Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

Key Words
activity The effective concentration of a solute in solu-
tion. (p. 675)
binding rate constant The rate constant that indicates the
association of two molecules. (p. 670)
chromatography A chemical technique involving the
partition of a solute between a stationary phase and a
mobile phase. The technique can be used to separate
or purify mixtures of solutes. (p. 678)
contact process An industrial method used to produce
sulfuric acid from elemental sulfur. (p. 676)
distribution constant The equilibrium constant that
describes the partitioning of a solute between two
immiscible phases. (p. 678)
equilibrium A system of reversible reactions in which
the forward and reverse reactions occur at equal
rates, such that no net change in the concentrations
occurs, even though both reactions continue.
(p. 670)
equilibrium constant (K) The value of the equilibrium ex-
pression when it is solved using the equilibrium con-
centrations of reactants and products. (p. 672)
equilibrium expression The ratio of product to reactant
concentrations raised to the power of their stoichio-
metric coefficients. This expression relates the equi-
librium concentrations to the equilibrium constant.
It is also known as the mass-action expression.
(p. 672)
gas chromatography A specific chromatography tech-
nique in which the mobile phase is a gas and the sta-
tionary phase is a solid. (p. 678)
heme group A compound that, when bound to hemo-
globin and iron cations, is responsible for binding
oxygen. (p. 669)
heterogeneous equilibrium An equilibrium that results
from reactants and products in different phases or
physical states. (p. 682)
homogeneous equilibrium An equilibrium that results
from reactants and products in the same phase, or
physical state. (p. 682)
Le Châtelier’s principle If a system at equilibrium is
changed, it responds by returning toward its original
equilibrium position. (p. 700)
mass-action expression The ratio of product to reactant
concentrations raised to the power of their stoichio-
metric coefficients. This expression relates the equi-
librium concentrations to the equilibrium constant.
It is also known as the equilibrium expression.
(p. 672)
mobile phase In chromatography, the phase that moves.
(p. 678)
myoglobin (Mb) A biochemical compound responsible
for storing and releasing oxygen in a living organism.
(p. 669)
quadratic equation A mathematical equation written in
the form ax
2
+ bx + c = 0. (p. 699)
quadratic formula The method used to solve for x from
the quadratic equation,
x =
−b ±
√
b
2
− 4ac
2a
(p. 699)
reaction quotient (Q) The ratio of product concentra-
tions to reactant concentrations raised to the power
of their stoichiometric coefficients for a reaction that
is not at equilibrium. (p. 688)
release rate constant The rate constant of the reaction in
which oxygen is released from the myoglobin-oxygen
reaction. (p. 671)
stationary phase In chromatography, the phase that does
not move. (p. 678)
708 Chapter 16 Chemical Equilibrium
Focus Your Learning
The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
Section 16.1 The Concept of Chemical Equilibrium
Skill Review
1. The word dynamic refers to changes. Explain how the de-
scriptive term dynamic equilibrium can be applied to a chem-
ical system where the concentrations of reactants and
products do not change.
2. Match each of these conditions of a chemical system to the
appropriate description of the Gibbs free energy: +, −, or 0.
a. System reacting toward products
b. System at equilibrium
c. System reacting toward reactants
3. When describing a reacting system, a scientist may say that
the reaction “does not go to completion.” Use free energy and
equilibrium to explain the meaning of that phrase.
4. Assume the expression “rate
1
” represents the rate of a reac-
tion, and “rate
2
” represents the expression for the rate of the
reverse of that reaction. Which of these statements is not true
of a reaction at equilibrium, and why?
a. rate
1
/rate
2
= 1 c. rate
1
/rate
2
= K
2
b. rate
2
/rate
1
=−1 d. rate
2
= rate
1
× K
5. Write the equilibrium expression for each of these reactions:
a. HCl(g) + C
2
H
4
(g)
C
2
H
5
Cl(g)
b. CH
4
(g) + 2O
2
(g)
CO
2
(g) + 2H
2
O(g)
c. 2H
2
(g) + O
2
(g)
2H
2
O(l)
6. Write the equilibrium expression for each of these reactions:
a. CaCO
3
(s)
CaO(s) + CO
2
(g)
b. SO
3
(aq) + H
2
O(l)
H
2
SO
4
(aq)
c. 4NH
3
(g) + 7O
2
(g)
4NO
2
(g) + 6H
2
O(g)
Chemical Applications and Practices
7. Phosphoric acid (H
3
PO
4
) is used in soft drinks and in pro-
ducing fertilizers. As shown in the following reaction, phos-
phoric acid can be produced by the action of sulfuric acid on
rocks that contain calcium phosphate.
