Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

The nitrogen dioxide that is produced then reacts with water vapor to produce
nitrous and nitric acids:
2NO
2
(g) + H
2
O(l)
HNO
2
(aq) + HNO
3
(aq)
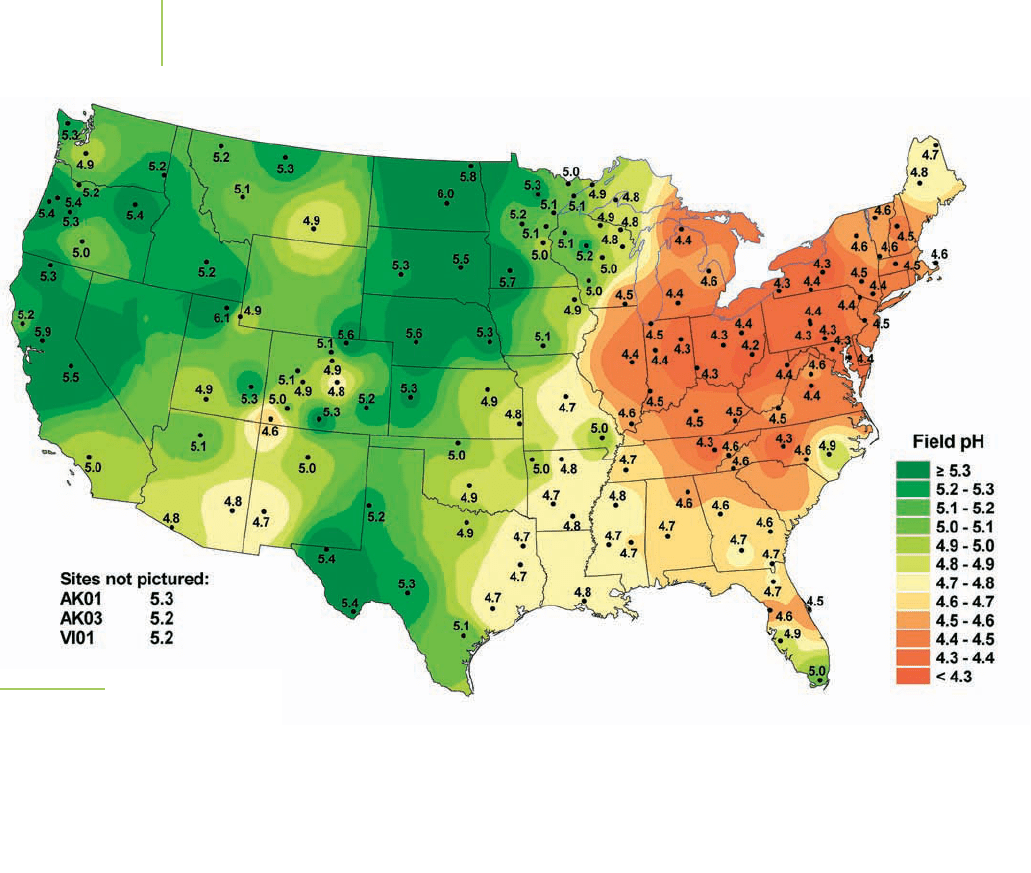
The nitric acid adds to the problem of acid deposition. As can be seen in Fig-
ure 17.21, a lot of deposition that occurs throughout the United States has an un-
usually low pH. However, some places (for example, in the western United States)
seem to be less affected by acid deposition than other places. Why?
The atmosphere acts as a large mixing chamber, and we’ve considered only
the acidic inputs so far. Ammonia gas from agriculture and animal-feeding oper-
ations can react with water vapor to form aqueous ammonia, increasing the pH
of the precipitation:
NH
3
(g) + H
2
O(l)
NH
4
+
+ OH
−
(aq)
There are more natural sources of ammonia gas in the western United States than
sources of sulfur dioxide and nitrogen dioxide, so the pH of precipitation in this
region is higher. The ability of some lakes to mitigate the effects of acid deposi-
tion has to do with
acid-neutralizing capacity. This represents the theme of our
next chapter: how and why acid–base and other types of reactions in aqueous so-
lution are used in chemical analysis. This discussion will include acid–base neu-
tralization, buffers, and titrations. Home is where the heart is. But we have shown
that the home, and the people within it, are, chemically speaking, where the acids
and bases also reside. Our global home, Spaceship Earth, is ever changing. It and
we who occupy it owe much of this change to the acids and bases we have dis-
cussed here. In the next chapter, we will learn how.
758 Chapter 17 Acids and Bases
FIGURE 17.21
The pH of materials that deposit on the
United States varies across the country,
1999. The low pHs centered over Ohio
are probably due to industrial processes.
The Bottom Line
Key Words 759
■
Acids and bases can be defined using three different
models. (Section 17.1)
■
Acids and bases come in different strengths.
(Section 17.2)
■
Both the strength and the initial concentration of
the acid affect the acidity in solution at equilibrium.
(Section 17.2)
■
Acids and bases have conjugates pairs whose behav-
ior is related to that of the acid or base from which
they are derived. (Section 17.1)
■
We use pH as our common measure of acidity.
(Section 17.3)
■
We can interconvert among H
+
,OH
−
, pH, and pOH
for a given acidic or basic solution. (Section 17.3)
■
We can calculate the pH of strong and weak acids
and bases in aqueous solutions. (Sections 17.4
and 17.5)
■
We can solve for the pH of a polyprotic acid or base,
including salts. (Sections 17.6 and 17.7)
■
K
a
and K
b
are related via K
w
. (Section 17.7)
■
The reaction of an acid anhydride with water results
in an acid, and the reaction of a basic anhydride with
water results in a base. (Section 17.8)
Key Words
acid According to the Arrhenius model, a species that
produces hydrogen ions in solution. Compare the
definitions of Brønsted–Lowry acid and Lewis acid.
