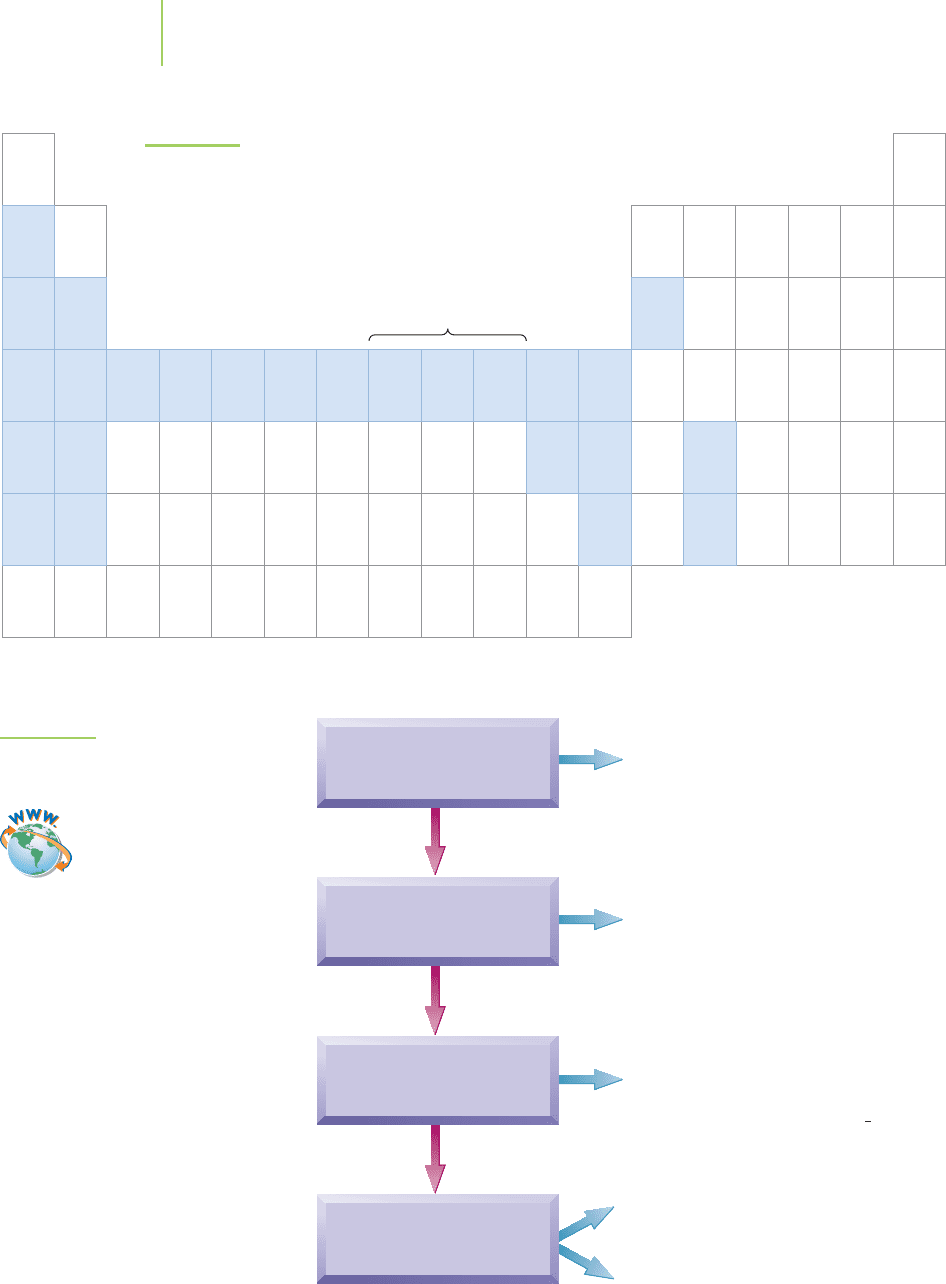
FIGURE 19.4
Oxidation states of the metals.
* How to recognize hydrides:
Hydrides have a metal first in their chemical formula.
For example: CaH
2
, MgH
2
, LiAlH
4
, NaH
Hydrides contain no nonmetals.
For example, these are not hydrides: CH
4
, NaHCO
3
, NH
3
, HCl
Is the chemical an element
or a simple ion?
Assign elements as zero (Cu
0
= 0),
simple ions as their charge (Cu
2+
= +2).
Assign H in hydrides as –1, and
assign all other H as +1.*
No
Ye s
Is hydrogen one of
the elements present?
Are any elements present that
show just one oxidation
state? If so assign them.
How many elements remain
to be assigned?
No
Ye s
No
1
2 or
more
Ye s
Assign them as follows:
All Group IA metals +1
All Group IIA metals +2
Zn, Cd +2
Group IIIA metals +3
O (except H
2
O
2
= –1; K
2
O = –
1
) –2
F –1
Subtract the total of the other oxidation
states from the charge on the overall
compound.
For each element, subtract eight (8)
from its group number.
2
FIGURE 19.5
Decision rules for assigning oxidation
states.
