Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

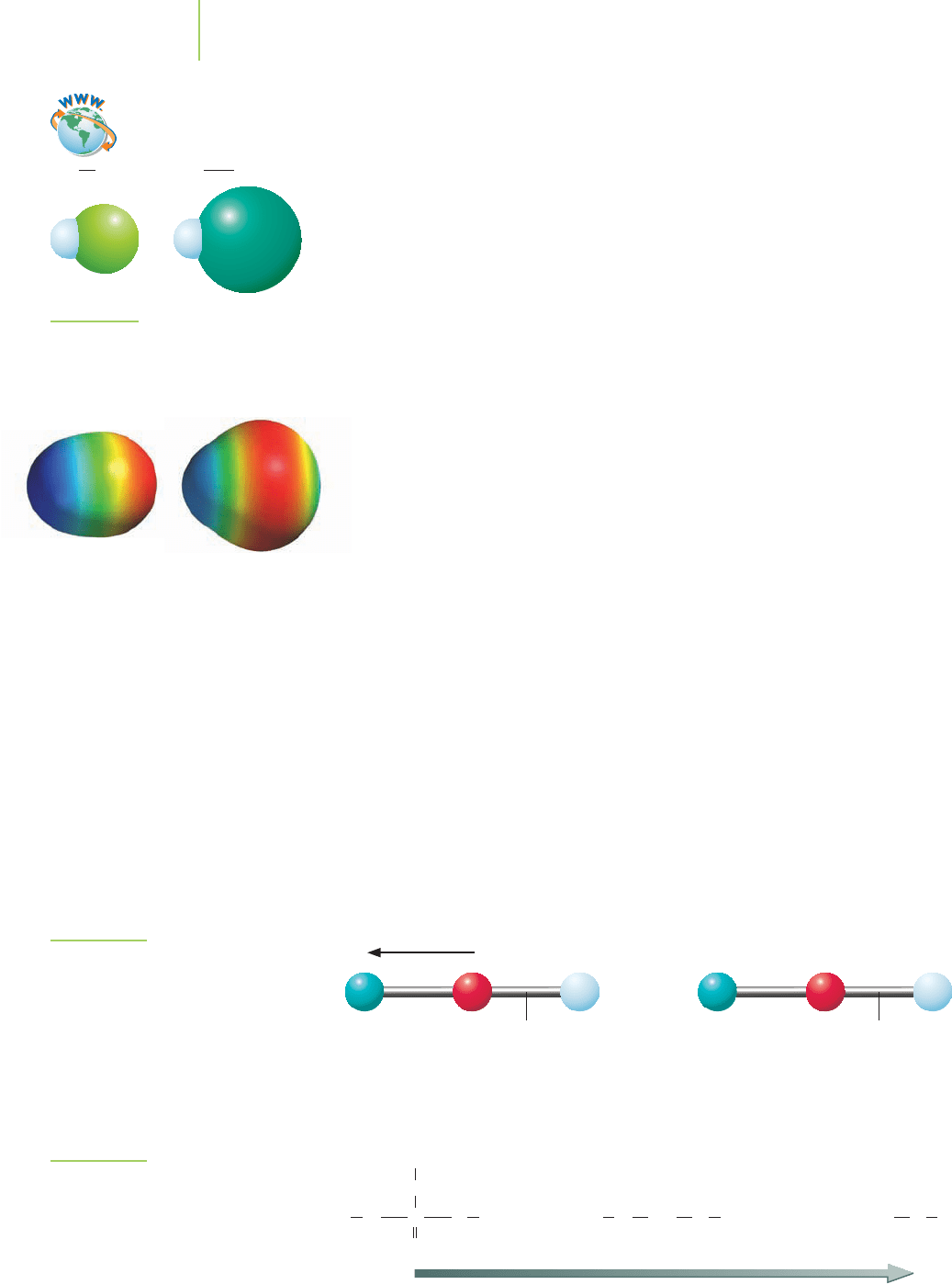
H — F H — Cl
Electrostatic potential maps for HF and
HCl indicate the location of electron den-
sity in the molecules. Note the color of
the map near the hydrogen end of each
molecule. The more intense blue color in-
dicates less electron density around the
hydrogen. How does this compare to the
relative acidities for HF and HCl?
Why Do Acids Have Different Strengths?
As with so many answers to chemical questions, the key to differing acid strengths
lies in structure. For binary acids such as HCl or HF (shown in Figure 17.9),
where the electronegative atom is bonded directly to the hydrogen, smaller atoms
have the valence electrons present in a smaller space. This higher electron density
results in stronger bonds between the electronegative atom and hydrogen, which
makes these acids weaker (less likely to donate the proton). That is why HF is
weaker than HCl. However, if the sizes of the atoms bonded to hydrogen are
about the same, the acidity increases with increasing electronegativity of the atom
bonded to hydrogen, because the polarity of the bond also increases. This is why
HF is a stronger acid than H
2
O, which, though it is not binary, has two H—O
bonds.
Consider a “generic” oxygen-containing compound with a central atom, A, as
shown in Figure 17.10. If A has a high electronegativity, then it will have a ten-
dency to form a covalent bond with oxygen, which is also highly electronegative,
while weakening the bond between the oxygen and hydrogen. The hydrogen can
then be easily removed, which means the compound is acidic. The more elec-
tronegative the central atom (A), the more acidic the compound (see Figure 17.11).
Chlorine is more electronegative than sulfur, which, in turn, is more electroneg-
ative than phosphorus. This means that perchloric acid (HClO
4
) is inherently
stronger than sulfuric acid (H
2
SO
4
), which is stronger than phosphoric acid
(H
3
PO
4
). We do not see the difference between perchloric and sulfuric acids in
aqueous solution, but phosphoric acid is noticeably weaker in water than either
of these other compounds.
For the same central atom (sulfur, for example), the higher the oxidation
state, the higher the attraction for electrons and the stronger the covalent bond
between the sulfur and oxygen atom. This tends to weaken the O—H bond in
these compounds, as described above. This is why H
2
SO
4
(with sulfur in the +6
oxidation state) is a stronger acid than H
2
SO
3
(where sulfur is in the +4 oxida-
tion state). For the same reason, HNO
3
is stronger than HNO
2
, and the strength
of so-called chlorine “oxoacids” is HClO
4
> HClO
3
> HClO
2
> HClO.
