Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

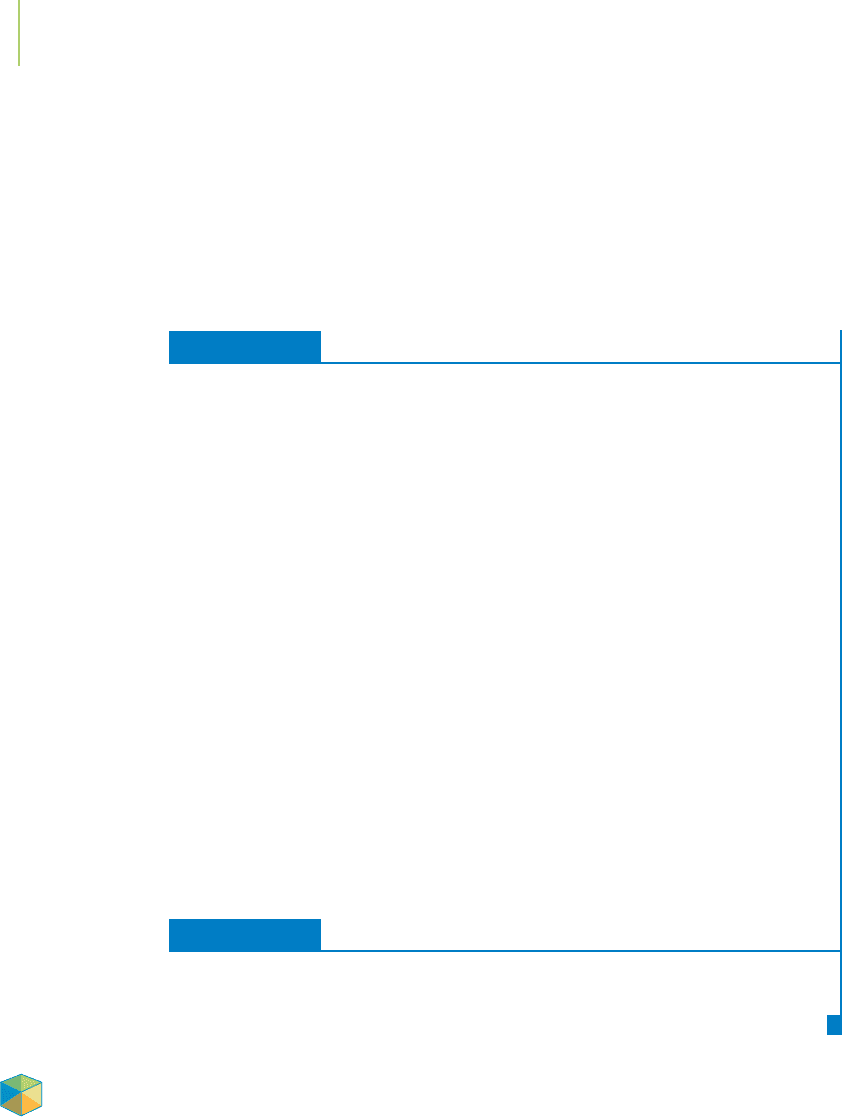
The K
sp
value for AgCl is 1.6
×
10
−10
. This value is based on the equilibrium con-
ditions of the sparingly soluble salt. The reaction quotient we calculated is 60
times greater than the equilibrium constant for the precipitation, so the AgCl
precipitate forms. If the reaction quotient were smaller than the equilibrium
value, no precipitate would form. To reiterate:
■
If Q
sp
> K
sp
, a precipitate forms and continues to form until Q
sp
= K
sp
.
■
If Q
sp
< K
sp
, no precipitate forms.
EXERCISE 18.13 Will It Make a Solid?
A chemical technician wishes to precipitate the lead ions in 100 mL of a water
sample. If the water sample contains 3.10
×
10
−10
M Pb
2+
, and 100 mL of a
solution containing 7.0
×
10
−4
M Cl
−
is added, will a precipitate form? K
sp
for
PbCl
2
= 1.6
×
10
−5
.
Solution
The solubility equilibrium is
PbCl
2
(s)
Pb
2+
(aq) + 2Cl
−
(aq)
The mass-action expression is
K
sp
= [Pb
2+
] [Cl
−
]
2
When the two solutions are mixed, the total volume is doubled, to 200 mL. There-
fore the final concentrations of the respective ions after the two solutions are mixed
become
[Pb
2+
] = 1.55
×
10
−10
M
[Cl
−
] = 3.5
×
10
−4
M
and Q
sp
is
Q
sp
= (1.55
×
10
−10
) (3.5
×
10
−4
)
2
= 1.90
×
10
−17
No, the chloride solution will not precipitate the lead in the sample.
PRACTICE 18.13
Will a precipitate form when equal volumes of the lead solution in the exercise
above and 3.5
×
10
−4
M sulfide ion are mixed? K
sp
for PbS = 7.0
×
10
−29
.
See Problems 55 and 56.
Acids, Bases, and Solubility
The chemical technician who works at the water treatment center is often re-
sponsible for treating waste water before it is returned to the environment.
One such treatment is
sedimentation, in which aluminum sulfate, Al
2
(SO
4
)
3
and
calcium hydroxide, Ca(OH)
2
, are added to help clarify and purify the wastewater
(Figure 18.19). The aluminum and hydroxide ions in the solution form a gelati-
nous precipitate.