Video Lesson: Balancing Redox
Reactions by the Oxidation
Number Method
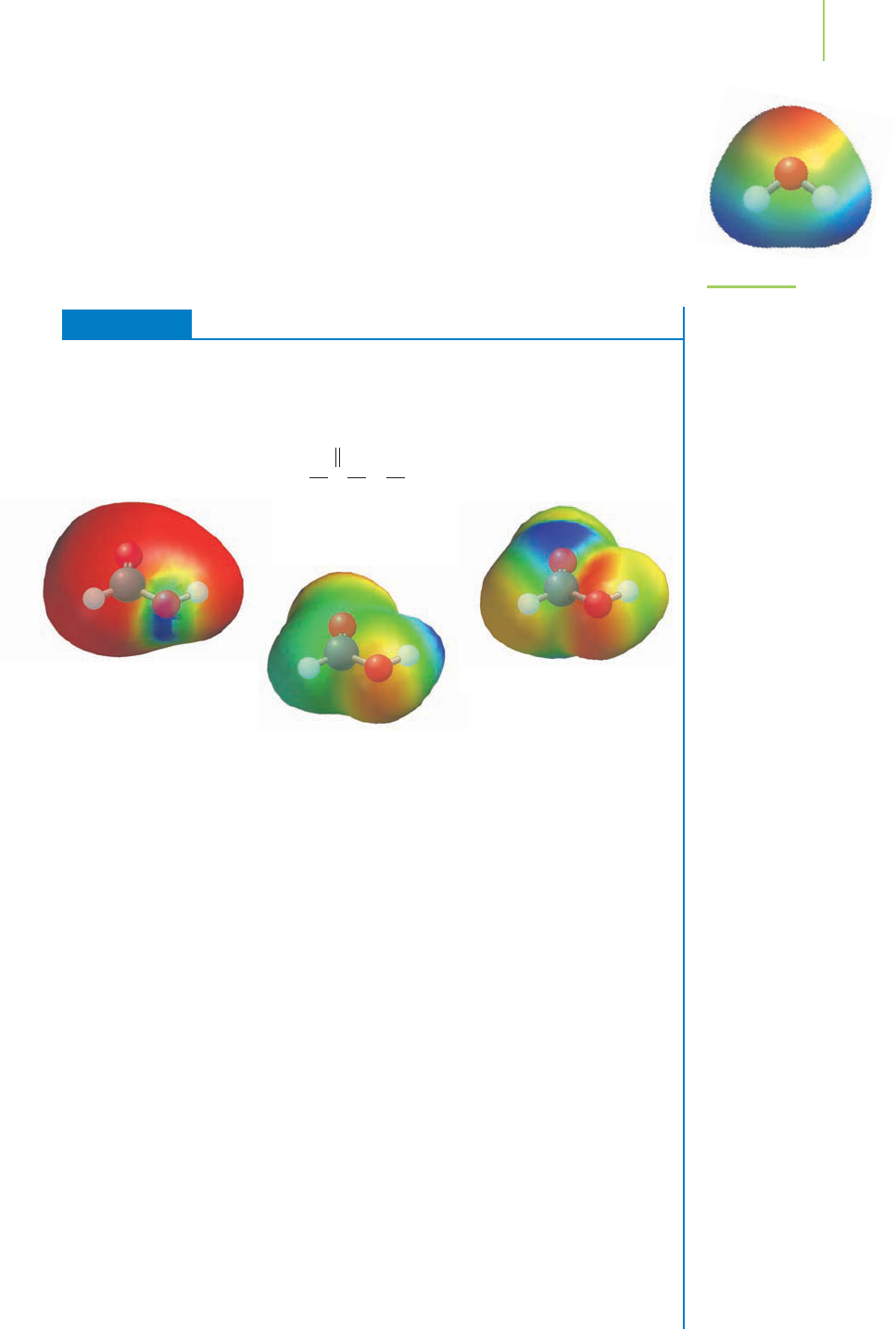
FIGURE 19.6
The electrostatic potential
map for water.
19.2 Oxidation States—Electronic Bookkeeping
829
have shifted away from hydrogen toward the oxygen, as shown in the electrosta-
tic density map in Figure 19.6. This is consistent with our understanding of elec-
tronegativity (Chapter 7). The oxidation states do not mean that hydrogen has a
full +1 charge and oxygen a full −2 charge. Remember from Chapter 7 that water
is a covalent molecule. We can talk more comfortably about oxidation states as
representations of the charge of atoms in ionic compounds. Whether in covalent
or ionic compounds, we will use oxidation state as a bookkeeping tool to see
where electrons shift in chemical reactions.
EXERCISE 19.1 Oxidation States
Assign an oxidation state to each of the elements in formic acid (HCOOH). Given
the Lewis structure shown below, which of the electrostatic density maps that follow
represents formic acid?
H C O H
O
First Thoughts
We have two questions here: a technical one about the oxidation states and a more
conceptual one concerning what the oxidation states imply about the electron
distribution. In both cases, though, the assignment of the oxidation state and
the electrostatic density map should be consistent with our understanding of
electronegativity.
Solution
According to our rules for assigning oxidation states in Figure 19.5, each oxygen is
assigned an oxidation state of −2, and each hydrogen is +1. For the neutral formic
acid molecule, in which the sum of all of the oxidation states must be 0, we have
2H =+1 × 2 =+2
2O =−2 × 2 =−4
The total is +2 + (−4) =−2. The carbon atom must therefore have an oxidation
state of +2. This means that the electron density in this molecule is focused more
on the oxygen atoms than on the carbon or hydrogen atoms. Given the assignments
of the oxidation states, the second electron density map is the most reasonable rep-
resentation of the molecule. In the other two maps the charge density isn’t in the
correct location. In the first map, the electron density appears to be opposite of what
we’d expect, given that the red color on the map indicates regions of high electron
density. The third map again shows electron density that doesn’t appear to match
where we have predicted the electrons to reside in the molecule.
Further Insights
Carbon can have several oxidation states, depending on the atoms with which
it bonds. When we talk about “complete combustion” of carbon, we mean the
Oxidizing and Reducing Agents
Reactant What Happens Examples
Oxidizing agent Gains electrons Element: O
2
,O
3
, and halogens
Is reduced Compound: H
2
O
2
Ionic species (typically with a large
positive oxidation state): MnO
4
−
Reducing agent Loses electrons Element: H
2
and metals
Is oxidized Compound: BH
3
Ionic species: NaH, LiAlH
4
TABLE 19.1
conversion to its highest possible oxidation state, +4, as is true in CO
2
. For example,
when glucose (C
6
H
12
O
6
) is burned in air, the carbon atoms can undergo complete
combustion to CO
2
. However, they can also form partial combustion products,such
as carbon monoxide (CO), in which the oxidation state of the carbon atom differs.
PRACTICE 19.1
Determine the oxidation states of carbon in glucose and carbon monoxide, the
compounds discussed in the “Further Insights” section just above.
See Problems 5–8 and 44.