(p. 719)
acid deposition The precipitation of acidic compounds
from the atmosphere. This includes wet deposition as
rain and dry deposition of particles. (p. 757)
acid dissociation constant (K
a
) The equilibrium constant
for the dissociation of an acid. (p. 724)
acid-neutralizing capacity The capacity of a solution,
such as lake water, to neutralize acidity. (p. 758)
acidic Having a pH less than 7.0 in aqueous solution at
24°C. (p. 735)
acid anhydrides Binary compounds formed between
nonmetals and oxygen that react with water to form
acids. (p. 757)
alkaloid Nitrogen-containing bases found in vegetables
and other plants. (p. 746)
amphiprotic Having the ability to act as either an acid or
a base in different circumstances. (p. 733)
autoprotolysis Proton transfer between molecules of the
same chemical species, as in the autoprotolysis of
water. (p. 733)
base According to the Arrhenius model, a species that
produces hydroxide ions in solution. Compare the
definitions of Brønsted-Lowry base and Lewis base.
(p. 719)
base hydrolysis The process in which a base reacts with
water to produce its conjugate acid and hydroxide
ion. (p. 753)
basic Having a pH greater than 7.0 in aqueous solution
at 24°C. (p. 735)
basic anhydrides Binary compounds that are formed be-
tween metals with very low electronegativity and oxy-
gen and that react vigorously with water. (p. 757)
Brønsted–Lowry acid Any species that donates a hydro-
gen ion (proton) to another species. (p. 720)
Brønsted–Lowry base Any species that accepts a hydro-
gen ion (proton) from another species. (p. 720)
common-ion effect The addition of an ion common to
one of the species in the solution, causing the equilib-
rium to shift away from production of that species.
(p. 737)
conjugate acid The acid that results after accepting a
proton. (p. 720)
conjugate base The base that results after donating a
proton. (p. 720)
dibasic salt A salt that can accept two hydrogen ions.
(p. 748)
diprotic acid An acid that contains two acidic protons.
(p. 736)
gypsum Calcium sulfate dihydrate, CaSO
4
·2H
2
O.
(p. 748)
isoelectric pH pH value at which an amino acid is in the
zwitterion form and so is electrically neutral overall.
(p. 756)
Lewis acid Accepts a previously nonbonded pair of elec-
trons (a lone pair) to form a coordinate covalent
bond. (p. 722)
Lewis base Donates a previously nonbonded pair of
electrons (a lone pair) to form a coordinate covalent
bond. (p. 722)
monobasic salt A salt that can accept one hydrogen ion.
(p. 748)
monoprotic acid An acid that contains one acidic
proton. (p. 736)
neutral pH A pH of 7.0 in aqueous solution at 24°C.
(p. 734)
pH A numerical value related to hydrogen ion concen-
tration by the relationship pH = –log[H
+
] or pH =
−log[H
3
O
+
]. (p. 730)
polyprotic acid An acid that can release more than 1 mol
of hydrogen ions per mole of acid. (p. 747)
strong acid An acid that fully dissociates in water,
releasing all of its acidic protons. (p. 724)
superacids Amazingly strong acids that can be used to
add hydrogen ions to organic molecules that are oth-
erwise impervious to such reaction. (p. 729)
tribasic salt A salt that can accept three acidic hydrogen
atoms. (p. 748)
triprotic acid An acid that contains three acidic protons.
(p. 747)
weak acid An acid that only partially dissociates in
water. (p. 724)
zwitterion A molecular ion carrying both an acid group,
which generates a negative ion, and a basic group,
which generates a positive ion. (p. 755)
760 Chapter 17 Acids and Bases
Focus Your Learning
The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
Section 17.1 What Are Acids and Bases?
Skill Review
1. Explain how ammonia (NH
3
) qualifies as a base in both the
Brønsted–Lowry and Arrhenius acid–base models.
2. Explain how HCl and NaOH are classified in both the
Brønsted–Lowry and Arrhenius acid–base models.
3. List the conjugate base of each of these acids.
a. HNO
3
b. HBr c. H
2
Od.HClO
4
4. List the conjugate base of each of these acids.
a. HCl b. NH
4
+
c. CH
3
OH d. H
2
SO
4
5. List the conjugate acid of each of these bases.
a. NaOH b. NH
3
c. H
2
Od.NaF
6. List the conjugate acid of each of these bases.
a. KBr b. CH
3
OH c. KNO
2
d. KSH
7. Acids react with active metals to produce hydrogen gas.
Complete, balance, and name each of the products for these
reactions.
a. Al(s) + HCl(aq)
→
_____ + _____
b. Ca(s) + HNO
3
(aq)
→
_____ + _____
c. Na(s) + HCl(aq)
→
_____ + _____
d. K(s) + HNO
3
(aq)
→
_____ + _____
8. Many bases react with metals to form insoluble hydroxides.
Write the formula for each of these hydroxides.
a. aluminum hydroxide b. copper(II) hydroxide
c. barium hydroxide d. strontium hydroxide
e. iron(III) hydroxide f. calcium hydroxide
9. Being strong usually refers to the ability to carry out a task or
produce an effect. (For example, a spice may have a strong
flavor, and a weightlifter may display a lot of strength.) How
is this term used when applied to acids? When an acid is said
to be strong, what effect is being described?
10. What is the definition of a Lewis acid? Why are many metal
ions capable of behaving as Lewis acids? Write out the reac-
tion that depicts an aluminum cation behaving as a Lewis
acid in an aqueous solution.
11. Balance and identify each of the species in the following re-
actions as either acid, base, conjugate acid, or conjugate base.
a. HCl + H
2
O
→
Cl
−
+ H
3
O
+
b. NaOH + CH
3
COOH
→
CH
3
COONa + H
2
O
c. H
2
SO
4
+ Mg(OH)
2
→
MgSO
4
+ H
2
O
12. Balance and identify each of the species in the following re-
actions as either acid, base, conjugate acid, or conjugate base.
a. HCOOH + NH
3
→
NH
4
+
+ HCOO
−
b. KOH + CH
3
OH
→
CH
3
OK + H
2
O
c. H
3
PO
4
+ Ca(OH)
2
→
Ca
3
(PO
4
)
2
+ H
2
O
Chemical Applications and Practices
13. One common antacid used in relief of upset stomachs is
Mg(OH)
2
. It helps to neutralize excess hydrochloric acid.
