Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

718
“Home is where the heart is.” Home is also
where the phosphoric acid is and where other
important acids are, including nitric, sulfuric, acetic,
and even acetylsalicylic and ascorbic acids. Most homes
also have products containing bases, among which we count
ammonia and sodium hydroxide as the two most important.
Given the continuing increase in population, the resulting need for
more food, the frantic pace of new home construction (see Figure 17.1),
and the increase in sales of consumer products, we can understand why many
acids and bases are among the top 20 chemicals produced in the United States, as
shown in Table 17.1.
Why are acids and bases worth knowing about? As we will see in this chapter, they are a
part of every household, the people in it, and the house itself. Acid–base reactions in
our blood keep us alive. Amino acids combine to form proteins that are enzymes,
hormones, and structural materials in
our bodies. DNA and RNA are formed
from nucleotides that are made by the
chemical combination of phosphoric
acid, a sugar, and a base. Our food is
mostly acidic. Many of us take daily
supplements of vitamin C (ascorbic
acid) for its health benefits. We
clean our homes with products that
include both acids, such as acetic acid
(CH
3
COOH) and sulfuric acid (H
2
SO
4
)
and bases, such as ammonia (NH
3
) and
Application
C
HEMICAL
ENCOUNTERS:
Common Uses of
Acids and Bases
Acids and Bases in Top Chemicals Produced in U.S. Industry in 2004
Substance Key Commercial Uses Billions of kg Produced
Sulfuric acid Fertilizer 37.5
Calcium oxide Cement, paper 18.4*
Phosphoric acid Fertilizers, animal feed 11.5
Ammonia Fertilizers, plastics 10.8
Sodium carbonate Glass, cleansers 10.3*
Sodium hydroxide Industrial synthesis 9.5
Nitric acid Fertilizers, explosives 6.7
*2002 data.
Source: Chemical & Engineering News, July 11, 2005, pp. 69–75.
TABLE 17.1
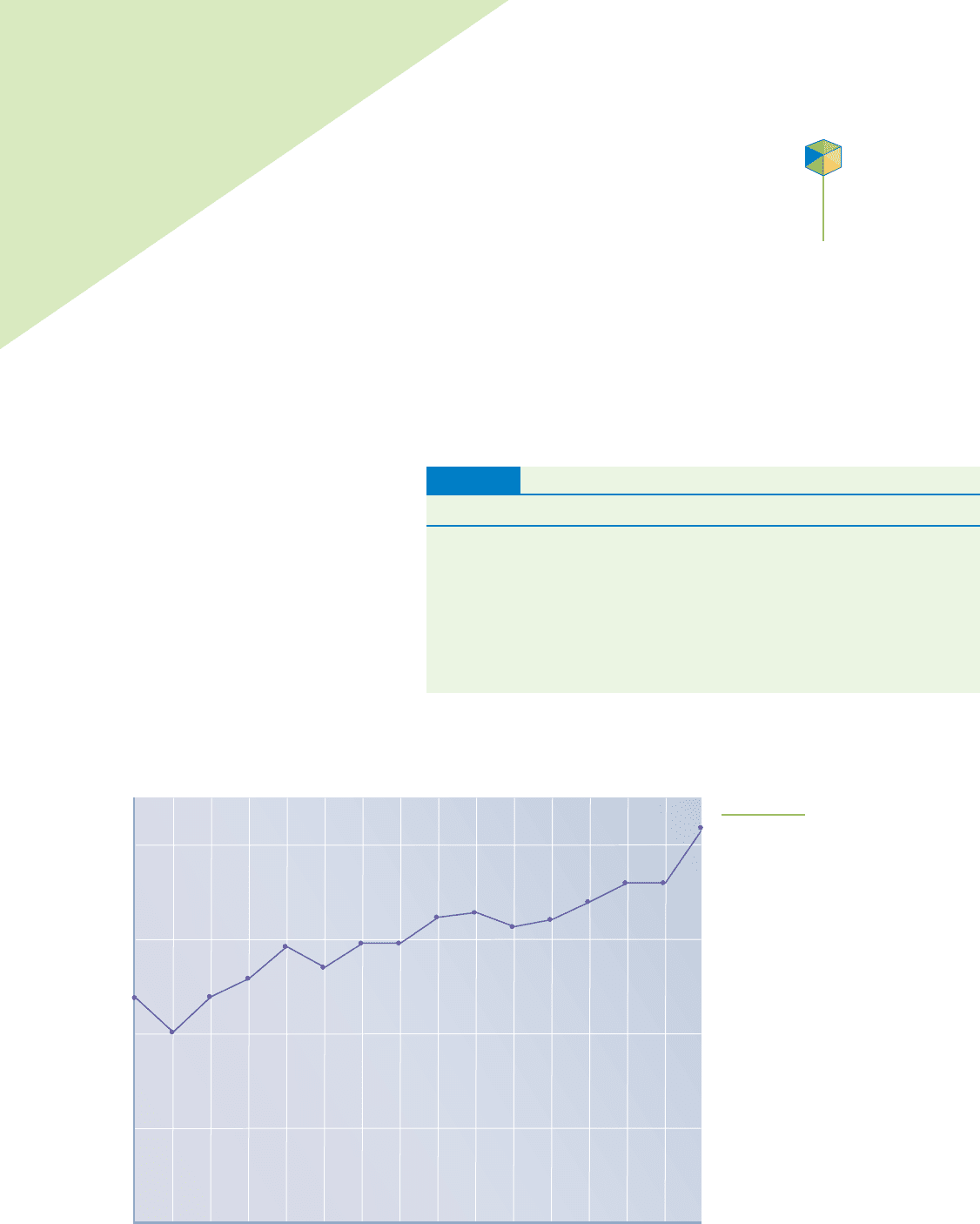
0.5
1.0
1.5
2.0
1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001
U.S. housing starts (in millions)
2002 2003 2004 2005
FIGURE 17.1
Housing starts in the United States
for the 16-year period ending in
2005. Acids and bases are a part of
home construction, household fur-
nishings, and appliances. This trend
in new home construction indicates
a robust demand for the industrial
production of acids and bases.

