Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

Sørenson’s “new” pH term, taking the negative log of both sides of Henderson’s
equation to give
pH = –log(K
a
) – log
[weak acid]
[conjugate base]
This can be slightly modified by using pK
a
(the same as –log(K
a
)) and changing
the sign before the log term, which inverts the concentrations within the log
term. This gives the final form of what is commonly known as the
Henderson–
Hasselbalch equation
:
pH = pK
a
+ log
[conjugate base]
[weak acid]
The equation is not necessary; we can solve any buffer problem without it, in-
cluding the ammonia–ammonium ion system and the KHP buffer system we dis-
cussed earlier in this section. However, scientists in the medical and biological
professions use the equation because it can be a timesaver for calculating the pH.
We added the phrase “we proceed, but with caution” to the title of this sub-
section because, in spite of how common the use of the Henderson–Hasselbalch
equation is, there are two reasons why you must be cautious with its use. First, a
system must contain both an acid and its conjugate base in order for the equation
to be valid. If you don’t have a buffer system, using this equation will lead you to
incorrect results. Second, and more troublesome for many students, is the ten-
dency to invert the ratio of acid to base within the log term. Proceed with caution!
EXERCISE 18.5 The Henderson–Hasselbalch Equation
Use the Henderson–Hasselbalch equation to find the ratio of conjugate base to weak
acid in an acetic acid–acetate buffer solution with a pH of 5.0. K
a
= 1.8 × 10
−5
.
Solution
pH = pK
a
+ log
[base]
[acid]
5.0 = 4.74 + log
[CH
3
COO
−
]
[CH
3
COOH]
0.26 = log
[CH
3
COO
−
]
[CH
3
COOH]
10
0.26
=
[CH
3
COO
−
]
[CH
3
COOH]
= 1.8
This means there is 1.8 times as much acetate ion as acetic acid in a solution with a
pH just above the pK
a
.
PRACTICE 18.5
Determine the pH of an acetic acid–acetate buffer containing 0.500 M acetic acid
and 0.250 M sodium acetate. K
a
= 1.8 × 10
−5
.
See Problems 19–22.
778 Chapter 18 Applications of Aqueous Equilibria
The conclusion to Exercise 18.5 is important, because it says that in order for
the pH of the buffer to be higher than the pK
a
, there must be more conjugate
base than weak acid. The opposite statement is also true (as illustrated in Prac-
tice 18.5); that is, pH values lower than the pK
a
value require more conjugate acid
than base. Take a quick look back at Exercises 18.1 through 18.3 to see that this is
so. In Exercise 18.2, the concentrations of the weak acid and conjugate base are
equal. When this is so, the pH is equal to the pK
a
, and the buffer that results has
the greatest possible capacity to neutralize strong acids and bases.
Buffer Capacity
The job of the chemical technician may include monitoring the emissions from
a smoke stack. Recent laws enacted to protect the environment have made this a
priority. To reduce the emissions, businesses often make use of a scrubber at-
tached to the smoke stack.
Scrubbing is used to remove sulfur dioxide from the
smoke emitted by combustion of sulfur-rich coal. Chemically, the process can be
accomplished by
flue-gas desulfurization, in which a limestone slurry is combined
with sulfur dioxide in a multistep process:
CaCO
3
(s) + SO
2
(g)
CaSO
3
(s) + CO
2
(g)
The calcium sulfite that is formed is further oxidized and hydrated to give gyp-
sum (CaSO
4
·2H
2
O), which is used to make wallboards for home construction, as
discussed in Chapter 17.
CaSO
3
(s) +
1
⁄
2O
2
(g) + 2H
2
O(l)
CaSO
4
· 2H
2
O
To make this conversion of sulfur dioxide to calcium sulfate dihydrate more
efficient, a mixture of formic acid (HCOOH) and sodium formate (HCOONa) is
added to the scrubber. The low pH of this buffer (Exercise 18.3) enhances the
reaction efficiency by converting some of the sulfite in solution to bisulfite,
(HSO
3
−
). Doing so allows the more soluble calcium bisulfite (CaHSO
3
) to form,
as well as minimizing calcium sulfite buildup on the processing machinery.
How
can the formic acid–formate ion buffer system keep the system pH within a small
range?
The buffer capacity of a system is a measure of the number of moles of
strong acid or strong base that can be added while keeping the pH relatively
constant. IUPAC defines “relatively constant” as between +/– 1 pH unit. Others
have different parameters. Because this discussion serves as an introduction to
acid–base titrations, we will define “relatively constant” as that point at which all
of the conjugate acid (or base) is reacted.