We began this section discussing the reaction of hydrogen and oxygen gases in
a fuel cell. By comparing the reactants and products, we see that both H and O
show a change in their oxidation state. The change indicated that a transfer of
electrons from one species to another has occurred. Any chemical reaction in
which atoms change their oxidation states is classified as an oxidation–reduction
reaction, or redox reaction for short. When O
2
and H
2
react to form water, O
2
is
reduced (it gains electrons) and its oxidation state decreases from 0 to −2.
Similarly, H
2
is oxidized (it loses electrons), and its oxidation state increases from
0 to +1:
We can look at the compounds in this reaction in a different way. Oxygen
(O
2
) causes the oxidation of the hydrogen (H
2
), so it is an oxidizing agent. In fact,
oxygen stands as the premier oxidizing agent on planet Earth, both because of its
abundance and because of its strong ability to accept electrons. We observe oxy-
gen’s effect every day when we metabolize glucose or explore a rusty, old ship-
wreck (rust results from the oxidation of Fe to Fe
3+
). The hydrogen (H
2
) in the
fuel cell causes the reduction of the oxygen and is therefore a
reducing agent (see
Table 19.1). Looking at this another way, we can say that the oxidizing agent itself
is reduced and the reducing agent itself is oxidized.
Hydrogen peroxide is sometimes used to bleach hair or disinfect
wounds. Is its decomposition into oxygen and water a redox reac-
tion? If so, which species is the oxidizing agent and which is the
reducing agent?
2H
2
O
2
(l) n O
2
(g) + 2H
2
O(l)
Occasionally, a chemical reaction such as this one employs a single
reactant as both the oxidizing and the reducing agent. Such a reac-
tion is known as a
disproportionation. Using our decision rules in Fig-
ure 19.5, we can assign the following oxidation states to each atom.
+1 −10+1 −2
2H
2
O
2
(l) n O
2
(g) + 2H
2
O(l)
830 Chapter 19 Electrochemistry
2H
2
(g) + O
2
(g) 2H
2
O(g)
Oxidized
ReducedReduced
2H
2
O
2
(l) O
2
(g) + 2H
2
O(l)
Oxidation
ReductionReduction
–1 0 –2
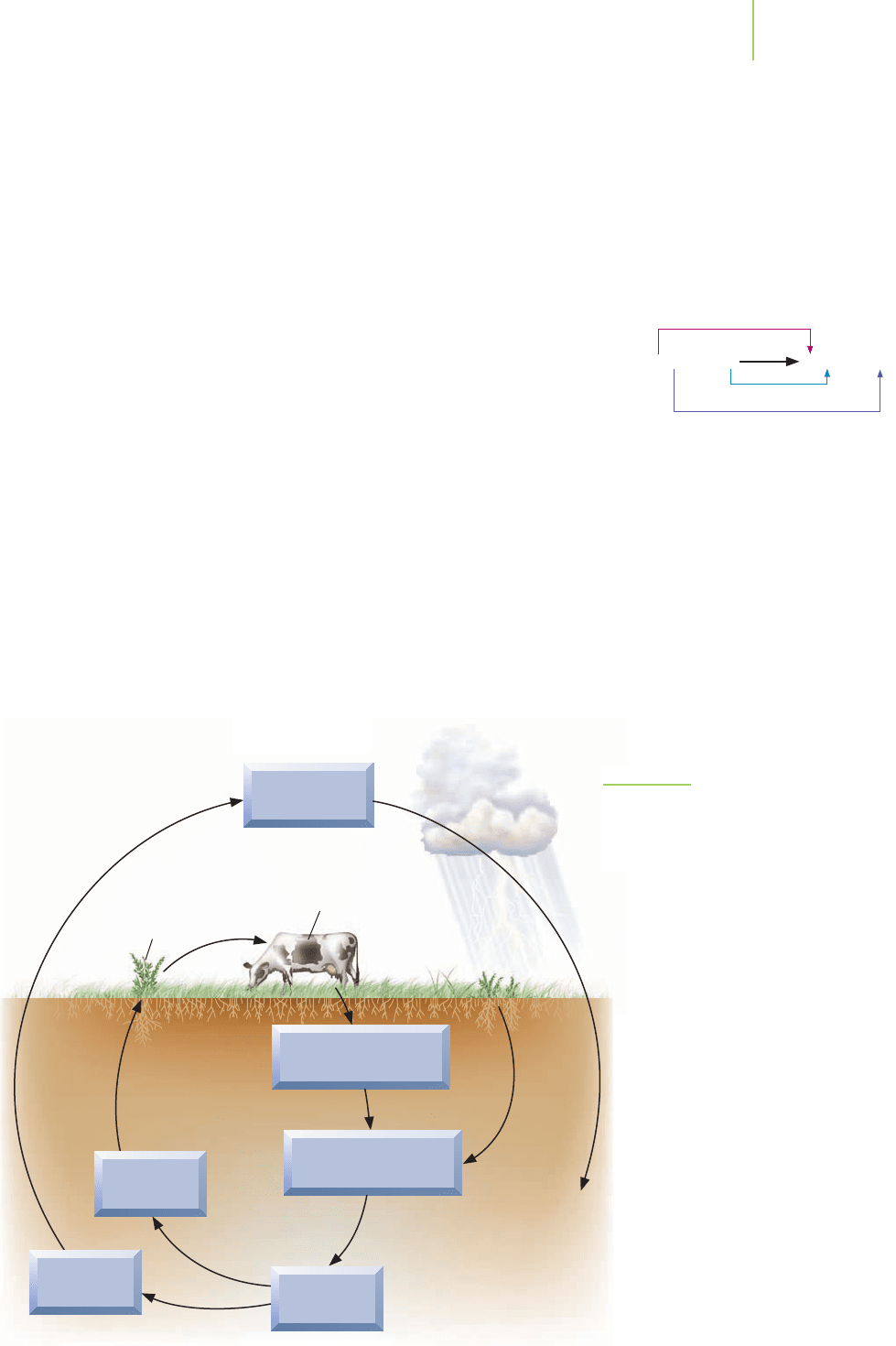
Nitrogen Cycle
Atmospheric
nitrogen (N
2
)
Dead plants and
animals, animal waste
Decomposers
Nitrogen-
fixing
bacteria
Nitrogen
fixation
Nitrifying
bacteria
Nitrification
Denitrification
Plant
protein
Animal
protein
Ammonia (NH
3
), or
Ammonium (NH
4
+
)
Nitrite
(NO
2
–
)
Nitrate
(NO
3
–
)
Denitrifying
bacteria
NaOH + HCl NaCl + H
2
O
+1 in both compounds
–1 in both compounds–1 in both compounds
–2 in both com
p
ounds
In this reaction, the oxygen atoms in hydrogen peroxide (like any peroxide) have
an oxidation state of −1. The two products of the reaction contain oxygen atoms
with different oxidation states, O
2
with a higher oxidation state (zero) and H
2
O
with oxygen in a lower oxidation state (−2). We say that the hydrogen peroxide is
both the oxidizing agent and the reducing agent.