sodium hypochlorite (NaOCl). The walls of our house or
apartment are likely to be made of gypsum, another name
for calcium sulfate (CaSO
4
), formed from the reaction of
phosphate rock and sulfuric acid. Municipal water supplies
are monitored, and (when required) chemically treated, to
maintain safe acid concentrations.
Acids, such as aluminum sulfate (Al
2
(SO
4
)
3
) and bases, such as sodium hydroxide
(NaOH) and sodium carbonate (Na
2
CO
3
), are used in the manufacture of the paper
on which you are now reading these ideas. Other acids and bases are used in the
manufacture of plastics and polymer-based clothing, such as polyester and rayon.
Computer chips are etched with hydrofluoric acid. Fertilizer for the garden is often
made with ammonia-based compounds. Gold is isolated from its ores with a base
known as sodium cyanide (NaCN). Toothpaste contains a base known as tetra-
sodium pyrophosphate (Na
4
P
2
O
7
). The list goes on and on and on. Whether the
context we discuss is biological, medical, agricultural, environmental, or industrial,
acids and bases are a part of it (see Table 17.2). How much of a part? As we will learn
in Chapter 18, acids and bases are used to answer that as well.
17.1 What Are Acids and Bases? 719
Sodium hydroxide
NaOH
Magnesium hydroxide
Mg(OH)
2
Ascorbic acid
C
6
H
8
O
6
Acetic acid
CH
3
OOH
Acetylsalicylic acid
C
9
H
8
O
4
Acids and bases are worth knowing about because they
are ubiquitous in the world both around us and within
our bodies. Those household products containing bases
are listed in blue; those containing acids are listed in red.
Common Acids and Bases and Examples of Their Uses
Acid or Base Example of Use
Acetylsalicylic acid Aspirin
Ammonia Household cleaners, fertilizer,
manufacture of fertilizers
Lauric acid, CH
3
(CH
2
)
10
COOH Key ingredient in toothpaste
manufacture
Nitric acid Key ingredient in acid precipitation
Nicotine (a base) Tobacco products
Sodium hydroxide Manufacture of soap, many industrial
compounds
TABLE 17.2
17.1 What Are Acids and Bases?
There are several models we can use to define an acid. One of these is the Arrhenius
model, named after the Swedish chemist Svante Arrhenius (1859–1927), in which
an
acid is any species that produces hydrogen ions in solution and a base is any
speciesthatproduceshydroxide ions in solution.Forexample,nitricacidcanbeclas-
sified as an Arrhenius acid because it produces hydrogen ions in aqueous solution.
HNO
3
(aq)
H
+
(aq) + NO
3
−
(aq)
Similarly, sodium hydroxide is an Arrhenius base because it produces hydroxide
ions in aqueous solution.
NaOH(aq) n Na
+
(aq) + OH
−
(aq)
In truth, hydrogen ions (H
+
) do not exist free in solution. Rather, they always
interact with the solvent, often water. Just as metal cations such as Na
+
are sur-
rounded by water molecules through ion–dipole interactions, hydrogen cations
(which are just protons because they have no neutrons or electrons) similarly
Video Lesson: Arrhenius/
Brønsted–Lowry Definitions of
Acids and Bases
H
3
O
+
H
2
O
interact with water molecules that surround them. In fact, protons interact so
strongly that they become directly incorporated into the fabric of one or more
water molecules, and are easily passed among them. Experimental data show that
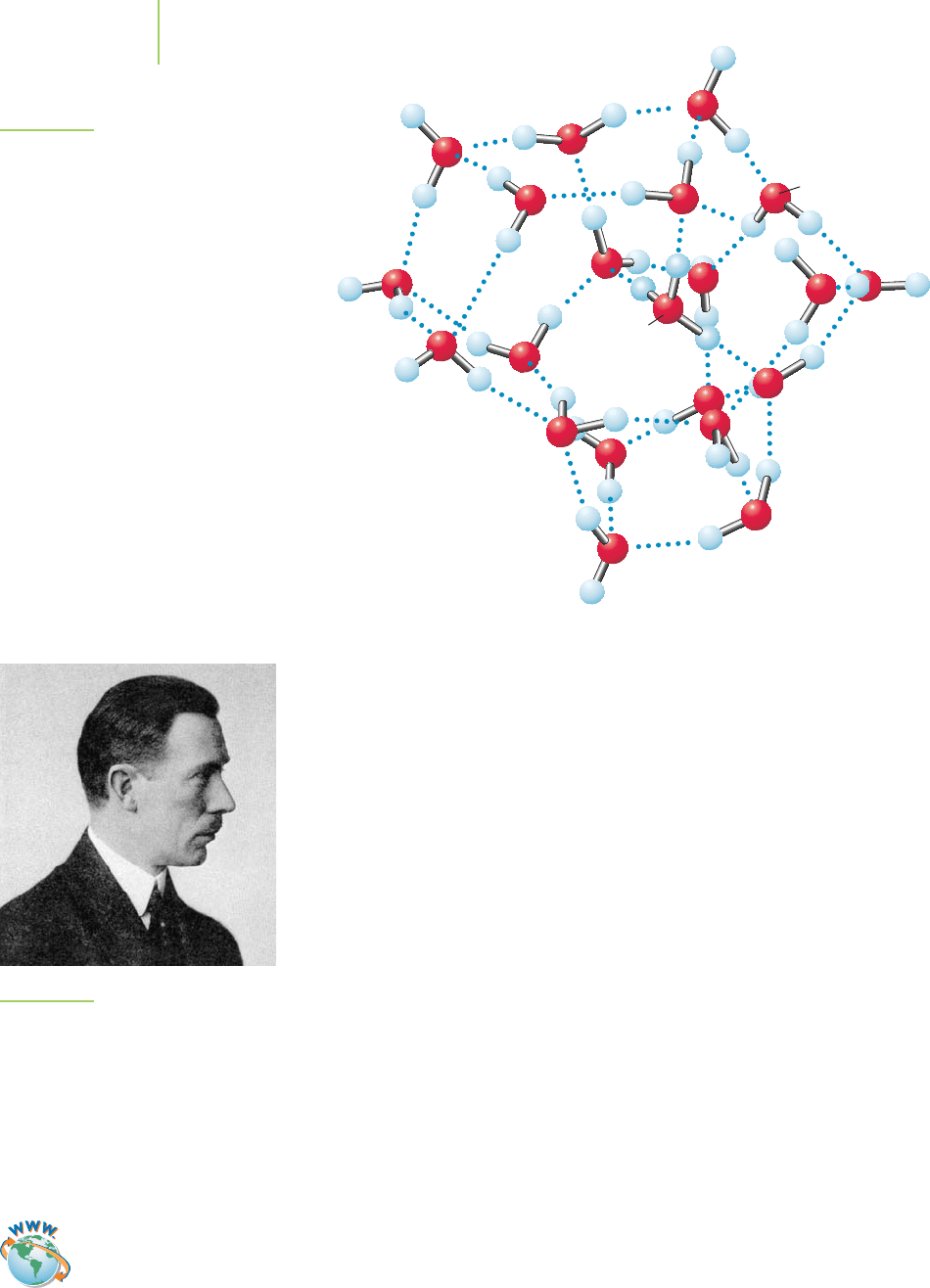
each proton is immediately surrounded by at least four water molecules and is
most likely surrounded by many more. Recent research by several groups in the
U.S. suggests that, when H
+
is surrounded by twenty-one (21) water molecules,
a most wonderful structure, shown in Figure 17.2, is formed! However, in room
temperature aqueous solution, no single well-defined structure exists, because