Suppose we wish to determine the buffer capacity of 100.0 mL of a solution
containing 0.200 M HCOOH and 0.400 M HCOONa. We first address this prob-
lem by noting all of the relevant equilibria in aqueous solution.
2H
2
O(l)
H
3
O
+
(aq) + OH
−
(aq) K = 1.0 × 10
−14
HCOOH (aq)
HCOO
−
(aq) + H
+
(aq) K
a
= 1.8 × 10
−4
Again, the autoprotolysis of water is insignificant, based on the relative size of the
equilibrium constant. Considering only the formic acid–formate equilibrium,
the pH of this system is 4.05, shown by our calculation:
[H
+
] =
K
a
[HCOOH]
[HCOO
−
]
=
1.8 ×10
−4
(0.200)
(0.400)
[H
+
] = 9.0 × 10
−5
M pH = 4.05
Let’s look at the impact on the pH of adding strong acid and then strong base.
18.1 Buffers and the Common-Ion Effect 779
Application
C
HEMICAL
ENCOUNTERS:
Scrubbing Sulfur
Dioxide Emissions
Video Lesson: Acidic Buffers
Video Lesson: Basic Buffers
Change 1: Addition of a strong acid*
What happens to the pH of our buffer if we add 10.0 mL of a 1.00 M HCl solu-
tion to 100.0 mL of buffer (total solution volume = 110.0 mL)? We can set the
stage by calculating the moles of each component initially in solution.
mol HCOOH
initial
=
0.1000 L HCOOH ×
0.200 mol HCOOH
L HCOOH solution
= 0.0200 mol HCOOH
mol HCOO
−
initial
=
0.1000 L HCOO
−
×
0.400 mol HCOO
−
L HCOO
−
solution
= 0.0400 mol HCOO
−
We continue by finding out how many moles of HCl were added.
mol HCl
added
=
0.0100 L ×
1.00 mol
L
= 0.0100 mol HCl
The addition of the strong acid supplies H
+
to the solution, and this H
+
essen-
tially completely reacts with HCOO
−
to form HCOOH. (K =5.6 ×10
3
).
How much HCOOH will be formed? We can organize our thinking using a
table. At the top of our table we will write the equation that indicates the reaction
of the added HCl (a source of H
+
) with the conjugate base of formic acid
(HCOO
−
). We note that this is a limiting-reactant calculation in which the HCl is
limiting and the HCOO
−
is in excess.
HCOO
−
(aq) + H
+
(aq)
HCOOH(aq) K = 5.6 × 10
3
moles initial 0.0400 0.0200
moles added 0.0100
change −0.0100 −0.0100 +0.0100
moles at equilibrium 0.0300 ≈0 0.0300
After addition of the HCl, which threw the system out of equilibrium, it returns
again to its new equilibrium position, shown by
HCOOH(aq)
HCOO
−
(aq) + H
+
(aq)
We still have lots of conjugate acid and base—a buffer system, for which we can
find the pH:
[H
+
]
=
K
a
[HCOOH]
[HCOO
−
]
=
1.8 ×10
−4
(0.300)
(0.300)
= 1.8 × 10
−4
M pH = pK
a
= 3.74
The buffer has responded to the addition of HCl by having only a slightly lowered
pH, from 4.05 to 3.74.
780 Chapter 18 Applications of Aqueous Equilibria
*The value for the equilibrium constant for the reaction of the strong acid with the formate anion
(HCOO
−
) to form HCOOH can be calculated by combining the following two equations, as we did in
Section 16.4.
HCOO
−
(aq) + H
2
O(aq)
HCOOH(aq) + OH
−
(aq) K
b
= K
w
/K
a
= 5.6 × 10
−11
H
+
(aq) + OH
−
(aq)
H
2
O(aq) K = 1/K
w
= 1.0 × 10
−14
HCOO
−
(aq) + H
+
(aq)
HCOOH(aq) K = (K
b
× 1/K
w
) = 1/K
a
= 5.6 × 10
3
We use this method several times in this chapter to determine the equilibrium constant for the addition of
strong acids or bases to weak bases or acids in a titration.
Visualization: Adding an Acid to
a Buffer

Change 2: Addition of a strong base
What happens to the pH of our original solution if we add 15.0 mL of a 1.00 M
NaOH solution (total volume = 115.0 mL)? We already know how much formic
acid (0.0200 mol) and formate ion (0.0400 mol) we started with. How many
moles of NaOH were added?
mol NaOH
added
= 0.0150 L NaOH solution ×
1.00 mol NaOH
L NaOH solution
= 0.0150 mol NaOH
The addition of the strong base supplies OH
−
to the solution, and this OH
−
essentially completely reacts with HCOOH to form HCOO
−
(K = 1.8 × 10
10
).
