Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


buffers to help us sort it all out. For example, the initial concentration of our
strong acid is
[H
+
] = 0.1000 M =
(0.1000 mol)
(1.000 L)
=
(0.1000 mmol)
(1.000 mL)
How much H
+
is in the solution? Using the concentration in moles per liter and
the volume in liters, we find that
(0.1000 mol)
(1.000 L)
× 0.05000 L
= 0.005000 mol H
+
We can calculate the amount of strong base, OH
−
, added to the solution in the
same way.
(0.2000 mol)
(1.000 L)
× 0.00500 L
= 0.001000 mol OH
−
Putting our values in tabular format shows that we still have plenty of strong acid
in the solution. Note that because water is the solvent, it will not enter into the
mass-action expression, and we can ignore it in our calculations.
H
+
(aq) + OH
−
(aq)
H
2
O(l) K = 1.0
×
10
14
moles initial 0.005000
moles added 0.001000
change −0.001000 −0.001000
moles at equilibrium 0.004000
≈
0
We can calculate the pH, keeping in mind the total solution volume of
55.00 mL (50.00 mL of HCl solution + 5.00 mL of added NaOH solution).
[H
+
] =
(0.004000 mol)
(0.05500 L)
= 0.0727 M
pH = 1.14
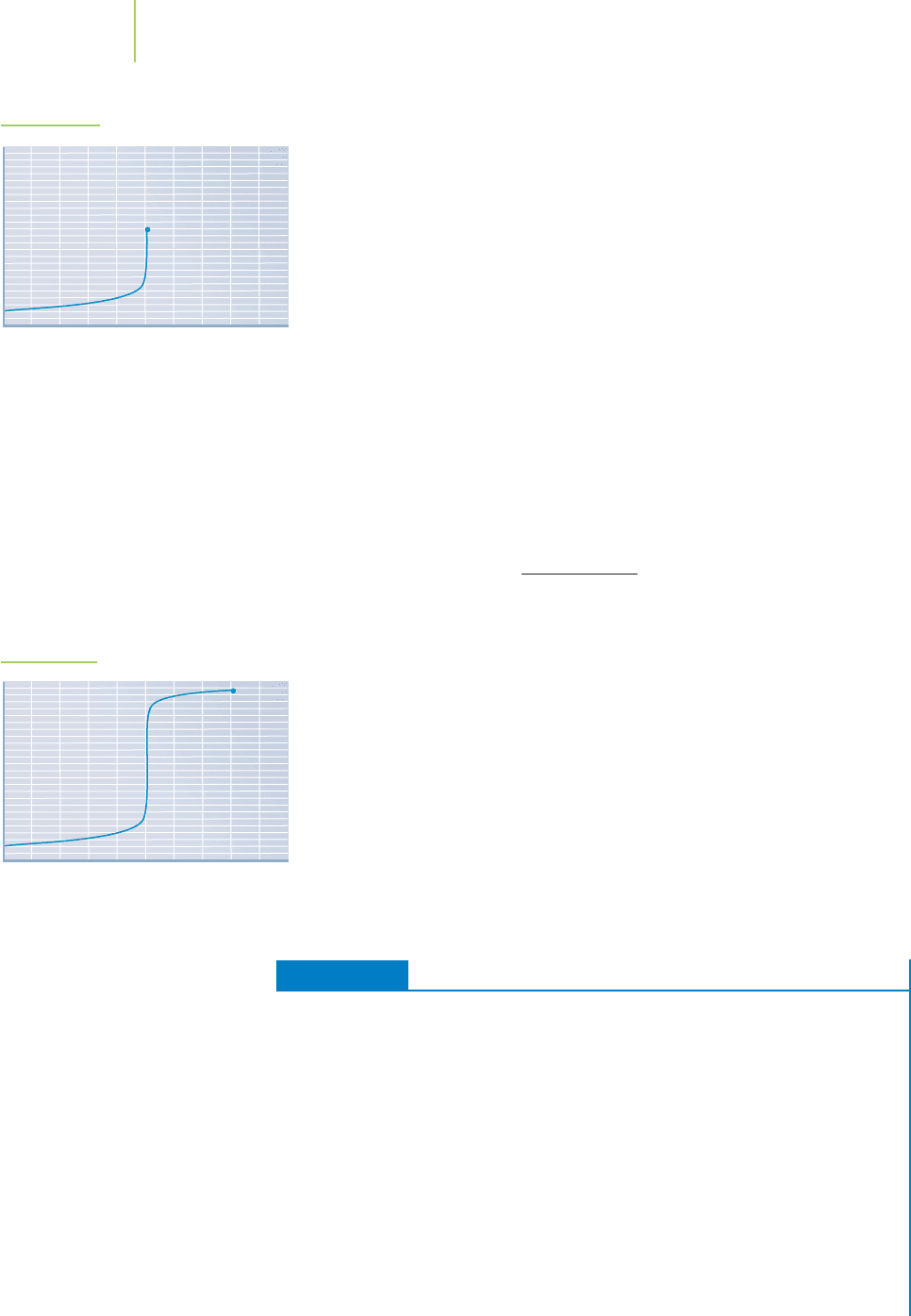
The addition of the strong acid has raised the pH, but not much, because we
still have plenty of excess acid. We have entered the data onto our graph in
Figure 18.8b.
Part 3: Addition of a total of 12.50 mL of NaOH solution
Half of the H
+
is neutralized at this point.
H
+
(aq) + OH
−
(aq)
H
2
O(l) K = 1.0
×
10
14
moles initial 0.005000
moles added 0.002500
change −0.002500 −0.002500
moles at equilibrium 0.002500
≈
0
The total volume is 62.50 mL (50.00 mL of HCl solution + 12.50 mL of NaOH
solution), which results in a pH of 1.4, as shown here and in Figure 18.8c.
[H
+
] =
(0.0025000 mol)
(0.06250 L)
= 0.0400 M
pH = 1.40
788 Chapter 18 Applications of Aqueous Equilibria
1
3
5
7
9
11
13
0 5 10 15 20 25 30 35 40 45 50
Volume of NaOH
(
mL
)
pH
FIGURE 18.8b

Part 4: Addition of a total of 24.00 mL of NaOH solution
By the time we add 24.00 mL of 0.2000 M NaOH to the acid, we have neutralized
nearly all of the hydrogen ion, as shown in the following data table.
H
+
(aq) + OH
−
(aq)
H
2
O(l) K = 1.0
×
10
14
moles initial 0.005000
moles added 0.004800
change −0.004800 −0.004800
moles at equilibrium 0.000200
≈
0
The total volume is 74.00 mL (50.00 mL of HCl solution + 24.00 mL of
NaOH solution), which results in a pH of 2.57, as shown.