hydrogen bonds are being continually broken and formed. For simplicity,
chemists typically refer to the hydrogen ion as either H
+
or H
3
O
+
(the hydro-
nium ion), understanding all the while that H
+
actually exists surrounded by sev-
eral water molecules.
A more useful way of describing acids and bases is the Brønsted–Lowry
model (named after the Danish scientist Johannes Nicolaus Brønsted (1879–
1947, Figure 17.3) and the English chemist Thomas Lowry (1874–1936), who
proposed the definition in 1923). In his model, a
Brønsted–Lowry acid is defined
as any species that donates a hydrogen ion (proton) to another species. This defi-
nition of an acid is applicable to all solvents in which protons can be exchanged,
and, being more far-reaching than the Arrhenius model, it is the definition we will
use. A
Brønsted–Lowry base is any species that can accept ahydrogenion(proton)
from an acid. As an example of a Brønsted–Lowry acid and base, consider what
happens when hydrogen chloride gas is dissolved in water.
HCl(g) + H
2
O(l)
Cl
−
(aq) + H
3
O
+
(aq)
Hydrochloric acid Hydronium ion
Figure 17.4 shows the Lewis structures of the reactants and products. In the
Brønsted–Lowry model, HCl is the acid and water is the base. The chloride ion
(Cl
−
) is called the conjugate base of HCl because it is the base that results after the
HCl donates a proton to water. Similarly, H
3
O
+
is the conjugate acid of water
because it is the acid that results after the water accepts the proton from HCl.
The word conjugate comes from the Latin word conjugare, which means “join
together.”
HCl and Cl
−
are a conjugate acid–base pair.
H
3
O
+
and H
2
O are a conjugate acid–base pair.
720 Chapter 17 Acids and Bases
FIGURE 17.2
Data show that hydrogen ions interact with several
water molecules. One possible structure involves a
shell of 21 water molecules surrounding a H
3
O
+
ion.
(Source: Mark Johnson, Yale University.)
FIGURE 17.3
Johannes Nicolaus Brønsted (1879–1947)
was a Danish physical chemist. In addi-
tion to studying quantum mechanics and
developing an acid–base theory, he be-
came interested in politics during the
German occupation of Denmark in World
War II. He was elected to the Danish
parliament in 1947 but became ill and
died before he could take office.
Visualization: Brønsted–Lowry
Reaction
Note that a conjugate base is paired with an acid (Cl
−
and HCl) and a conjugate
acid is paired with a base (H
3
O
+
and H
2
O).
[Base] [Conjugate acid]
HCl(g) + H
2
O(l)
Cl
−
(aq) + H
3
O
+
(aq)
[Acid] [Conjugate base]
EXERCISE 17.1 Acids, Bases, and Their Conjugates
Complete the equation for the reaction of formic acid (HCOOH) and water. Iden-
tify the conjugate acid–base pairs.
HCOOH(aq) + H
2
O(l)
First Thoughts
Formic acid can donate a hydrogen ion to water. Is water an acid or a base in this
process? The products will be the formate and hydronium ions. When deciding on
the conjugate pairs, think about what each reactant forms when it either donates or
accepts a hydrogen ion. We will denote the conjugate pairs as “1” and “2.”
Solution
[Base
2
] [Conjugate acid
2
]
HCOOH(aq) + H
2
O(l)
HCOO
−
(aq) + H
3
O
+
(aq)
[Acid
1
] [Conjugate base
1
]
Further Insights
Conjugate acid–base pairs can exist in nonaqueous solvents (that is, the solvent is
something other than water). For example, phenol (C
6
H
6
OH) acts as an acid when
it reacts with the basic solvent ethylenediamine (H
2
NCH
2
CH
2
NH
2
) in the follow-
ing way:
[Base
2
] [Conjugate acid
2
]
C
6
H
6
OH + H
2
NCH
2
CH
2
NH
2
C
6
H
6
O
−
+ H
2
NCH
2
CH
2
NH
3
+
[Acid
1
] [Conjugate base
1
]
PRACTICE 17.1
Complete the equation for the reaction of ethylenediamine with water. Identify the
conjugate acid–base pairs.