Not all reactions require the transfer of electrons. Many of the reactions we’ve
discussed in this text thus far are not redox reactions. For example, the neutral-
ization of sodium hydroxide by hydrochloric acid is not a redox reaction. We can
tell because the oxidation states for each of the atoms remain the same on both
sides of the equation:
+1 −2 +1 +1 −1 +1 −1 +1 −2
NaOH + HCl n NaCl + H
2
O
Many elements show a variety of oxidation states, depending on the chemical
species in which they are found. An example of this can be found in the nonmetal
nitrogen, which has a range of possible oxidation states, as shown in Table 19.2 on
page 832. The gases found in our atmosphere, both naturally and as pollutants,
have different positive oxidation states of nitrogen, since the oxygen atom in each
compound is more electronegative than nitrogen and is assigned an oxidation
state of −2. For the same reason, nitrates (NO
3
−
) and nitrites (NO
2
−
) have ni-
trogen with positive oxidation states. However, when combined with a less elec-
tronegative element such as hydrogen, nitrogen is assigned a negative oxidation
state. Accordingly, look for a negative oxidation state of N in ammonia and in
compounds containing the ammonium ion, NH
4
+
.
The
nitrogen cycle (Figure 19.7) illustrates how nitrogen moves through its
different oxidation states on our planet. You can find similar cycles where the
oxidation states of sulfur and carbon change as these elements form different
19.2 Oxidation States—Electronic Bookkeeping 831
FIGURE 19.7
The nitrogen cycle. The nitrogen in the environ-
ment is found in many different compounds
and with many different oxidation states.
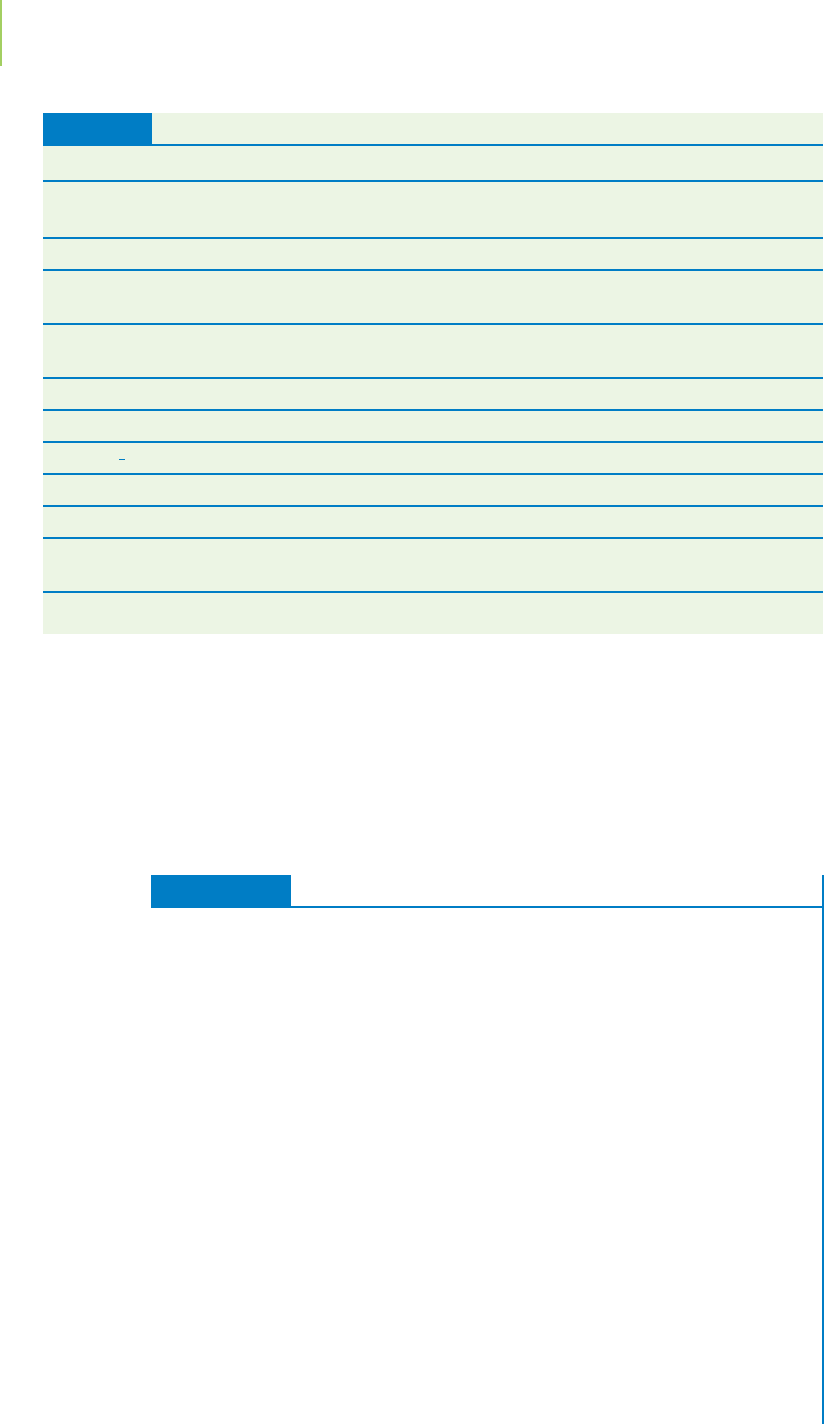
compounds. What is the point? Whereas oxidation states are fixed for a particu-
lar compound at a particular moment in time, chemicals in our world constantly
undergo change. Oxidation states help us keep track of the changes that involve
the important processes of oxidation and reduction.