Al
3+
(aq) + 3OH
−
(aq)
Al(OH)
3
(s) K
sp
= 2
×
10
−32
The solid settles, carrying with it some dissolved organic material, microorgan-
isms and other undesirable substances in a process called
coagulation. Iron(III)
hydroxide can also be used in this way.
On the other hand, increasing the acidity of waterways can increase the con-
centration of undesirable metals. For example, lead can be found naturally as the
insoluble sulfide, PbS. When acidic waters contact the natural lead sulfide, hydro-
gen ions compete via Le Châtelier’s principle to form hydrogen sulfide, H
2
S. This
808 Chapter 18 Applications of Aqueous Equilibria
Application

allows the lead ion to enter the waterway as Pb
2+
. The equilibrium constant for
the process is not especially high (K ≈ 10
−7
), but the leaching of metals, includ-
ing lead, mercury, cadmium and aluminum into waterways, even at low concen-
trations, is of concern.
18.4 Complex-Ion Equilibria
We noted before that adding a common ion to a solution of a sparingly soluble
salt affects its solubility. We said that if too much of the common ion were added,
the sparingly soluble salt could dissolve instead of precipitate. This interesting
phenomenon arises because of the formation of a chemical complex, which typ-
ically consists of one or more metal cations bonded to one or more Lewis bases
known as
ligands (recall that such bases donate electrons). Examples of ligands
include Cl
−
,F
−
,OH
−
,CN
−
,NH
3
, and H
2
O. The complexes can be anions, as with
AgCl
4
−3
, or cations, such as Co(NH
3
)
6
3+
. Each of these ionic complexes has one
or more ions of opposite charge to balance the total charge in the solution.
Introducing the Formation Constant
Our first example of a chemical complex begins with the zinc cation, which exists
in aqueous solution bonded to four water molecules, written as Zn(H
2
O)
4
2+
.In
an ammonia–ammonium ion buffer, an ammonia molecule replaces a water
molecule.
Zn(H
2
O)
4
2+
(aq) + NH
3
(aq)
ZnNH
3
(H
2
O)
3
2+
(aq) + H
2
O(l)
We will next simplify the expression by assuming the presence of water, as we
often do in acid–base reactions, and simplify the expression.
Zn
2+
(aq) + NH
3
(aq)
Zn(NH
3
)
2+
(aq)
K
f
1
= 190
The equilibrium constant for the formation of the zinc–ammonia complex
is called its
formation constant (K
f
) or stability constant, and conceptually, it means
the same thing as any other equilibrium constant. Le Châtelier’s principle teaches
us that as we add more ammonia, more NH
3
ligands will form coordinate cova-
lent bonds with the central atom, each step having its own formation constant.
Zn(NH
3
)
2+
(aq) + NH
3
(aq)
Zn(NH
3
)
2
2+
(aq)
K
f
2
= 220
Zn(NH
3
)
2
2+
(aq) + NH
3
(aq)
Zn(NH
3
)
3
2+
(aq)
K
f
3
= 250
Zn(NH
3
)
3
2+
(aq) + NH
3
(aq)
Zn(NH
3
)
4
2+
(aq)
K
f
4
= 110
18.4 Complex-Ion Equilibria 809
FIGURE 18.19
The process of sedimentation can be
used to clarify water for drinking.
Video Lesson: The Formation of
Complex Ions
810 Chapter 18 Applications of Aqueous Equilibria
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
0 –1–2–3–4–5
log [NH
3
]
Fraction Present
FIGURE 18.20
Here are the changes that occur in the equilibrium
concentrations of the various zinc–ammonia complex
ions as we increase the concentration of ammonia.
The x-axis displays log [NH
3
], so that each factor of
10 by which we change [NH
3
] occupies equal space
in the plot.
Application
C
HEMICAL ENCOUNTERS:
Commercial Uses of
Aminopolycarboxylic
Acid Chelating Agents
H
N
N
H
O
O
O
O
O
O
Na
+
Na
+
O
–
O
–
Na
+
Zn
2+
Zn(NH
3
)
2+
Zn(NH
3
)
2
2+
Zn(NH
3
)
3
2+
Zn(NH
3
)
4
2+
Figure 18.20 shows the distribution of the various zinc–ammonia complexes as
the ammonia concentration is increased. Because the formation constants of the
steps are so similar, several different zinc–ammonia species are typically present
in solution, as shown in the figure. Going from Zn
2+
to Zn(NH
3
)
4
2+
does not give
a clear, sharp endpoint, and in general, metal-ion concentrations cannot be ana-
lyzed by titration that involves a process with multiple formation constants.
Is it
possible to have a titrant that will completely combine with a single reaction in a 1-
to-1 mole ratio with the metal ion, in order to determine its concentration?
This is
where EDTA, introduced as a titrant in Section 18.1, comes in.
Extending the Discussion to EDTA
EDTA is a very useful compound for titrations. It is typically used in its most basic
form, shown in Figure 18.21. It has six pairs of electrons, one pair on each of the
two nitrogen atoms and one pair on each of four oxygen atoms, which form be-
tween four and six coordinate covalent bonds to a single metal ion such as Ca
2+
.
