Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

0
2
4
6
8
10
12
14
0 5 10 15 20 25 30
Volume added (mL)
pH
Strong base added
to strong acid
Strong base added
to weak acid
Strong acid added
to weak base
Strong acid added
to strong base
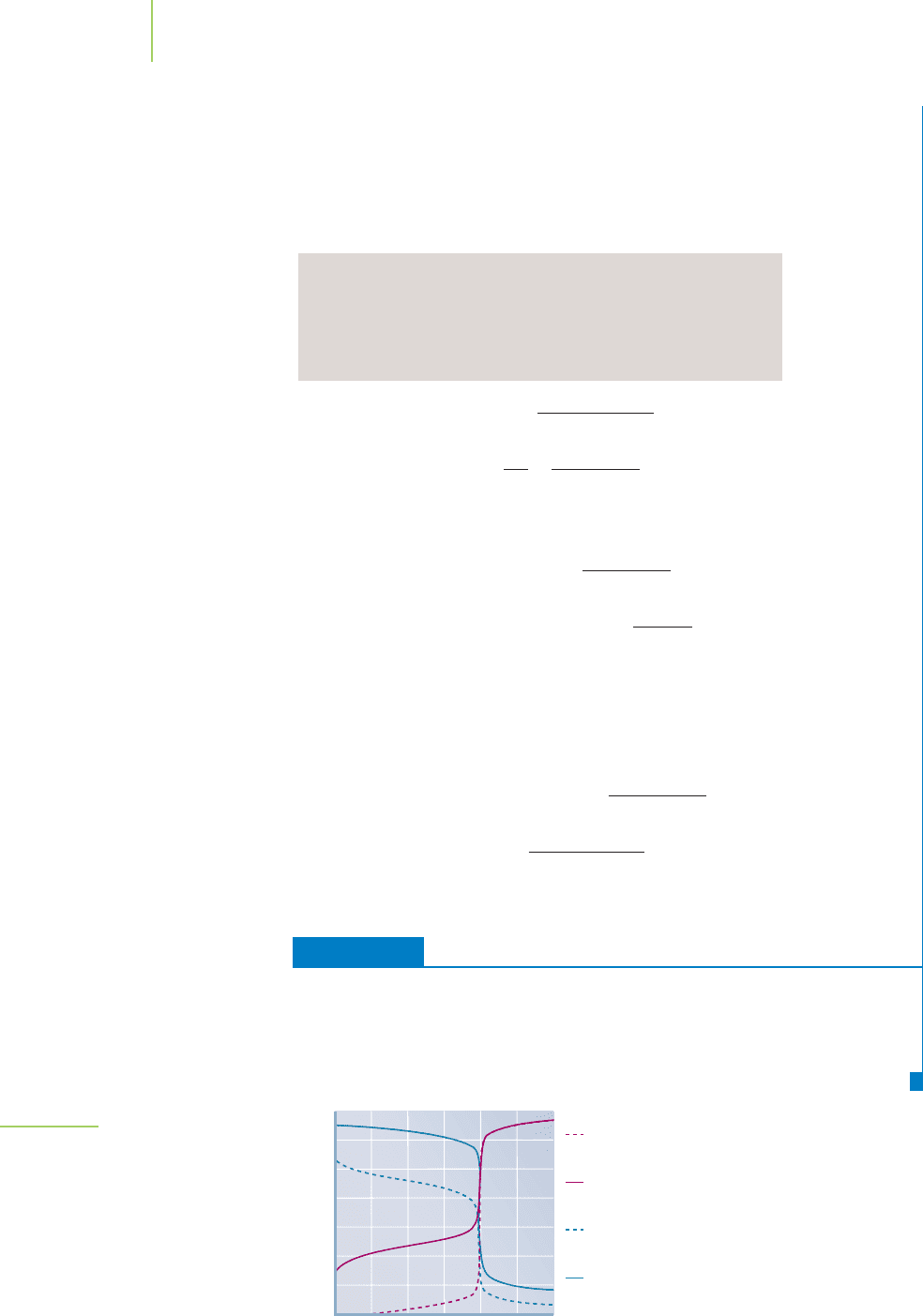
FIGURE 18.12
This plot shows a comparison of
the pH for strong-acid–strong-base,
weak-acid–strong-base, and weak-
base–strong-acid titrations using equal
concentrations of acid and base.
e. Addition of a total of 25.00 mL of HCl solution. We are at the equivalence point,
at which we have added exactly the same number of moles of HCl as there
were moles of ammonia at the start of the titration. We now have a solution
that is weakly acidic as a consequence of the conversion of ammonia to the
ammonium ion.
NH
3
(aq) + H
+
(aq)
NH
4
+
(aq) K =1.8
×
10
9
moles initial 0.005000
≈
0
moles added 0.005000
change −0.005000 −0.005000 +0.005000
moles at equilibrium
≈
0
≈
0 0.005000
[NH
4
+
] =
(0.005000 mol)
(0.07500 L)
= 0.06667 M
K
a
=
K
w
K
b
=
1.0 ×10
−14
1.8 ×10
−5
= 5.6
×
10
−10
We can now solve for the pH of the weak acid.
K
a
=
[H
+
][NH
3
]
[NH
4
+
]
5.6
×
10
−10
=
x
2
0.06667
x = [NH
3
] = [H
+
] = 6.1
×
10
−6
M
pH = 5.21
f. Addition of a total of 40.00 mL of HCl solution.
mol excess H
+
= 0.01500 L
×
(0.2000 mol)
(1.000 L)
= 0.003000 mol
[H
+
] =
(0.003000 mol)
(0.09000 L)
= 0.03333 M
pH = 1.48
PRACTICE 18.9
Calculate the pH values and draw the curve for the titration of 25.00 mL of
0.2500 M acetic acid with 0.2500 M sodium hydroxide using the same volumes of
titrant as in our previous titrations: a. 0 mL; b. 5.00 mL; c. 12.50 mL; d. 24.00 mL; e.
25.00 mL; f. 40.00 mL. K
a
(acetic acid) = 1.8 × 10
−5
See Problems 35, 36, and 38.