Balance the following representative equation, and identify
the conjugate acid–base pairs.
Mg(OH)
2
+ HCl
→
MgCl
2
+ H
2
O
14. Prizes in cereal boxes used to include little submarines that,
when filled with baking soda (NaHCO
3
) and placed in a cup
of water containing a little vinegar (CH
3
COOH), would rise
and fall as though by magic. Balance the reaction of baking
soda and vinegar, and identify the conjugate base and acid
pairs.
CH
3
COOH + NaHCO
3
→
CH
3
COONa + H
2
CO
3
Section 17.2 Acid Strength
Skill Review
15. Describe the characteristics of a strong acid with regard to
each of the following:
a. The numerical value of its K
a
.
b. The ability of its conjugate base to regain a H
+
.
c. The approximate percent dissociation of a 0.1 M solution.
16. Describe the characteristics of a weak acid with regard to
each of the following:
a. The numerical value of its K
a
.
b. The ability of its conjugate base to regain a H
+
.
c. The approximate percent dissociation of a 0.1 M solution.
17. Judging on the basis of electron density and electronegativity,
which acid, HBr or HI, would you expect to be stronger?
Explain your choice.
18. Which conjugate base, Br
−
(aq) or I
−
(aq), would you expect
to be stronger? Explain your choice.
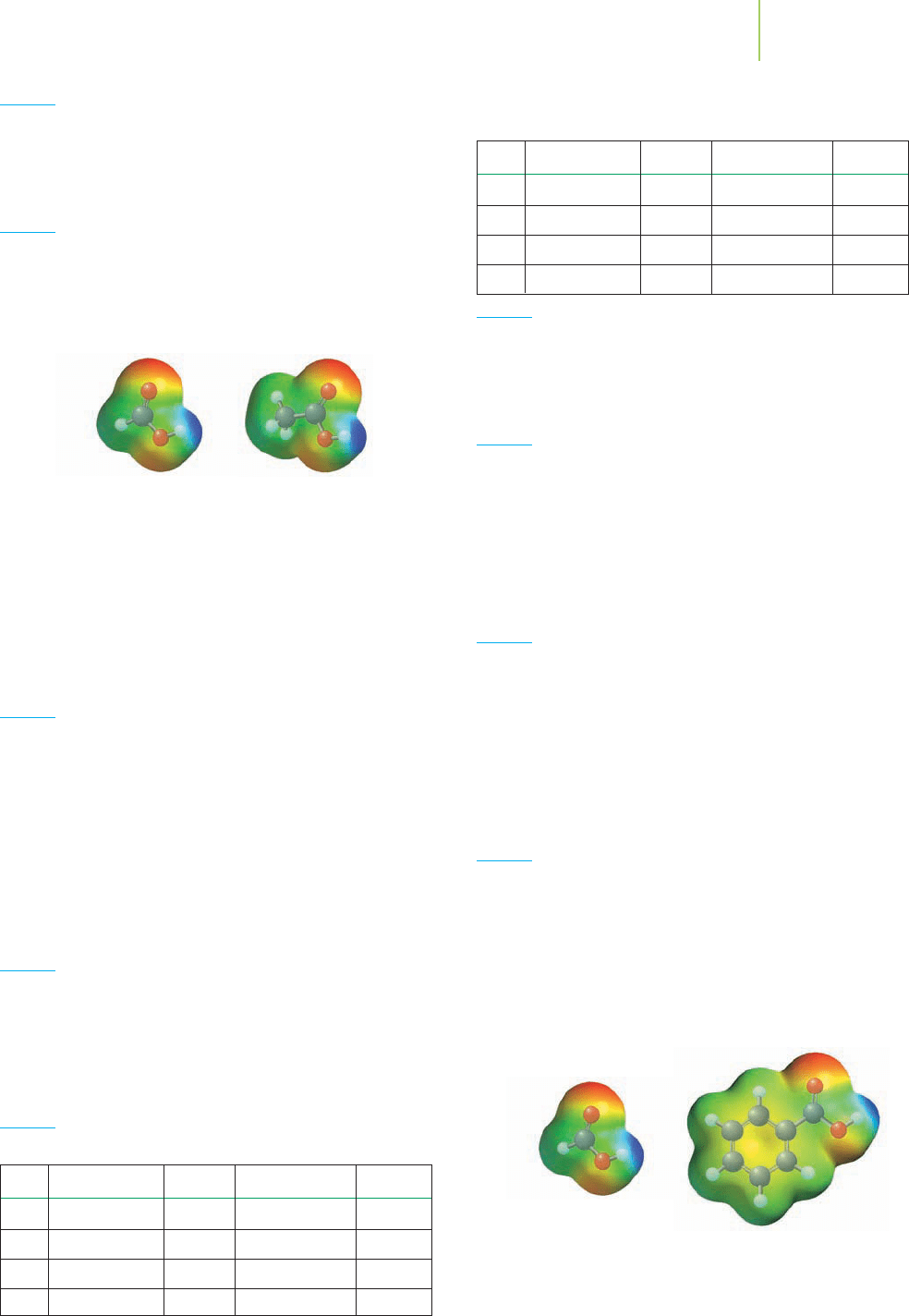
Formic acid Benzoic acid
19. Using molecular structure and electronegativity, explain
which acid, HClO
4
or HBrO
4
, would be the weaker.
20. Which acid, phosphoric (H
3
PO
4
) or phosphorous (H
3
PO
3
),
would you expect to be stronger? Explain your choice.
Chemical Applications and Practices
21. Formic acid, found in ants, and acetic acid, found in vinegar,
have the K
a
values 1.8 × 10
−4
and 1.8 × 10
−5
, respectively.
a. Which acid has the stronger conjugate base?
b. Which acid, if both were in 0.10 M solutions, would have
the higher percent dissociation?
29. If the pH value in an aqueous sample were doubled, what ef-
fect would be detected in the hydronium ion concentration?
30. What would be the effect on the hydroxide ion concentration
if the pH were doubled?