This model explains why a compound such as NaOH is basic. Let’s look again
at Figure 17.10, where “A” is Na. Sodium has a relatively low electronegativity and
therefore will not form a strong covalent bond with the oxygen atom. The bond
728 Chapter 17 Acids and Bases
ClHFH
FIGURE 17.9
Compare the relative sizes of the atoms
in HCl and HF.
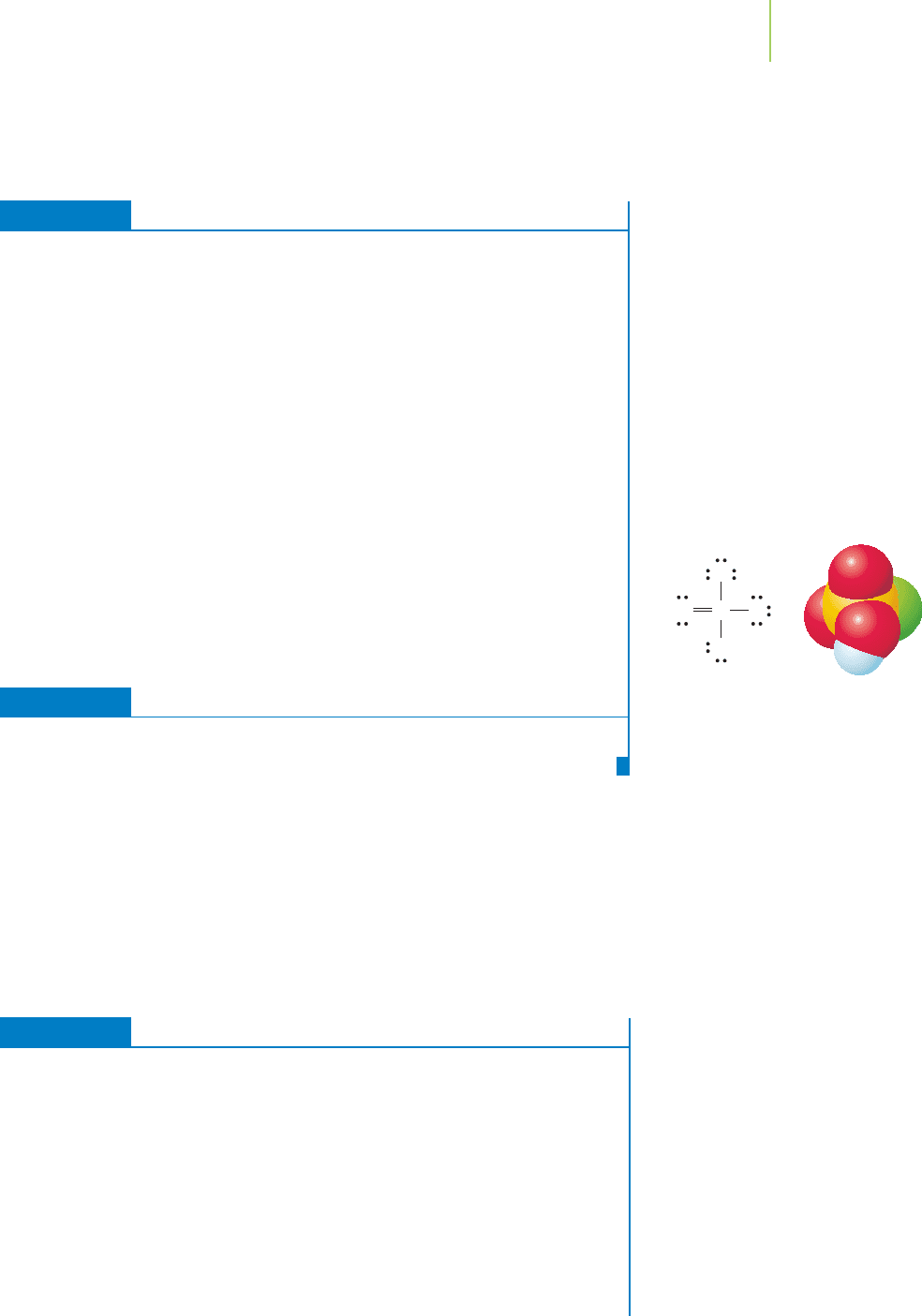
A
OH
A
OH
e
–
density pulled toward A e
–
density not
pulled toward A
Weaker
bond
Electronegative A Less electronegative A
Stronger
bond
FIGURE 17.10
In this “generic” oxygen-containing com-
pound the central atom, A, is bonded to
an oxygen atom, which is itself bonded
to a hydrogen atom. If A is highly elec-
tronegative, it will weaken the bond be-
tween oxygen and hydrogen.
Stronger
HHOO
O
O
H
PHHOOSO
2
HOO
3
Cl
FIGURE 17.11
Chlorine is more electronegative than
sulfur, which, in turn, is more electroneg-
ative than phosphorus. The result is that
HClO
4
is inherently stronger than sulfuric
acid (H
2
SO
4
), which is stronger than
H
3
PO
4
. The listed structures are examples
of resonance structures of each molecule.
Video Lesson: Trends in Acid
and Base Strengths
between the oxygen and hydrogen is stronger and will remain intact. The hy-
droxide ion is therefore readily released to the solution, making NaOH a strong
base in water. The same model holds for all Group IA bases.
EXERCISE 17.5 Which Acid Is Stronger?
Given the two acids HIO and HIO
3
and two values for K
a
, 0.17 and 2 × 10
−11
, indi-
cate which K
a
value goes with which acid.
First Thoughts
How do we judge which of the two acids will be stronger? HIO
3
has more oxygen
atoms than HIO. These additional oxygen atoms will draw electrons away from the
O—H bond, weakening it. The acidic hydrogen will come off more easily from
HIO
3
than the one in HIO.
Solution
HIO
3
is therefore the stronger acid. Its K
a
value is 0.17. The K
a
value for HIO is
2 × 10
−11
.
Further Insights
It is possible to make acids that are amazingly strong by the addition of very elec-
tronegative groups to the central atom. For example, HSO
3
F, shown at right, is one
of several superacids that can be used to add hydrogen ions to organic molecules
that are otherwise impervious to such reaction. Other superacids include HSO
3
CF
3
and the recently synthesized HCB
11
H
6
X
6
,where X = Cl or Br.
PRACTICE 17.5
Explain your answer to the following question:“Which acid is stronger, H
2
SorHBr?”