798 Chapter 18 Applications of Aqueous Equilibria
OH
OH
+
C
HO
2
C
–
O
2
C
pH
Orange-red
(formed in 65–98% H
2
SO
4
)
Colorless Pink
Colorless
(formed above pH 11)
<–1
–1 8.5
8.5 11 >11
OH
OHC
O HO
O
O
–
OC
–
O
2
C
O
–
O
–
C
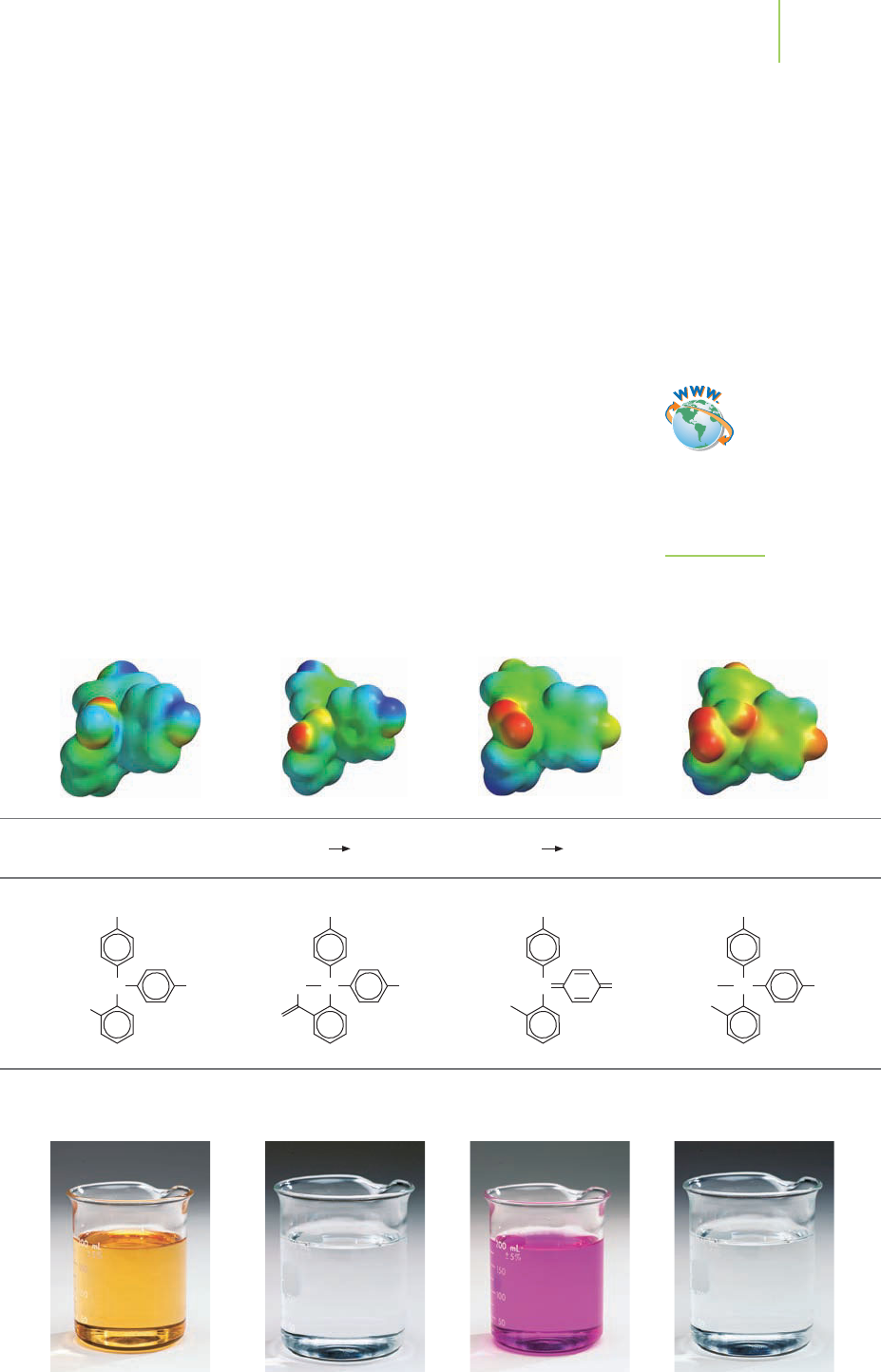
FIGURE 18.13
The structure of phenolphthalein
changes as we increase the pH
from very low to very high.
We have seen that a successful monoprotic acid–base titration has an analyte
(what you are titrating) that, when titrated with a strong acid or strong base, has
a large K. The reaction is typically fast and reproducible. Polyprotic acid and base
titrations are common, and the analysis of their titration curves presents inter-
esting challenges, although we will not deal with these here.
Indicators
The pH meter, discussed in Chapter 19, can provide very accurate readings of the
pH. Making successful measurements using a pH meter, however, relies on its
having been properly maintained, calibrated, and operated. For speed and
simplicity in a titration, the chemical technician often relies on a chemical pH
reporter. This class of compounds, known as
acid–base indicators or pH indicators,
visually indicates the change in pH as we approach, reach, and pass the equiva-
lence point. Indicators themselves are conjugate acid–base pairs of organic
molecules that change color as they change between their acid and base forms.
Perhaps the best-known example is phenolphthalein, which changes from color-
less to rose-pink as the pH changes from 8.5 to 9.5. Figure 18.13 shows that
phenolphthalein actually has several structural changes (and therefore color
changes) from very low to very high pH.