EXERCISE 19.2 The Electrochemistry of Smog
Although oxygen and nitrogen do not react at low temperatures, they combine in
a hot automobile engine to form the pollutant NO, with subsequent oxidation by
atmospheric oxygen to form the poisonous brown gas NO
2
, which is largely re-
sponsible for the smog found in urban areas on hot, sunny days:
N
2
(g) + O
2
(g) n 2NO(g)
2NO(g) + O
2
(g) n 2NO
2
(g)
Assign oxidation states to all the atoms involved in these two reactions. Which
species are oxidized? Which are reduced? Do your answers make sense in terms of
the relative electronegativity values of nitrogen and oxygen?
First Thoughts
Which of the two atoms, nitrogen or oxygen, is more likely to be reduced (gain elec-
trons)? Oxygen is the more electronegative, so in this system, nitrogen will be
oxidized. That is how we evaluate whether our answers make sense.
Solution
According to Figure 19.5, we may assign all atoms in elements (such as N
2
and O
2
)
an oxidation state of zero. In the compounds, we may assign oxygen as −2, because
the compounds are not peroxides or superoxides. We assign nitrogen so that the
sum of the oxidation states is zero, because NO and NO
2
, as compounds, carry no
charge.
832 Chapter 19 Electrochemistry
The Oxidation State of Nitrogen
Oxidation State Formula Name Produced in Nature
+5 HNO
3
Nitric acid From NO
2
−
by nitrifying bacteria
NO
3
−
Nitrate ion
+4NO
2
Nitrogen dioxide In air by oxidation of NO
+3 HNO
2
Nitrous acid From NH
3
by nitrifying bacteria
NO
2
−
Nitrite ion
+2 NO Nitrogen monoxide From N
2
by lightning or volcanoes
(nitric oxide)
+1N
2
O Nitrous oxide From NO
2
−
by denitrifying bacteria
0N
2
Nitrogen From N
2
O by denitrifying bacteria
−
1
3
N
3
−
Azide ion (not found in nature)
−1NH
2
OH Hydroxylamine (not found in nature)
−2N
2
H
4
Hydrazine (not found in nature)
−3NH
3
Ammonia From biological decay of proteins
NH
4
+
Ammonium ion
NH
4
OH Ammonium hydroxide —
TABLE 19.2
Saccharine
19.3 Redox Equations 833
Compound Oxidation Number of N Oxidation Number of O
N
2
0 −
O
2
− 0
NO +2 −2
NO
2
+4–2
The nitrogen has been successively oxidized going from N
2
to NO to NO
2
, and
the oxygen has been reduced as it changes from O
2
to its products.
Further Insights
We noted in Figure 19.5 that hydrogen, which is normally assigned an oxidation
state of +1 in compounds, is assigned an oxidation state of −1 when combining
with Group 1A metals. This can result in a very reactive reducing agent that is quite
useful in organic chemical reactions. One example is NaH, mentioned earlier, which
is used in the manufacture of many pharmaceuticals, perfumes, and other organic
chemicals.
PRACTICE 19.2
Rank these chemicals in order of increasing oxidation state of sulfur: SO
2
,H
2
SO
4
,
S
8
, and Li
2
S.
See Problems 9–12.
19.3 Redox Equations
Ira Remsen (1846–1927), one of the co-discoverers of the artificial sweetener
saccharine, was particularly interested in chemistry as a boy. As an adult, he told
of a childhood visit to the doctor’s office where he, when left alone in the exami-
nation room, set out to discover what was meant by something he had read in a
chemistry book: “Nitric acid acts upon copper.” To discover this for himself, he
placed a pure copper penny on the exam table and poured nitric acid from the
doctor’s bottle onto the penny. Remsen continues,
But what was this wonderful thing which I beheld? The cent was already changed,
and it was no small change either. A greenish blue liquid foamed and fumed over
the cent and over the table. The air in the neighborhood of the performance be-
came dark red.A great colored cloud arose. This was disagreeable and suffocating—
how should I stop this? I tried to get rid of the objectionable mess by picking it up
and throwing it out the window, which I had meanwhile opened. I learned an-
other fact—nitric acid not only acts upon copper but it acts upon fingers. The
pain led to another unpremeditated experiment. I drew my fingers across my
trousers and another fact was discovered. Nitric acid also acts upon trousers. Tak-
ing everything into consideration, that was the most impressive experiment, and,
relatively, probably the most costly experiment I have ever performed.