Ca
3
(PO
4
)
2
(s) + 3H
2
SO
4
(aq)
3CaSO
4
(aq) + 2H
3
PO
4
(aq)
Describe the system at equilibrium, using each of these three
concepts:
a. Reaction rates
b. Concentration conditions
c. Gibbs free energy
8. Batteries in cars, watches, and the like all depend on a drive
from reactants to products that produces electricity. When
the production of electricity stops, we typically say that the
battery is “dead.” A chemical way to express this is to say that
the battery has attained equilibrium. Explain why this
chemical statement also describes why the battery no longer
produces electricity.
9. Hydrogen chloride gas (used in the production of hydrochlo-
ric acid) can be produced directly by the combination of
hydrogen and chlorine gas as follows:
H
2
(g) + Cl
2
(g)
2HCl(g)
At equilibrium, k
1
[H
2
][Cl
2
] = k
−1
[HCl]
2
. Write the mass-
action expression (that is, the equilibrium expression) for the
reaction.
10. Chlorofluorocarbons, including Freon-12, have been used in
air conditioning units. However, their use is being phased out
in most countries as a consequence of their breakdown into
chlorine atoms that attack our planet’s protective ozone layer.
Atmospheric scientists studied the following reaction to un-
derstand the breakdown process:
CCl
2
F
2
(g) (Freon-12)
CClF
2
(g) + Cl(g)
At equilibrium, k
1
[CCl
2
F
2
] = k
2
[CClF
2
][Cl]. Write the
mass-action expression, i.e. equilibrium expression for the
reaction.
Section 16.2
Why Is Chemical Equilibrium a Useful Concept?
Skill Review
11. In your own words, explain the economic outcome to the
petroleum industry if equilibria could not be controlled.
12. What types of interactions might exist in a chromatographic
system to make a solute interact strongly with the mobile
phase instead of the stationary phase?
Chemical Applications and Practices