Therefore, we will set up a table based on the reaction of OH
−
with HCOOH.
HCOOH(aq) + OH
−
(aq)
HCOO
−
(aq) K = 1.8 ×10
10
moles initial 0.0200 0.0400
moles added 0.0150
change −0.0150 −0.0150 +0.0150
moles at equilibrium 0.0050 ≈0 0.0550
We still have both acid and conjugate base in the buffer, although the acid con-
centration is a little low. We can solve for the pH.
[H
+
] =
K
a
[HCOOH]
[HCOO
−
]
=
1.8 ×10
−4
(0.0050)
(0.0550)
= 1.64 ×10
−5
M pH = 4.79
The pH of the buffer has gone up, but the solution still has the capacity to keep
the pH close to the original value of 4.05.
Change 3: Exceeding the buffer capacity
At what point will the buffer no longer have the capacity to keep the pH reasonably
close to the original value? In other words, when will the pH rise or fall sharply?
How much will the pH change before stabilizing? The addition of 50.0 mL 1.00 M
HCl solution to the original buffer solution will stress the system. Let’s see how
much, again using our table.
HCOO
−
(aq) + H
+
(aq)
HCOOH(aq) K = 5.6 ×10
3
moles initial 0.0400 0.0200
moles added 0.0500
change −0.0400 −0.0400 +0.0400
moles at equilibrium ≈0 0.0100 0.0600
How did we calculate the moles at equilibrium in this case? This is still a limiting-
reactant problem, with the HCOO
−
instead of the H
+
limiting the amount of
product formed. We have 0.0500 mol of H
+
, but only 0.0400 mol of HCOO
−
with which to react! This means that 0.0400 mol will react to form the HCOOH
product, and there will be 0.0100 mol of H
+
in excess.
What is the final pH of the resulting solution? At equilibrium, we have a solu-
tion that contains 0.0600 mol of HCOOH and 0.0100 mol of H
+
. The formic acid
is so much weaker than the hydrochloric acid (judging on the basis of their K
a
values)
that its presence will not affect the final hydrogen ion concentration. Only the H
+
18.1 Buffers and the Common-Ion Effect 781

from the HCl is important. Therefore, the pH is based solely on the [H
+
]dueto
the ionization of the strong acid. The total volume of 150.0 mL (0.1500 L) is the
sum of the initial 100.0 mL of solution and the 50.0 mL HCl solution added to it.
[H
+
] =
(0.0100 mol)
(0.1500 L)
= 0.0667 M pH = 1.18
This reveals that the buffer’s ability to resist a change in pH has been exceeded;
the pH has changed dramatically. In the original solution, the formate ion
(HCOO
−
) could react with up to 0.0400 mol of the strong acid without having
any excess H
+
to greatly lower the pH. This is its buffer capacity toward strong acid.
Similarly, the formic acid could react with up to 0.0200 mol of strong base with-
out having any excess strong base to greatly raise the pH. This is its buffer capacity
toward strong base. As we saw with the addition of too much HCl, when the buffer
capacity is exceeded, the pH changes quite sharply. For a monoprotic acid or base,
the buffer capacity is greatest when [HA] = [A
−
]. When this occurs, the buffer
can neutralize equal amounts of both strong base and strong acid.
EXERCISE 18.6 Buffer Capacity
Let’s look at the buffer capacity of an ammonia–ammonium buffer system like the
one we used for our calcium–EDTA analysis at the beginning of this chapter. We will
start with 200.0 mL of a solution containing 0.500 M NH
3
and 0.200 M NH
4
+
. What
will be the initial pH of the buffer? Will we exceed the buffer capacity by adding
75.0 mL of 2.00 M NaOH? What will be the pH after that addition?
Solution
We have a total of 0.100 mol of NH
3
and 0.0400 mol NH
4
+
. The solution has more
conjugate base than acid, so the pH should be higher than the pK
a
for ammonium,
9.26. We use the expression for K
a
of NH
4
+
so that we can solve directly for [H
+
]:
NH
4
+
(aq)
NH
3
(aq) + H
+
(aq) K = 5.6 ×10
−10
[H
+
] =
K
a
[NH
4
+
]
[NH
3
]
=
5.6 ×10
−10
(0.200)
(0.500)
[H
+
] = 2.2 × 10
−10
M pH = 9.65
We now take the system out of equilibrium by adding 75.0 mL of 2.00 M NaOH
(0.150 mol of OH
−
added). The OH
−
will react with the weakly acidic ammonium
ion to give more ammonia. Given the amount of OH
−
added, what is the limiting
reactant?