[H
+
] =
(0.000200 mol)
(0.07400 L)
= 2.70
×
10
−3
M
pH = 2.57
Adding a bit more, so that the volume of base added is 24.95 mL, results
in a pH of 3.87, shown in Figure 18.8d. As we get very close to neutraliz-
ing the acid, the pH starts to rise sharply.
Part 5: Addition of a total of 25.00 mL of NaOH solÏution
At this point, all of the strong acid has been neutralized by the strong base. This
is called the
equivalence point of the titration, the exact point at which the reac-
tant has been neutralized by the titrant.
H
+
(aq) + OH
−
(aq)
H
2
O(l) K = 1.0
×
10
14
moles initial 0.005000
moles added 0.005000
change −0.005000 −0.005000
moles at equilibrium
≈
0
≈
0
We say that there are “
≈
0” hydrogen and hydroxide ions in the solution. This
really says that the amount is insignificant when compared to the original amounts
of acid and base that were mixed. How much is “
≈
0” in this neutral solution?
We have 75.00 mL of (ideally) pure water at equilibrium. The only reaction
that is important in our solution at this point is the autoprotolysis of water.
Finally, the equilibrium constant for this reaction has become very important.
18.2 Acid–Base Titrations 789
1
3
5
7
9
11
13
0 5 10 15 20 25 30 35 40 45 50
pH
Volume of NaOH (mL)
1
3
5
7
9
11
13
0 5 10 15 20 25 30 35 40 45 50
pH
Volume of NaOH (mL)
FIGURE 18.8c
FIGURE 18.8d
Using the equation for this reaction, and the corresponding mass-action
expression, we can solve for the concentration of H
+
in the solution.
H
2
O(l)
H
+
(aq) + OH
−
(aq) K
w
= 1.0
×
10
−14
[H
+
] = [OH
−
] = 1.0
×
10
−7
M
pH = 7.00
The answer to our question here is [H
+
] = 1.0
×
10
−7
M, which is in-
significant compared to the initial concentration of strong acid and base.
However, in the absence of other sources of H
+
, this is very significant.The
solution now has a neutral pH, as shown in Figure 18.8e. Note the sharp
rise to the equivalence point, which makes it easy to identify.
Part 6: Addition of a total of 40.00 mL of NaOH solution
At this point, the additional strong base is just being added to water (neglecting
the spectator ions Na
+
and Cl
−
), so we would expect the pH to rise sharply. We
are adding 15.00 mL of 0.2000 M NaOH, or 0.00300 mol, past the equivalence
point, and our pH is calculated as shown for the total solution volume of 90.00 mL
(50.00 mL of HCl solution + 40.00 mL of NaOH added).
[OH
−
] =
(0.003000 mol)
(0.09000 L)
= 0.03333 M
pOH = 1.48
pH = 12.52
The solution has become quite basic. The complete strong-acid–strong-
base
titration curve is shown in Figure 18.8f.
Summarizing, we have shown that a strong base will only slightly in-
crease the pH of a strong acid until very close to the equivalence point,
where it will rise sharply to pH =7. After the equivalence point, the pH will
continue its sharp increase to a point that depends on the concentration of
the strong-base titrant.