H
2
NCH
2
CH
2
NH
2
(aq) + H
2
O(l)
See Problem 7.
17.1 What Are Acids and Bases? 721
++
++
+
+
–
–
Cl ClHHO
H
(a)
(b)
Acid
1
Acid
2
Base
2
Base
1
HO
H
H
HCl(aq) + H
2
O(l)H
3
O
+
(aq) + Cl
–
(aq)
FIGURE 17.4
When hydrochloric acid gas is added to water,
the HCl molecule ionizes and interacts with the
solvent as shown here via Lewis structures (a)
and space-filling models (b).
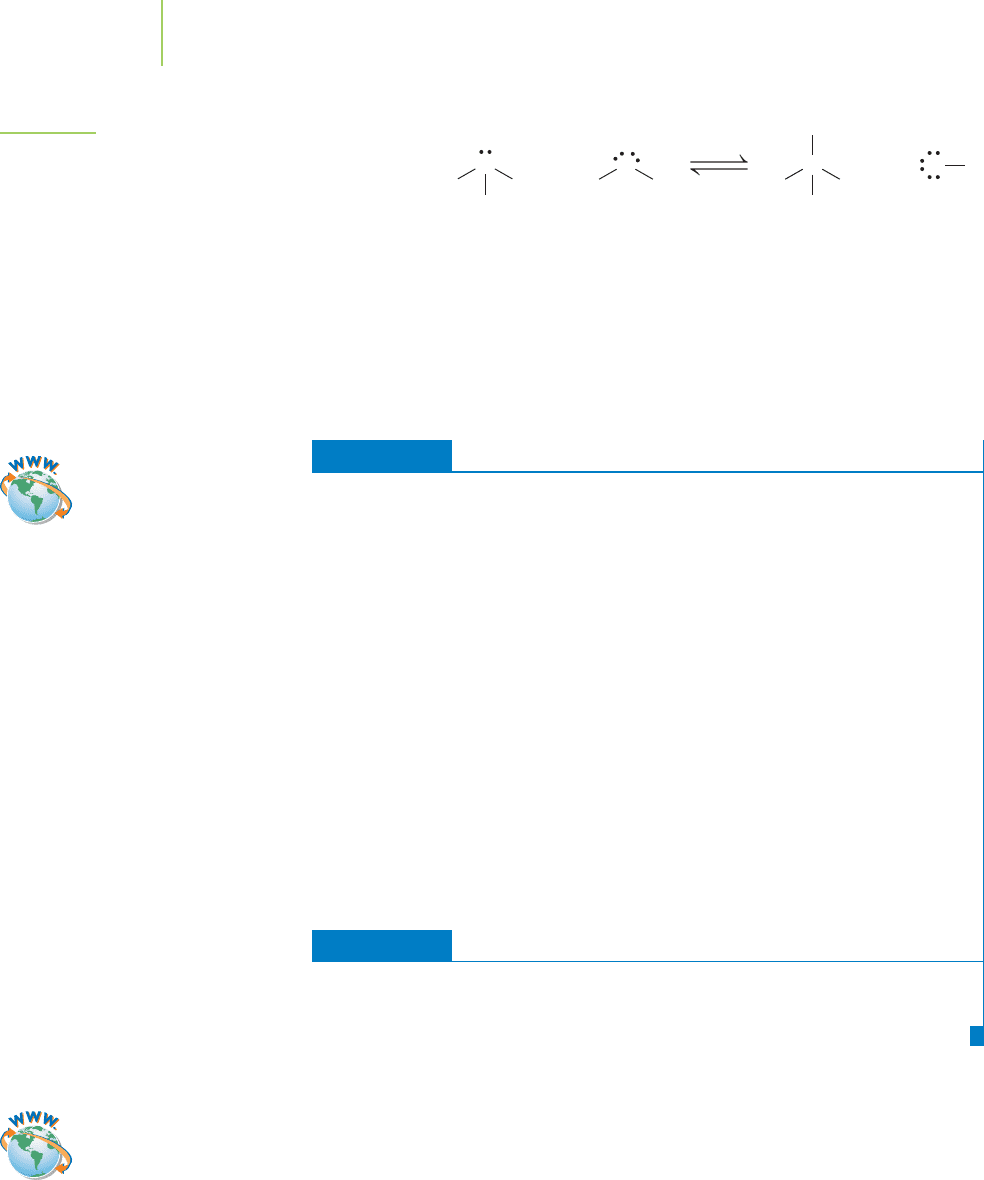
We previously mentioned that ammonia (NH
3
) is an important chemical, and
the 2004 worldwide production figure of 10.8 billion kg would seem to confirm
this. Ammonia is a Brønsted–Lowry base because it accepts a hydrogen ion from
water. The unshared pair of electrons on the nitrogen can form a covalent bond
with the hydrogen, forming the ammonium ion, as shown in Figure 17.5.
EXERCISE 17.2 Ammonia as a Base
Identify the conjugate acid–base pairs in the reaction of ammonia with water. How
does water behave differently in this reaction than when it reacts with HCl?
First Thoughts
This contrasts with Exercise 17.1 because ammonia (NH
3
) is a base. Water takes on
a very different role than in the previous exercise, that of an acid. Yet the concept of
the conjugates is still the same—both ammonia and water will have conjugate pairs.
Solution
[Acid
2
] [Conjugate base
2
]
NH
3
(aq) + H
2
O(l)
NH
4
+
(aq) + OH
−
(aq)
[Base
1
] [Conjugate acid
1
]
Further Insights
We devote many words in this text to the important properties of water. Here we see
another one. As the “universal solvent,” water can be an acid or, as in Exercise 17.1,
a base, depending on the solute. Any substance that can be an acid or a base is called
amphiprotic. We will explore this property later in the chapter.