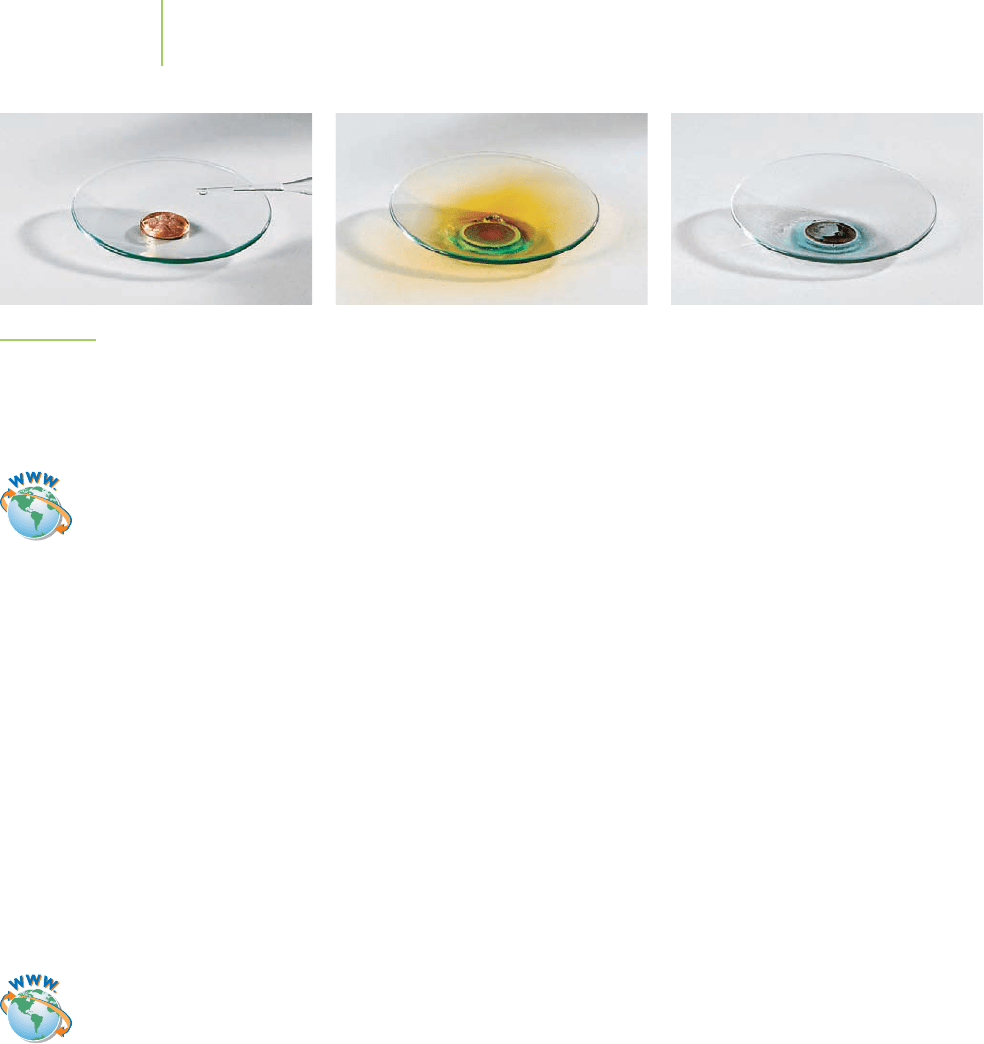
The reaction that Remsen describes, which is illustrated in Figure 19.8 on page
834, is a redox reaction. Shown on the right is the unbalanced equation. How do
we know that it is a redox reaction? Examine the oxidation state of copper. Cop-
per metal (oxidation state =0) is being oxidized to the copper(II) ion.
Simultaneously, nitrogen in nitric acid is being reduced from +5 to
+2 in forming nitrogen monoxide. The NO gas released by the reac-
tion rapidly reacts with O
2
in the air to make NO
2
(g), the brown fumes
that so alarmed the budding chemist.
HNO
3
(aq) + Cu(s)
Cu
2+
(aq) + NO(g)
2NO(g) + O
2
(g) 2NO
2
(g)
Half-Reactions
Ira Remsen noted that this reaction proceeded spontaneously to generate a cloud
of noxious fumes. Can we predict this spontaneity by examining the reaction
equation, which tells us whether the driving force (the potential) is favorable for
this reaction? To assist us in answering this question, we need to extract the oxi-
dation and reduction reactions from the overall equation. These half-reactions,
like so many half-reactions, are so well known that the potential for each has been
measured and the results collected into a Table of Standard Reduction Potentials,
such as Table 19.3. A more comprehensive table can be found in the Appendix.
What do you notice about these tables? One of the things is that all of the
reactions are written as reduction reactions. That is, the reactions show the con-
sumption of electrons to make products with less positive oxidation states.
Standard Reduction Potentials tables can be used to determine the potential of a
reaction, be it for the silver oxide battery found in a pacemaker or for the action of
nitric acid on a copper penny. Moreover, knowing the potential of the half-
reactionshelps usdetermine the spontaneityof aredoxreaction,aswewill seelater.
Each half-reaction in the table is balanced both atomically and electrically.
Half-reactions are simply what they appear to be: half of an oxidation–reduction
reaction that is occurring in aqueous solution. The half-reaction listed in the
table for the reduction of copper shows the reactants (copper ions and electrons),
the product (copper metal), and the
standard potential (E°) of the half-reaction.
Cu
2+
(aq) + 2e
−
n Cu(s) E° =+0.34 V
The value of E° is a measure of how strongly the reduced species on the right-
hand side of the reduction half-reaction pulls electrons toward itself. The
standard potential is measured in
volts, the SI unit of electrical potential. It is
sometimes referred to as the
electromotive force (emf) of the half-cell or, more
commonly, as the
voltage.
The values listed for E° are measured under a specific set of conditions:
■
Any aqueous ion is present at a concentration (technically, activity) of 1.0 M.
All gases are at a pressure of 1 bar (approximately 1 atm).
■
The temperature is 25°C (298 K).
These conditions are “standard” for half-reactions and are indicated by the “°”
in E°. If the conditions are not standard, the voltage will be different from that
listed in the table (see Section 19.7), and the potential will be equal to E.Keep
in mind that there are several “standards”! For example, standard conditions
of temperature and pressure of gases (STP) refer to 0°C (273 K) as the standard
temperature.
834 Chapter 19 Electrochemistry
FIGURE 19.8
Nitric acid acts on copper. The spontaneous reaction is evident from the generation of a blue solution and
a cloud of noxious brown gas. The gas results from the reaction of NO with oxygen in the air.