See Problems 17, 19, and 20.
Will the hydrogen ion concentration always be greater in a solution of a
strong acid than in a solution of a weak acid? We might envision that a solution
of a strong acid generates a greater hydrogen ion concentration than a similar
solution of a weak acid. However, recall our previous discussion of the terms
strong, weak, concentrated, and dilute. The concentration of the acid, be it weak or
strong, must be considered if we are to determine the concentration of hydrogen
ions in solution. In fact, a concentrated solution of a weak acid can produce more
hydrogen ions in solution than a dilute solution of a strong acid.
EXERCISE 17.6 Concentrated Versus Dilute Strong Acids
Fish and other aquatic organisms are very sensitive to the acid concentration in
their surroundings. Lake trout, for example, can flourish when the hydrogen ion
concentration in a lake is 1 × 10
−5
M, but they cannot survive if the hydrogen
ion concentration becomes greater than 1 × 10
−4
M. Indicate whether a 1000.0 L
tank, filled to capacity, would have too great a hydrogen ion concentration for lake
trout to survive if the tank contained:
a. 0.0365 g of HCl
b. 6180 g of boric acid (H
3
BO
3
). This much boric acid in 1000.0 L of water
ionizes about 0.008% = 0.008 mol of H
+
produced per 100 mol of H
3
BO
3
added. Note: We are strictly concerned with the acid concentration here, not
with the possible impact of the boric acid itself on the fish.
17.2 Acid Strength 729
OFS
OH
O
First Thoughts
In order for the Lake trout to survive in the tank, the [H
+
] must be less than or equal
to 1 × 10
−4
M. Because there is 1000.0 L of water in the tank, the number of moles
of hydrogen ion supplied by the acid in each case must not be more than
1 ×10
−4
mol H
+
1 L solution
×
1000.0 L solution = 0.1 mol H
+
Which of our acids supply more than this quantity of hydrogen ions to the solution
(that is, which ones will kill the fish)? Keep in mind that HCl essentially completely
ionizes in solution.
Solution
a. 0.0365 g HCl
×
1 mol HCl
36.5gHCl
×
1 mol H
+
1 mol HCl
= 0.00100 mol H
+
As this result shows, the strong acid solution is so dilute that the fish can
survive.
b. 6180 g H
3
BO
3
×
1 mol H
3
BO
3
61.8gH
3
BO
3
×
0.008 mol H
+
100 mol H
3
BO
3
= 8 × 10
−3
mol H
+
In this case, the fish would not die from the acid level caused by the addition of
this very weak acid.
Further Insights
Aquatic life is very sensitive to acid concentration. Reproduction in salmon is af-
fected when the hydrogen ion concentration is greater than 1 × 10
−6
M, and snails
cannot live in waterways that exceed that hydrogen ion concentration.
PRACTICE 17.6
What is the hydrogen ion concentration of a solution in a 500.0 L tank filled to
capacity with an aqueous solution that contains 2.38 g of the strong acid HNO
3
?
See Problems 21–24.
As we saw in the previous exercise, just because an acid is strong does not nec-
essarily mean that the resulting hydrogen ion concentration due to this acid will
be substantial. Instead, the equilibrium hydrogen ion concentration will depend
on both the strength and the initial concentration of the acid. The acidity of so-
lutions, whether food, shampoos, or samples of acid precipitation, is properly
discussed in measures of equilibrium hydrogen ion concentration.
17.3 The pH Scale
Although acid concentrations can be expressed in terms of their molarities, we
can describe them more conveniently with a mathematical operator that gets rid
of the exponent. This is done via a term, “p,” that is the first part of the common
expression
pH. You may have heard pH used to describe the acidity of a waterway,
as in “The pH of the water in the lake is about 4.5,”or in advertisements for sham-
poo: “It will protect your hair; it is pH-balanced.”
What is pH? We can use this
standard definition:
pH = −log [H
+
]
The term “p” is a mathematical operator that in practice means “take the negative
base-10 logarithm of.” The combined term “pH” is interpreted as “the negative
730 Chapter 17 Acids and Bases
Visualization: pH Scale
Video Lesson: Hydronium,
Hydroxide, and the pH Scale

base-10 logarithm of the hydrogen ion concentration.” For example, if [H
+
] =
6.4 × 10
−2
M, then pH = −log (6.4 × 10
−2
M). To solve this on the calculator,
you would do the following:
1. Enter 6.4 × 10
−2
into your calculator.
2. Press the “log” button. (The calculator display will now read “−1.1938 ....”)
3. Press the “+/−” key, to give the final answer of 1.19, rounded to two signifi-
cant figures past the decimal point.
17.3 The pH Scale 731
This means that for a solution in which [H
+
] = 6.4 × 10
−2
M, the pH is 1.19. In
subsequent chapters we will deal with related terms such as pCl, which is roughly
equal to –log [Cl
−
], and pCa, which approximately represents –log [Ca
2+
]. The
next exercise helps illustrate why “p” is a convenient operator with which to ex-
press concentrations.
The equation (pH
=–log [H
+
]) can also be used in reverse. For instance, if we
know that the pH of a solution is 1.19, we can calculate the [H
+
]. Mathematically,
the conversion is
pH =−log[H
+
]
1.19 = −log[H
+
]
−1.19 = log[H
+
]
10
−1.19
= [H
+
]
[H
+
] = 6.4 × 10
−2
M
In general, we can rearrange our equation for calculating pH into a form that will
enable us to calculate the [H
+
].
[H
+
] = 10
−pH
We need to make a note about significant figures and logarithms. The expo-
nent on a number written in scientific notation is not significant, as we have seen.
The numbers to the left of the decimal point in a logarithm carry the same mean-
ing as the exponent in scientific notation. That is, they are not considered signif-
icant figures. For instance, from our discussion above, each of
6.4 × 10
−2
M and pH = 1.19
possesses only two significant figures.