Chemical Applications and Practices
31. Paper is produced from the processed fibers of trees. Part of
the process of sulfate pulping uses sodium hydroxide. What
would be the pH of a solution in a wood-pulping mill if it
contained 10.0 g of OH
−
ions for every 10.0 L of solution?
32. One method to increase oil production in areas drilled
through limestone deposits is to use hydrochloric acid to in-
crease drainage channels through the stone. If such a solution
had a pH of 2.59, what would you calculate as the grams of
HCl dissolved per liter of solution?
33. Two samples of rainwater are being analyzed for an environ-
mental impact study. What is the hydronium concentration
in each sample? What is the pH of each sample?
a. 500.0 mL containing 1.55 × 10
−5
mol of H
+
b. 250.0 mL containing 7.25 × 10
−6
mol of H
+
34. The pH of human blood must be maintained within a very
narrow range to ensure proper health. The following blood
samples were analyzed to determine their pH values. What
would you calculate as the hydronium concentration in each?
a. pH = 7.42 b. pH = 7.38 c. pH = 7.51
35. Assuming a negligible change in volume, how many moles of
either OH
−
or H
+
would have to be added to change the pH
of 1.00 L of a solution from 4.35 to 5.85?
36. Formic acid has a pK
a
value of 3.74. Benzoic acid has a pK
a
value of 4.20. Compare the electrostatic potential maps of
formic acid and benzoic acid. Which is the stronger acid?
Which of the two acids would have the weaker conjugate
base?
Focus Your Learning
761
Formic acid Acetic acid
22. The K
a
value for an acid provides information about one type
of reaction of the acid, its ability to provide H
+
.However,a
weak acid may have other very important reactions. For ex-
ample, hydrofluoric acid, K
a
= 7.2 × 10
−4
, has the ability to
etch glass. Phenol, K
a
= 1.6 × 10
−10
, can be used as a disin-
fectant.
a. Which of these two is the weaker acid?
b. Which has the stronger conjugate base?
c. If both were 0.10 M, which would produce the greater
concentration of H
+
?
23. Propanoic acid is a weak acid that can be used to prepare a
type of mold retardant. What is the value for K
a
of the acid if,
in a solution, the following equilibrium concentrations
were found: [acid] = 0.10 M, [conjugate base] and [H
+
] =
0.0011 M?
24. At 25
o
C the K
b
for ammonia (NH
3
) is 1.8 × 10
−5
. What is
the hydroxide concentration when [NH
3
] = 0.103 M and
[NH
4
+
] = 0.00205 M?
Section 17.3 The pH Scale
Skill Review
25. Calculate the pH of each of these solutions.
a. [H
+
] = 4.55 × 10
−3
M
b. [H
+
] = 3.27 × 10
−6
M
c. [H
+
] = 8.11 × 10
−9
M
26. Calculate the [H
+
] for each of these solutions.
a. pH = 1.50 b. pH = 10.25
c. pH = 5.38 d. pH = 7.00
27. Calculate the values missing from the following table.
[H
+
] (M)pH[OH
−
] (M) pOH
a. 4.42
b. 0.0056
c. 0.000078
d. 10.10
[H
+
] (M)pH [OH
−
] (M) pOH
a. 12.50
b. 0.000035
c. 0.00388
d. 3.75
28. Calculate the values missing from the following table.

Section 17.4 Determining the pH of Acidic Solutions
Skill Review
37. Determine the pH of each of these solutions of strong acids.
a. 0.45 M HCl b. 0.045 M HCl
c. 0.000487 M HNO
3
d. 0.00026 M HBr
38. Determine the pH of each of these solutions of strong bases.
a. 0.550 M NaOH b. 0.00089 M KOH
c. 0.00388 M KOH d. 0.015 M KOH
39. Determine the pH of each of these solutions. (Use the table in
the text to find the values for the appropriate K
a
.)
a. 0.45 M HOCl b. 0.0250 M CH
3
COOH
c. 0.18 M HF d. 0.0010 M HCOOH
40. Determine the pH of each of these solutions. (Use the table in
the text to find the values for the appropriate K
a
.)
a. 0.299 M HOCl b. 0.18 M CH
3
COOH
c. 0.45 M lactic acid (HC
3
H
5
O
3
) d. 0.050 M HCN
41. Determine the value of pK
a
for:
a. K
a
= 3.75 × 10
−5
b. K
a
= 1.84 × 10
−2
c. K
a
= 4.59 × 10
−8
42. Determine the value of K
a
for:
a. pK
a
= 3.50 b. pK
a
= 4.74 c. pK
a
= 6.17
43. If the pH of a 0.015 M solution of codeine, a drug used in
some pain relievers, is 10.19, what is the value of K
b
for
codeine (C
18
H
21
NO
3
)?
44. What would be the resulting pH when a solution was made
that was 0.0100 M in HCl and 0.100 M in HCN (hydrocyanic
acid, a deadly poison, which has a K
a
value of 6.2 × 10
−10
)?
Chemical Applications and Practices
45. Among the growth requirements for bacteria is the proper
range of aqueous hydrogen ion concentration. Suppose a
microbiologist was preparing growth media to study a spe-
cific bacterium. Examine both of the following situations and
determine in which case the H
+
(aq) would be greater than
1.0 × 10
−6
M?
a. A 0.500-L solution containing 1.00 g of benzoic acid
(K
a
= 6.5 × 10
−5
; molar mass = 122 g)
b. 100.0 mL of 0.0001 M sulfuric acid
46. Benzoic acid is often used to prepare a preservative known
as sodium benzoate. If the K
a
value of benzoic acid is
6.5 × 10
−5
, what is the hydrogen ion concentration when the
acid concentration is 0.0040 M and the conjugate base con-
centration is 0.0024 M?
47. The following reaction depicts an industrial process to man-
ufacture gaseous hydrogen fluoride.
CaF
2
(s) + H
2
SO
4
(aq) n CaSO
4
(aq) + 2HF(g)
a. How many grams of HF can be made from 1.00 kg of
fluorospar (CaF
2
)?
b. HF can then be used to prepare fluorocarbon compounds.