A word of caution: The pH at the equivalence point will equal 7 only in
monoprotic strong-acid–strong-base titrations. We will show why this is so
immediately after Exercise 18.8.
EXERCISE 18.8 Titrating Sodium Hydroxide with Hydrochloric Acid
Calculate and draw a graph showing the relationship between the pH and the vol-
ume of HCl for the titration of 25.00 mL of 0.2500 M NaOH with the following
total volumes of 0.1250 M HCl: a. 0 mL; b. 20.00 mL; c. 49.80 mL; d. 50.00 mL;
e. 60.00 mL.
Solution
The net ionic reaction is the same as in the addition of NaOH to HCl, except that
the reactant (now OH
−
) and the titrant (now H
+
) have switched roles.
OH
−
(aq) + H
+
(aq)
H
2
O(l) K = 1.0
×
10
14
a. 0 mL of acid added: The pH of this strong base can be found from the hy-
droxide ion concentration, which is equal to the initial concentration of the
NaOH solution.
[OH
−
] = 0.2500 M pOH = 0.60 pH = 13.40
790 Chapter 18 Applications of Aqueous Equilibria
1
3
5
7
9
11
13
0 5 10 15 20 25 30 35 40 45 50
pH
Volume of NaOH
(
mL
)
1
3
5
7
9
11
13
0 5 10 15 20 25 30 35 40 45 50
pH
Volume of NaOH
(
mL
)
FIGURE 18.8e
FIGURE 18.8f
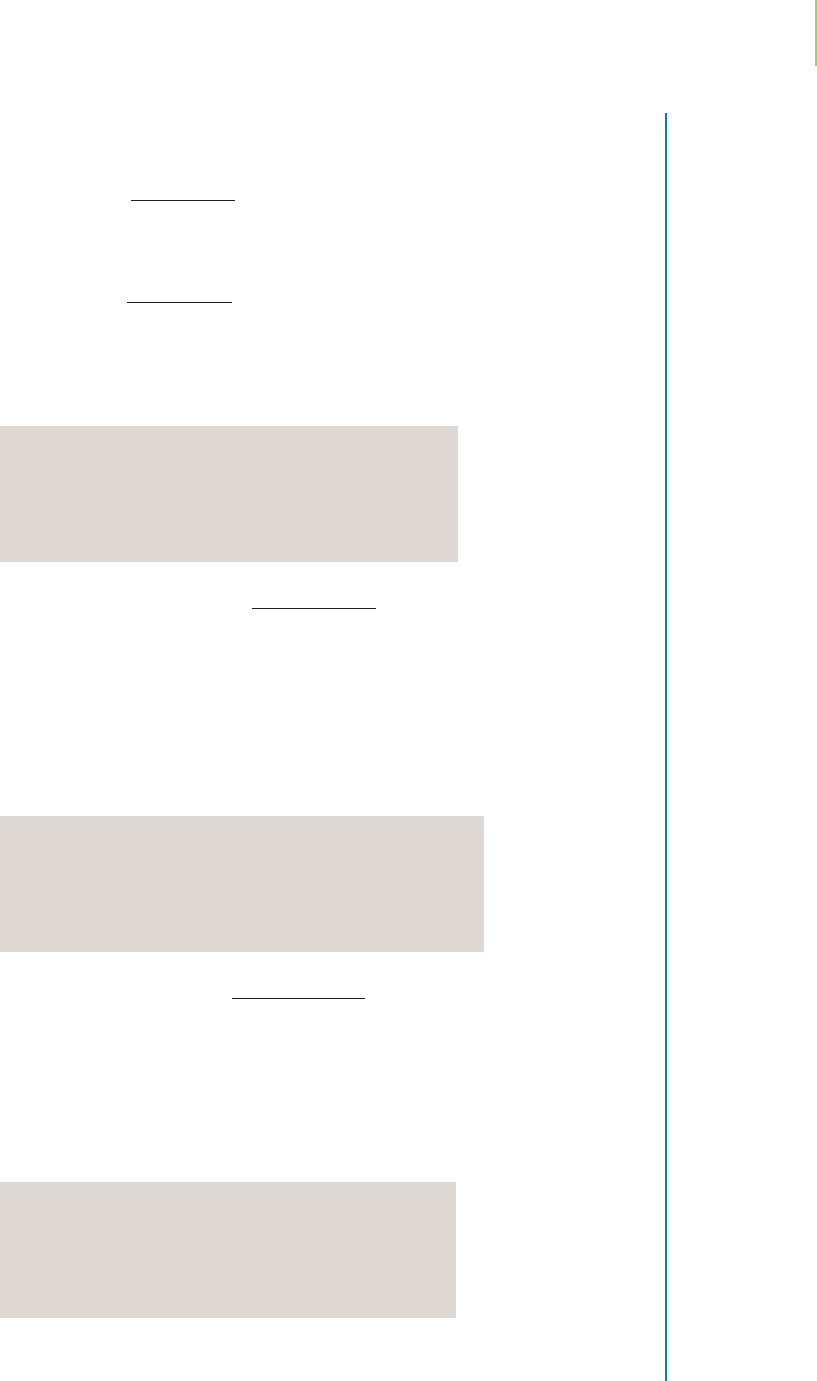
b. 20.00 mL of acid added: We can use the same table setup that we used previously
to calculate the moles of each after reaction. There are initially
(0.2500 mol)
(1 L)
× 0.02500 L
= 0.006250 mol OH
−
in the solution. We add
(0.1250 mol)
(1.000 L)
× 0.02000 L
= 0.002500 mol of H
+
in a total solution volume of 45.00 mL (25.00 mL of base +20.00 mL of acid) =
0.04500 L.
OH
−
(aq) + H
+
(aq)
H
2
O(l) K = 1.0
×
10
14
moles initial 0.006250
moles added 0.002500
change −0.002500 −0.002500
moles at equilibrium 0.003750
≈
0
[OH
−
]
=
(0.003750 mol)
(0.04500 L)
= 0.08333 M
pOH = 1.08
pH = 12.92
c. 49.8 mL of acid added: Using the same type of calculations, we find that
OH
−
(aq) + H
+
(aq)
H
2
O(l) K = 1.0
×
10
14
moles initial 0.006250
moles added 0.006225
change −0.006225 −0.006225
moles at equilibrium 0.000025
≈
0
[OH
−
] =
(0.0000250 mol)
(0.07480 L)
= 3.34
×
10
−4
M
pOH = 3.48
pH = 10.52
d. 50.00 mL of acid added:
OH
−
(aq) + H
+
(aq)
H
2
O(l) K = 1.0
×
10
14
moles initial 0.006250
moles added 0.006250
change −0.006250 −0.006250
moles at equilibrium
≈
0
≈
0
The solution is at the equivalence point, and pH = 7.0 for a strong-base–
strong-acid titration.
18.2 Acid–Base Titrations 791
e. 60.00 mL of acid added: We have added 10.00 mL of excess acid, which
is a total of 0.00125 mol.The total solution volume is 85.00 mL (25.00 mL
of base + 60.00 mL of added acid) = 0.08500 L. We can calculate the
pH from this information.
[H
+
] =
(0.001250 mol)
(0.08500 L)
= 0.01471 M
pH = 1.83
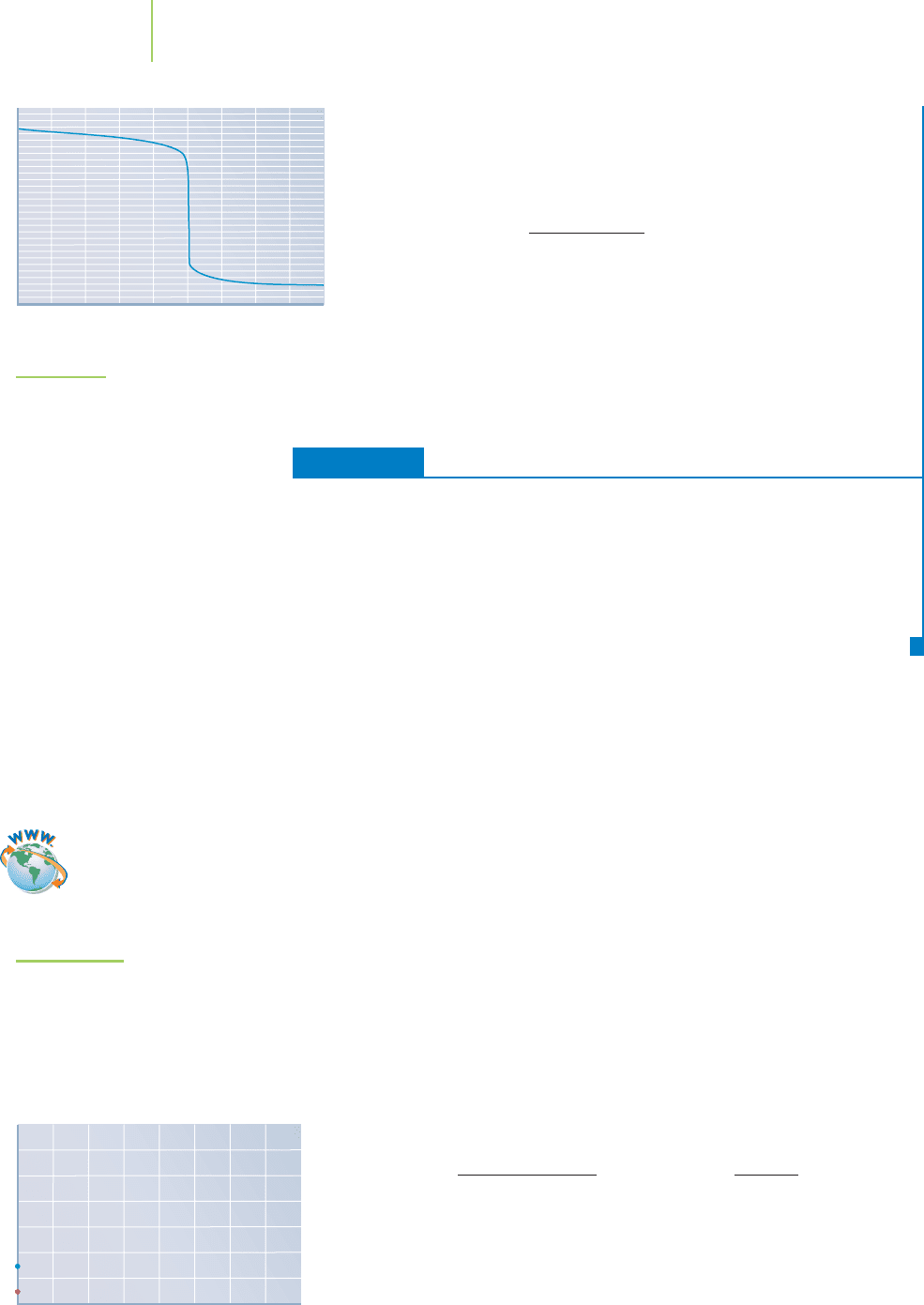
The titration curve shown in Figure 18.9 has a shape similar to what we
expected. It is fairly flat until close to the equivalence point, where it
drops quite sharply and over a large pH range, so it is easily detectable.