Video Lesson: Balancing Redox
Reactions Using the Half-
Reaction Method
Video Lesson: Standard
Reduction Potentials
Video Lesson: Electromotive
Force
We speak of standard reduction potential in terms of how strongly the species
pulls electrons toward itself. Yet just as in a tug-of-war, we must consider what we
are pulling against. We need a commonly used reference half-reaction with which
to compare our reduction. Our reference is called the
standard hydrogen electrode
reaction (SHE)
. This half-reaction, which is assigned the potential of zero volts, is
also shown in the table as the reduction of H
+
to H
2
. To say that the reduction of
Cu
2+
to Cu
0
has a voltage of +0.34 V, as in Table 19.3, really is to say that it has
this voltage compared to the reduction of H
+
described by the SHE reaction.All po-
tentials that we use in our discussions will be compared to the SHE reaction.
The potential of a half-reaction can be used to assess the spontaneity of the
half-reaction. For instance, fluorine has a strong attraction for electrons. Recall-
ing our discussion of ionization energy and electron affinity from Chapter 7, we
might predict that adding electrons to F
2
should be more thermodynamically
favorable than adding electrons to a Group IA metal cation such as Li
+
. The
half-reaction potentials for each reduction bear this out. Fluorine has a large
positive reduction potential (+2.87 V), and lithium has a large negative reduc-
tion potential (−3.04 V). Michael Faraday (1791−1867), an English electro-
chemist, worked hard to illustrate how a favorable reaction could be related to the
potential. Gibbs later was able to show this relationship mathematically as
∆G° = −nFE°
where n is the number of moles of electrons transferred in the reaction, and F is
called Faraday’s constant, which we’ll discuss in a moment. A key feature of this
equation is that the change in free energy, ∆G
°, and the cell potential, E°, have
19.3 Redox Equations 835
Selected Standard Reduction Potentials
The selected potentials shown here were obtained under standard conditions (in aqueous solution, 25°C, all
solutions 1.0 M, all gases 1.0 atm).
Shorthand Notation Half-Cell Reaction Standard Potential, E°(V)
Li
+
(aq) | Li(s)Li
+
(aq) + e
–
n Li(s) –3.04
Na
+
(aq) | Na(s)Na
+
(aq) + e
–
n Na(s) –2.71
Mg
+2
(aq) | Mg(s)Mg
+2
(aq) + 2e
–
n Mg(s) –2.38
Al
3+
(aq) | Al(s)Al
3+
(aq) + 3e
–
n Al(s) –1.66
H
2
O(l) | H
2
(g)2H
2
O(l) + 2e
–
n H
2
(g) + 2OH
−
(aq) –0.83
Cd(OH)
2
(s) | Cd(s) Cd(OH)
2
(s) + 2e
–
n Cd(s) + 2OH
–
(aq) –0.81
Fe
2+
(aq) | Fe(s)Fe
2+
(aq) + 2e
–
n Fe(s) –0.44
H
+
(aq) | H
2
(g)2H
+
(aq) + 2e
–
n H
2
(g) 0.00
Fe
3+
(aq) | Fe(s)Fe
3+
(aq) + 3e
–
n Fe(s) +0.04
Cu
2+
(aq) | Cu(s)Cu
2+
(aq) + 2e
–
n Cu(s) +0.34
O
2
(g) | OH
−
(aq)O
2
(g) + 2H
2
O(l) + 4e
−
n 4OH
–
(aq) +0.40
NiO
2
(s) | Ni(OH)
2
(s)NiO
2
(s) +2H
2
O(l) +2e
−
n Ni(OH)
2
(s) +2OH
−
(aq) +0.49
Ag
+
(aq) | Ag(s)Ag
+
(aq) + e
−
n Ag(s) +0.80
HNO
3
(aq) | NO(g)3H
+
(aq) + HNO
3
(aq) + 3e
–
n NO(g) + 2H
2
O(l) +0.96
Br
2
(l) | Br
−
(aq)Br
2
(g) + 2e
−
n 2Br
–
(aq) +1.07
O
2
(g) | H
2
O(l)O
2
(g) + 4H
+
(aq) + 4e
−
n 4H
2
O(l) +1.23
Cl
2
(g) | Cl
−
(aq)Cl
2
(g) + 2e
−
n 2Cl
–
(aq) +1.36
Au
3+
(aq) | Au(s)Au
3+
(aq) + 3e
−
n Au(s) +1.50
F
2
(g) | F
−
(aq)F
2
(g) + 2e
−
n 2F
−
(aq) +2.87
TABLE 19.3
Video Lesson: Using Standard
Reduction Potentials
opposite signs (n and F are always positive). Because a negative value of free en-
ergy indicates a spontaneous process, a positive value of cell potential must also
indicate spontaneity.
Faraday’s constant is a unit of electric charge equal to the magnitude of charge
on a mole of electrons:
1 faraday = 1F= 96,485
coulombs
mol
= 9.6485
×
10
4
C
mol
On the basis of the relationship shown by Faraday, we can determine that 1 joule
equals 1 coulomb·volt, and 1 volt = 1
joule
coulomb
:
1 J = 1 C · V
1V=
J
C
EXERCISE 19.3 Spontaneity and Potential
Copper ions undergo reduction according to the following half-reaction:
Cu
2+
(aq) + 2e
−
n Cu(s) E° =+0.34 V
What is the free energy change associated with this process? Is this a spontaneous
half-reaction?
Solution
The free energy change is negative; the half-reaction is spontaneous. However,
this is only half of a redox reaction.
G
◦
=−nFE
◦
=−(2 mol e
−
)
96485 C
mol e
−
+0.34 J
C
=−65609.8J=−66 kJ
PRACTICE 19.3
The silver cell battery used in pacemakers utilizes the following reaction with a
measured potential of 1.86 V. What is
G
o
for this reaction? Is this reaction
spontaneous?
Ag
2
O(s) + Zn(s)
→
2Ag(s) + ZnO(s)
See Problems 25 and 26.