Video Lesson:
Common
Mathematical
Functions

1.00 x 10
+0
1.00 x 10
–1
1.00 x 10
–2
1.00 x 10
–3
1.00 x 10
–4
1.00 x 10
–5
1.00 x 10
–6
1.00 x 10
–7
1.00 x 10
–8
1.00 x 10
–9
1.00 x 10
–10
1.00 x 10
–11
0510
pH
[H
+
]
EXERCISE 17.7 Using the Operator “p” to Determine Useful Quantities
Calculate the desired term.
a. pH for [H
+
] = 3.2 × 10
−11
M d. pH for [H
+
] = 3.2 × 10
−10
M
b. pK
a
for K
a
= 1.8 × 10
−5
e. [H
+
] for pH = 12.73
c. pOH for [OH
−
] = 8.8 × 10
−13
M f. [OH
−
] for pOH = 5.08
Solution
In parts a–d, we use the operator “p” and take “−log of” the indicated value. Note
that the log of a value has no units; it is dimensionless. In parts e and f, we use 10
−p
to determine the answer.
a. pH = −log (3.2 × 10
−11
M) = 10.49
b. pK
a
= −log (1.75 × 10
−5
M) = 4.74
c. pOH = −log (8.8 × 10
−13
M) = 12.06
d. pH = −log (3.2 × 10
−10
M) = 9.49
e. [H
+
] = 10
−12.73
= 1.9 × 10
−13
M
f. [OH
−
] = 10
−5.08
= 8.3 × 10
−6
M
PRACTICE 17.7
Determine each of the desired terms.
a. pH for [H
+
] = 4.61 × 10
−3
M d. [H
+
] for pH = 3.92
b. pK
a
for K
a
= 2.77 × 10
−9
e. [H
+
] for pH = 1.49
c. pOH for [OH
−
] = 3.22 × 10
−6
M f. [OH
−
] for pOH = 9.93
See Problems 25, 26, 33, and 34.
Converting from [H
+
] to pH enables us to express acid concentration in a
way that does not require exponents. The use of the log scale has another impor-
tant implication. If we look again at parts a and d of Exercise 17.7, we see that the
value of [H
+
] changes by a factor of 10 and the pH changes by one unit. This is
the impact of taking the log of a number. The pH will change by one unit for each
power-of-10 change in [H
+
] concentration. A decrease in [H
+
] from 1 × 10
−
5
to
1 ×10
−
6
M is indicated by an increase in pH from 5 to 6. This power-of-10 rela-
tionship is shown in Figure 17.12, in which we begin with [H
+
] = 1 × 10
−
8
M
in water and increase by powers of 10 until we get to [H
+
] = 1 M.
732 Chapter 17 Acids and Bases
FIGURE 17.12
Relationship between pH and [H
+
]. The
pH unit is a logarithm term. Therefore,
a 1-unit increase represents a 10-fold
increase in [H
+
].
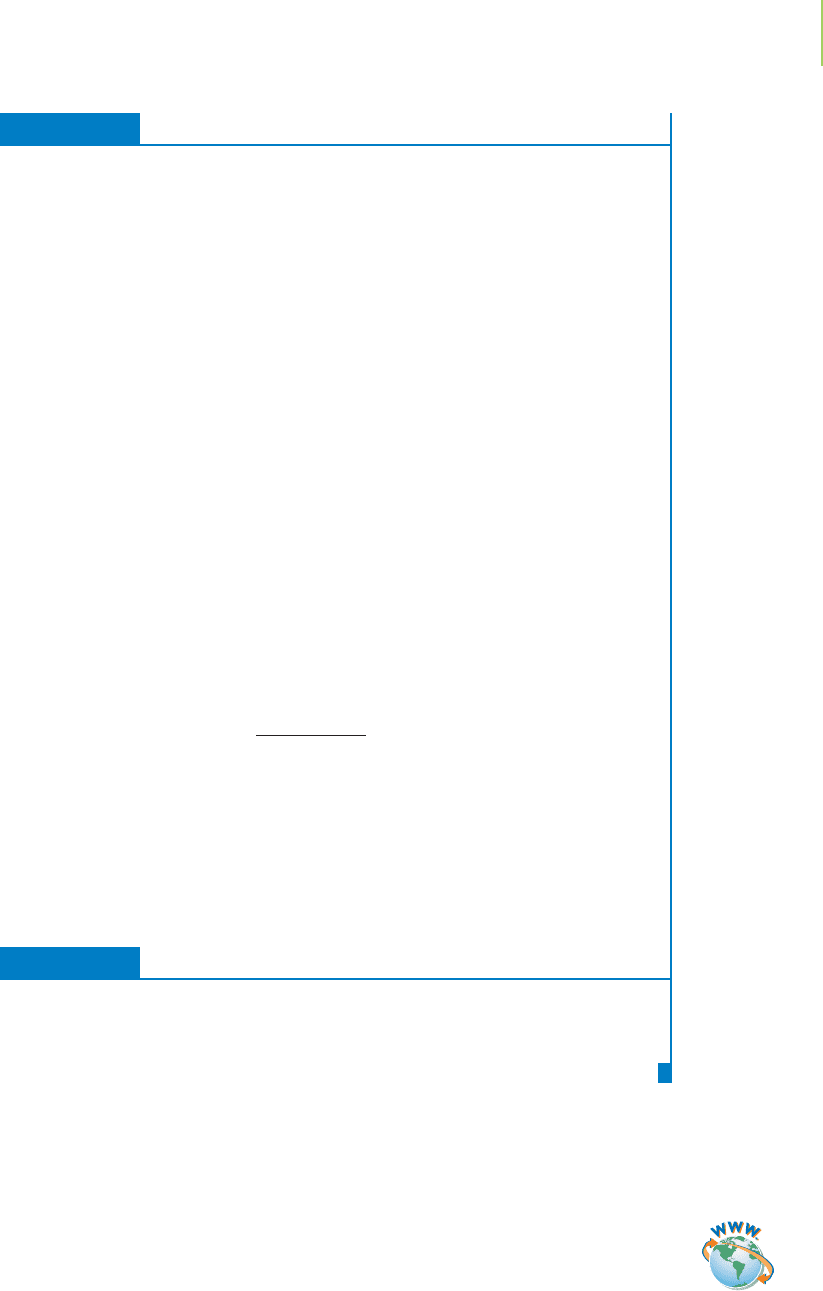
EXERCISE 17.8 [H
+
] and pH: One Implication
In Exercise 17.6, we discussed the sensitivity of aquatic species to the hydrogen ion
concentration. Let’s extend this discussion. Mussels can survive in waterways with a
pH of 6.8. They cannot survive at pH 5.2. What is the ratio of the hydrogen ion con-
centrations in the two solutions? That is, how many times greater is one than the
other?