What would be the H
+
,F
−
,and OH
−
concentrations in a
solution that was 0.25 M in HF? (K
a
of HF = 7.2 × 10
−4
)
48. An aspirin tablet may contain 325 mg of acetylsalicylic acid
(pK
a
= 3.522, 180.16 g/mol). What would be the approxi-
mate pH when two tablets were dissolved in 275 mL of water?
49. A vitamin C tablet may contain 500.0 mg of ascorbic acid
(C
6
H
8
O
6
). What would be the pH of a solution made from
dissolving one such tablet in 355 mL of solution? (K
a
1
of
ascorbic acid = 8.0 × 10
−5
) Does the 5% rule assumption
apply in this example? Show your proof.
50. Benzoic acid (K
a
= 6.5 × 10
−5
) and propionic acid (K
a
=
1.3 × 10
−5
) can both be used to produce food preservatives.
A 0.10 M solution of one of the acids has [H
+
] = 0.00255 M.
Which acid was used?
Section 17.5 Determining the pH of Basic Solutions
Skill Review
51. Determine the pH of each of these solutions.
a. 0.100 M aniline (K
b
= 3.8 × 10
−8
)
b. 0.0100 M NaOH
c. 0.250 M ammonia
52. Determine the pH of each of these solutions.
a. 0.0333 M methylamine (K
b
= 5.9 × 10
−4
)
b. 0.0150 M Ca(OH)
2
c. 0.016 M ammonia
Chemical Applications and Practices
53. Pyridine is a weak base that can be used to make a product
used in some mouthwash preparations. The K
b
value of
pyridine is approximately 1.4 × 10
−9
. What would be the
hydroxide ion concentration, the pOH, and the pH of a
solution that was 0.0010 M pyridine?
54. The base Ca(OH)
2
is known as slaked lime. It is widely used
in the paper industry and in steel making. When properly
heated, it gives off a bright light. In the 1800s, this light was
used to illuminate some theaters. Actors began appearing in
the “limelight.”
a. Write out the equation representing the dissociation of
lime in water.
b. Ca(OH)
2
is not very soluble in water. If 0.025 mol could
dissolve per liter, what would you calculate as the pH of
the solution?
55. The typical “fish aroma” is due to the production of amine
compounds. What would be the K
b
value of ethylamine
(CH
3
CH
2
NH
2
) if a 500.0 mL solution that contained 1.90 g
of ethylamine had a pH of 11.87?
56. Metacaine is used to anesthetize groups of fish when scien-
tific studies on them are conducted. The active ingredient of
metacaine is a base known as ethyl 3-aminobenzoate. What
would be the K
b
value of ethyl 3-aminobenzoate (C
9
H
11
NO
2
)
if a 750.0 mL solution containing 1.00 g had a pH of 9.30?
Section 17.6 Polyprotic Acids
Skill Review
57. Write out the three hydrogen dissociation steps for phospho-
rous acid (H
3
PO
3
). What is the oxidation number of phos-
phorus in H
3
PO
3
?
58. Write out the two hydrogen dissociation steps for carbonic
acid (H
2
CO
3
). What is the oxidation number of carbon in
H
2
CO
3
?
762 Chapter 17 Acids and Bases
59. The respective K
a
values for the three dissociation steps of
phosphoric acid are 7.4 × 10
−3
, 6.2 × 10
−8
, and 4.8 × 10
−13
.
What would be the pH and HPO
4
2−
concentration of a 1.0 M
solution of H
3
PO
4
?
60. What are the pH and the HSO
4
−
concentration of a 0.750 M
solution of H
2
SO
4
?
Chemical Applications and Practices
61. Sulfurous acid (H
2
SO
3
) is a by-product of burning sulfur-
containing coal. SO
2
is produced during the process and,
when combined with water in the air, can produce H
2
SO
3
.
Write the balanced equations that show the two-stage ioniza-
tion of sulfurous acid. Identify the conjugate base produced
in each stage.
62. Carbonic acid can be found in carbonated drinks. It forms
when CO
2
reacts with water.
a. Write the balanced equation that shows the formation of
carbonic acid from dissolved carbon dioxide.
b. Write out the equilibrium expressions for both ionization
steps for this diprotic acid.
c. Use Le Châtelier’s principle to explain why increasing the
pressure of CO
2
produces a lower pH in the solution.
63. Nicotine is dibasic because of the presence of two nitrogen
atoms, each of which may accept hydrogen ions. The respec-
tive K
b
values, at 25
o
C, are approximately 7.0 × 10
−7
and
1.1 × 10
−10
. What would be the pH of a solution that was
0.045 M in nicotine.
64. Tartaric acid (H
2
C
4
H
4
O
6
) is a diprotic acid used in some bak-
ing preparations. For the successive hydrogen ionizations,
K
a
1
= 9.2 × 10
−4
and
K
a
2
= 4.3 × 10
−5
. What would be
the pH of a solution that was 0.10 M in tartaric acid?
Section 17.7 Assessing the Acid–Base
Behavior of Salts in Aqueous Solution
Skill Review
65. Arrange the following 0.10 M solutions in order of decreas-
ing pH: NH
4
Cl, NaCl, NaC
2
H
3
O
2
.
66. Which of these ions could produce a basic aqueous solution?
S
2−
,Cl
−
,NO
3
−
,NO
2
−
,CO
3
2−
,OCl
−
.
67. Two acids, HX and HY, have pK
a
values of 4.55 and 5.44, re-
spectively. Which salt, NaX or NaY, will produce the more
basic aqueous solution when prepared as 0.10 M?
68. Two sodium salts, symbolized as NaW and NaY, are com-
pletely dissolved to produce 0.20 M solutions. The respective
pH values of the two solutions are 8.55 and 9.55. Which acid,
HW or HY, is stronger? Prove your choice.
69. What is isoelectric pH? Why is it useful information to know
about amino acids?
70. If the isoelectric pH for an amino acid were above 7, what
would that indicate about the relative values of its K
a
and K
b
?
71. The K
a
value for the dissolved cation Zn(H
2
O)
6
2+
is approx-
imately 2.4 × 10
−10
. What is the pH of a solution that is
0.10 M ZnCl
2
? (Hint: What happens when ZnCl
2
dissolves?)