Substances that form multiple bonds in this way are called
chelates (the Greek
word chele means “claw”) or chelating agents because they grab the metal ion like
a set of claws. These substances are further characterized by the number of coor-
dinate covalent bonds they make to the metal ion. Ammonia (NH
3
) is a mon-
odentate ligand
(one-toothed ligand). EDTA can be a tetradentate ligand
(four-toothed ligand) or a hexadentate ligand (six-toothed ligand). In Chap-
ter 16, we saw the very high equilibrium constants (formation constants)
for the reactions of EDTA with metals, shown in Table 18.4. Note in the table
how the higher charge on the Fe
3+
results in a dramatically higher formation
constant with EDTA compared to that of Fe
2+
. We now see that the great sta-
bility of such metal–chelate complexes is a result of the
polydentate nature of
the ligand. Several industrially important polydentate chelating agents are
listed in Table 18.5. These compounds are so useful that well over 150 million
kg are used annually in a host of different products and applications, some of
which are listed in Table 18.6.
Complex formation with EDTA and its chemical relatives can be used to
determine the concentration of many metals, such as zinc, aluminum, nickel,
cobalt, iron, and, in the study of the hardness of water, calcium, and magne-
sium. We can write the reaction of the calcium ion with EDTA in ionic equa-
tion form:
Ca
2+
(aq) + EDTA
4−
(aq)
CaEDTA
2−
(aq)
or we can give it more visual clarity by giving Lewis structures as well as
space-filling models, as shown in Section 18.1. Because the equilibrium
FIGURE 18.21
EDTA is a most important chelating agent.
Solutions of EDTA are typically prepared as
the disodium salt (Na
2
EDTA), with EDTA
2−
shown here as a Lewis dot structure (top)
and in a free-energy-minimized configura-
tion (bottom), including the two sodium
ions.
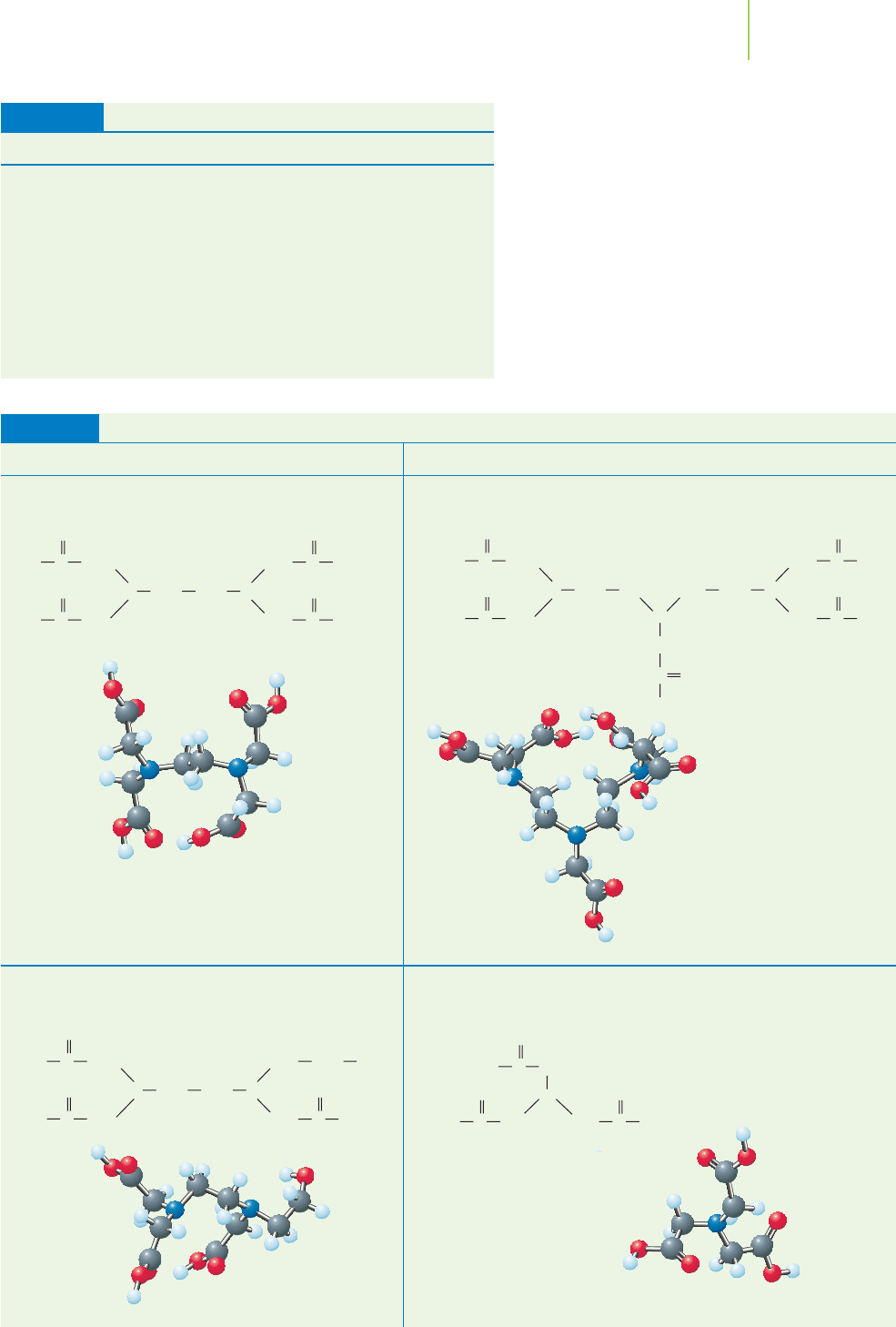
18.4 Complex-Ion Equilibria 811
Formation Constants of Some Metal–EDTA Complexes
Element Cation K
f
silver Ag
+
2.1
×
10
7
calcium Ca
2+
5.0
×
10
10
cobalt Co
2+
2.0
×
10
16
zinc Zn
2+
3.0
×
10
16
iron(II) Fe
2+
2.1
×
10
14
nickel Ni
2+
3.6
×
10
18
bismuth Bi
3+
8.0
×
10
27
iron(III) Fe
3+
1.7
×
10
24
vanadium V
3+
8.0
×
10
25
TABLE 18.4
Industrially Important Aminopolycarboxylic Acid Chelating Agents
Name and Abbreviation Name and Abbreviation
Ethylenediaminetetraacetic acid, EDTA Diethylenetriaminepentaacetic acid, DTPA
TABLE 18.5
C
O
OH
CH
2
C
NN
O
OH
CH
2
C
O
HO
CH
2
C
O
HO
CH
2
CH
2
CH
2
C
O
OH
CH
2
C
N
N
N
O
OH
CH
2
C
O
HO
CH
2
C
O
HO
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
C
OH
O
C
O
OH
CH
2
N
C
O
HO
CH
2
C
O
HO
CH
2
N-(hydroxyethyl)-ethylenediaminetriacetic acid, Nitrilotriacetic acid, NTA
HEDTA
C
O
OH
CH
2
NN
CH
2
CH
2
OH
C
O
HO
CH
2
C
O
HO
CH
2
CH
2
CH
2

812 Chapter 18 Applications of Aqueous Equilibria
Products and Applications of Chelating Agents
Application Benefits of Using Chelating Agents
TABLE 18.6
Protects the natural flavor, color, texture, and nutritive value of your food
products
Improves shelf life and consumer appeal
Better foaming, detergency, and rinsing in hard water
Helps remove metal oxides and salts from fabrics
Enhances shelf life by inhibiting rancidity, clouding, and discoloration
Improved consumer appeal and product value
Improved germicidal action