Balancing Redox Reactions
To balance a redox reaction such as the one describing the action of nitric acid on
copper, we first determine the identity of the half-reactions. It can be hard (if not
seemingly impossible!) to balance a redox equation correctly using a trial-and-
error approach (Chapter 3), so we often use a series of steps to accomplish the job
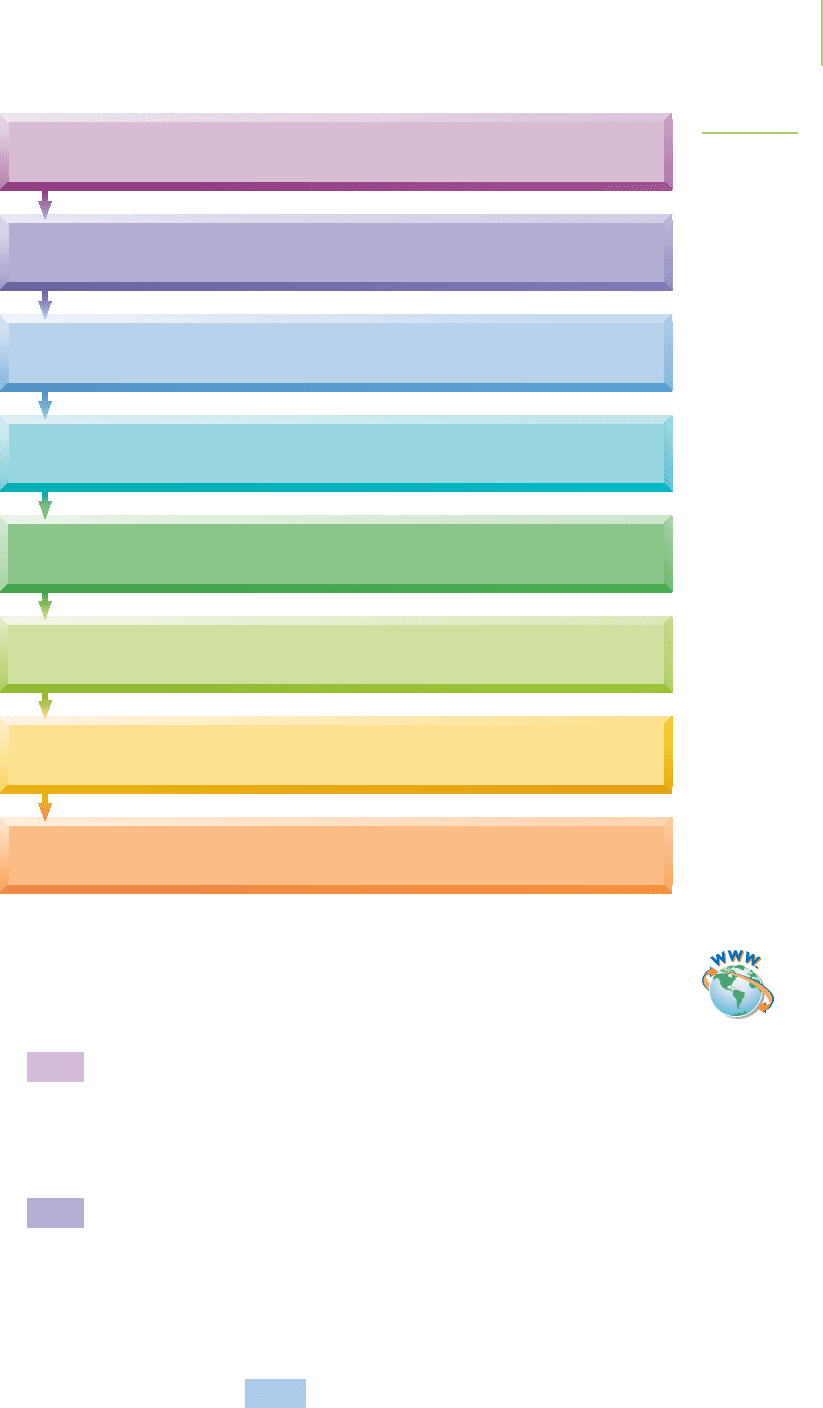
(see Figure 19.9). To be fair, this method is just a device to make the balancing go
more quickly, rather than a representation of what actually happens at the mole-
cular level. In the nanoworld, electron transfer processes not only occur simulta-
neously rather than sequentially but also occur in a fairly complex way, with
charges building up at the phase changes (the so-called interfaces) in solution.
Here we will focus just on the technical aspects of balancing equations.
836 Chapter 19 Electrochemistry
Let’s follow the algorithm in Figure 19.9 as we balance the copper–nitric acid
redox reaction:
HNO
3
(aq) + Cu(s) n Cu
2+
(aq) + NO(g)
Step 1 in Figure 19.9 indicates that we should determine whether we have a
redox reaction. That is, does our oxidation state bookkeeping indicate that one
species is undergoing oxidation and another is undergoing reduction? We deter-
mine that the equation describes a redox process by noting that the oxidation
state of copper increases (from 0 to +2) while that of nitrogen decreases (from
+5 to +2).
Step 2 indicates that we should separate the redox reaction into the two half-
reactions. These half-reactions show the oxidation (copper to copper ion) and
the reduction (nitric acid to nitrogen monoxide).
Cu(s) n Cu
2+
(aq)
HNO
3
(aq) n NO(g)
The numbers of atoms (not including H’s and O’s) are then balanced on both
sides of each half-reaction in Step 3. No modification of our reactions is needed
for this step.
19.3 Redox Equations 837
Step 8: Add the reactions together and simplify.
Step 7: As needed, multiply one or both of the half-reactions by some coefficient so
that both half-reactions will have the same number of electrons.
Step 6: Balance the charges in each half-reaction by adding electrons to one side.
Step 5: Balance the hydrogen atoms by adding H
+
to one side in each half-reaction.
Step 4: Balance the oxygen atoms by adding H
2
O to one side of each half-reaction.
Step 3: Balance all atoms that are neither oxygen nor hydrogen.
Step 2: Separate the redox reaction into two parts: an oxidation reaction and a reduction
reaction. Include only the compounds that have a change in the oxidation state.
Step 1: Determine the oxidation state on each element in each compound in the
chemical equation. Do we in fact have a redox reaction?
FIGURE 19.9
Algorithm for balancing redox reactions
in acidic solution.
Visualization: Copper Metal in
Nitric Acid