72. Will each of these salts be acidic, basic, or neutral?
a. KI b. NH
4
F c. (NH
4
)
3
PO
4
73. Determine the value of K
a
if:
a. K
b
= 4.26 × 10
−5
b. K
b
= 8.36 × 10
−9
c. pK
a
= 2.85
74. Determine the value of K
b
if:
a. K
a
= 6.90 × 10
−3
b. K
a
= 1.77 × 10
−12
c. pK
a
= 4.74
75. Determine the pH of a solution that is 0.050 M in HCOONa.
76. Determine the pH of a solution that is 0.136 M in KNO
2
.
Chemical Applications and Practices
77. The salt ammonium chloride is used in some chemical “cold
packs” to absorb heat and cool muscle wounds. Ammonium
chloride can be produced from an acid–base reaction.
a. Write out the reaction using an acid and a base that would
produce ammonium chloride.
b. Would the resulting solution of ammonium chloride be
acidic, basic, or neutral? Explain.
c. Determine the pH of a solution of ammonium chloride
that is 0.136 M.
78. The salt sodium hypochlorite (NaOCl) can be used in some
bleaching actions needed for disinfecting aqueous systems.
a. Write out the balanced equation that represents the
complete dissociation of the salt in water.
b. Would this be likely to produce an acidic, basic, or neutral
solution?
c. Determine the pH of a solution that is 0.250 M NaOCl.
79. Sodium carbonate, sometimes called soda ash, is used indus-
trially in the manufacture of glass, paper, and soaps. What
would be the pH of a 0.15 M solution of sodium carbonate?
80. Sodium bicarbonate, also known as baking soda or sodium
hydrogen carbonate, is produced industrially by the addition
of carbon dioxide to soda ash. Sodium bicarbonate has wide-
spread uses in antacids, paper manufacturing, and some fire
extinguishers, as well as to remove some harmful gases dur-
ing coal combustion. What would be the pH of a 0.15 M
solution of sodium bicarbonate?
81. Sodium benzoate, a salt of benzoic acid sometimes used as a
food preservative, dissolves in water to produce the benzoate
ion, C
6
H
5
CO
2
−
. The K
a
value for benzoic acid is approxi-
mately 6.5 × 10
−5
.
a. Write out the reaction of the benzoate ion in water and
calculate the K
b
value for the reaction.
b. What would be the pH of a 0.010 M solution of sodium
benzoate?
82. Novocain is often used as a local anesthetic. The compound
is actually a salt of the base procaine. Procaine has a K
b
value
of 7.13 × 10
−6
.
a. What would be the K
a
of Novocain?
b. What would be the pH of a 0.010 M solution of Novocain?
83. Lysine is considered an essential amino acid. Essential amino
acids are those that are not synthesized by humans and must,
therefore, be part of a healthful diet. Lysine can be found in
Focus Your Learning
763

HX
HX
HX
HX
HX
HX
HX
HX
(
a
)
(
b
)
HX
HX
X
–
X
–
X
–
X
–
X
–
X
–
X
–
X
–
X
–
X
–
X
–
H
+
H
+
H
+
H
+
H
+
H
+
H
+
H
+
H
+
H
+
H
+
beans. The formula of lysine is given below. Rewrite the for-
mula showing lysine as a zwitterion.
84. Glycine has the simplest structure of the amino acids.
a. Write out the reactions that show glycine acting as an acid
and acting as a base.
b. What would be the pH of a 0.10 M solution of glycine?
(The approximate
K
a
2
and
K
b
2
values, at 25
o
C, needed are
2.0 × 10
−10
and 2.2 × 10
−12
.)
17.8 Anhydrides in Aqueous Solution
Skill Review
85. Give the structure of the anhydride of sulfuric acid.
86. Indicate the structure of the substance that would be the an-
hydride of Ba(OH)
2
.
Comprehensive Problems
87. The hydrogen ion donated by Brønsted–Lowry acids is typi-
cally represented in aqueous solutions as H
3
O
+
(aq).
a. Explain the origin of the positive charge on this ion.
b. What other forms could the hydrogen ion take in water?
c. Would the shape of H
3
O
+
be more likely to be flat or
pyramidal? Explain.
88. Acetic acid, found in vinegar, has a small equilibrium con-
stant. Hydrochloric acid is known as a strong acid. Which of
these two representations depicts acetic acid? (Note: In the
boxes, HX represents a general acid structure where X
−
rep-
resents the conjugate base.)
C COOHH
H
NH
2
C NH
2
CH
2
CH
2
CH
2
CH
2
H
2
N
COOH
H
Explain why acetic acid would make a better solvent than
water for comparing acid strength among strong acids.
92. At 25
o
C the K
w
value for water is 1.0 × 10
−14
. Using that
value and the autoprotolysis of water, determine what per-
cent of water molecules actually dissociate under these con-
ditions.
93. Explain why the second ionization constant of a diprotic acid
is typically much smaller than the first.
94. Only very pure phosphoric acid is used in food products. An
older term for soft drinks is phosphates. This name was ap-
plied because pure phosphoric acid was used to produce a
tart taste in some beverages and to help dissolve the other
ingredients.
a. What is the pH of a solution that is known to be 5.44 M
in H
3
PO
4
?
b. What would be the approximate concentration of
PO
4
3−
(aq) in that solution?
95. a. Using the Internet reference http://www.fda.gov/
medwatch/safety/1997/ephedr.htm explain why caffeine
should not be mixed with stimulants that contain
ephedrine alkaloids.
b. Use other searches to obtain the formula of an ephedrine
alkaloid.
c. Is caffeine an alkaloid? What is meant by the term alkaloid?
96. Lactic acid is produced in muscle tissue during vigorous ex-
ercise. It is also found in spoiled milk. The K
a
value of lactic
acid (CH
3
CHOHCOOH), at 25
o
C, is 1.3 × 10
−4
. What is the
H
+
(aq) concentration in a sample of cellular fluid that is
0.0000033 M in lactic acid and 0.0027 M in lactate ion?
97. You can answer the following question by looking at this link
from the National Atmospheric Deposition Program
(NADP), which contains a variety of data from its nationwide
network of monitoring sites: http://nadp.sws.uiuc.edu/
isopleths/annualmaps.asp. Look up the maps of pH from
1994, and then from 2005. How are the maps similar and
different? Can you explain the reasons for any trends you see?