NH
4
+
(aq) + OH
−
(aq)
NH
3
(aq) +H
2
O(l) K =5.6×10
4
moles initial 0.0400 0.100
moles added 0.150
change −0.0400 −0.0400 +0.0400
moles at equilibrium ≈0 0.110 0.140
NH
4
+
is the limiting reactant, and we will have 0.110 mol of OH
−
and 0.140 mol of
NH
3
in excess. The [OH
−
] will determine the final pH because it is a much stronger
base than NH
3
. The total solution volume consists of the 200.0 mL with which we
started and the 75.0 mL of strong base that we added, for a total of 275.0 mL.
[OH
−
] =
(0.0110 mol OH
−
)
(0.2750 L OH
−
solution)
= 0.0400 M
pOH = 1.40 pH = 12.60
782 Chapter 18 Applications of Aqueous Equilibria

The excess of OH
−
, and the resulting sharp increase in the pH, indicate that we have
exceeded the buffer capacity.
PRACTICE 18.6
What is the pH of the ammonia–ammonium ion buffer in this exercise after the ad-
dition of 30.0 mL of 0.100 M HCl? . . . after the addition of 50.0 mL of 1.50 M HCl?
See Problems 29 and 30.
EXERCISE 18.7 Keeping the pH Within Specified Limits
Our goal in buffer preparation is often to keep the pH within specific limits upon
the addition of a strong acid or base. How many milliliters of 0.100 M HCl may be
added to 100.0 mL of a buffer containing 0.150 M each of formic acid (HCOOH)
and formate ion (HCOO
−
) so that the pH will not change by more than 0.20?
First Thoughts
Our goal is to find out how many moles of HCl we can add to the system. We can
then convert to milliliters of HCl via molarity. We can add only as many moles of
HCl as will change the pH by only 0.20 unit. We can find this by determining the ini-
tial pH and the final pH and then calculating how the amounts of formic acid and
formate ion change.
Solution
The initial amounts of formic acid and formate ion are equal:
mol HCOOH = mol HCOO
−
= 0.1000 L
×
(0.150 mol)
(1.00 L)
= 0.0150 mol of each
[H
+
]
=
K
a
[HCOOH]
[HCOO
−
]
=
1.8 ×10
−4
(0.0150)
(0.0150)
[H
+
] = K
a
= 1.8 ×10
−4
M
pH = pK
a
= 3.74
When we add HCl solution, the pH is not to drop below 3.54 (0.20 from the origi-
nal pH). The concentration of H
+
at this pH is [H
+
] =10
−3.54
=2.88×10
−4
M.
We
can solve for the ratio of acid to base:
[H
+
]
K
a
=
2.88 ×10
−4
1.8 ×10
−4
=
[HCOOH]
[HCOO
−
]
=
1.60
1
Therefore, retaining an extra figure in this immediate calculation,
[HCOOH] = 1.60[HCOO
−
]
Because the volumes are equal,
mol HCOOH = 1.60(mol HCOO
−
)
The total amount of formic acid and formate ion equals 0.0300 mol (it was origi-
nally 0.01500 mol each). We can substitute for HCOOH and solve.
1.60(HCOO
−
) + HCOO
−
= 0.0300 mol
2.60(HCOO
−
) = 0.0300 mol
mol HCOO
−
=
0.0300 mol
2.60
=
0.0115 mol
mol HCOOH = 1.60(0.0115 mol HCOO
−
) = 0.0184 mol
18.1 Buffers and the Common-Ion Effect 783
Because our original amounts of HCOOH and HCOO
−
were 0.01500 mol each, we
can have an addition of 0.0035 mol of HCl and still have the pH stay within 0.20 of
the original pH. We can now determine the maximum HCl solution volume.
mL HCl = 0.0035 mol HCl ×
1.000 L
0.1000 mol
= 0.035 L = 35 mL
Further Insights
We see the importance of working in moles with buffer calculations. Keep in mind
that this is possible only because the volumes of the conjugates cancel. This will be
the case only when we are dealing with buffers.
PRACTICE 18.7
Solve the same problem using the Henderson–Hasselbalch equation instead of the
mass-action expression for the buffer.
See Problems 29 and 30.
In Summary: What are the criteria for a suitable buffer?