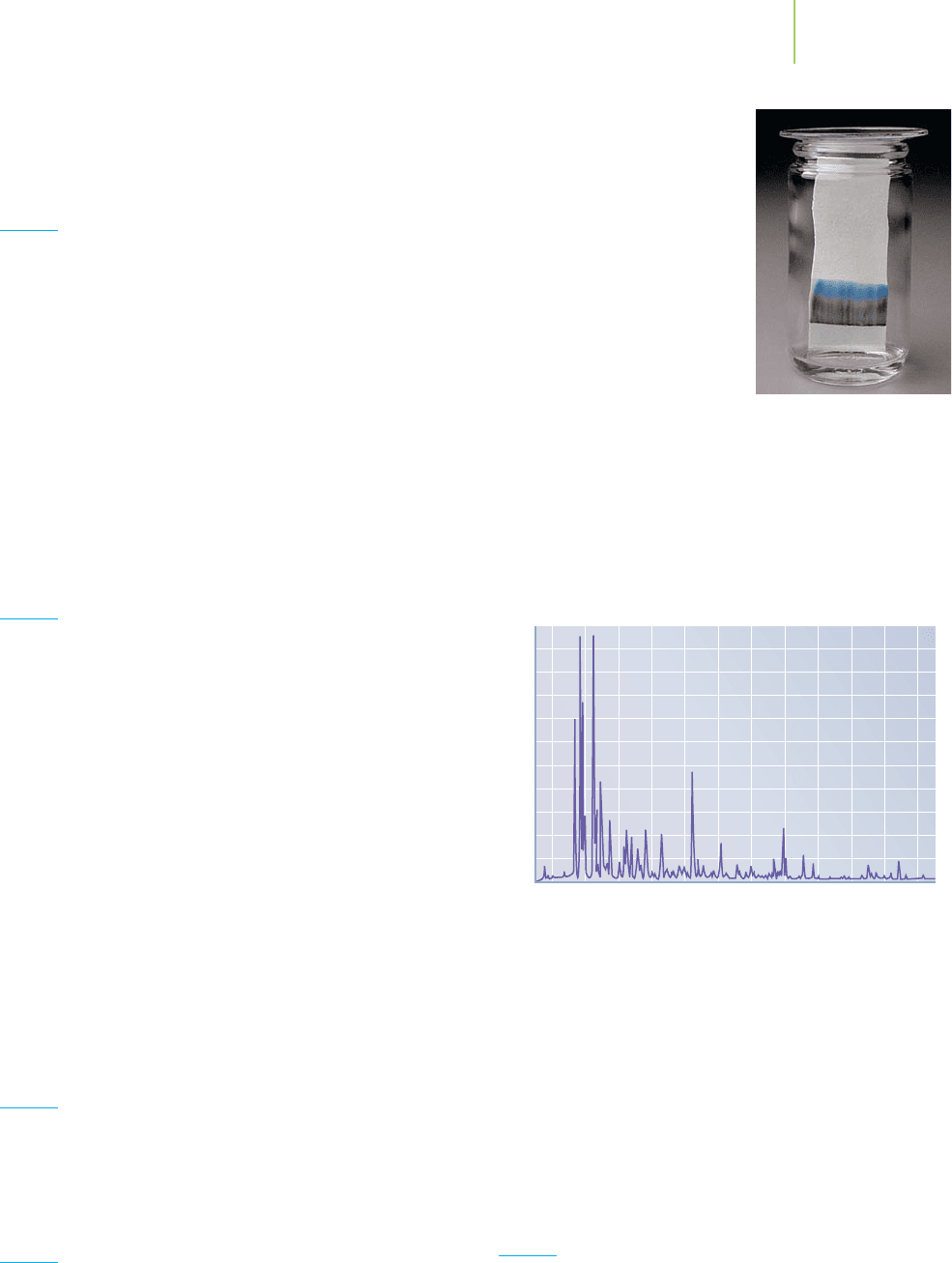
13. The following chromatogram was developed when ink from
a black felt-tip pen was drawn on a plate containing a sta-
tionary phase. The plate was then dipped in solvent, and the
solvent moved up the plate by capillary action. After develop-
ment of the plate, it was obvious that the original black ink
had separated into the dif-
ferent dyes used to make the
ink black.
a. Which color dye in the
ink has the largest value
of K
D
?
b. Which color dye interacts
least with the stationary
filter paper?
14. The following is a chromatogram from a gas chromatogra-
phy analysis of the vapor above a sample of gasoline. Each
peak corresponds to a component in the gasoline vapor mix-
ture. It is important to blend gasoline components for better
performance at certain altitudes. From the chromatogram,
which component has the lower value for K
D
? Which has the
most interaction with the stationary phase?
Focus Your Learning
709
Time (minutes)
0
20
100
1 Butane
2 2-Methylbutane
3 Pentane
4 2-Methylbutane
5 Hexane
6 Methylcyclopentane
7 Benzene
8 Methylcyclopentane
9 Toluene
10 Octane
11 Ethylbenzene
12 Xylene
13 Xylene
14 Xylene
15 Nonane
16 1-Ethyl-2-methylbenzene
17 1, 2, 4-Trimethylbenzene
1
2
4
5
6
7
8
9
10
11
12
13
14
15
16
17
3
Section 16.3 The Meaning of the Equilibrium Constant
Skill Review
15. For each of the following systems, an equilibrium expression
is given. If any errors are present in the expressions, correct
them and rewrite the equilibrium expression.
a. 2PbO(s) +O
2
(g)
2PbO
2
(s) K =[PbO
2
]/[PbO]
2
[O
2
]
b. H
2
O(l) + SO
3
(g)
H
2
SO
4
(aq) K =1/[SO
3
]
16. For each of the following systems, an equilibrium expression
is given. If any errors are present in the expressions, correct
them and rewrite the equilibrium expression.