(1994 data: http://nadp.sws.uiuc.edu/isopleths/maps1994/
phlab.gif.) (2005 data: http://nadp.sws.uiuc.edu/isopleths/
maps2005/phlab.gif.)
Thinking Beyond the Calculation
98. A salt of sorbic acid (HC
6
H
7
O
2
) has been used as mold in-
hibitor. The salt most commonly used in this practice is
potassium sorbate (KC
6
H
7
O
2
).
a. Will a solution of potassium sorbate produce a neutral,
acidic, or basic solution?
b. Write a balanced chemical reaction that describes the
processes that take place when potassium sorbate is
dissolved in water.
c. The K
b
value of the sorbate ion is 5.88 × 10
−10
. What is
the pH of a 0.0100 M solution of potassium sorbate?
d. If a researcher wanted to make 500.0 mL of a solution of
potassium sorbate with a pH of 9.44, how many grams of
this compound would need to be added to the water?
e. A solution is prepared that contains both 0.01000 M NH
3
and 0.01000 M potassium sorbate. What is the pH of
the solution? What effect, if any, does the ammonia have
on the pH of the solution (compare the answers to parts c
and e).
764 Chapter 17 Acids and Bases
89. Is it possible for a concentrated solution of a weak acid to
have the same level of hydronium ion concentration as a
dilute solution of a strong acid such as HCl?
90. Water, which has hydrogen bonded to oxygen, can form both
H
+
ions and OH
−
ions. KOH, which also has hydrogen
bonded to oxygen, produces only OH
−
ions in solution.
Using structure and electronegativity, explain why this situa-
tion occurs.
91. When comparing the strengths of strong acids, one must use
a solvent other than water. One such solvent is acetic acid.

765
Contents and Selected Applications
Chemical Encounters: Industrial and Environmental Applications
of Titrations
18.1 Buffers and the Common-Ion Effect
Chemical Encounters: Scrubbing Sulfur Dioxide Emissions
18.2 Acid–Base Titrations
Chemical Encounters: Anthocyanins and Universal Indicators
18.3 Solubility Equilibria
18.4 Complex-Ion Equilibria
Chemical Encounters: Commercial Uses of Aminopolycarboxylic Acid
Chelating Agents
Applications
of Aqueous
Equilibria
A chemical technician often performs
titrations to analyze solutions for specific
analytes.
Go to college.hmco.com/pic/kelterMEE for online learning resources.

766
Water, so essential to life, makes up 70%
of the Earth’s surface and a nearly equal pro-
portion of our own body mass. Water in living
organisms is useful as a medium in which to dissolve
the compounds necessary for life, and it also acts as a bar-
rier to keep some compounds out of our bodies. As fundamen-
tal as it is to life, there are some everyday processes in which the
presence of water can be harmful. Small amounts of water in your gas
tank can reduce the efficiency of your car’s engine; water in your motor oil
increases the rate of decomposition of the lubricating properties (which can lead
to the breakdown of the interior of your engine); and water in the coolant used in
the manufacture of metal parts can damage the tooling machines, resulting in im-
perfections in the parts.
One job of people working as chemical technicians is to perform tests
many times each day to determine the quality of the coolant, oil, or gaso-
line that their company uses or sells. Indeed, one of the many measures
of quality oil is that it contains only a negligible amount of water. Tech-
nicians use the analytical method of titration, which we first discussed in
Section 4.3, to measure the concentration of water in oil and to measure
the quantity of a whole host of compounds and ions in water samples.
A
titration, shown in the photograph at the beginning of this chapter, is
the controlled addition of just enough solution of known concentration,
called a
titrant, to react with essentially all of an analyte (the substance of
interest) so that we can determine its concentration. Titrating oil to de-
termine the amount of water present is only one of the multitudes of
applications of titrations. These applications fall into several different
categories, including those that
■
cause a reduction or oxidation to occur in an analyte
■
result in the formation of a precipitate
■
form a complex ion
■
involve an acid and a base reacting
Food and pharmaceutical manufacturers use titrations
for
quality control—making sure that the product con-
tains what it is supposed to, and in the proper amounts.
Environmental chemists use titrations for analyzing
trace (very small) amounts of hazardous metals and
other potentially harmful substances. Table 18.1 lists
some analyses that are commonly accomplished using
titrations. At the core of most titrations are many of the
principles of aqueous equilibrium that we discussed in
Chapters 16 and 17, and we will put those concepts to
good use here.A good starting point is buffer solutions
because they are commonplace both in industrial titra-
tion analyses and, more broadly, in biochemical systems
(including us!).
Application
C
HEMICAL
E
NCOUNTERS:
Industrial and
Environmental
Applications of
Titrations
Water, essential for life, is not as desirable
in some consumer products such as
motor oil, where it adversely affects the
oil’s lubricating properties.