A buffer should not react with the system it is buffering.
■
The pK
a
of a buffer should be as close as possible to the pH you want to
maintain.
■
The buffering capacity of a buffer must be sufficient to accommodate the ad-
dition of a strong acid or a strong base.
Food for Thought:
Are Strong Acids and Bases Buffers?
A buffer, as we noted before, is a mixture of a weak acid and its conjugate base or
of a weak base and its conjugate acid. Is it possible that a solution containing only
a strong acid (such as HCl) or a strong base (such as NaOH) could be a buffer?
For example, 1 L of 0.10 M HCl has a pH of 1.0. The solution pH remains close
to 1.0 even when some NaOH is added. Similarly, the pH of 0.10 M NaOH re-
mains close to 13.0 even when some strong acid is added. In that sense, they meet
the criterion of keeping the pH of the solution fairly constant upon addition of
strong acid or base. However, buffers should also keep their pH constant when di-
luted, and this is where strong acids and bases fail as buffers. Dilution of a strong
acid or a strong base solution causes the pH to change by roughly 1 unit
with every 10-fold dilution. On the other hand, buffers made from conjugate
acid–base pairs show very little change in pH with dilution. This is critical in bio-
chemical processes, which require a fairly constant pH whatever the solution
concentration.
The importance of buffers in medicine is illustrated by a class of drugs called
antacids. These drugs are compounds that neutralize gastric acid and exert a
buffering effect in the stomach for temporary relief of acid indigestion. Similarly,
magnesium carbonate is added to certain brands of aspirin specifically to alter
stomach pH. The magnesium carbonate, by neutralizing the “gastric juice,” in-
creases stomach pH. The net result is that the aspirin exists predominantly in its
ionized form, as shown in Figure 18.5, and reduces the risk of aspirin-induced
stomach bleeding or ulcers. Although this is desirable in some cases, it can reduce
the amount of drug absorption. Unfortunately, antacids alone may also raise the
pH of the stomach above the pK
a
of other drugs, resulting in their ionization and
in reduced absorption through the stomach. This reduced absorption means that
the drug treatment is not effective. Consequently, many drugs bear the warning
784 Chapter 18 Applications of Aqueous Equilibria
18.1 Buffers and the Common-Ion Effect 785
Enzymes, which catalyze nearly all the
chemical reactions that occur in living or-
ganisms, are often highly sensitive to the
hydrogen ion concentration of the envi-
ronment in which they are found. Slight
increases or decreases in pH can have a
dramatic effect on an enzyme’s ability to
carry out its unique function. Conse-
quently, living organisms typically have
buffering systems in place to maintain a
relatively constant pH. Technological ad-
vances in biochemistry and molecular biol-
ogy have enabled chemists to prepare, pu-
rify, and study just about any enzyme they
desire outside of its normal cellular envi-
ronment, as long as they can maintain the
pH at around 7. For example, members of
the class of enzymes called cytochrome
P450 are chemically active only between
pH 6.5 and pH 8.5, with optimum activi-
ties usually occurring between pH 6.8 and
pH 7.5. These enzymes are present in the
human liver, where they help the body rid
itself of foreign chemicals, including most
pharmaceuticals.
In 1966, Norman Good and coworkers
reported on the design of a dozen new
buffers for use in biological research. These
so-called Good (or Good’s) buffers are now
widely used, because they are fairly chemi-
cally stable in the presence of enzymes or
visible light and do not interact with bio-
logical compounds. Moreover, they are easy
to prepare. Several of the Good buffers
(those shown with asterisks in Table 18.2)
and boric acid are among the buffers most
commonly used in the study of enzyme be-
havior. Their abbreviated names, struc-
tures, and pK
a
values are listed in Table 18.2.
An additional set of highly effective biologi-
cal buffers were synthesized in the late
1990s and are now commercially available.
Not too surprisingly, they are called“better
buffers.”