Why is phenolphthalein such a popular acid–base indicator? Its color change
occurs in the pH region where many acids titrated with strong bases reach their
18.2 Acid–Base Titrations 799
Video Lesson: Acid–Base
Indicators
Video Lesson: CIA
Demonstration: Natural
Acid–Base Indicators
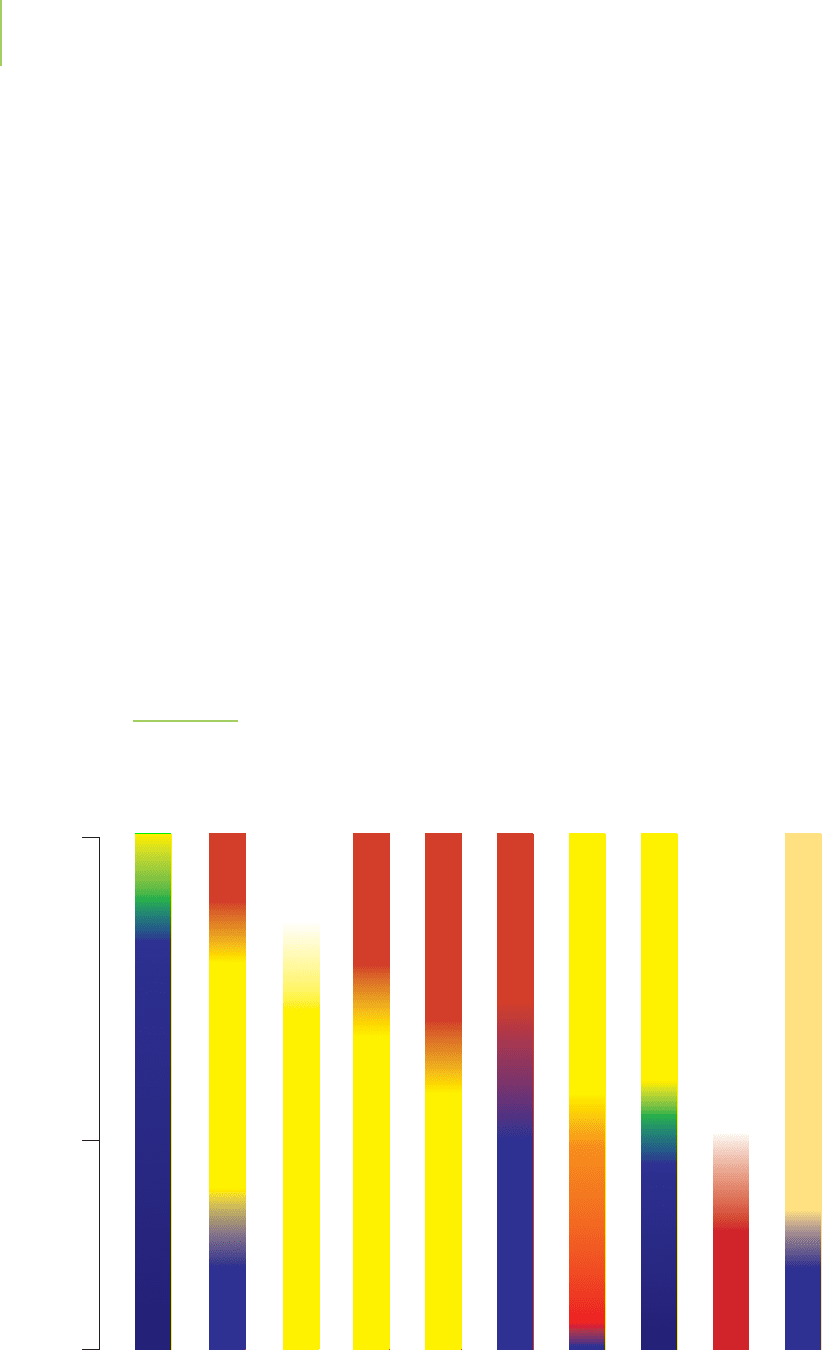
equivalence point. For example, acetic acid has an equivalence point, at which it
is essentially all changed to acetate ion, at about pH 9, and the pH goes from
about 6 to 11 within a small volume of titrant on either side of the equivalence
point. The titration of a strong acid with base (or vice versa) changes very rapidly
between the region of pH 6 to 11. An indicator that itself changes color anywhere
in this area would, generally speaking, be a suitable reporter of the equivalence
point.
In a given titration, how do we know which acid–base indicator to choose? We
want to choose an indicator with a pK
a
as close as possible to the pH at the equiv-
alence point. A distinct color change is also useful. For example, the pK
a
of
phenolphthalein is about 9.5. This is well within the region of the pH at the
equivalence point of titration of acetic acid by sodium hydroxide (pH ≈ 9, de-
pending on acetic acid concentration). As a rule of thumb, the color changes of pH
indicators are visible to
+/−1 pH unit on either side of the indicator pK
a
. This
means that phenolphthalein will be completely colorless below a pH of 8.5 and
will then be rose-pink from pH 8.5 to about pH 11. It will turn colorless again
above pH 11. A selection of common pH indicators, their color changes, and their
pH ranges is given in Figure 18.14.
During the chemical technician’s analysis, only a few drops of an indicator so-
lution are added to the analyte solution. Why so little? Because the indicator also
undergoes an acid–base reaction. This means that in addition to adding the re-
quired volume to neutralize the analyte, we add just a bit more to cause the color
change in the indicator. For example, if we titrate a solution of acetic acid
800 Chapter 18 Applications of Aqueous Equilibria
0
1
2
3
4
5
6
7
8
9
10
11
12
Methyl violet
Thymol blue
Bromphenol blue
Methyl orange
Methyl red
Eriochrome black T
Alizarin
Bromthymol blue
Phenolphthalein
Alizarin yellow R
pH
FIGURE 18.14
A selection of common pH indicators, their color changes, and their pH ranges. Our key criterion in se-
lecting the proper indicator is that its pK
a
should be as close as possible to the equivalence point of the
titration in which it is used. Note that most indicators exhibit a color change over less than 2 pH units.
containing phenolphthalein indicator, we might need 35.27 mL of a sodium hy-
droxide solution to react with the acetic acid itself. Changing the indicator’s
structure (and therefore its color) might require an additional 0.02 mL of the
strong base. The equivalence point is at 35.27 mL, but the point at which you see
the change in indicator color that tells you the titration is finished, which is called
the
titration endpoint, is at 35.29 mL. Therefore, we would use 35.29 mL as our
number for calculating the concentration of analyte. This could lead to very large
errors if we had a lot of extra indicator in the solution. These errors are reduced
if the endpoint is as close as possible to the equivalence point.
EXERCISE 18.10 Picking an Indicator
What would be a reasonable indicator for the titration of 0.10 M ammonia with
0.10 M HCl?
Solution
The key question is “What is the pH of the solution at the equivalence point?”When
essentially all of the ammonia is converted to ammonium ion, the pH will be
around 5.2, as we saw in Exercise 18.9, part e. As shown in Figure 18.14, several in-
dicators change color around pH =5.2, and methyl red appears to be a good choice.
PRACTICE 18.10
What would be a reasonable indicator for the titration of 0.10 M NaOH with
0.10 M HCl?
See Problems 39–42.