PRACTICE 17.2
Identify the conjugate pairs in the reaction of lauric acid (CH
3
(CH
2
)
10
COOH) with
water.
See Problems 3–6, 11, and 12.
G. N. Lewis (remember him from Chapter 8 for his work representing sub-
stances via Lewis structures) proposed a third model of acids and bases in which
a
Lewis base donates a previously nonbonded pair of electrons (a lone pair) to
form a coordinate covalent bond. Electrons are not transferred entirely but,
rather, are shared to make a bond. Reduction and oxidation are not taking place
when a Lewis base donates a pair of electrons. All Brønsted–Lowry acids are also
Lewis acids, and all Brønsted–Lowry bases are also Lewis bases. However, many
metal ions, such as Al
3+
,are Lewis acids, because they can accept electrons,
although they do not donate protons. For example, when Al
3+
is in aqueous
solution, it combines with water:
Al
3+
(aq) + 6H
2
O(l)
Al(H
2
O)
6
3+
(aq)
It then can act as a Brønsted–Lowry acid:
Al(H
2
O)
6
3+
(aq) + H
2
O(l)
Al(H
2
O)
5
OH
2+
(aq) + H
3
O
+
(aq)
722 Chapter 17 Acids and Bases
H
N
HH
O
HH
H
H
N
HH
HO
FIGURE 17.5
Ammonia is an Arrhenius base because it generates OH
−
in water. It is also considered a Brønsted–Lowry base
because it accepts a proton from water.
Visualization: Ammonia
Fountain
Video Lesson: CIA
Demonstration: The Ammonia
Fountain
Video Lesson: Lewis Acids
and Bases

Ascorbic acid
In another example, we could identify borane (BH
3
) in the reaction below as
a Lewis acid and ammonia as a Lewis base. This reaction and the specific bond-
ing patterns were discussed in detail in Chapter 8.
BH
3
(g) + NH
3
(g)
H
3
BONH
3
(s)
Lewis acid Lewis base
We mentioned in the opening of the chapter that aluminum sulfate is added
to wood pulp as it is formed into paper. The compound is used as a “sizing agent,”
a substance that prevents ink from spreading. Unfortunately, this acid slowly
breaks down the cellulose in paper, causing it to turn light brown with age. That
is why many newspapers and paperback books look old even after only weeks or
months.
We will have more to say about Lewis acids in the next chapter. In our present
discussion, because we are dealing primarily with water as a solvent, and because
Lewis acids become Brønsted–Lowry acids in water, the Brønsted–Lowry model
will be the most useful to us.
How do we know an acid when we see one? Even though
nearly all common foods (including milk!) are acidic, we often
associate acids specifically with citrus juices. Orange juice and
grapefruit juice can taste sour. This is a characteristic property of
acids. Bases, on the other hand, are slippery and often taste bitter.
We learned in Chapter 12 that hydrochloric acid reacts with
zinc to produce hydrogen gas:
Zn(s) + 2HCl(aq) n Zn
2+
(aq) + 2Cl
−
(aq) + H
2
(g)
This is typical of another characteristic of acids: that they
react with many metals to form solutions and often release
hydrogen gas. In this sense, acids corrode metals. Bases often
react with metal ions to produce insoluble hydroxides, such as
Fe(OH)
3
. A vital property that acids and bases share is that they
modify the structure of some types of organic molecules, often
found in plants, to cause color changes. In the next chapter, we
will discuss how we use these color changes to help us analyze
the amount of substances present in a sample. All of these
things—sour or bitter, slimy, reactions with metals, color
changes, and more—are properties that signal that a material is
acidic or is basic (see Table 17.3).
17.2 Acid Strength
We now know what acids and bases are, and we have seen some typical acid–base
reactions. We have shown that acids and bases are present throughout our world
and within ourselves. However, just as people come in all shapes and sizes, acids
and bases come in different strengths. These differences have a profound impact
on their chemical behavior and their uses.
We can begin to understand acid (and, by extension, base) strength by recall-
ing that nearly all of our foods are acidic. Let’s turn that statement around and
see whether it still makes sense. If nearly all foods are acidic, are nearly all acids
suitable as foods? That is, we know that citrus fruits contain citric acid (C
6
H
8
O
7
)
and that ingesting it in reasonable amounts is safe. Oranges and grapefruits also
contain ascorbic acid (H
2
C
6
H
6
O
6
), which is also called vitamin C. Aspirin
(C
8
H
8
O
4
) is known chemically as acetylsalicylic acid. We eat these things. Some
are necessary for good health (vitamin C); some can relieve headaches and may
17.2 Acid Strength 723
Aluminum is a Lewis acid because it
combines with water. Although alu-
minum sulfate is useful as a sizing agent
in books and newspapers to prevent ink
from spreading, the aluminum ion acting
as an acid breaks down cellulose in
paper, causing the pages to become
yellow and brittle after only months.
Properties of Acids and Bases
Acids Bases
Taste sour Taste bitter
Donate protons during Accept protons during
an acid–base reaction an acid–base reaction
React with some metals Form insoluble
to produce hydrogen hydroxides with
gas and metal ions many metal ions
TABLE 17.3
Tutorial: Acid Strength
even help prevent heart attacks (aspirin). Yet ingesting sulfuric acid (H
2
SO
4
)
at the concentration found in a car battery would be lethal. This is also true of
the hydrochloric acid that is used as a 30% solution in water to clean stains on
concrete.
There are two issues here. The first is that sulfuric acid and hydrochloric acid
are strong acids (a term defined below). Many acids found in foods are weak acids.
The other point is that in batteries and concrete cleaners, the acids are relatively
concentrated, whereas in an orange or other food, the acids are fairly dilute.
Diluting the hydrochloric acid by a factor of 1000 with water would render
it ineffective for cleaning concrete, even though it would remain a strong
acid. Some acids are inherently strong and some are weak. Solute concentra-
tion, whether 10 M or 1 × 10
−6
M—that is, whether relatively concentrated or
dilute—doesn’t change the inherent strength of the acids or bases.