NanoWorld / MacroWorld
Big effects of the very small:
Buffers in biochemical studies and medicine
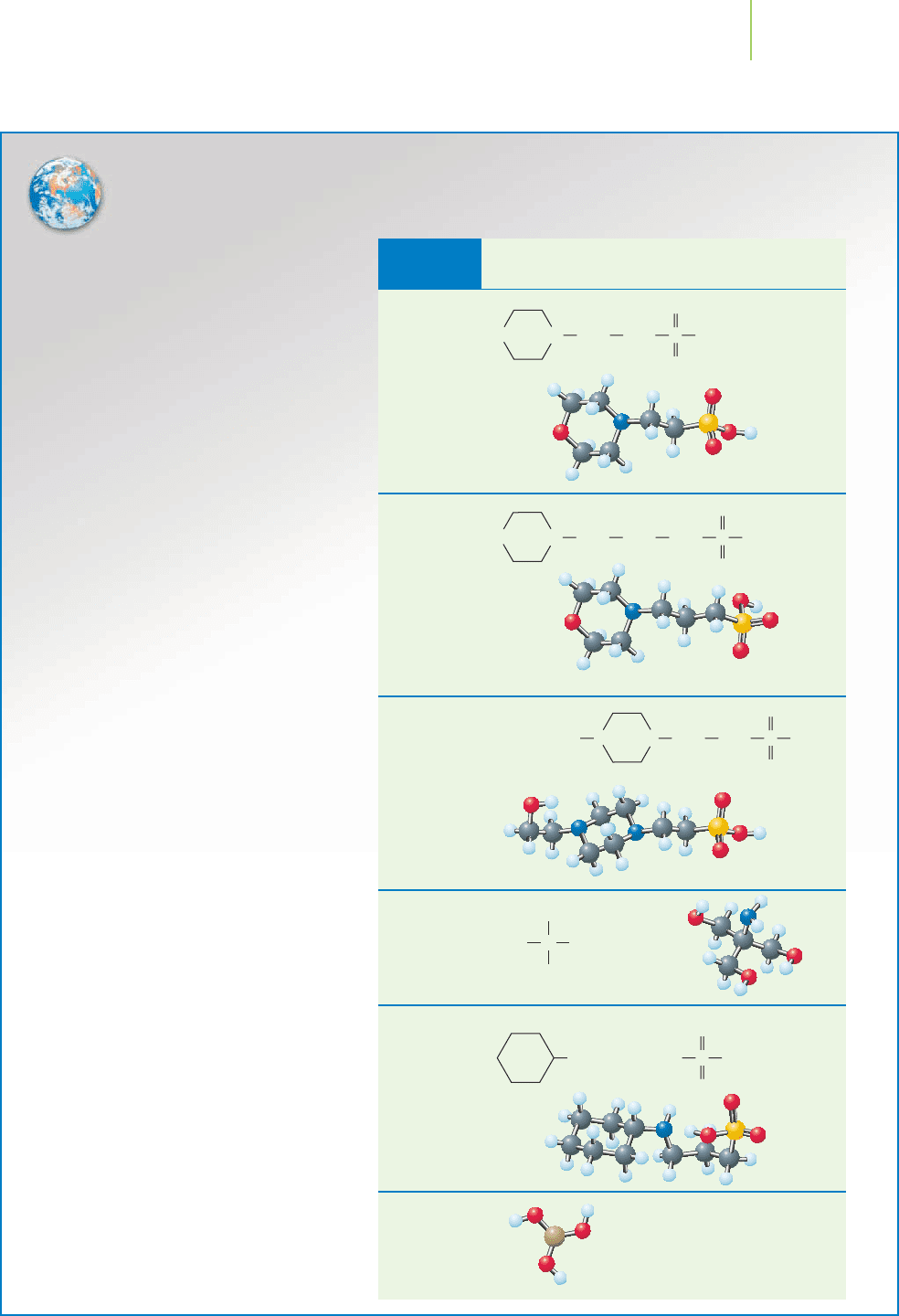
Common Biological Buffers:
Good Buffers and Boric Acid
MES
pK
a
: 6.15
MOPS
pK
a
: 7.20
HEPES
pK
a
: 7.55
Tr i s
pK
a
: 8.30
CAPS
pK
a
: 10.40
Boric acid
pK
a
: 9.24
H
3
BO
2
TABLE 18.2
ON S
O
O
OHCH
2
CH
2
ON S
O
O
OHCH
2
CH
2
CH
2
NNS
O
O
OHCH
2
HOCH
2
CH
2
CH
2
CH
2
N
CH
2
OH
CH
2
OH
CH
2
OH
S
O
O
OH
NHCH
2
CH
2
CH
2
TABLE 18.2
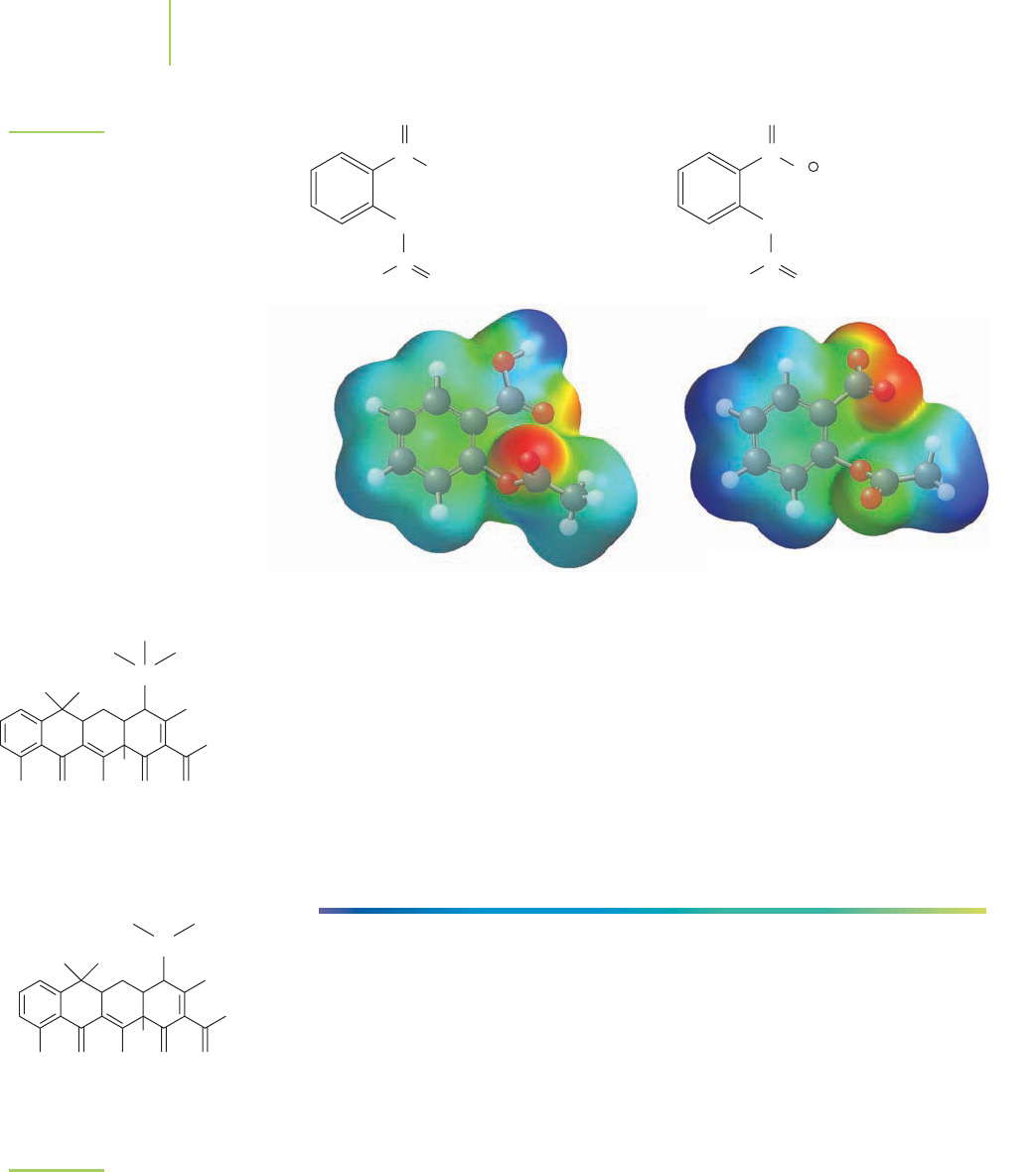
786 Chapter 18 Applications of Aqueous Equilibria
C
O
OH
O
O
C
CH
3
C
O
O
O
O
C
CH
3
–
Neutral form
pH < 4
Ionic form
pH > 4
FIGURE 18.5
The neutral and ionic forms of aspirin.
OH
OH
N
H
NH
2
CH
3
CH
3
OOH OOOH
OH
CH
3
Protonated form
pH < 8.3
OH
OH
N
NH
2
CH
3
CH
3
OOH OOOH
OH
CH
3
Neutral form
pH > 8.3
FIGURE 18.6
The ionic and neutral forms of
tetracycline.
“Do not take antacids containing (hydroxides of) aluminum, calcium, or magne-
sium (e.g., Mylanta, Maalox) while taking this medication.”
The buffering effects of antacids and other buffered medicines can give rise to
a number of undesirable drug interactions with prescription medications. Tetra-
cycline, for example, is an antibiotic drug that is absorbed primarily through the
stomach lining in its protonated form, shown in Figure 18.6, thanks to the nor-
mally low pH of the stomach.