First Thoughts
The pH scale is logarithmic. That is, each pH unit represents a difference in [H
+
] of
a factor of 10. The pH values of 6.8 and 5.2 therefore have very different hydrogen
ion concentrations. In order to find the ratio of the concentrations, we must convert the
pH of each solution into its hydrogen ion concentration. We cannot compare the pH
values, themselves, as a measure of the hydrogen ion concentration ratio. Which one
has the higher hydrogen ion concentration, [H
+
]? The lower the pH, the higher the
[H
+
], so the pH 5.2 solution has a greater [H
+
] than the pH 6.8 solution.
Solution
[H
+
] = 10
−pH
, so for the waterway that has a pH = 6.80,
[H
+
] = 10
−6.80
= 1.6 × 10
−7
M
For the waterway that has a pH = 5.20,
[H
+
] = 10
−5.20
= 6.3 × 10
−6
M
The ratio of the hydrogen ion concentrations is the quotient of the two values:
6.3 ×10
−6
M
1.6 ×10
−7
M
= 39
Further Insights
The waterway at pH 5.20 has an acid concentration that is about 40 times greater
than that of the pH 6.80 waterway. This shows that seemingly small changes in pH
can translate into large changes in hydrogen ion concentration. This is of particular
importance in discussions involving the chemistry of life, where small changes in
the pH of blood, for example, can be hazardous or fatal.
PRACTICE 17.8
If the hydrogen ion concentration of a body of water is 500 times that of another
waterway, and the pH of the less acidic water is 8.84, what is the pH of the more
acidic water?
See Problems 29 and 30.
Water and the pH Scale
Pure water can undergo autoprotolysis, proton transfer within only the solvent
itself. Such a proton transfer would indicate that one of the water molecules is
acting as a base and the other is acting as an acid.
H
2
O(l) + H
2
O(l)
H
3
O
+
(aq) +OH
−
(aq) K
w
= 1.00 × 10
−14
at 24°C
The ability of a compound to act both as an acid and as a base (not necessarily
in the same reaction) isn’t unique to water. Those compounds capable of such a
feat are called
amphiprotic. They include, among many others, water, ammonia
(NH
3
), and, as we shall discover in Section 17.7, the amino acids that make up the
proteins in the human body. In the autoprotolysis of water, the reaction is often
simplified as
H
2
O(l)
H
+
(aq) + OH
−
(aq) K
w
= 1.00 × 10
−14
at 24°C
17.3 The pH Scale 733
Visualization: Self-Ionization
of Water
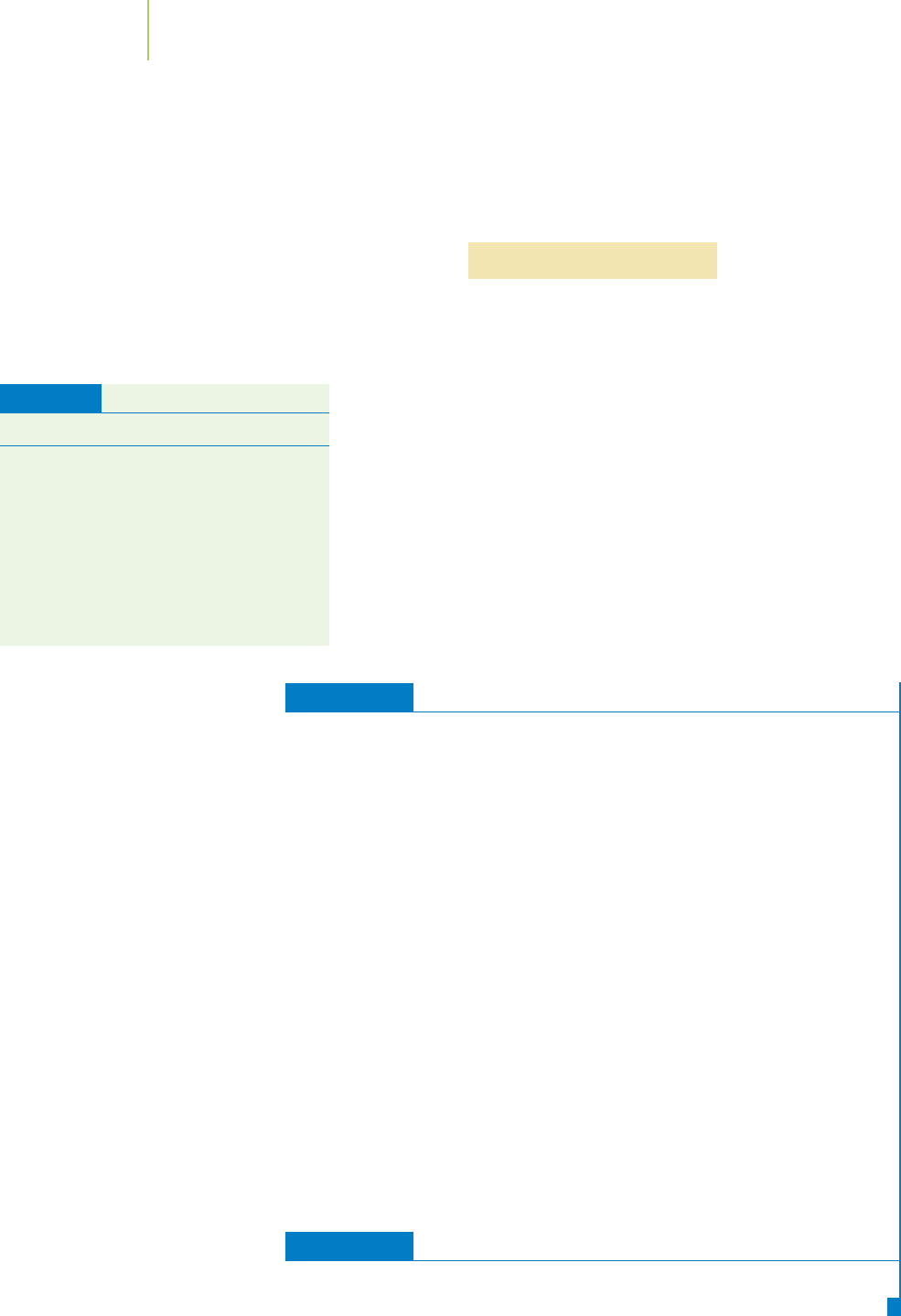
It is understood that the H
+
and OH
−
really aren’t naked ions; they are solvated
by the aqueous solution. However, we can write the equation this way to simplify
our equation. In any case, this equation has the mass-action expression
K
w
= [H
+
][OH
−
] = 1.00 × 10
−14
Taking the log of both sides gives
pK
w
= pH + pOH = 14.00
In pure water, the only source of [H
+
] and [OH
−
] is water itself, so the concen-
trations of H
+
and OH
−
(formed in a 1:1 ratio) will be equal. What are the con-
centrations of H
+
and OH
−
in pure water? If we call each “x,” then
[H
+
][OH
−
] = x
2
= 1.00 × 10
−14
x = [H
+
] = [OH
−
] = 1.00 × 10
−7
M
This means that in pure water at 24
o
C, [H
+
] = [OH
−
] = 1.00 ×10
−7
M,
and the solution will have pH = 7.0. We define this as
neutral pH when
the solvent is water and the system is at 24°C. This pH value also equals
pOH because both [H
+
] and [OH
−
] are equal to 1.0 × 10
−7
M under
these conditions. The K
w
value is temperature dependent, as shown in
Table 17.5.