Strong and Weak Acids
In aqueous solution, a strong acid is one that at 1 molar concentration dissociates
(or, in some cases, ionizes) essentially completely. “Dissociates” means separates or,
in this case, loses a hydrogen cation—a proton. Molecules in which the hydrogen
atom is covalently bonded actually do ionize when they go into solution. Whether
the process is called dissociation or ionization, the result is the same: the production
of H
+
in the solution. A weak acid only partially ionizes (or dissociates) at 1 molar
concentration in aqueous solution. We can reinforce these ideas by comparing a
strong acid, nitric acid (HNO
3
), to a weak acid, acetic acid (CH
3
COOH). The
ionization equations for 1 molar aqueous solutions of these two acids can be
written as follows:
HNO
3
(aq) + H
2
O(l)
NO
3
−
(aq) + H
3
O
+
(aq)
CH
3
COOH(aq) + H
2
O(l)
CH
3
COO
−
(aq) + H
3
O
+
(aq)
The ionization of nitric acid proceeds essentially all the way to products, so it is a
strong acid. The reverse reaction will not occur to any extent because nitric acid
donates a proton to water (forward direction) much more effectively than the hy-
dronium ion (H
3
O
+
) donates a proton to nitrate ion (reverse direction). Acetic
acid only slightly dissociates (typically less than 1%, depending on its initial con-
centration), so it is a weak acid. This occurs because H
3
O
+
can donate a proton
to the acetate ion (CH
3
COO
−
), the conjugate base of acetic acid, regenerating the
initial reactants (see Figure 17.6).
The equilibrium constant for the dissociation of an acid is called the
acid
dissociation constant (K
a
) and has the same relationship to the extent of an
acid–base reaction as any other equilibrium constant to that of its own reac-
tion. This is a key point:
The principles of equilibrium are consistent no matter what system we are
working with.
724 Chapter 17 Acids and Bases
Application
C
HEMICAL
ENCOUNTERS
:
Acids in Foods
Sulfuric acid Citric acid
Acid and base strength is an inherent prop-
erty of a substance and is unaffected by dilu-
tion. Sulfuric acid, found in car batteries, is
inherently strong, whereas citric acid, found
in oranges, is inherently weak.
Video Lesson: Weak Bases
Video Lesson: Strong Acids
and Bases
Video Lesson: Weak Acids
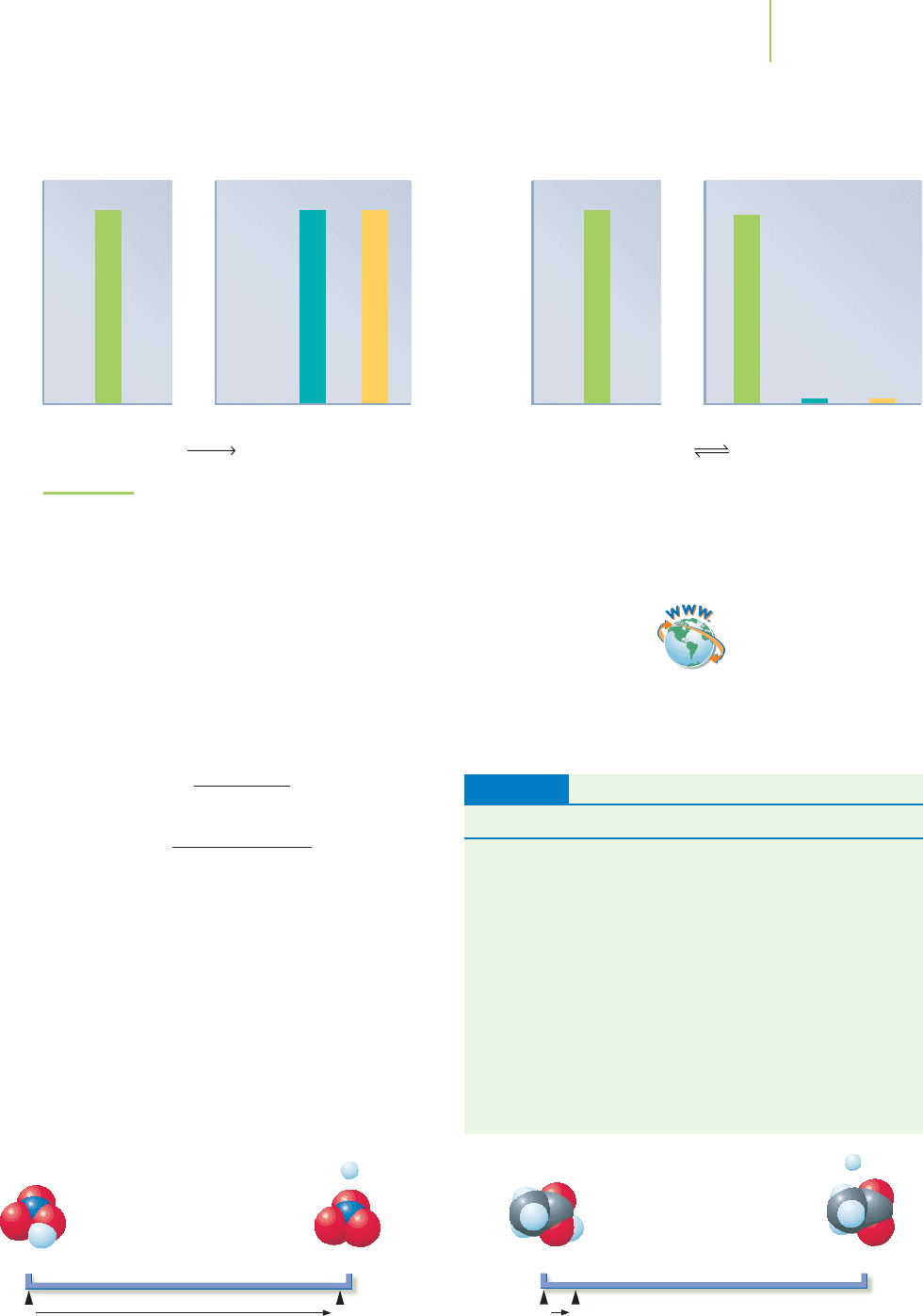
Reactants
SE
Products
Weak acid: acetic acid dissociation.