Better lathering in shampoos and soaps, particularly in the presence of
hard water
Improves shelf life and consumer appeal
Prevents softening, brown spotting, and cracking in bar soaps
Improves stability of fragrances, fats, oils, and other water-soluble ingredients
EDTA is approved by the FDA for use in treatment of heavy-metal poisoning
Deactivates metal ions that interfere with drug performance
Higher brightness and/or lower bleaching costs
Less need to overbleach to ensure specified brightness level
Dissolves common types of scale during normal operation
Improves process efficiency and reduces downtime
Works over a wide range of temperatures, pH levels, and pressures
Improved product performance in hard water
Improved high-temperature performance
Less need to overbleach to ensure specified brightness level
Dye shade stability
Excellent water solubility makes metal chelants more readily utilized
by plants than the inorganic forms of metals
Stable polymerization rates
Reduced polymer buildup in reactors
Better polymer stability and shelf life
Higher-quality prints and negatives
Enhanced silver recovery
Increased longevity of prints and negatives
Prevents plugging, sealing, precipitation by deactivating metal ions
Foods and Beverages
Canned seafood products
Dressings, sauces, spreads
Canned beans
Beverages
Cleaning Products
Heavy-duty laundry detergents
Hard-surface cleaners
Personal Care Products
Creams, lotions
Bar and liquid soaps
Shampoos
Hair preparations
Pharmaceuticals
Treatment for lead poisoning
Drug stabilization
Pulp and Paper
Mechanical pulp bleaching
Chemical pulping
Reduction of paper yellowing
Water Treatment
Boilers
Heat exchangers
Metalworking
Surface preparation
Metal finishing and plating
Textiles
Preparation
Scouring
Bleaching
Agriculture
Chelated micronutrients
Polymerization
Styrene–butadiene polymerization
PVC polymerization
Photography
Developers
Bleaches
Oilfield Applications
Drilling
Production
Recovery
Source: Dow Chemical, http://www.dow.com (accessed September 2005).

18.4 Complex-Ion Equilibria 813
constant is so high, the reaction is essentially complete. This is important when
designing a titration. It is also vital in another of EDTA’s important uses: com-
bining with metals in food products so that they are unavailable to participate in
spoilage processes. To put it in a more formal way, the metal ions are
sequestered
(tied up) by EDTA.
The hard-water analysis at pH 10 allows EDTA to react with both calcium and
magnesium ions in the water, which is a measure of “total hardness.” At pH val-
ues above 12, magnesium ions precipitate as the hydroxide, and the analysis al-
lows determination of the calcium ion concentration alone. Here again, as is our
theme in this discussion, pH control is vital. Let’s look more closely at the rela-
tionship of pH to the reaction.
Why should the titration of calcium with EDTA be
more complete at basic than at acidic pH values?
The Importance of the Conditional Formation Constant
Equilibrium represents a competition—a wrestling match between substances to
acquire and release ions and molecules in their quest for energetic stability, as
measured by the minimum system free energy. In an aqueous solution of calcium
and EDTA, the primary competitor is hydrogen ion. The acidic H
4
EDTA can lose
four protons in stepwise fashion to form EDTA
4−
.