Unless otherwise stated, we will assume a temperature of 24
o
C for
the remainder of our discussion, so that K
w
= 1.00 × 10
−14
. In aqueous
solution, we can always determine pH, [H
+
], pOH, or [OH
−
] in a solu-
tion if any one of these factors is known.
EXERCISE 17.9 Water in a Pristine Lake
Pure water has a pH = 7.0. But “pristine” rainwater (unaffected by pollutants from
sources such as nitrogen oxides from automobile tailpipe emissions or sulfur oxides
from industrial smokestack emissions) generally has a pH of between 5.5 and 6.0.
This results from the dissolving and equilibration of carbon dioxide from the at-
mosphere and the subsequent release of a hydrogen ion to the water.
CO
2
(g) + H
2
O(l)
H
2
CO
3
(aq)
H
+
(aq) + HCO
3
−
(aq)
Many waterways in the United States have pH values significantly lower than this
range as a consequence of acid precipitation, in which the stronger acids HNO
3
and H
2
SO
4
combine with the water. We discussed the implications of this in Exer-
cises 17.6 and 17.8. If the pH of the water in a pristine lake is 5.90, determine the
value of [H
+
], [OH
−
], and pOH in this water.
Solution
There are several ways to do this problem. We’ll show just one.
pOH = 14.00 − pH = 14.00 − 5.90 = 8.10
[H
+
] = 10
−
pH
= 10
−5.9
= 1.3 × 10
−
6
M
[OH
−
] = 10
−
pOH
= 10
−8.10
= 7.9 × 10
−9
M
We can check the result by noting that [H
+
][OH
−
] = K
w
and
(1.3 × 10
−6
)(7.9 × 10
−9
) = 1.0 × 10
−14
.
PRACTICE 17.9
What are the pH, [H
+
], and [OH
−
] values for a solution with pOH = 12.35?
See Problems 27 and 28.
734 Chapter 17 Acids and Bases
K
w
at Several Temperatures
Temperature K
w
pK
w
0°C 1.14 × 10
−15
14.94
10°C 2.92 × 10
−15
14.54
20°C 7.81 × 10
−15
14.11