The equilibrium constant may be called K
a
for acids, K
b
for bases, or K
sp
for
solids; and there are others. But their fundamental meaning as equilibrium con-
stants, and how we use them in our problem solving, are the same.
Table 17.4 lists several weak acids and their K
a
values.
The mass-action expressions for the reactions shown for nitric and acetic
acids with water can be written in the following “shorthand” form (neglecting the
presence of water because it is the solvent, and neglecting activity effects).
K
a
=
[NO
−
3
][H
+
]
[HNO
3
]
K
a
=
[CH
3
COO
−
][H
+
]
[CH
3
COOH]
Nitric and other strong acids have K
a
values much
greater than 1, often regarded as infinity in aqueous
solution.Weak acids,suchas acetic acid (K
a
=1.8 × 10
−5
),
have K
a
values less than 1. We can use the equilibrium
line chart that we introduced in Chapter 16 to see the ex-
tent of the reaction for HNO
3
, the strong acid, compared
to CH
3
COOH, the weak acid.
There are differences in acid strength among strong
acids. For example, HClO
4
is inherently stronger than
HCl. However, this difference not observable in water, in
which HCl, or any other strong acid, appears to be just as
17.2 Acid Strength 725
HNO
3
(aq) + H
2
O(l)H
3
O
+
(aq) + NO
3
–
(aq)
HNO
3
HNO
3
1.0 M
Strong acid
H
3
O
+
H
3
O
+
NO
3
–
Relative number
of molecules
Before dissociation
100 100 100 100
99.5
0.5 0.5
After dissociation
CH
3
COOH(aq) + H
2
O(l)H
3
O
+
(aq) + CH
3
COO
–
(aq)
CH
3
COOH CH
3
COOH
1.0 M
Weak acid
CH
3
COO
–
Relative number
of molecules
Before dissociation After dissociation
FIGURE 17.6
There is a competition between acetic acid (CH
3
COOH) and hydronium ion (H
3
O
+
) to get rid of a hydrogen
ion. The hydronium ion is a stronger acid, so the acetic acid does not dissociate to a great extent. The single-
headed arrow in the ionization of nitric acid indicates that the reaction goes essentially to completion.
K
a
of Selected Weak Acids
Formula Name K
a
HSO
4
−
Hydrogen sulfate ion 1.2 × 10
−2
HClO
2
Chlorous acid 1.2 × 10
−2
HF Hydrofluoric acid 7.2 × 10
−4
HNO
2
Nitrous acid 7.0 × 10
−4
HC
3
H
5
O
3
Lactic acid 1.3 × 10
−4
HC
2
H
3
O
2
Acetic acid 1.8 × 10
−5
(CH
3
COOH)
[Al(H
2
O)
6
]
3+
Hydrated aluminum ion 1.4 ×10
−5
HOCl Hypochlorous acid 3.5 × 10
−8
HCN Hydrocyanic acid 6.2 × 10
−10
NH
4
+
Ammonium ion 5.6 ×10
−10
HOC
6
H
5
Phenol 1.6 × 10
−10
TABLE 17.4
Reactants
SE
Products
Strong acid: nitric acid dissociation.
Visualization: Acid Ionization
Equilibrium
strong as HClO
4
, because their strength makes their reaction with water essen-
tially complete. It is possible to use nonaqueous solvents to show differences in
strength even among strong acids.
EXERCISE 17.3 Relative Acid Strength
Arrange the following acids in order from weakest to strongest. How is your place-
ment of them related to their K
a
values?
Acid K
a
HClO
2
1.2 × 10
−2
CH
3
COOH 1.8 × 10
−5
H
2
SO
4
>1
HCN 6.2 × 10
−10
First Thoughts
Recall that a strong acid is one that dissociates (or ionizes) completely at 1 molar
concentration, whereas a weak one does not. This is measured by the value of K
a
.
Solution
The larger the value of K
a
, the stronger the acid. Based on our values of K
a
, the order
of acids from weakest to strongest is HCN < CH
3
COOH < HClO
2
< H
2
SO
4
.
Further Insights
The significance of the magnitude of the equilibrium constant is the same for acids
(K
a
) and bases (called “K
b
”) in a given solvent. For example, an acid with K
a
= 1 ×
10
−5
has the same meaning for the extent of reaction in water as does a base with an
equilibrium constant, K
b
,of1 × 10
−5
, even though the specific reactions are differ-
ent. This shows that understanding the concept of equilibrium goes well beyond a
particular situation—a consistent theme in this discussion.
PRACTICE 17.3
The Appendix of this textbook has a list of many acids. On the basis of the K
a
val-
ues, pick three acids that are weaker than hydrofluoric acid (HF) and three that are
stronger than hypochlorous acid (HOCl).
See Problems 15 and 16.
Given the relative acid strengths of nitric acid and acetic acid, what might we
conclude about the strengths of their conjugate bases, nitrate ions and acetate
ions? Nitric acid is strong, so its ionization equilibrium to produce H
+
will lie all
the way to the right. In other words, the nitrate ion is such a weak base that it stays
as is and essentially doesn’t go back to form nitric acid at all. Another way of ex-
pressing this is to say that in the tug of war for protons between water (which acts
as a base on the reactant side) and nitrate (which acts as a base on the product
side), the water wins because water is a much stronger base than the nitrate ion.