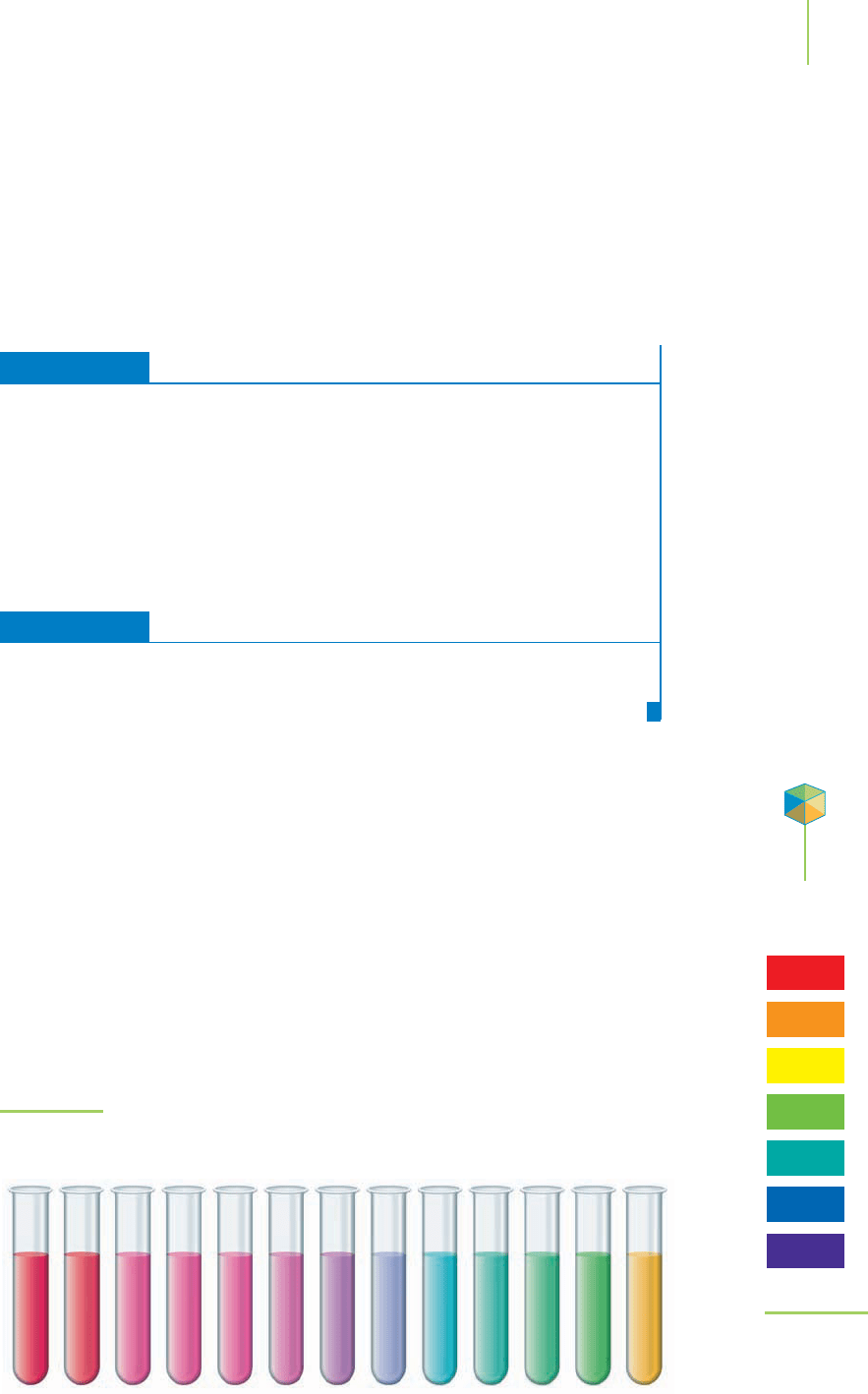
Some natural and commercially prepared indicators are made up of several
colorful organic molecules, and they change color throughout the pH range. No-
table among the natural indicators are the
anthocyanins, which are responsible for
most of the different colors found in vegetables and flowers. There are over 150
naturally occurring anthocyanins in foods such as the red cabbage that we cook
and serve with dinner. The juice from the red cabbage can therefore be used as an
indicator. Figure 18.15 shows the wide range of colors that can be obtained by ad-
justing the pH of red cabbage juice. Commercially prepared solutions of mixtures
of indicators can mimic these color changes, but far more intensely, so far less is
required. For instance, the commercially prepared “universal indicator” is a mix-
ture of thymol blue, methyl red, bromthymol blue, and phenolphthalein. Because
each indicator gains or loses protons at a different pH, the universal indicator has
color changes over a wide pH range, as shown in Figure 18.16.
18.2 Acid–Base Titrations 801
1234567
pH
8 9 10 11 12 13
FIGURE 18.15
The spectrum of colors in each sample of red cabbage juice is caused by the
changes in structure of the anthocyanins as the pH moves from 1 to 13.
pH = 4
pH = 5
pH = 6
pH = 7
pH = 8
pH = 9
pH = 10
FIGURE 18.16
A typical universal in-
dicator is a mixture of
several common
indicators.
Application
C
HEMICAL ENCOUNTERS:
Anthocyanins and
Universal Indicators
HERE’S WHAT WE KNOW SO FAR
■
Strong-acid–strong-base titrations show a relatively level pH until near the
equivalence point, where the pH rises dramatically.
■
Titration curves in which one component is weak and the other is strong con-
tain four regions, including the initial pH, the buffer region, the equivalence
point region and the post–equivalence point region.
■
The buffer region contains a point at which one-half of the analyte has been
converted to its conjugate. This is called the titration midpoint, and the pH at
this point is equal to the pK of the analyte.
■
The larger the pK of the analyte, the sharper will be the change in pH at the
equivalence point.
■
We can use an indicator to “see” the equivalence point of a titration.
■
We add only a few drops of an indicator to the titration solution so that the
equivalence point and titration endpoint can be as close together as possible.