a. H
2
CO
3
(aq)
H
2
O(l) + CO
2
(g) K = 1/[CO
2
]
b. H
2
O(l) + NH
3
(g)
NH
4
+
(aq) + OH
−
(aq)
K = [NH
4
+
][OH
−
]/[NH
3
][H
2
O]
17. Using the equilibrium line chart for the reaction of carbon
monoxide and water:
CO(g) + H
2
O(g)
CO
2
(g) + H
2
(g) K = ????
a. Estimate the value of K.
b. Based on this information, what can you say about the
relative concentrations of carbon monoxide and carbon
dioxide when the reaction has reached equilibrium?
18. Based on the equilibrium line charts for the following hypo-
thetic reactions, which reaction would produce fewer moles
of product? Explain why this is possible. (Assume that each
reaction has the same initial conditions.)
Chemical Applications and Practices
19. Some camp stoves and portable burners operate via the com-
bustion of propane (C
3
H
8
). Balance the following combus-
tion equation, and write the equilibrium expression for the
reaction.
C
3
H
8
(g) + O
2
(g)
CO
2
(g) + H
2
O(g)
20. Ethanol (C
2
H
5
OH) is widely used as a fuel additive in
gasoline.
a. Balance the following combustion equation, and write the
equilibrium expression for this important reaction.
C
2
H
5
OH(g) + O
2
(g)
CO
2
(g) + H
2
O(g)
b. Would you expect the value for K for this reaction, at
combustion temperatures, to be large or small? Explain.
21. Hydrogen peroxide, in a dilute solution, is a very commonly
used disinfectant. The following balanced equation
shows the decomposition reaction for gas-phase hydrogen
peroxide.
2H
2
O
2
(g)
2H
2
O(g) + O
2
(g)
Given the following initial and final concentrations, deter-
mine the equilibrium concentration of O
2
.
[H
2
O
2
]
0
= 0.100; [H
2
O]
0
= 0.050; [O
2
]
0
= 0.025
[H
2
O
2
] = 0.050; [H
2
O] = 0.100; [O
2
] = ?
22. The Haber process, at 450°C, has an equilibrium constant
K = 0.30. What is the equilibrium concentration of hydrogen
in a system containing the following equilibrium concentra-
tions of nitrogen and ammonia?
[N
2
] = 0.100; [NH
3
] = 0.100; [H
2
] = ?
23. Molybdenum, element 42, has many uses, perhaps most no-
tably in the production of a strong type of steel. The reaction
shown here is involved in one of the steps in recovering
molybdenum from its natural ore.
2MoS
2
(s) + 5O
2
(g)
2MoO
3
(s) + 4SO(g)
Write the proper mass-action expression for the equilibrium
constant for this reaction.
24. The following reaction shows the dissociation of magnesium
hydroxide into ions. This compound can be found in several
commercial antacids.
Mg(OH)
2
(s)
Mg
2+
(aq) + 2OH
−
(aq)
Write the proper mass-action expression for the equilibrium
constant for this reaction.
25. At 450°C, the Haber process has a K value of approximately
0.30. At 25°C, the reaction between NO from airplane ex-
haust and ozone has a K value of approximately 3.0 × 10
34
.
The ionization reaction of acetic acid in vinegar, at 25°C, is
approximately 1.8 × 10
−5
. Complete the following table and
compare the three systems.
710 Chapter 16 Chemical Equilibrium
Reactant Product
System K Favored Favored Mixture
Ionization 3.5 × 10
−4
of HF
Decomposition 0.133
Phosgene 3.7 × 10
9
reaction
Reactant Product
System K Favored Favored Mixture
Ammonia 0.30
synthesis
Ozone depletion 3.0 × 10
34
Acid ionization 1.8 × 10
−5
Reactants
A(aq) + B(aq)C(aq)
E
Products
Reactants
X(aq) + Y(aq) 2C(aq)
E
Products
26. Aqueous solutions of hydrofluoric acid (HF) have the unique
property of being able to dissolve glass. The ionization of HF
in water has an equilibrium value, at 25°C, of approximately
3.5 ×10
−4
. The decomposition of gaseous dinitrogen tetrox-
ide (N
2
O
4
(g)), rocket fuel used on the lunar landers of the
Apollo missions, has a K value of 0.133. The reaction of chlo-
rine gas (Cl
2
) and carbon monoxide (CO) to form phosgene
(COCl
2
), a deadly nerve gas, has a K value of 3.7 × 10
9
at
25°C. Complete the following table and compare the three
systems.