PRACTICE 18.8
Calculate and draw a graph of the relationship between the pH and the volume of
NaOH solution for the titration of 25.00 mL of 0.2500 M HCl with the following
total volumes of 0.2500 M NaOH:
a. 0 mL d. 25.00 mL
b. 10.00 mL e. 30.00 mL
c. 20.00 mL f. 40.00 mL
See Problems 33, 34, and 37.
Acid–Base Titrations in Which One Component
Is Weak and One Is Strong
Although the general problem-solving strategy is the same when we titrate a weak
acid with a strong base (or a weak base with a strong acid) as when we perform a
strong-acid–strong-base titration, there is a critical difference, the K
a
(or K
b
) of
the analyte, that affects both the pH and the pH change at the equivalence point.
Our ability to do a titration analysis depends on having a large, sharp break in
the equivalence point. We can best see this by comparing the data from our
HCl–NaOH titration with those from the titration of 50.00 mL of a 0.1000 M
acetic acid solution (CH
3
COOH, K
a
= 1.8
×
10
−5
) with a 0.2000 M
sodium hydroxide solution. The amounts and concentrations are the
same. Only the acid has been changed from strong to weak.
Part 1: Initial pH
We can determine pH in this weak acid as we would in any other weak
acid, using principles we learned in Chapter 17.
CH
3
COOH(aq)
CH
3
COO
−
(aq) + H
+
(aq) K
a
= 1.8
×
10
−5
K
a
=
[H
+
][CH
3
COO
−
]
[CH
3
COOH]
1.8
×
10
−5
=
x
2
(0.1000)
x = [CH
3
COO
−
] = [H
+
] = 1.3
×
10
−3
M
pH = 2.87
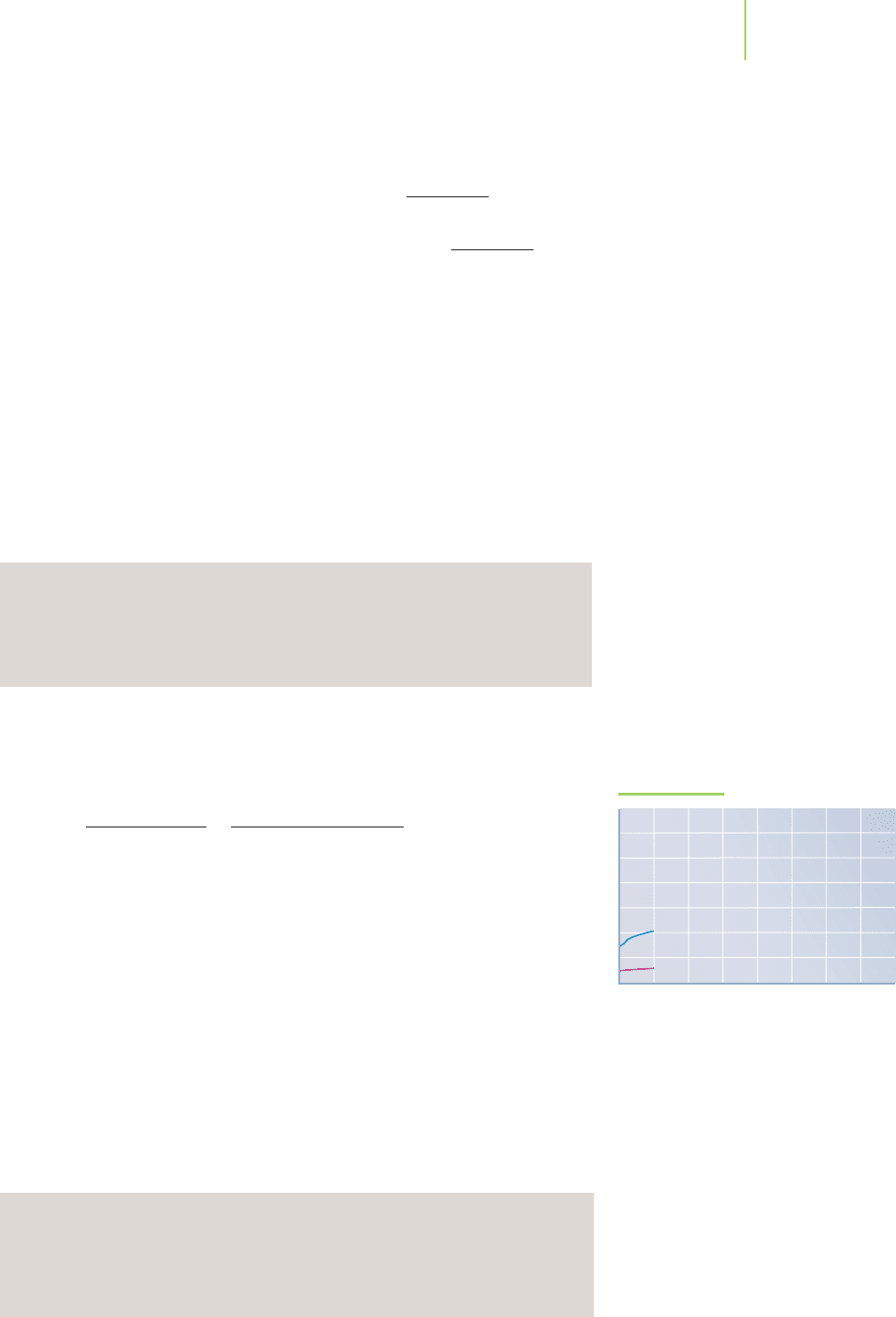
We already see a difference in the titration curve (Figure 18.10a) because
this weak acid pH is nearly 2 units higher than the initial pH of the strong
acid, HCl, of the same concentration.