H
4
EDTA
H
3
EDTA
−
+ H
+
K
a
1
= 1.0
×
10
−2
H
3
EDTA
−
H
2
EDTA
2−
+ H
+
K
a
2
= 2.2
×
10
−3
H
2
EDTA
2−
HEDTA
3−
+ H
+
K
a
3
= 6.9
×
10
−7
HEDTA
3−
EDTA
4−
+ H
+
K
a
4
= 5.5
×
10
−11
The higher the pH, the greater the fraction of EDTA
−4
in the aqueous solution as
hydrogen ions are sequentially removed from the molecule. Figure 18.22 shows
that as the pH goes down (the solution is made more acidic), the fraction of
EDTA present as EDTA
4−
(highlighted in the figure) diminishes drastically. The
tendency to form protonated EDTA reduces the stability of the calcium–EDTA
complex with reactions such as this:
CaEDTA
2−
+ H
+
Ca
2+
+ HEDTA
3−
We show this reduction in stability via the conditional forma-
tion constant (K
)
, which takes into account the fractions of the
free (uncomplexed) metal ion and the EDTA
4−
.
K
= K
f
Ca
2+
EDTA
4−
where
Ca
2+
= fraction of free Ca
2+
(1, in this case)
EDTA
4−
= fraction of EDTA present as EDTA
4−
For example, with our buffer system at pH = 10.0, the forma-
tion constant, K
f
, for the titration reaction is 5
×
10
10
and
Ca
2+
= 1. It is possible to calculate
EDTA
4−
, but we will simply
estimate it from Figure 18.22 as being roughly 0.10.
K
= K
f
Ca
2+
EDTA
4−
= 5
×
10
10
(1)(0.10) = 5
×
10
9
The conditional formation constant is still sufficiently high for
the titration to be essentially complete. If, however, we calcu-
late the conditional formation constant at pH = 3.0, at which
point very little of the EDTA is present as EDTA
4−
and
EDTA
4−
= 2
×
10
−11
, we get
K
= K
f
Ca
2+
EDTA
4
−
= 5
×
10
10
(1)(2
×
10
−11
) = 1
The conditional formation constant is far too low for the titra-
tion to be feasible.
0.0
0.2
0.4
0.6
0.8
1.0
02468101214
pH
Fraction Present
FIGURE 18.22
As the pH is lowered, the fraction of
EDTA
4−
sharply decreases, reducing
the conditional stability constant of
any metal–EDTA titration. This is one
of several factors that is important to
consider when selecting the best pH
for this type of analysis.
EDTA
4–
HEDTA
3–
H
2
EDTA
2–
H
3
EDTA
–
H
4
EDTA
H
5
EDTA
+
H
6
EDTA
2+
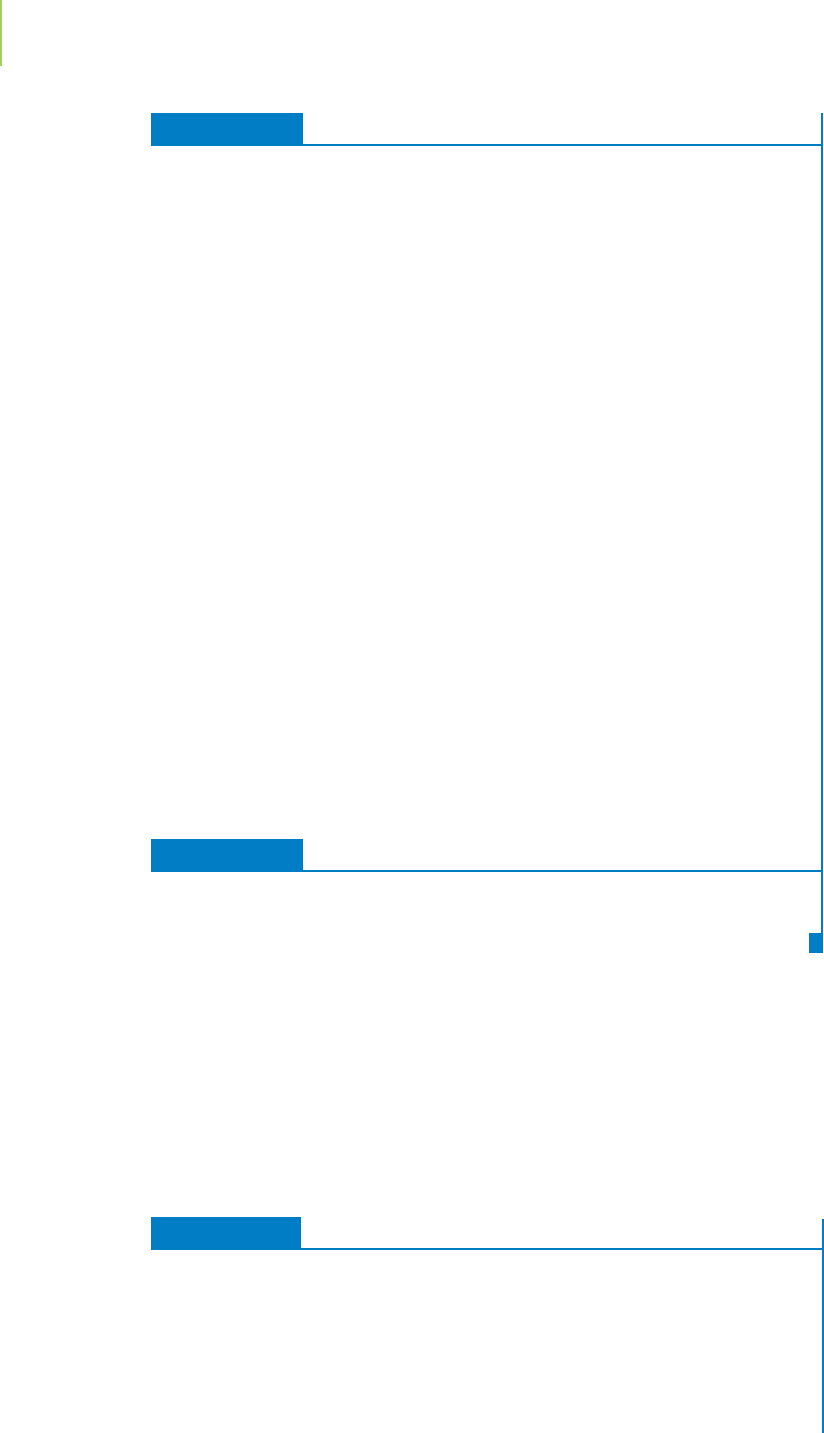
EXERCISE 18.14 Conditional Formation Constant
With some metals, the competition between EDTA and ammonia to bond to the
metal ion can become important when we are judging the feasibility of a titration.