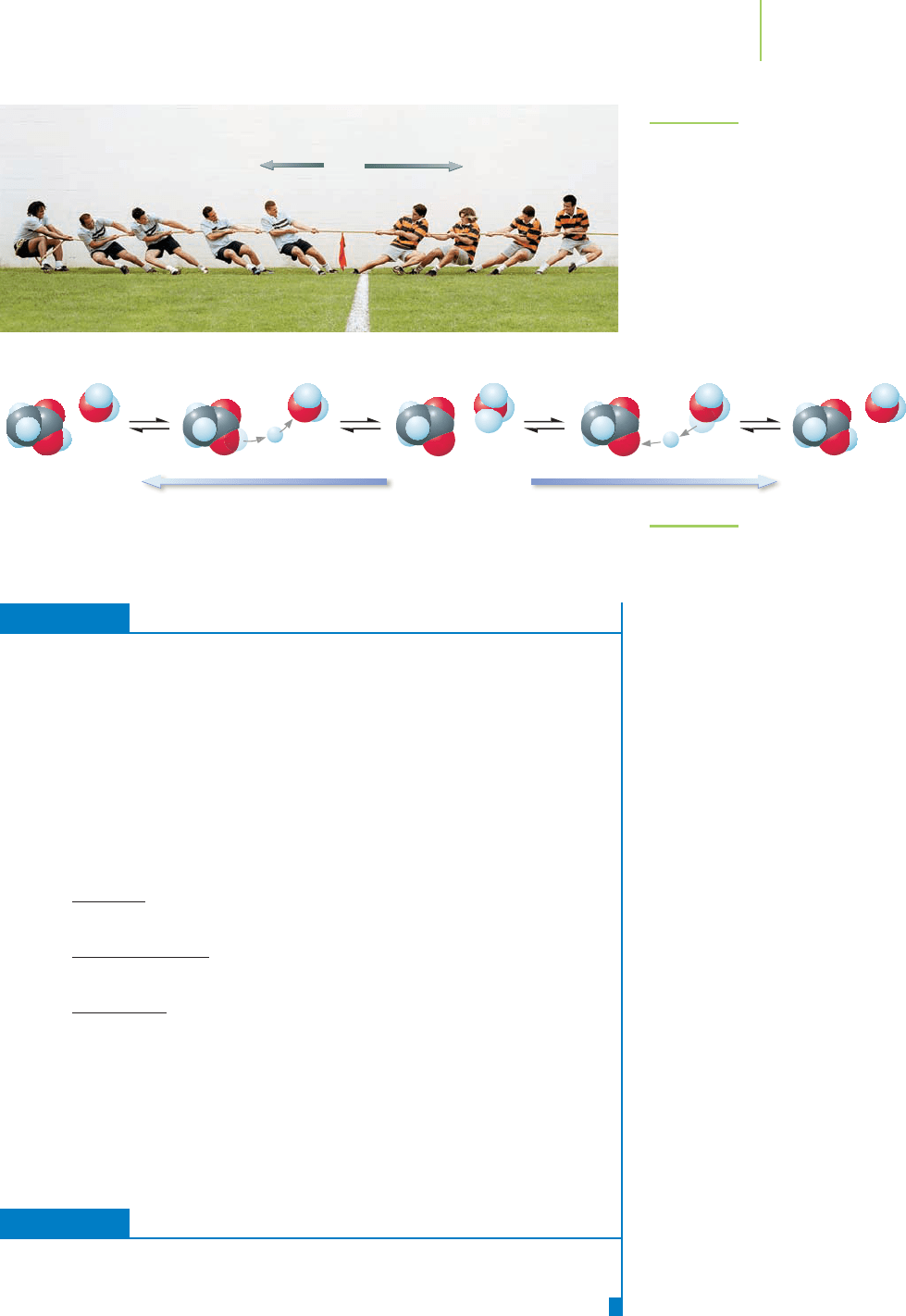
This battle, and its outcome, are shown in Figure 17.7.
Let’s consider acetic acid, which we call a weak acid because its acid ionization
reaction doesn’t go very far to the right in water. This means that most of the
acetic acid stays as acetic acid when it is added to water. When water (on the re-
actant side) and acetate ion (on the product side) enter into the tug of war for
protons, the acetate ion wins because it is a much stronger base than water. These
reactions are shown in Figure 17.8. The bottom line is that strong acids have very
weak conjugate bases and weak acids have somewhat stronger conjugate bases.
726 Chapter 17 Acids and Bases
17.2 Acid Strength 727
EXERCISE 17.4 Strength of the Conjugates
For each of the following acids in water—hydrofluoric acid (HF, K
a
= 7.2 × 10
−4
),
acetic acid (CH
3
COOH, K
a
= 1.8 × 10
−5
), and hypochlorous acid (HOCl, K
a
=
3.5 × 10
−8
):
a. Write the mass-action expression for dissociation of each acid.
b. Place the conjugate bases of these acids in order from strongest to weakest.
c. State which of the conjugate bases are stronger than water and which are
weaker than water.
Solution
a. K
a
=
[F
−
][H
+
]
[HF]
K
a
=
[CH
3
COO
−
][H
+
]
[CH
3
COOH]
K
a
=
[OCl
−
][H
+
]
[HOCl]
b. Based on the K
a
values, the acid strengths are ordered as follows:
HF > CH
3
COOH > HOCl
The relative strengths of the conjugate bases are therefore in reverse order:
OCl
−
> CH
3
COO
−
> F
−
c. All of these bases are stronger than water.
PRACTICE 17.4
Using Table 17.4, pick two acids with conjugate bases that are weaker than the
acetate ion (CH
3
COO
−
).
See Problem 18.
H
2
ONO
3
H
+
–
FIGURE 17.7
There is a “tug of war” for hydrogen ions
between water and the nitrate ion. The
water wins because water is a much
stronger base than the nitrate ion.
CH
3
COOH + H
2
O CH
3
COOH + H
2
O
CH
3
COO
–
+ H
3
O
+
+ +
– –
FIGURE 17.8
In the reaction of acetate (CH
3
COO
−
)
and water, the acetate wins because it is
a stronger base than water.