792 Chapter 18 Applications of Aqueous Equilibria
1
3
5
7
9
11
13
15
0 102030405060708090
Volume of HCl (mL)
pH
FIGURE 18.9
The titration curve for the addition of 0.125 M
HCl to 25.00 mL of 0.250 M NaOH.
0
2
4
6
8
10
12
14
0 5 10 15 20 25 30 35 40
NaOH added (mL)
pH
Strong acid, strong base
Weak acid, strong base
FIGURE 18.10a
Each plot follows the pH changes as we
add 0.2000 M NaOH solution to 50.00 mL
of 0.1000 M acetic acid solution. The pH
values at each volume are superimposed on
those from Figure 18.9 to show the differ-
ence in the nature of the titration curve be-
tween the strong acid and the weak acid.
Tutorial: Titration Curves: Weak
Base with Strong Acid
Video Lesson: Weak-
Acid–Strong-Base Titration
Video Lesson: Weak-
Base–Strong-Acid Titration
Part 2: Addition of 5.00 mL of NaOH solution
We will use the same thinking—and ask the same questions—to assess the
chemistry here as in the strong-acid–strong-base titration. We begin with
0.005000 mol of acetic acid
50.00 mL ×
0.1000 mol
L
,
to which we add 0.001000 mol of OH
−
5.000 mL ×
0.2000 mol
L
.
Question 1: What will be the reaction of the acetic acid with the NaOH solution?
The net ionic form of the reaction is useful because spectator ions are not part
of the acid–base chemistry.
CH
3
COOH(aq) + OH
−
(aq)
CH
3
COO
−
(aq) + H
2
O(l) K = 1.8
×
10
9
The equilibrium constant is the same whether we describe a reaction by giving
its molecular or net ionic form. For this reaction, K is quite high and the reac-
tion is fast.
Question 2: What, and how much, will be left over after addition of the titrant? We
can use a table to clarify the amounts involved when the system reaches its new
equilibrium position.
CH
3
COOH(aq) + OH
−
(aq)
CH
3
COO
−
(aq) + H
2
O(l)
moles initial 0.005000 0
moles added 0.001000
change −0.001000 −0.001000 +0.001000
moles at equilibrium 0.004000
≈
0 0.001000
Because we have both a weak acid and its conjugate base, we have produced a
buffer, and we can solve for the pH as with any buffer system. Note that we have
substituted moles into the equation instead of molarities because the total
volume of the solution will cancel out.
[H
+
] =
K
a
[CH
3
COOH]
[CH
3
COO
−
]
=
(1.8 ×10
−5
)(0.004000)
(0.001000)
= 7.2
×
10
−5
M
pH = 4.14
We have entered the data point onto our graph in Figure 18.10b. How is
the curve shaping up compared to that of the strong-acid–strong-
base titration? Because the solution is a buffer at this point, this part of the
titration is called the
buffer region. We expect the pH to be relatively con-
stant within the buffer region.
Part 3: Addition of 12.50 mL of NaOH solution
Proceeding as we did in part b, we can generate the following table and solve for
pH. Again, in our calculation of the hydrogen ion concentration, we needn’t
worry about the volumes of acid and base because they will cancel out during our
calculation.
CH
3
COOH(aq) + OH
−
(aq)
CH
3
COO
−
(aq) + H
2
O(l)
moles initial 0.005000 0
moles added 0.002500