Selected Titration-Based Analyses
What the Titration Primary
Determines Reagent
Acidity Sodium hydroxide
Alkalinity Hydrochloric acid
Vitamin C Iodine
Chloride ion Silver nitrate
Water hardness EDTA
Dissolved oxygen Sodium thiosulfate
Salinity Mercuric nitrate
Water Iodine, sulfur dioxide,
primary amines
TABLE 18.1
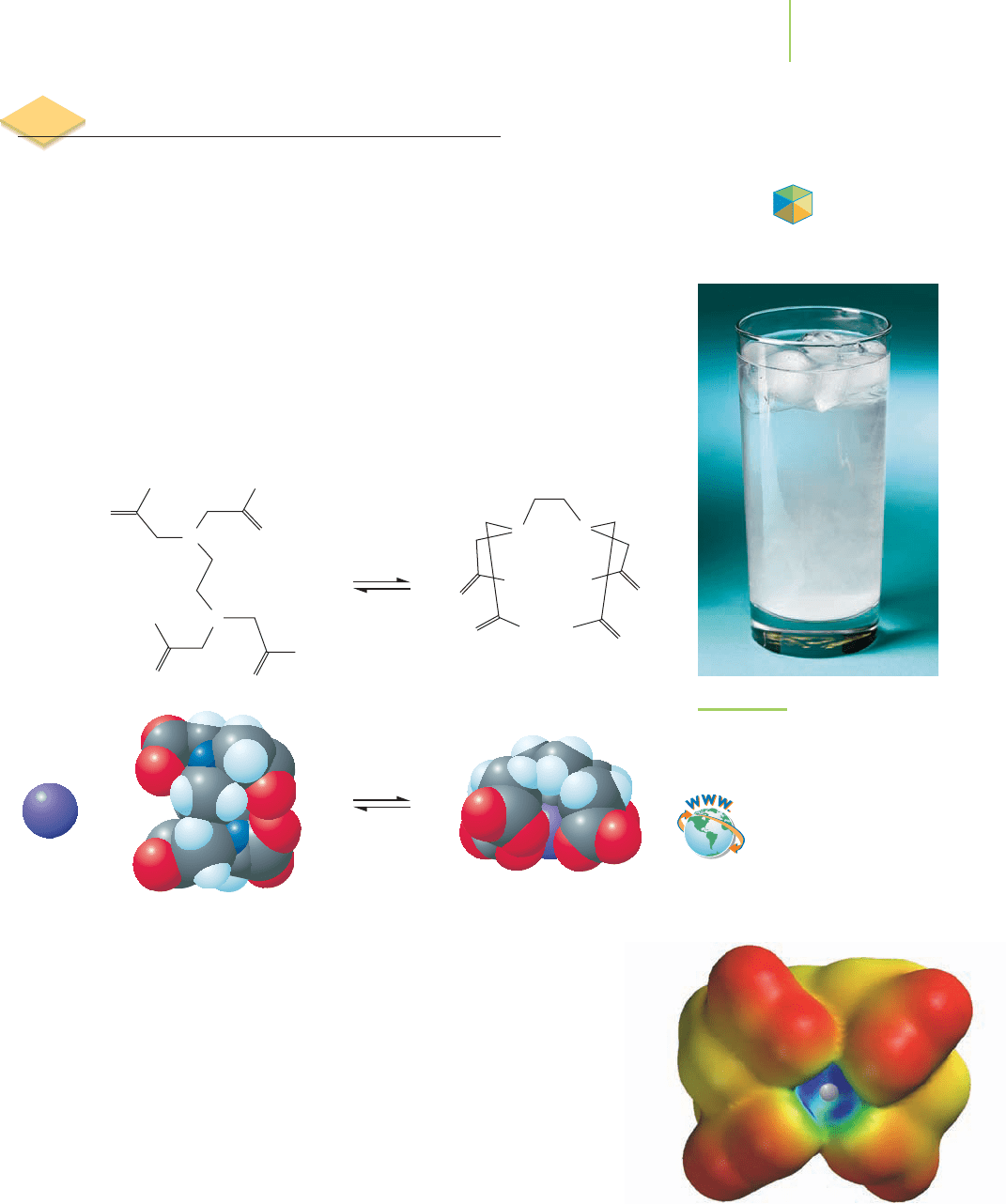
EDTA
4−
complexes with a sodium ion.
The complex involves each of the car-
boxylate oxygens (COO
−
) and the two
nitrogen atoms in the EDTA molecule.
The resulting structure creates a pocket
surrounding the sodium ion.
18.1 Buffers and the Common-Ion Effect 767
18.1 Buffers and the Common-Ion Effect
The degree of “hardness” of water, a measure of the concentration of calcium
(sometimes including magnesium and iron) in household water supplies, is de-
termined by titration. When heated, the calcium ions in hard water form rock-
hard carbonates and sulfates. The resulting solids, known as
boiler scale, build up
on the inside of pipes. In addition, ice cubes made with “hard” water melt to give
an ugly-looking precipitate, as shown in Figure 18.1. Furthermore, very hard
water has a bitter taste that many homeowners find unappealing.
The concentration of calcium ions in water can be determined by titration
with ethylenediaminetetraacetic acid (EDTA), which we first discussed in Chap-
ter 16 and will consider in greater detail later in this chapter as well as in
Chapter 20. The equation describing the titration is
Ca
2+
(aq) + EDTA
4−
(aq)
CaEDTA
2−
(aq) K = 5.0 × 10
10
FIGURE 18.1
Hard water can deposit a white
precipitate in your icy glass of water.
O
O
–
O
–
O
–
O
–
O
–
O
–
O
–
O
–
O
O
O
O
O
O
O
N
N
Ca
2+
Ca
2+
(aq) EDTA
4–
(aq) CaEDTA
2–
(aq)
Ca
2+
N
N
In order to ensure that the EDTA exists as the tetraanion, the entire system must
remain basic during the titration. An added buffer maintains the alkaline solu-
tion. As you will remember from our previous discussions, a
buffer is a chemical
system that is able to resist changes in pH. A buffer is the combination of a weak
acid and its conjugate base or of a weak base and its conjugate acid. Buffers do not
exist if a strong acid or strong base is paired with its conjugate. However, buffers
can accommodate the addition of strong acid and base, and can also withstand dilu-
tion, without large changes in the solution pH.
One standard hard-water analysis protocol requires the addition of a buffer
made from ammonia (a weak base) and ammonium chloride (a source of am-
monia’s conjugate acid). The required pH of the buffer for this analysis is 10.
According to the protocol, this can be achieved when the initial concentrations
are [NH
3
]
0
=8.44 M and [NH
4
+
]
0
=1.27 M. How do these initial concentrations
result in a solution with a pH close to 10?
To answer this question, we proceed as we do with any equilibrium process,
by first examining the possible reactions that can take place in the aqueous solu-
tion. In doing so, we recognize that ammonium chloride (NH
4
Cl) will dissociate
Application
Visualization: Buffers
Video Lesson: An Introduction to
Buffers
Tutorial: Buffered Solutions