If we were titrating zinc instead of calcium in our pH = 10 ammonia buffer, we
would find that the zinc would form complex ions ranging from Zn(NH
3
)
2+
to
Zn(NH
3
)
4
2+
. This would make the fraction of free Zn
2+
,
α
Zn
2+
, less than 1, lowering
the conditional formation constant.
Zn
2+
(aq) + EDTA
4−
(aq)
ZnEDTA
2−
(aq) K
f
= 3
×
10
16
Calculate the conditional formation constant for the titration of zinc with EDTA in
the pH =10 buffer in which
Zn
2+
= 8
×
10
−6
, and
EDTA
4−
=0.10. Is this titration
still feasible in spite of the low fraction of uncomplexed zinc?
First Thoughts
We can solve for the conditional formation constant, K
f
, just as we did with the
calcium–EDTA system. Here, however, the fraction of free metal ion is very low.
Solution
K
= K
f
Zn
2+
EDTA
4−
= 3
×
10
16
(8
×
10
−6
)(0.10) = 2.4
×
10
10
This value for
K
is still quite large, and the titration works well.
Further Insights
Keep in mind that in spite of the various and K terms we are working with, a key
question with which we are concerned is “How much of each reactant is available to
react?” In the case of the zinc ion, the answer is “Not much at all.” With the EDTA,
there is a much higher fraction available. To reinforce the point we have brought up
before, it is the beauty of EDTA that even when the fraction of available reactant is
low, the titration is still practical.
PRACTICE 18.14
Calculate the conditional formation constant, K
f
, for the system in Exercise 18.14 at
pH = 3.0.
See Problems 63 and 64.
In the titration of metals with EDTA, we have seen equilibrium principles all
come together in one of the most important types of aqueous analyses at the dis-
posal of the chemical technician. Recall Table 18.1 at the beginning of the chap-
ter, in which we listed the calcium–EDTA titration-based hard-water analysis
among several ones that are commonly done. Equilibrium principles make these
processes ideal for use in many everyday venues. That EDTA titrations are so very
common in industrial and academic settings is testimony to the universal utility
of equilibrium theory and practice.
EXERCISE 18.15 Data for a Calcium–EDTA Analysis
Here are some data obtained from the titration of calcium ion in water with EDTA.
How many milligrams of calcium are there per liter of water?
The EDTA solution was prepared by combining the disodium salt Na
2
H
2
EDTA
with water. Its molarity is 0.01944 M. About 5 mL of an ammonia–ammonium ion
buffer are combined with a 50.00 mL aliquot of the water. The resulting solution is
titrated with EDTA, and it requires 31.88 mL to reach the Eriochrome Black T indi-
cator endpoint, shown by a color change from wine red to blue.
814 Chapter 18 Applications of Aqueous Equilibria
Solution
We begin by finding the mass of calcium in the 50.00 mL aliquot.
Grams of Ca in the 50.00 mL aliquot
= 0.03188 L EDTA solution
×
0.01944 mol EDTA
1 L EDTA sol’n
×
1 mol Ca
1 mol EDTA
×
40.08 g Ca
1 mol Ca
= 0.02484 g Ca in the 50.00 mL aliquot
To convert to the 1 L sample, we use dimensional analysis to scale up to the 1 L volume:
0.02484 g
×
1000 mL
50 mL
= 0.497 ≈ 0.500 g/L calcium
This is equal to 500 mg/L, which is the same as 500 parts per million, or 500 ppm
(recall the discussion about ppm in Section 4.2), of calcium in the solution. This
corresponds to fairly hard water.
PRACTICE 18.15
How many milliliters of the EDTA solution described in this exercise would be
needed to titrate a 50.00 mL sample of water containing 123.8 ppm Ca?
See Problems 65 and 66.