change −0.002500 −0.002500 +0.002500
moles at equilibrium 0.002500
≈
0 0.002500
18.2 Acid–Base Titrations
793
0
2
4
6
8
10
12
14
0 5 10 15 20 25 30 35 40
pH
Strong acid,
strong base
Weak acid,
strong base
NaOH added (mL)
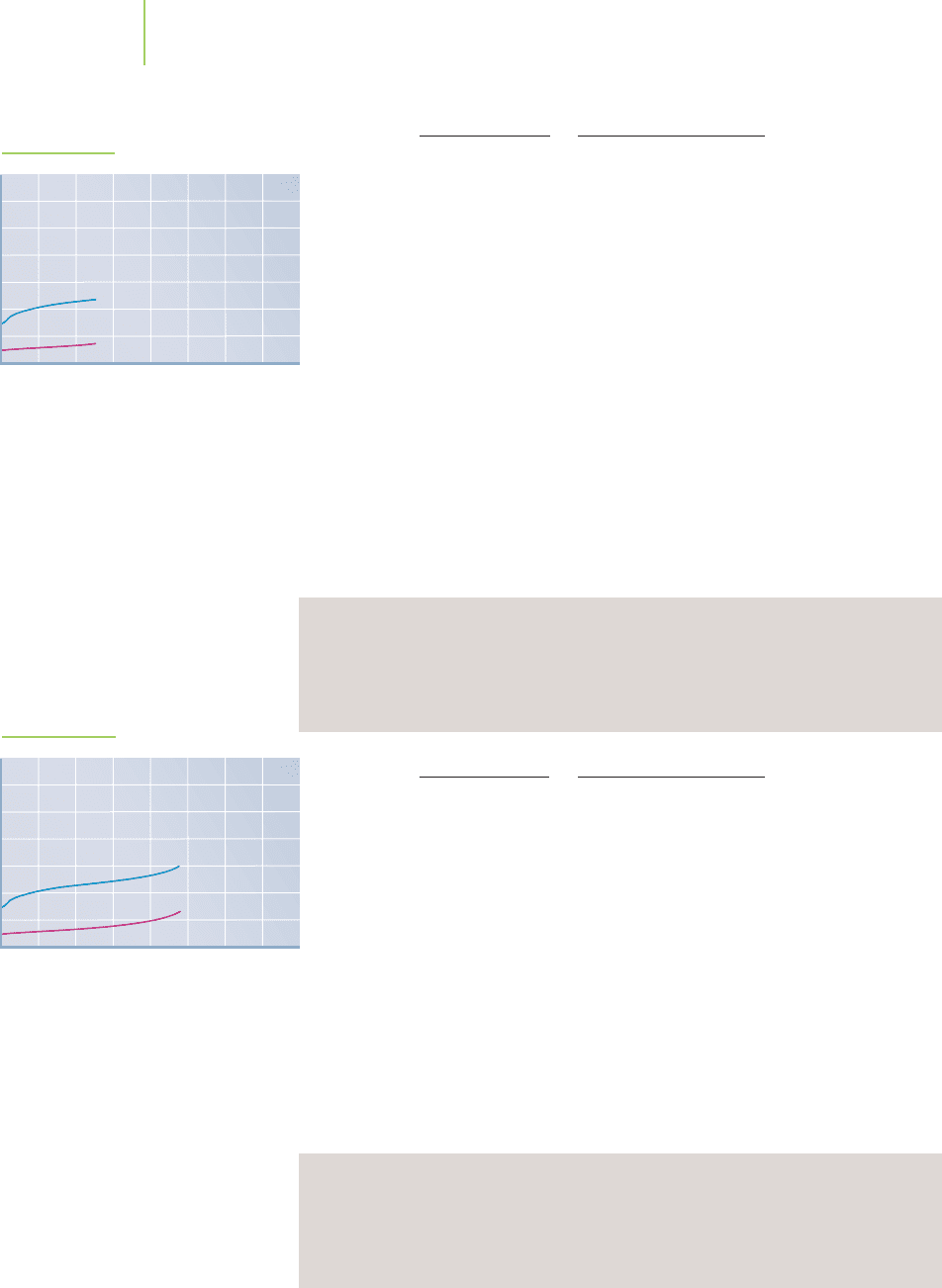
FIGURE 18.10b
0
2
4
6
8
10
12
14
0 5 10 15 20 25 30 35 40
pH
Strong acid,
strong base
Weak acid,
strong base
NaOH added (mL)
FIGURE 18.10d
[H
+
] =
K
a
[CH
3
COOH]
[CH
3
COO
−
]
=
(1.8 ×10
−5
)(0.002500)
(0.002500)
= 1.8
×
10
−5
M
pH = 4.74
We are still in the buffer region, having neutralized precisely one-half of
the acetic acid and forming an equal amount of acetate ion, the conjugate
base. This is called the
titration midpoint, at which the pH equals the pK
a
of
acetic acid. It is also possible to work backward, finding the pK
a
of an acid
from pH at the titration midpoint. As before, we have entered the data
point onto our graph (see Figure 18.10c). How is the curve shaping up
compared to that of the strong-acid–strong-base titration?
Part 4: Addition of a total of 24.00 mL of NaOH solution
Continuing, we generate the following table, which shows that we are still—
though barely—in the buffer region. The pH is beginning to rise on the way to
the equivalence point.
CH
3
COOH(aq) + OH
−
(aq)
CH
3
COO
−
(aq) + H
2
O(l)
moles initial 0.005000
≈
0
moles added 0.004800
change −0.004800 −0.004800 +0.004800
moles at equilibrium 0.000200
≈
0 0.004800
[H
+
] =
K
a
[CH
3
COOH]
[CH
3
COO
−
]
=
(1.8 ×10
−5
)(0.000200)
(0.004800)
= 7.5
×
10
−7
M
pH = 6.12
Continue to keep an eye on the data comparison (Figure 18.10d) between
this titration and the strong-acid–strong-base titration.
794 Chapter 18 Applications of Aqueous Equilibria
0
2
4
6
8
10
12
14
0 5 10 15 20 25 30 35 40
pH
Strong acid,
strong base
Weak acid,
strong base
NaOH added (mL)
FIGURE 18.10c
Part 5: Addition of a total of 25.00 mL of NaOH solution
The completed table shows that we are at the equivalence point. We converted all
of the acetic acid to acetate ion.
CH
3
COOH(aq) + OH
−
(aq)
CH
3
COO
−
(aq) + H
2
O(l)
moles initial 0.005000
≈
0
moles added 0.005000
change −0.005000 −0.005000 +0.005000
moles at equilibrium
≈
0
≈
0 0.005000
How do we calculate the pH of this solution? To answer that, we need to go back
to our most important questions: “What is in solution?” and “What equation
describes their behavior?”
The answer to the first question is “0.005000 mol of acetate ion in a total of
75.00 mL of solution.” Using the chemist’s shorthand,
[CH
3
COO
−
] =
(0.005000 mol)
(0.07500 L)
= 0.06667 M
We have neutralized all of the weak acid, and we now have a solution of its con-
jugate base. This acetate ion will hydrolyze, if only barely, to acetic acid.
CH
3
COO
−
(aq) + H
2
O(l)
CH
3
COOH(aq) + OH
−
(aq)
We can calculate the equilibrium constant for the hydrolysis of this conjugate
base of acetic acid, K
b
,from K
w
and the acetic acid K
a
.
K
b
=
K
w
K
a
=
1.0 ×10
−14
1.8 ×10
−5
= 5.6
×
10
−10
We can now solve for the pH of the weak base.