24°C 1.00 × 10
−14
14.00
25°C 1.01 × 10
−14
14.00
30°C 1.47 × 10
−14
13.83
50°C 5.47 × 10
−14
13.26
60°C 9.71 × 10
−14
13.01
TABLE 17.5
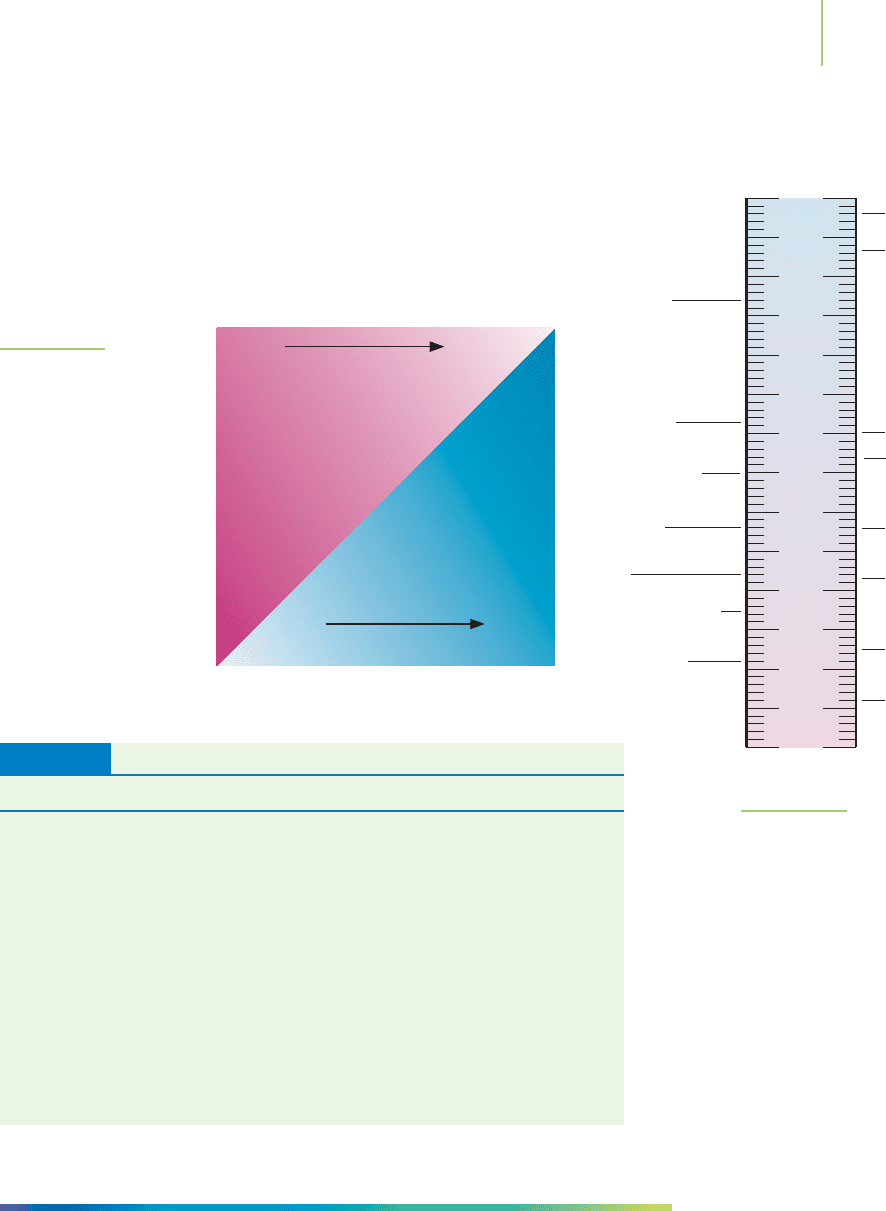
Very basic
Very acidic
Ammonia
Egg whites
Distilled water
Swimming
pool water
Household lye
Bleach
14.0
12.0
11.0
10.0
9.0
8.0
7.0
6.0
5.0
4.0
3.0
2.0
1.0
0.0
13.0
Seawater
Egg yolks
Orange juice
Vinegar
Battery acid
Pure rain
Beer
Pickle processing
Lemon juice
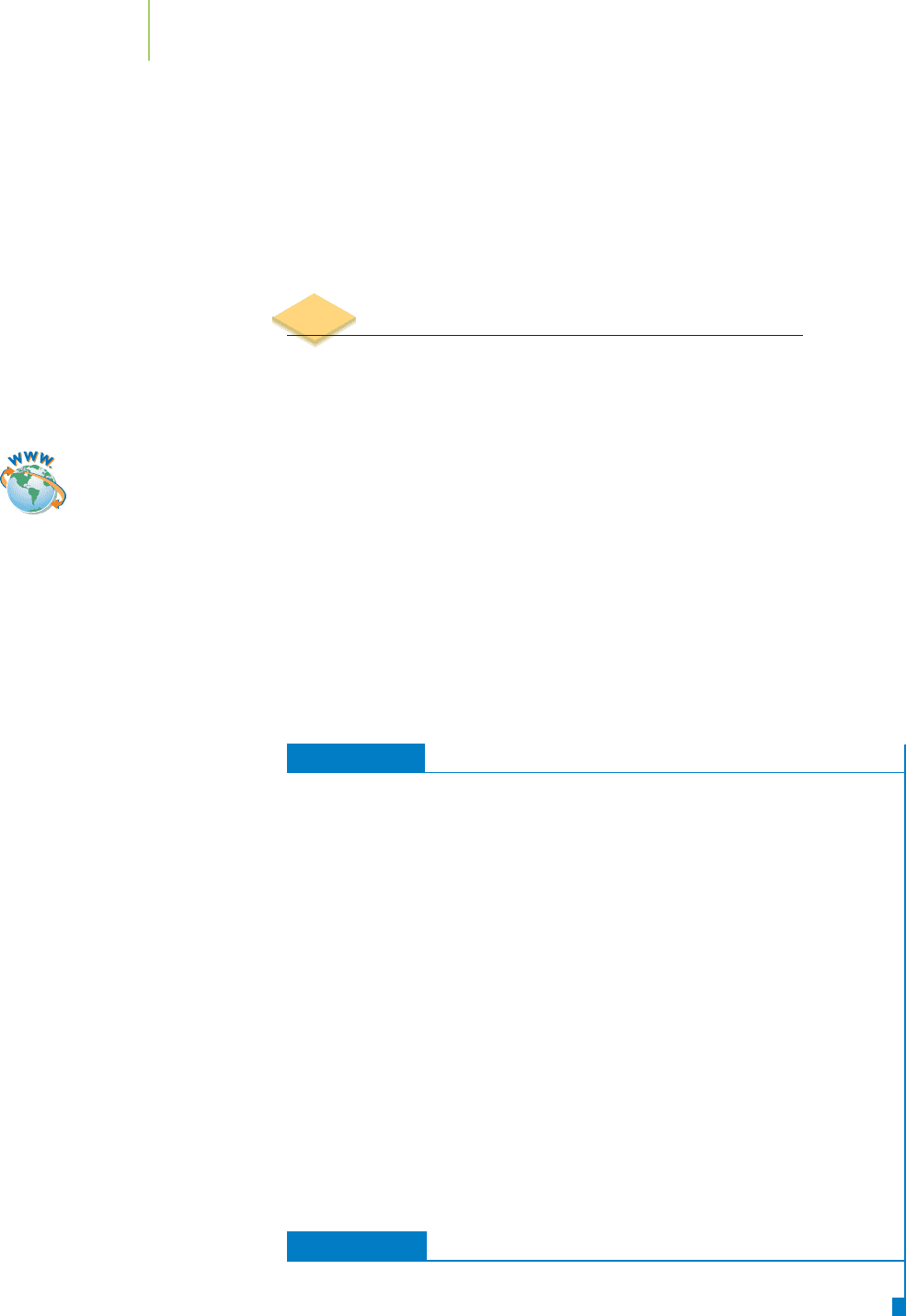
The sum of the pH and pOH values is 14.00. This is the basis of the common
pH scale, shown in Figure 17.13. Note that the hydrogen ion and hydroxide ion
concentrations are inversely related. Aqueous solutions with a pH less than 7.0
are said to be
acidic, and those with a pH greater than 7.0 are basic. Also, as the
solutions become more acidic or basic, their pH moves farther away from 7.0,
as shown in the figure. The pH values of some common substances are also listed
in Table 17.6 and illustrated graphically in Figure 17.14.
17.3 The pH Scale 735
0714
pH
Log scale: Concentration (M)
[H
3
O
+
]
Large
[H
3
O
+
]
Small
[OH
–
]
Small
[OH
–
]
Large
FIGURE 17.13
The sum of the pH and
pOH values is 14.00 in
water at 24°C. This is the
basis of the common pH
scale, shown here. Note
the inverse relationship
between the concentra-
tion of hydrogen and
that of the hydroxide ion.
FIGURE 17.14
pH of some common
substances.
HERE’S WHAT WE KNOW SO FAR
■
We now understand what is meant when we read or hear about acids and
bases.
■
We know the difference between strong and weak acids and the difference
between concentrated and dilute acids.
■
We have a frame of reference—the pH scale—with which we can categorize
solutions of acids and bases.