The Bottom Line 815
The Bottom Line
■
A titration is a technique used to find out how much
of a substance is in a solution. (Section 18.2)
■
There are several types of titrations, including
reduction–oxidation, precipitation, complex-
formation, and acid–base titration. (Section 18.2)
■
Many reactions are pH-sensitive and require buffers
to control pH. (Section 18.1)
■
A buffer resists change in pH upon addition of a
strong acid or strong base or upon dilution.
(Section 18.1)
■
Buffers are typically composed of weak conjugate
acid–base pairs. (Section 18.1)
■
We can solve for the pH of buffers in a straight-
forward way by recognizing the importance of Le
Châtelier’s principle and the common-ion effect.
(Section 18.1)
■
We can calculate the approximate ratio of conjugate
acid to base in order to prepare a buffer of a known
pH. Activity effects are important, and our acid-to-
base ratio will probably need to be slightly adjusted
to be at the desired pH. (Section 18.1)
■
Solving for the pH of a buffer upon addition of
strong acid or base is really solving a limiting-
reactant problem. (Section 18.1)
■
It is possible to exceed the buffer capacity, in which
case the pH will go sharply higher (with excess base)
or lower (with excess acid). (Section 18.1)
■
Strong-acid–strong-base titrations show a relatively
level pH until near the equivalence point, where the
pH dramatically changes. (Section 18.2)
■
Titration curves in which one component is weak
and the other is strong contain four regions: the
initial pH, the buffer region, the equivalence-point
region and the post–equivalence point region.
(Section 18.2)
■
The buffer region contains a point at which one-half
of the analyte has been converted to its conjugate.
This is called the titration midpoint, and the pH is
equal to the pK
a
of the analyte. (Section 18.2)
■
The larger the pK of the analyte, the sharper will
be the change in pH at the equivalence point.
(Section 18.2)
■
The higher the concentration of the weak acid (or
base) and the strong base (or acid), the sharper the
endpoint. (Section 18.2)
■
An indicator is used to visually detect the
equivalence point of a titration. (Section 18.2)
■
Only a few drops of an indicator are added to the
titration solution so that the equivalence point and
endpoint can be as close together as possible.
(Section 18.2)
■
Solubility equilibria can often be complex, involving
several side reactions and molecular-level processes
that make calculations challenging. (Section 18.3)
■
The effects of ion-pairing, activity, and other
thermodynamic considerations add to the challenge
of properly calculating the concentration of
dissolved salts in aqueous solution. (Section 18.3)
■
Gravimetric analysis is based on weighing the
precipitate that includes the substance of interest.
(Section 18.3)
■
The pH of an aqueous solution can significantly af-
fect the solubility of the substances in that solution.
(Section 18.3)
816 Chapter 18 Applications of Aqueous Equilibria
■
A chemical complex typically consists of one or
more metal cations bonded to one or more Lewis
bases. (Section 18.4)
■
The formation constant is a measure of the extent
of reaction between a Lewis base and metal ion in
aqueous solution. (Section 18.4)
■
EDTA is the primary example of a highly effective
chelating agent. (Section 18.4)
■
The reaction of chelating agents and metal ions has a
very high formation constant. (Section 18.4)
■
The analysis of calcium in hard water by EDTA
titration is an important application of complex-ion
equilibrium. (Section 18.4)
Key Words
acid–base indicator A compound that changes color on
the basis of the pH of the solution in which it is dis-
solved. The color change is often a result of structural
changes due to protonation or deprotonation of
acidic groups within the compound. (p. 799)
analyte A solute whose concentration is to be measured
by a laboratory test. (p. 766)
anthocyanins A naturally occurring class of compounds
responsible for many of the colors of plants. These
compounds often act as acid–base indicators.
(p. 801)
boiler scale A buildup of calcium and magnesium salts
within pipes and water heaters. Typically composed
of calcium carbonate and magnesium carbonate.
(p. 767)
buffer A solution containing a weak acid and its
conjugate base or a weak base and its conjugate acid.
Buffers resist changes in pH upon the addition of
acid or base or by dilution. (p. 767)
buffer capacity The degree to which a buffer can
“absorb” added acid or base without changing pH.
(p. 779)
buffer region The region of a titration indicated by the
presence of a weak acid or base and its conjugate. The
pH changes little within this region. (p. 793)
calcareous oozes The calcium-containing detritus from
dead single-celled, calcium-based sea life. (p. 802)
chelates Substances capable of associating through co-
ordinate covalent bonds to a metal ion. Also known
as chelating agents. (p. 810)
coagulation The precipitation of a solid along with
some dissolved organic material, microorganisms
and other undesirable substances (p. 808)
conditional formation constant (K
) The formation
constant that accounts for the free metal ion and its
associated ligand. (p. 813)
equivalence point The exact point at which the reactant
in a titration has been neutralized by the titrant.
(p. 789)
flue-gas desulfurization A process used to remove sulfur
dioxide (and other sulfur oxides) from combustion
smoke. (p. 779)
formation constant (K
f
) The equilibrium constant
describing the formation of a stable complex.
Typically, K
f
values are large. Also known as the
stability constant. (p. 809)
Good (Good’s) buffers Buffers typically used in bio-
chemical research because they are chemically stable
in the presence of enzymes or visible light and are
easy to prepare. (p. 785)
gravimetric analysis A laboratory technique in which the
concentration of substances in solution is determined
by forming insoluble salt precipitates and weighing
them or their related solids. (p. 806)
Henderson–Hasselbalch equation A shorthand equation
used to determine the pH of a buffer solution.
pH = pK
a
+ log(base/acid). (p. 778)
hexadentate ligand A ligand that makes six coordinate
covalent bonds to a metal ion. (p. 810)
ion pair Ions in solution that associate as a unit.