18.3 Solubility Equilibria
The Pacific Ocean is an incredibly complex heterogeneous system. The bottom
layers of this and other massive waterways are covered with a variety of soils and
sediments, including
calcareous oozes, calcium-containing detritus from dead
single-celled, calcium-based sea life. One of the important compounds within the
oozes is calcium carbonate (CaCO
3
), some of which is in contact with ocean
water, dissociating to form calcium and carbonate ions. The equation relating
this dissociation is written so that the solid is a reactant and the dissolved ions are
products:
CaCO
3
(s)
Ca
2+
(aq) + CO
3
2−
(aq)
The mass-action expression that can be used to determine the solubility of
CaCO
3
is called the solubility product when the equation is written as shown
above. The solubility product is equal to the product of the concentrations of
the ions (remember that the CaCO
3
is a solid and is not written as part of the
mass-action expression).
K
sp
= [Ca
2+
][CO
3
2−
]
This equilibrium constant is called the solubility product constant (K
sp
) and has the
same conceptual meaning as any other equilibrium constant along with its mass-
action expression. Because the values of K
sp
tend to be very small, the concentra-
tions of ions are quite low and that activities are not important here.
Table 18.3 lists representative K
sp
values for some of the sparingly soluble
salts.
The difficulty with describing solubility using a single mass-action
expression is that there are so many other processes that enter into the
chemistry that our typically simple mass-action expression often just
won’t do. The simple calculations we can perform do not always agree with
what we observe in real systems. Let’s take a look at the calcium carbonate
system in a somewhat nonmathematical approach as we discover the
factors that affect the solubility of solids.
Side Reactions That Affect Our Reaction of Interest
The solubility of many ions, when dissolved in an aqueous system, is
affected by side reactions. The solubility of calcium carbonate in a large
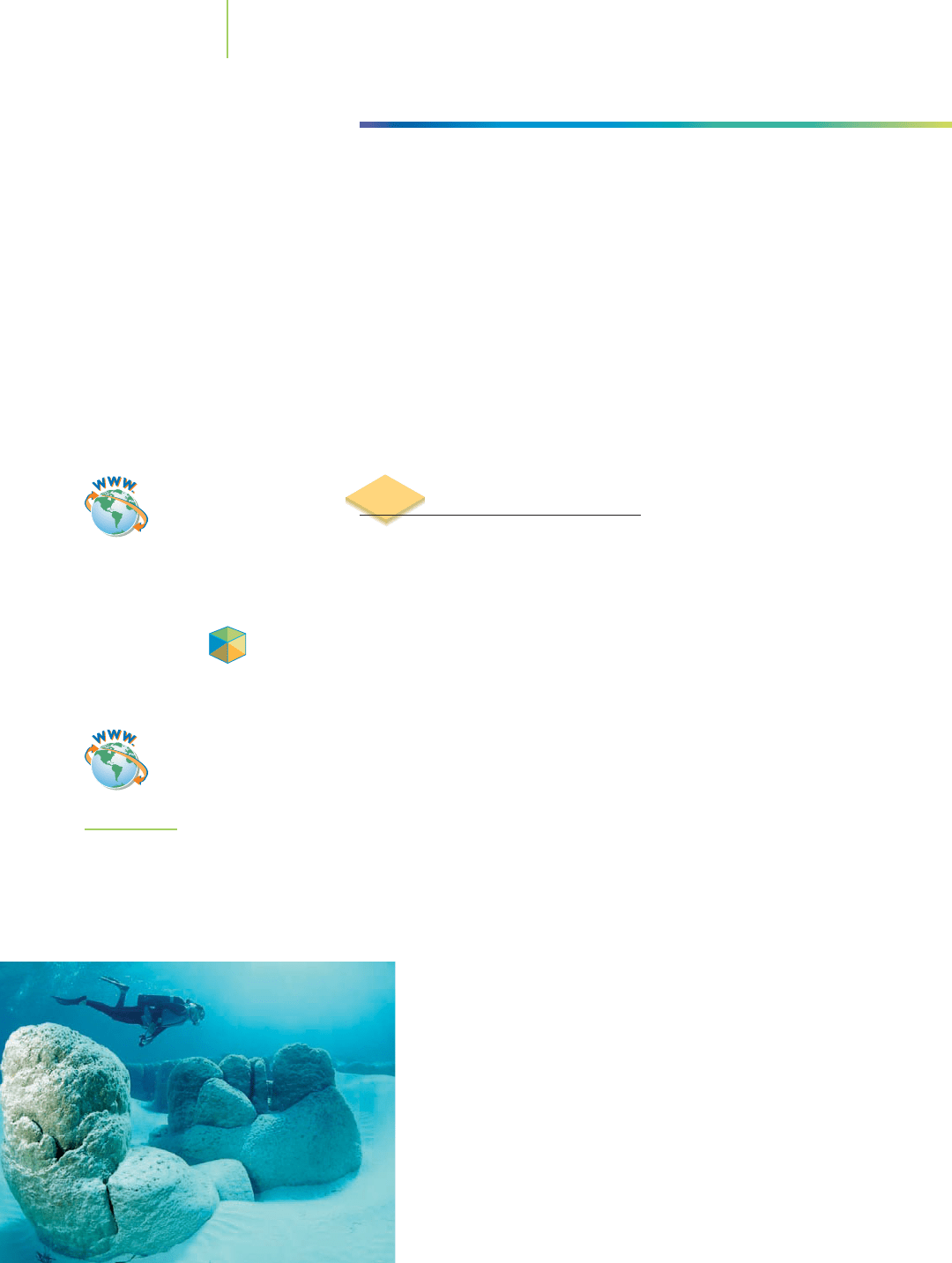
ocean-based system is no exception (Figure 18.17).
802 Chapter 18 Applications of Aqueous Equilibria
Application
FIGURE 18.17
Many processes, including the formation
of bicarbonate ion and the reaction of
hydrogen and hydroxide ions, affect the
solubility of calcium carbonate in the
ocean. These stromatolites are forma-
tions of calcium carbonate.
Video Lesson: The Effects of pH
on Solubility
Video Lesson: The Solubility
Product Constant

1. Hydrolysis of carbonate ion. The carbonate ion formed from the dissolution of
calcium carbonate is a base that reacts with water to form bicarbonate ion
and hydroxide ion.