K
b
=
[OH
−
][CH
3
COOH]
[CH
3
COO
−
]
5.6
×
10
−10
=
[x
2
]
[0.06667]
x = [CH
3
COOH] = [OH
−
] = 6.1
×
10
−6
M
pOH = 5.21
pH = 8.79
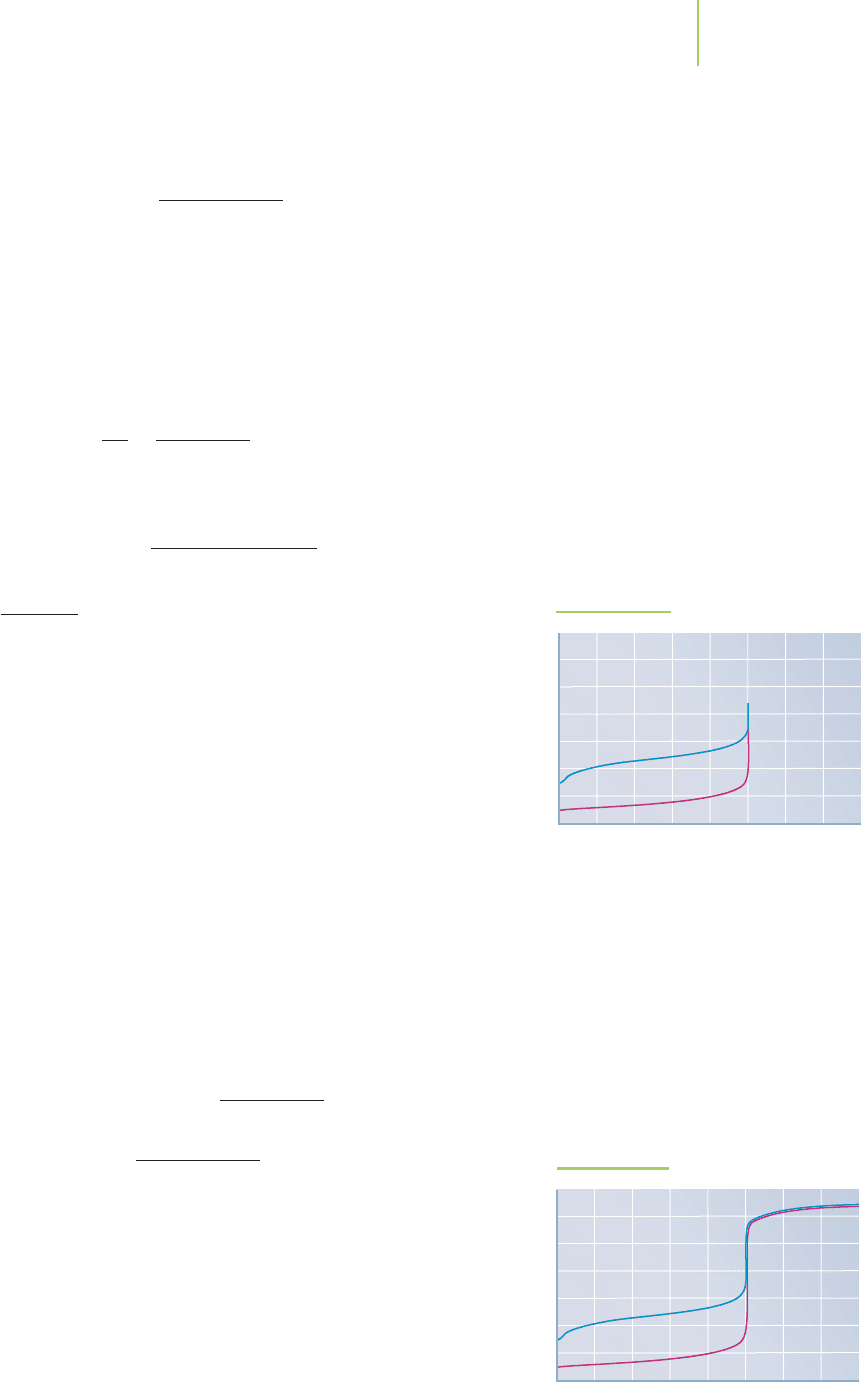
The pH of the weak base is, in fact, basic, as we would predict. Compare
this to the pH = 7 equivalence point of the strong-acid–strong-
base titration. There, only water was present at the equivalence point. In
this titration, however, we have an equivalence point solution of a weak
base. The pH for this titration did rise sharply near the equivalence point,
making it easy to determine, as shown in Figure 18.10e.
Part 6: Addition of a total of 40.00 mL of NaOH solution
We now add a 0.2000 M solution of a strong base to a very weak base. The weak
base will not be an important contributor to the total hydroxide ion concentra-
tion because it is so weak. The hydroxide ion concentration will be strictly deter-
mined by the excess moles of OH
−
and the total solution volume in which it is
contained.
excess mol OH
−
= 0.01500 L
×
(0.2000 mol)
(1.000 L)
= 0.003000 mol
[OH
−
] =
(0.003000 mol)
(0.09000 L)
= 0.03333 M
pOH = 1.48
pH = 12.52
The pH has increased sharply. Compare this data point with that from the
equivalent volume of strong base added to the strong acid in the previous
titration, shown in Figure 18.10f. They are the same! In both cases,
after the equivalence point, we added the same volume of the same con-
centration of strong base.
18.2 Acid–Base Titrations 795
0
2
4
6
8
10
12
14
0 5 10 15 20 25 30 35 40
pH
Strong acid,
strong base
Weak acid,
strong base
NaOH added (mL)
0
2
4
6
8
10
12
14
0 5 10 15 20 25 30 35 40
pH
Strong acid,
strong base
Weak acid,
strong base
NaOH added (mL)
FIGURE 18.10e
FIGURE 18.10f

0.2000 M NaOH added (mL)
10 20 30 40 50 60
2.0
4.0
6.0
8.0
10.0
12.0
0
K
a
= 10
–
2
K
a
= 10
–
4
K
a
= 10
–
6
K
a
= 10
–
8
K
a
= 10
–
10
Strong acid
pH
FIGURE 18.11
This plot shows the relationship between
K
a
value for 50.00 mL of a 0.2000 M acid
and the change in pH at the equivalence
point when titrated with a 0.2000 M
NaOH solution. Notice two items in the
plot. First, the concentration of the acid,
not its relative strength, determines the
equivalence point. Second, the larger the
K
a
value, the sharper the pH break at
the equivalence point.
Summarizing the Key Ideas of the
Weak-Acid–Strong-Base Titration Discussion
There are four key areas that define the titration curve and the information it
yields.
Initial pH: This will be closer to neutral for weaker acids, further away for
stronger acids.
Buffer region: This is where we have generated enough conjugate base, while
still having the weak acid available to produce a buffer. This part of the titra-
tion curve will have a gently sloping pH until quite near the equivalence
point. An important point in the buffer region is the titration midpoint (pH
= pK
a
). There is an inflection point at the titration midpoint that we do not
see with strong-acid–strong-base titrations (compare Figures 18.9 and 18.11
in this regard).
Equivalence point: The starting weak acid has been exactly neutralized, and the
solution is a weak base. The stronger the acid (the larger the K
a
value), the
sharper will be the break at the equivalence point. Figure 18.11 shows that when
the K
a
value of the weak acid is too small, the pH break at the equivalence point
is too small to be of practical use.
Post-equivalence point: Excess strong base sharply raises the pH of the system.
EXERCISE 18.9 Titrating a Weak Base with a Strong Acid
To further compare systems, calculate the pH values and draw the curve for the
titration of 50.00 mL of 0.1000 M ammonia with 0.2000 M of hydrochloric acid
using the same volumes of titrant as in our previous titrations:
a. 0 mL d. 24.00 mL
b. 5.00 mL e. 25.00 mL
c. 12.50 mL f. 40.00 mL
Solution
a. Initial pH. The important reaction is the hydrolysis of ammonia.