■
We now know where many common substances fit within this frame of
reference.
pH of Some Common Substances
Substance Contains This Acid or Base pH
Battery acid Sulfuric acid 1.3
Stomach acid Hydrochloric acid 1.5−3.0
Vinegar Acetic acid 2.5
Wines Tartaric acid 2.8−3.8
Apples Malic acid 2.9−3.3
Food preservative Benzoic acid 3.1
Cheese Lactic acid 4.8−6.4
Blood Carbonate ion and others 7.3−7.5
Baking soda Bicarbonate ion 8.3
Detergents Carbonate, phosphate ions 10−11
Milk of magnesia Magnesium hydroxide 10.5
Drain cleaner Sodium hydroxide 13+
TABLE 17.6
At its heart, chemistry is about the interactions of substances. The applications of
chemistry concern the changes that result from these interactions. To understand
the nature of change in acid–base chemistry, we first need to consider how to de-
termine the changes that occur when an aqueous acidic or basic solution is pre-
pared. This will be the focus of the remainder of this chapter. In the next chapter,
we will consider how changing a prepared acidic or basic solution affects its
chemical behavior.
17.4 Determining the pH of Acidic Solutions
We have learned that strong acids essentially completely dissociate in aqueous so-
lution (K
a
>>1) and that weak acids as a rule dissociate only partially (K
a
< 1).
We also noted that the concentration of [H
+
] (and therefore the pH) in solution
will be determined by both the strength and the initial concentration of the acid.
pH of Strong Acid Solutions
A monoprotic acid is an acid (such as HCl) that contains only one acidic hydrogen
ion, or proton. H
2
SO
4
, by contrast, contains two acidic protons and is called a
diprotic acid. In a strong monoprotic acid, the hydrogen ion concentration in
aqueous solution roughly equals the initial concentration of the acid (neglecting
activity effects). This is approximately true at any concentration greater than
about 10
−6
M. When the strong acid concentration is less than this, the autopro-
tolysis of water supplies relatively few hydrogen ions that exist in solution, and
the pH becomes more difficult to calculate, as we will discuss later in this section.
Even with an HCl solution of concentration 10
−8
M or less, the pH is still held
just below 7.0 because of the supply of H
+
from the autoprotolysis of water.
EXERCISE 17.10 Calculating the pH of a Strong Acid Solution
Your stomach contains hydrochloric acid. If we were going to prepare a solution
that had a hydrogen ion concentration within the range of that in the stomach, we
might work with a 3 × 10
−2
M aqueous solution of HCl. What is the pH of this
solution?
First Thoughts
This strong acid would essentially completely dissociate to give [H
+
] = 3 × 10
−2
M
and [Cl
−
] = 3 × 10
−2
M.
Solution
We can calculate the pH of this solution as follows:
pH = −log[H
+
] = −log(3 × 10
−2
M) = 1.5
Further Insights
Does the answer make sense? We have a moderately concentrated strong acid, so
we would expect the pH to be in the range of, perhaps, 1 to 3. We can narrow our ex-
pected answer down further by noting that [H
+
] is between 10
−1
and 10
−2
M, which
means that the pH will be between 1 and 2. Our answer to this problem does make
sense.
PRACTICE 17.10
What is the pH of a 0.0010 M HNO
3
solution? ...ofa 0.0000250 M HClO
4
solution?
See Problem 37.
736 Chapter 17 Acids and Bases
Tutorial: Calculating pH of
Strong Acid and Base Solutions

Le Châtelier’s Principle and the Supply
of Hydroxide Ion in Acidic Solutions
Consider a strong acid at pH 3.0. On the basis of our previous discussion, we
know that the pOH is 11.0, and [OH
−
] = 1 × 10
−11
M. Where does that small
amount of hydroxide ion come from? The only source is the autoprotolysis of
water:
H
2
O(l)
H
+
(aq) + OH
−
(aq) K
w
= 1.00 × 10
−14
If the liquid were pure water, [OH
−
] would equal 1 × 10
−7
M. However, the ad-
dition of H
+
from the strong acid imposed a stress on the aqueous system, to
which it responded by lessening the extent of dissociation of water. To put it
another way, when the acid was added, the reaction shifted to the left to com-
pensate. This is an example of Le Châtelier’s principle, which we discussed in Sec-
tion 16.6, and is known as the
common-ion effect. We added an ion common to
one of the products (H
+
), and the result was that the reaction did not proceed
to the right as much as it would have without the acid. We will look at many of
the practical outcomes of Le Châtelier’s principle and the common-ion effect in
the next chapter. The key in this introduction to acids and bases is that the addi-
tion to an aqueous solution of any substance that produces H
+
will reduce the
supply of H
+
and OH
−
from the autoprotolysis of water. Therefore, in all but the
dilute acid solutions, the autoprotolysis of water as a source of H
+
is generally
negligible.
For example, let’s consider the pH of a 1.0 × 10
−8
M solution of HCl, a strong
acid. We might predict that the HCl produces 1.0 × 10
−8
M H
+
in solution. We
would be correct in our prediction.
HCl(aq) n H
+
(aq) + Cl
−
(aq)
1.0 × 10
−8
M n 1.0 × 10
−8
M 1.0 × 10
−8
M
Determining the pH of this solution gives
pH =−log[H
+
]
pH =−log(1.0 × 10
−8
)
pH = 8.00
Does our answer make sense? No it doesn’t. Adding HCl to water, even a very small
amount, shouldn’t cause the pH to increase! What have we forgotten to consider?
The concentration of hydrogen ions in the solution is made up of all sources of
[H
+
].
[H
+
]
total
= [H
+
]
HCl
+ [H
+
]
water
This means we should add all of the sources of hydrogen ion together and then
determine the pH of the solution. What is the [H
+
] due to water? Recall our au-
toprotolysis equation:
H
2
O(l)
H
+
(aq) +OH
−
(aq) K
w
= 1.00 ×10
−14
On the basis of this, we might be inclined to say that [H
+
]
water
= [OH
−
]
water
=
1.0 × 10
−7
M. However, we must keep in mind the effect of Le Châtelier’s
principle, in which having hydrogen ions supplied by the HCl suppresses the
ionization of H
2
O. Therefore, [H
+
]
water
will be less than 1.0 × 10
−7
M. Although
we won’t go into the details here, it is possible to determine that in this solution,
[H
+
]
water
= 9.5 × 10
−8
M.
[H
+
]
total
= [H
+
]
HCl
+ [H
+
]
water
[H
+
]
total
= 1.0 × 10
−8
M + 9.5 × 10
−8
M
[H
+
]
total
= 1.05 × 10
−7
M
pH = 6.98
17.4 Determining the pH of Acidic Solutions 737