K
b
1
CO
3
2−
(aq) + H
2
O(l) HCO
3
−
(aq) + OH
−
(aq)
Because the carbonate ion is involved in this reaction, some of it is removed
from the calcium carbonate solubility equilibrium. The net result, in accor-
dance with Le Châtelier’s principle, is that more of the calcium carbonate dis-
solves than we would predict.
2. The interplay between the atmosphere and the ocean water. Dissolved CO
2
from
the air mixes with ocean water to form carbonic acid and, ultimately, hydro-
gen ion and bicarbonate ion.
K
K
a
1
CO
2
(aq) + H
2
O(l)H
2
CO
3
(aq)H
+
(aq) + HCO
3
−
(aq)
18.3 Solubility Equilibria 803
Selected K
sp
Values at 25°C
Ionic Solid K
sp
(at 25°C) Ionic Solid K
sp
(at 25°C) Ionic Solid K
sp
(at 25°C)
Fluorides Hg
2
CrO
4
*2
×
10
−9
Co(OH)
2
2.5
×
10
−16
BaF
2
2.4
×
10
−5
BaCrO
4
8.5
×
10
−11
Ni(OH)
2
1.6
×
10
−16
MgF
2
6.4
×
10
−9
Ag
2
CrO
4
9.0
×
10
−12
Zn(OH)
2
4.5
×
10
−17
PbF
2
4
×
10
−8
PbCrO
4
2
×
10
−16
Cu(OH)
2
1.6
×
10
−19
SrF
2
7.9
×
10
−10
Hg(OH)
2
3
×
10
−26
CaF
2
4.0
×
10
−11
Carbonates Sn(OH)
2
3
×
10
−27
NiCO
3
1.4
×
10
−7
Cr(OH)
3
6.7
×
10
−31
Chlorides CaCO
3
8.7
×
10
−9
Al(OH)
3
2
×
10
−32
PbCl
2
1.6
×
10
−5
BaCO
3
1.6
×
10
−9
Fe(OH)
3
4
×
10
−38
AgCl 1.6
×
10
−10
SrCO
3
7
×
10
−10
Co(OH)
3
2.5
×
10
−43
Hg
2
Cl
2
* 1.1
×
10
−18
CuCO
3
2.5
×
10
−10
ZnCO
3
2
×
10
−10
Sulfides
Bromides MnCO
3
8.8
×
10
−11
MnS 2.3
×
10
−13
PbBr
2
4.6
×
10
−6
FeCO
3
2.1
×
10
−11
FeS 3.7
×
10
−19
AgBr 5.0
×
10
−13
Ag
2
CO
3
8.1
×
10
−12
NiS 3
×
10
−21
Hg
2
Br
2
* 1.3
×
10
−22
CdCO
3
5.2
×
10
−12
CoS 5
×
10
−22
PbCO
3
1.5
×
10
−15
ZnS 2.5
×
10
−22
Iodides MgCO
3
1
×
10
−15
SnS 1
×
10
−26
PbI
2
1.4
×
10
−8
Hg
2
CO
3
* 9.0
×
10
−15
CdS 1.0
×
10
−28
AgI 1.5
×
10
−16
PbS 7
×
10
−29
Hg
2
I
2
* 4.5
×
10
−29
Hydroxides CuS 8.5
×
10
−45
Ba(OH)
2
5.0
×
10
−3
Ag
2
S 1.6
×
10
−49
Sulfates Sr(OH)
2
3.2
×
10
−4
HgS 1.6
×
10
−54
CaSO
4
6.1
×
10
−5
Ca(OH)
2
1.3
×
10
−6
Ag
2
SO
4
1.2
×
10
−5
AgOH 2.0
×
10
−8
Phosphates
SrSO
4
3.2
×
10
−7
Mg(OH)
2
8.9
×
10
−12
Ag
3
PO
4
1.8
×
10
−18
PbSO
4
1.3
×
10
−8
Mn(OH)
2
2
×
10
−13
Sr
3
(PO
4
)
2
1
×
10
−31
BaSO
4
1.5
×
10
−9
Cd(OH)
2
2.5
×
10
−14
Ca
3
(PO
4
)
2
1.3
×
10
−32
Pb(OH)
2
1.2
×
10
−15
Ba
3
(PO
4
)
2
6
×
10
−39
Chromates Fe(OH)
2
1.8
×
10
−15
Pb
3
(PO
4
)
2
1
×
10
−54
SrCrO
4
3.6
×
10
−5
TABLE 18.3
*Contains Hg
2
2
+
ions. K
=
[Hg
2
2
+
][X
−
]
2
for Hg
2
X
2
salts, for example.
This interaction generates the bicarbonate ion, which influences the equilib-
rium shown at the bottom of page 803. The net result is to reduce the effect of
the interaction of carbonate ions with water. The exact change depends on the
amount of carbon dioxide dissolved in seawater.
3. The formation of water from the reaction of hydrogen and hydroxide ions. These
are produced from the processes described in reactions 1 and 2.
1/K
w
H
+
(aq) + OH
−
(aq)H
2
O(l)
The effect of this reaction is an increase in the concentration of the bicarbon-
ate ion generated from the carbon dioxide equilibrium.
As you can see, these three equilibria interact with others in the ocean as part
of a remarkably complex system in which temperatures and concentrations
change, making the calculation of calcium carbonate solubility at a given tem-
perature most challenging.