NH
3
(aq) + H
2
O(l)
NH
4
+
(aq) + OH
−
(aq) K
b
= 1.8
×
10
−5
K
b
=
[NH
4
+
][OH
−
]
[NH
3
]
1.8
×
10
−5
=
x
2
0.1000
x = [OH
−
] = 1.34
×
10
−3
M
pOH = 2.87
pH = 11.13
b. Addition of 5.00 mL of HCl solution. The system is thrown out of equilibrium
by the addition of the HCl solution, much as the acetic acid system had to
compensate for the addition of NaOH. We will generate a buffer, shown in the
table.
796 Chapter 18 Applications of Aqueous Equilibria
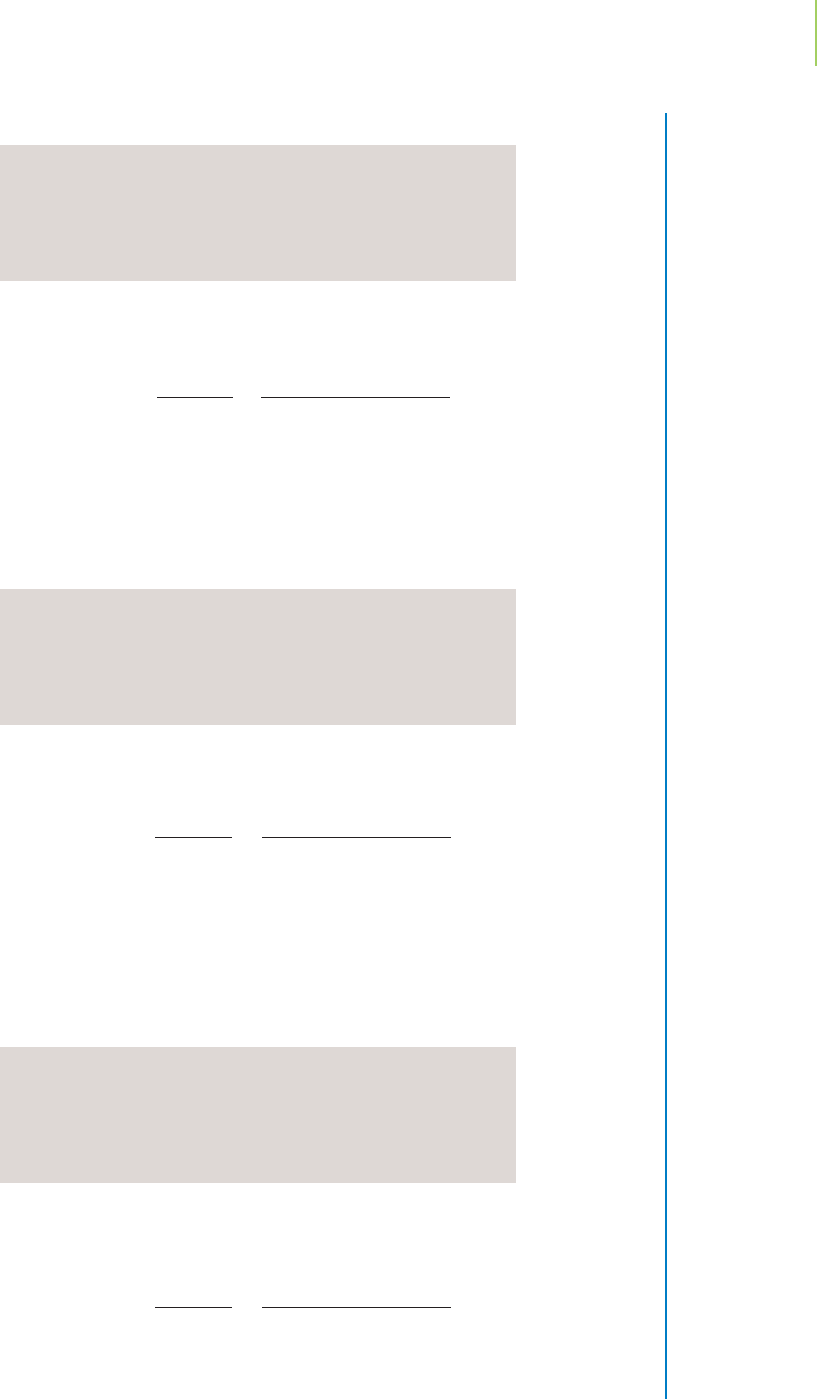
NH
3
(aq) + H
+
(aq)
NH
4
+
(aq) K
b
= 1.8
×
10
9
moles initial 0.005000
≈
0
moles added 0.001000
change −0.001000 −0.001000 +0.001000
moles at equilibrium 0.004000
≈
0 0.001000
When the system returns to equilibrium,
NH
3
(aq)
NH
4
+
(aq) + OH
−
(aq) K
b
= 1.8
×
10
−5
[OH
−
] =
K
b
[NH
3
]
[NH
4
+
]
=
(1.8 ×10
−5
)(0.004000)
(0.001000)
= 7.2
×
10
−5
M
pOH = 4.14
pH = 9.86
c. Addition of 12.50 mL of HCl solution. We can generate the following table and
solve for pH.
NH
3
(aq) + H
+
(aq)
NH
4
+
(aq) K
b
= 1.8
×
10
9
moles initial 0.005000
≈
0
moles added 0.002500
change −0.002500 −0.002500 +0.002500
moles at equilibrium 0.002500
≈
0 0.002500
When the system returns to equilibrium,
NH
3
(aq)
NH
4
+
(aq) + OH
−
(aq) K
b
= 1.8
×
10
−5
[OH
−
] =
K
b
[NH
3
]
[NH
4
+
]
=
(1.8 ×10
−5
)(0.002500)
(0.002500)
= 1.8
×
10
−5
M
pOH = pK
b
= 4.74
pH = 9.26
d. Addition of a total of 24.00 mL of HCl solution. We are nearing the equivalence
point. How do the various titration curves, shown in Figure 18.12 on page 798,
compare?
NH
3
(aq) + H
+
(aq)
NH
4
+
(aq) K = 1.8
×
10
9
moles initial 0.005000
≈
0
moles added 0.004800
change −0.004800 −0.004800 +0.004800
moles at equilibrium 0.000200
≈
0 0.004800
When the system returns to equilibrium,
NH
3
(aq)
NH
4
+
(aq) + OH
−
(aq) K = 1.8
×
10
−5
[OH
−
] =
K
b
[NH
3
]
[NH
4
+
]
=
(1.8 ×10
−5
)(0.000200)
(0.004800)
= 7.5
×
10
−7
M
pOH = 6.12
pH = 7.87
18.2 Acid–Base Titrations 797