HERE’S WHAT WE KNOW SO FAR
■
Many reactions are pH-sensitive and require buffers to control pH.
■
A buffer resists change in pH upon addition of a strong acid or a strong base.
■
The pH of a buffer is not changed when the solution is diluted.
■
Buffers are typically composed of weak conjugate acid–base pairs.
■
We can solve for the pH of buffers in a straightforward way by recognizing the
importance of Le Châtelier’s principle and the common-ion effect.
■
We can calculate the approximate ratio of conjugate acid to base in order to
prepare a buffer of a known pH.
■
Solving for the pH of a buffer upon addition of strong acid or base is really
solving a limiting-reactant problem.
■
It is possible to exceed the buffer capacity, in which case the pH will move
sharply higher (with excess base) or sharply lower (with excess acid).
We have seen that buffers are used to maintain the pH of all kinds of systems,
ranging from municipal scrubbers to your body. Our overarching application of
aqueous equilibria in this chapter is analysis of calcium in hard water via titration
with EDTA. We are one step closer to completing our task. We next focus on
titrations.

18.2 Acid–Base Titrations 787
18.2 Acid–Base Titrations
We discussed the wide range of titrations, of which acid–base titration is
one important type, in the opening section of this chapter. A small sample
of the commercial, environmental and biological uses of acid–base titra-
tions includes analysis of the acidity of food and drink, determination of
the pH of water supplies, measurement of the solubility of pharmaceuti-
cals, determination of amino acids in blood, and determination of the
acidity or basicity (called the “total acid” or “total base”number) of motor
oils.
In the lab, we typically set up an acid–base titration by monitoring the
pH as shown in Figure 18.7. This normally includes a buret to accurately
measure the volume of titrant delivered, a beaker or flask, and a calibrated
pH meter. Industrial laboratories often use automated titrators to increase
efficiency. The typical acid–base titrations fall into one of these main
categories:
■
strong-acid–strong-base titrations
■
strong-acid–weak-base titrations
■
weak-acid–strong-base titrations
A fourth type, weak-acid–weak-base titrations, is typically not used because the
equilibrium constant for the overall reaction is not nearly as large as with the
other systems, and the indication of the end of the titration is too gradual to
tell us when the titration is complete.
Strong-Acid–Strong-Base Titrations
The determination of HCl molarity based on titration with NaOH is a common
process throughout all levels of chemistry and from the academic to the
industrial laboratory setting. Let’s examine the changes that take place during a
strong-acid–strong-base titration by assuming that we wish to titrate 50.00 mL of
0.1000 M HCl by adding known amounts of 0.2000 M NaOH. The results of the
titration are graphically shown in Figures 18.8a–f, which illustrates the relation-
ship between pH and volume of OH
–
added.
Part 1: Initial pH
We can calculate the pH of the initial 0.1000 M solution of HCl as we
would that of any other strong acid:
pH =−log[H
+
] =−log(0.1000) = 1.0
We enter this on the graph to the right (Figure 18.8a).
Part 2: Addition of 5.00 mL of NaOH solution
What will be the reaction of the strong acid with the strong base? We can
write the reaction in molecular form:
HCl(aq) + NaOH(aq)
H
2
O(l) + NaCl(aq)
However, the net ionic form gives a better sense of what is going on in the
solution:
H
+
(aq) + OH
−
(aq)
H
2
O(l) K = 1.0 ×10
14
The equilibrium constant is very high and the reaction is fast, both good features
to have when doing a titration. What, and how much, will be left over after addi-
tion of the NaOH titrant to the HCl solution? This is a limiting-reactant
problem, and we can use the same type of table that we used when discussing
FIGURE 18.7
A typical set-up for an acid–base titra-
tion monitored by a pH meter includes a
buret to accurately measure the volume
of titrant delivered, a beaker, and the cal-
ibrated pH meter. An automatic titrator is
used when there are many titrations to
be done.
1
3
5
7
9
11
13
0 5 10 15 20 25 30 35 40 45 50
Volume of NaOH (mL)
pH
FIGURE 18.8a
Each plot follows the pH changes as we
add 0.2000 M NaOH solution to 50.00 mL
of 0.1000 M HCl solution. The initial pH
is shown here, followed in turn by the pH
after addition of the listed volumes.
Visualization: Acid–Base
Titration
Video Lesson: Strong-
Acid–Strong-Base Titration
Video Lesson: CIA
Demonstration: Barium
Hydroxide-Sulfuric Acid Titration
Tutorial: Titration Curves: Strong
Acid with Strong Base