Shifting our focus from the oceans to a more controlled setting, we find that
side reactions can still confound apparently simple systems. Consider the solubil-
ity of lead(II) iodide (PbI
2
) in distilled water. The amount of precipitated
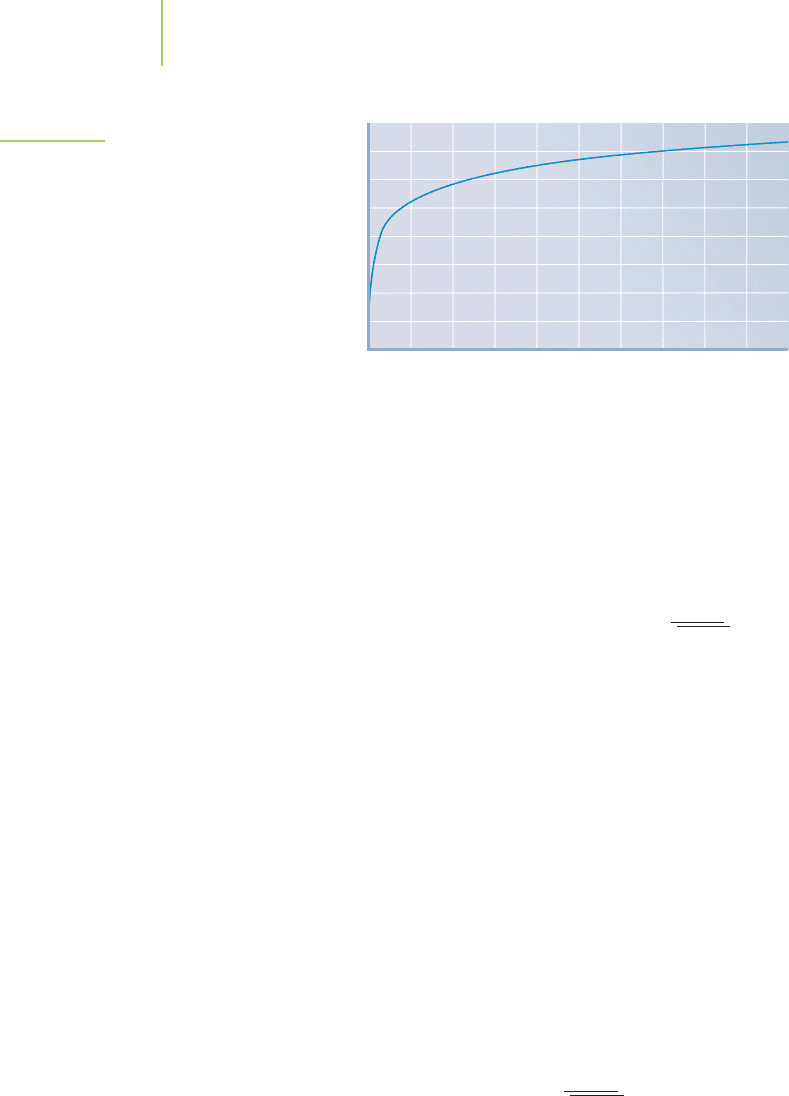
lead(II) iodide is related to the initial concentration of iodide as shown in Fig-
ure 18.18. Not accounting for these changes can lead to massive errors in calculating
the solubility of lead iodide.
Molecular-Level Processes
We can take a simple view of the solubility of a salt such as calcium sulfate by con-
sidering only the dissociation reaction:
K
sp
CaSO
4
(s)Ca
2+
(aq) + SO
4
2−
(aq)
Our mass-action expression is
K
sp
= [Ca
2+
][ SO
4
2−
]
However, Meites, Pode, and Thomas wrote as early as 1966 that even in the ab-
sence of side reactions, the concentrations we would calculate would be wrong by
about 60%, largely because of the tendency of the calcium and sulfate ions to stay
together as individual
ion pairs. When the small amount of calcium sulfate
dissolves, most of it does not form individual ions. Rather, the ions associate in-
timately with each other. This is especially important in salts of highly charged
(±2 or ±3) cations and anions. The bottom line is that even in the absence of side
reactions, such as the addition of H
+
to SO
4
2−
to make HSO
4
−
in acidic solution,
there are several factors that affect solubility at the molecular level, making many
such calculations challenging. These factors include
804 Chapter 18 Applications of Aqueous Equilibria
0
–1
–2
–3
–4
–5
–6
–7
–8
0 0.05 0.1 0.15 0.2 0.25 0.3 0.35 0.4 0.45 0.5
[I
–
]
Log [Pb
2+
]
FIGURE 18.18
Even a system that seems as simple as
the solubility of lead(II) iodide isn’t. Most
of the Pb
2
+
ions precipitate upon the ad-
dition of a small amount of iodide. How-
ever, a significant concentrate of Pb
2
+
remains.
1. Formation of ion pairs, as mentioned in the previous paragraph
2. Ion activities, a measure of the effective concentration of ions in solution
3. Thermodynamic measures, including enthalpy and entropy changes in the
solution process
Some simple univalent (both cation and anion singly charged) systems do
give reasonable answers when we do solubility calculations. In these cases, and
others as well, the total number of moles of solute that dissolve per liter of solu-
tion is often called the
molar solubility. For example, we can calculate the molar
solubility of silver bromide (AgBr) by using its solubility product constant and
mass-action expression.
AgBr(s)
Ag
+
(aq) + Br
−
(aq) K
sp
= 5.0
×
10
−13
K
sp
= [Ag
+
][Br
−
]
We can set up our table, as we’ve done before. Although the solid AgBr will not
enter into the mass-action expression, we’ll include it in the K
sp
ICE tables for the
reasons mentioned below. In any case, the number of moles of silver bromide that
will dissolve into solution and the number of moles of silver and bromide ions
produced will all be equal, because there is a 1-to-1-to-1 mole ratio in the reac-
tion. We can designate the molar solubility of AgBr as s, in which case the equi-
librium concentrations, [Ag
+
] and [Br
−
], will also be s.
AgBr(s)
Ag
+
(aq) + Br
−
(aq)
initial —
change −s +s +s
equilibrium — ss
K
sp
= [Ag
+
][Br
−
]
5.0
×
10
−13
= s
2
s = 7.1
×
10
−7
M = [Ag
+
] = [Br
−
]
s = molar solubility of AgBr =7.1
×
10
−7
M
Qualitatively, what would we expect to happen to the solubility if we added a
little sodium bromide, in which the added bromide is a common ion? According
to Le Châtelier’s principle, addition of an ion common to the product would push
the dissociation reaction back to the left, further decreasing the solubility. Cau-
tion: If we add too much bromide ion, the solubility of silver bromide could actu-
ally increase, as a consequence of the formation of soluble species such as AgBr
2
−
.
EXERCISE 18.11 How Much Dissolves?
One method of analyzing groundwater for nitrate requires that the chloride ions in
the water sample be removed first.This is typically done by adding a solution of silver
ions (Ag
+
) in order to precipitate the sparingly soluble silver chloride salt (AgCl).
What silver ion concentration is present when silver chloride is added to water?