(p. 804)
ligand A compound that associates with a metal ion
through coordinate covalent bonds. (p. 809)
metal recovery Recycling of metals from waste streams
by complexation with chelating agents. (p. 807)
molar solubility The total number of moles of solute
that dissolve per liter of solution. (p. 805)
monodentate ligand A ligand that makes one coordinate
covalent bond to a metal ion. (p. 810)
pH indicator A compound that changes color on the
basis of the acidity or basicity of the solution in
which it is dissolved. Also known as an acid–base
indicator. (p. 799)
polydentate Capable of forming several coordinate
covalent bonds from a single ligand to a metal.
(p. 810)
quality control The practice in industry of ensuring that
the product contains what it is supposed to, and in
the proper amounts. (p. 766)
scrubbing The process of removing harmful impurities
from smokestack gases. (p. 779)
sedimentation The process of removing dissolved
organic matter, heavy metals, and other impurities
from water. (p. 808)
sequester To tie up an ion or compound by chelation
and make it unavailable for use. (p. 813)
solubility product The mass-action expression for
the solubility reaction of a sparingly soluble salt.
(p. 802)
solubility product constant (K
sp
) The constant that is part
of the solubility product mass-action expression.
Typically, K
sp
values are much less than 1. (p. 802)
stability constant See formation constant. (p. 809)
tetradentate ligand A ligand that makes four coordinate
covalent bonds to a metal ion. (p. 810)
titrant The solution being added to a solution of an
analyte during a titration. (p. 766)
titration The process used to determine the exact
concentration of an analyte. (p. 766)
titration curve A plot of the pH of the solution versus
the volume (or concentration) of titrant. (p. 790)
titration endpoint The volume and pH at which the
indicator has changed color during a titration. The
endpoint is not always the same as the equivalence
point. (p. 801)
titration midpoint The pH of the titration where the
concentration of weak acid or base is equal to the
concentration of its conjugate. At this point, the pH
is equal to the pK
a
of the analyte. (p. 794)
Focus Your Learning 817
Focus Your Learning
The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
Section 18.1 Buffers and the Common-Ion Effect
Skill Review
1. Write the equations that would describe the equilibria pre-
sent in each of these solutions:
a. 0.10 M NH
3
b. 0.250 M Fe(OH)
3
c. 0.125 M HCOONa
2. Write the equations that would describe the equilibria pre-
sent in each of these solutions:
a. 0.30 M PbS b. 0.150 M NH
4
Cl
c. 0.050 M CH
3
COOH
3. Decide which, if any, of these pairs could be used to prepare a
buffer solution.
a. HF and NaF b. CH
3
COOH and NH
3
c. NH
4
Cl and HF d. H
2
SO
4
and NaHSO
4
e. NH
4
NO
3
and NH
3
4. Decide which, if any, of these pairs could be used to prepare a
buffer solution.
a. KBr and HBr b. NaOH and CH
3
COOH
c. HCOOH and HNO
3
d. NaNO
3
and HNO
3
e. CH
3
COONa and HCl
5. Without using the Henderson–Hasselbalch equation, calcu-
late the pH of a buffer made from each of these pairs. Assume
that the concentrations given are those in the final mixture.
a. 1.00 M NH
3
and 1.00 M NH
4
Cl
b. 4.50 M NH
4
Cl and 0.50 M NH
3
c. 2.50 M CH
3
COOH and 0.75 M CH
3
COONa
6. Without using the Henderson–Hasselbalch equation, calcu-
late the pH of a buffer made from each of these pairs. Assume
the concentrations given are those in the final mixture.
a. 2.33 M NH
3
and 1.00 M NH
4
Cl
b. 2.50 M HCOOH and 1.50 M HCOONa
c. 0.100 M CH
3
COOH and 0.75 M CH
3
COONa
7. Suppose an ammonia–ammonium buffer has pH of 10.1.
Indicate the effect, if any, of each of these changes:
a. Adding NH
3
b. Adding NH
4
+
c. Adding Cl
−
8. Suppose an acetic acid–acetate buffer has pH of 4.74. Indi-
cate the effect, if any, of each of these changes:
a. Adding NH
3
b. Adding Na
+
c. Adding HCl
9. Suppose you were to prepare a buffer solution using acetic
acid and sodium acetate. What would be the molar ratio of
acid to its conjugate when the pH was adjusted to each of
these values?
a. pH = 3.74 b. pH = 4.74 c. pH = 5.74
10. Suppose you were to prepare a buffer solution using ammo-
nia and ammonium chloride. What would be the molar ratio
of acid to its conjugate when the pH was adjusted to each of
these values?
a. pH = 10.10 b. pH = 9.26 c. pH = 8.40
11. How many milliliters of 0.20 M HCl would we have to add to
100.0 mL of 0.2500 M ammonia in order to prepare a buffer
that has each of these pH values?
a. pH = 9.26 b. pH = 10.5 c. pH =8.5
12. How many milliliters of 0.150 M NaOH would we have to
add to 100.0 mL of 0.100 M acetic acid in order to prepare a
buffer that has each of these pH values?
a. pH = 4.26 b. pH = 3.75 c. pH = 5.25