AgCl(s)
Ag
+
(aq) + Cl
−
(aq) K
sp
= 1.6 × 10
−10
Solution
Setting up the table, we get
AgCl(s)
Ag
+
(aq) + Cl
−
(aq)
initial — 00
change −s +s +s
equilibrium — ss
18.3 Solubility Equilibria
805
Application
Then, as usual, we can find our equilibrium concentrations by solving the mass-
action expression:
K
sp
= [Ag
+
][Cl
−
]
1.6
×
10
−10
= (s)(s) = s
2
s = 1.3
×
10
−5
M
PRACTICE 18.11
Calculate the molar solubility of barium fluoride (BaF
2
), K
sp
= 2.4
×
10
−5
.
See Problems 49 and 50.
EXERCISE 18.12 Calculating K
sp
The concentration of calcium ions in a saturated solution of calcium fluoride was
found to be 2.15
×
10
−4
M. What is the apparent value for the solubility product
constant, K
sp
?
First Thoughts
This problem is asking the same question as the previous exercise, but in the reverse
direction. The problem gives us the molar solubility of calcium ions, so we’ll exam-
ine the equilibrium expression to determine how to calculate K
sp
.
Solution
The equilibrium under consideration is
CaF
2
(s)
Ca
2+
(aq) + 2F
−
(aq)
The mole ratio of Ca
2+
to F
−
is 1-to-2, so if the equilibrium concentration of
Ca
2+
is 2.15 × 10
−4
, that of F
−
must be twice as large, or [F
−
] = 4.30 × 10
−4
.We
can substitute these values into the mass-action expression.
K
sp
= [Ca
2+
][F
−
]
2
K
sp
= (2.15
×
10
−4
) (4.30
×
10
−4
)
2
= 3.98
×
10
−11
Further Insight
The question asked us to calculate the apparent K
sp
value. This was done because
there may be some side reactions, activity considerations, or other factors that affect
the molar solubility of the calcium fluoride salt. In particular, we have neglected the
fact the fluoride ion (F
–
) is a Brønsted base, which will affect the solubility of the
calcium fluoride. (Can you propose how?) In this case, we’ve calculated a value for
K
sp
that is similar to the actual value, K
sp
= 4.00
×
10
−11
.
PRACTICE 18.12
Calculate the apparent value of K
sp
for lead bromide (PbBr
2
) if the concentration of
bromide in a saturated solution is 2.1
×
10
−2
M.
See Problems 53 and 54.
Solubility, Precipitation, and Gravimetric Analysis
Chemical technicians can determine the concentration of substances in solution
by causing them to form insoluble salt precipitates and weighing these precipitates
or their related solids in a technique called
gravimetric analysis. The quantitation
of a sample on the basis of its mass is among the most powerful tools at the dis-
posal of chemical technicians because good balances are both highly accurate and
806 Chapter 18 Applications of Aqueous Equilibria
Video Lesson: Solubility and the
Common Ion Effect
Video Lesson: Gravimetric
Analysis

precise, and weighing a sample is fast and inexpensive. For exam-
ple, the amount of chloride in a sample is routinely determined by
combining the chloride ion with silver ion, forming the solid silver
chloride as described in the Exercise 18.11.
Cl
−
(aq) +Ag
+
(aq)
AgCl(s)
Other substances can be routinely determined by gravimetric
analysis. Aluminum ion concentrations can be found via the for-
mation of aluminum hydroxide (Al(OH)
3
). Igniting (driving off
water at high temperature) the aluminum hydroxide forms alu-
minum oxide (Al
2
O
3
), which can then be weighed. Aluminum can
also be determined by reaction with 8-hydroxyquinoline
(C
9
H
7
ON) to form Al(C
9
H
6
ON)
3
without subsequent ignition.
Al
3+
(aq) + 3C
9
H
7
ON(aq)
Al(C
9
H
6
ON)
3
(s) + 3H
+
(aq)
The solid forms good crystals that can be weighed after drying.
Sulfur can be determined by reaction of the sulfate (SO
4
2−
)
with barium ion to form barium sulfate:
SO
4
2−
(aq) + Ba
2+
(aq)
BaSO
4
(s)
Calcium concentrations can be measured by reaction to form
calcium oxalate (CaC
2
O
4
).
Ca
2+
(aq) + C
2
O
4
2−
(aq)
CaC
2
O
4
(s)
In each of these analyses there are complicating factors, such as the presence
of other elements that can react with the precipitating agents, as well as the com-
plex nature of the precipitation process, so the procedures are a bit more involved
than the simple reactions suggest. In fact, acid–base and other equilibria are
nearly always a vital part of chemistry. Despite all of these concerns, a host of
elements can be determined via precipitation. Understanding solubility equilib-
ria and how we can affect them makes the analyses all the more meaningful.
Precipitation is also used in
metal recovery, in which dissolved metals are re-
claimed from processing wastes. Many metals in industrial effluents (runoff from
manufacturing) are worth recovering because they are environmental hazards or
they waste finite metal resources. Recycling these metals saves money and the
environment. Such metals, which include copper, mercury, lead, and zinc, are
typically recovered as their sulfide salts, although some metals can be precipitated
as their corresponding carbonate salt.
To Precipitate or Not to Precipitate
Often, the formation of a precipitate is not as obvious as simply mixing two solu-
tions containing ions that form a sparingly soluble salt. Let’s say our chemical
technician is interested in mixing two solutions, one containing silver ions and
one containing chloride ions, so that the final concentrations are [Ag
+
] = 1.0 ×
10
−4
M and [Cl
−
] = 1.0 × 10
−4
M. Will mixing these solutions cause the forma-
tion of the insoluble AgCl? To answer this question, we can perform a calculation
using our mass-action expression. However, because the concentrations do not
reflect equilibrium conditions, we will be calculating the reaction quotient (recall
this from Section 16.5) related to the solubility product. We’ll call this Q
sp
.
AgCl(s)
Cl
−
(aq) +Ag
+
(aq) K
sp
= 1.6
×
10
−10
Q
sp
= [Ag
+
]
0
[Cl
−
]
0
Q
sp
= (1.0
×
10
−4
M) (1.0
×
10
−4
M) = 1.0
×
10
−8
18.3 Solubility Equilibria 807
The concentration of aluminum in solution, like many
metals, can be determined by gravimetric analysis. Here,
aluminum is reacted to form the 8-hydroxyquinoline salt,
which is made pure by recrystallization and weighed.
Application