Reactants
CO(g) + H
2
O(g)CO
2
(g) + H
2
(g)
E
Products
27. Water hardness is a property of water that depends on the
concentration of calcium and magnesium ions. One method
used to determine these concentrations is titration with a

known solution of EDTA. The goal in the titration is to pro-
duce a reaction between the dissolved metal ions and EDTA
that converts essentially all the dissolved ions into com-
pounds involving EDTA.
a. Explain why this is a desirable outcome in the analysis of
water hardness.
b. Explain why K values on the order 10
10
for the reactions
between calcium and EDTA give us more confidence in the
analysis than we would have if the K values were on
the order of 10.
28. In aqueous solutions, acids dissociate to produce hydronium
ions and a negative ion. If the reverse reaction is favored, the
acid is considered weak. Judging on the basis of the following
two acid dissociation reactions, which acid is the weaker
acid?
Acetic acid (found in vinegar) K = 1.8 × 10
−5
Benzoic acid (found in berries) K = 6.5 × 10
−5
Section 16.4 Working with Equilibrium Constants
Skill Review
29. Water is a product in many chemical reactions. It can be di-
rectly made from its elements:
2H
2
(g) + O
2
(g)
2H
2
O(g)
a. Write the equilibrium expression for the reaction.
b. If the equilibrium concentrations for the compounds, at a
specific temperature, were as follows, what would be the
numerical value for K?
[H
2
] = 0.134; [O
2
] = 0.673; [H
2
O] = 1.00
c. What is the value for K for the reverse reaction?
d. What is the value of K for the reaction of only 1 mol of
hydrogen and 0.5 mol of oxygen to make 1 mol of water
(that is, if we halve the coefficients in this reaction)?
30. If a solid bar of zinc is placed in a solution containing 1 M sil-
ver ions (Ag
+
), a spontaneous reaction takes place producing
zinc ions and silver metal with an equilibrium constant value
of approximately 8 ×10
52
.
a. What would you calculate as the equilibrium constant for
the reverse reaction?
b. What would you calculate as the equilibrium constant if
the stoichiometric coefficients were doubled?
Chemical Applications and Practices
31. When scientists seek information about air pollution in large
cities, one of the reactions they study is
2NO(g) + O
2
(g)
2NO
2
(g)
If K for the reaction, at 25°C, is approximately 1.7 ×10
12
and
the specific rate constant for the reverse reaction is approxi-
mately 6.6 × 10
−12
, what would you calculate as the value of
the rate constant for the forward reaction? (Units have been
omitted for this problem.)
32. Ethyl acetate, a common laboratory solvent, can be prepared
by the following reaction of acetic acid and ethanol:
C
2
H
4
O
2
+ C
2
H
6
O
C
4
H
8
O
2
+ H
2
O
Aetic acid Ethanol Ethyl acetate
If K for the reaction, at 25°C, is 2.2 and the specific rate con-
stant for the forward reaction is 4.22 ×10
10
, what is the value
of the rate constant for the reverse reaction? (Units have been
omitted for this problem.)
33. Producing industrially useful amounts of acetylene (C
2
H
2
)
is important for more than acetylene torches. Acetylene is
also used as a starting compound for many important poly-
mers, such as vinyl chloride (used in PVC materials) and
acrylonitrile.
a. Using the equation CH
4
(g)
1
⁄2C
2
H
2
(g) +
3
⁄2H
2
(g), write
the appropriate mass-action equilibrium expression.
b. Rewrite the equation showing the stoichiometric ratios for
the production of 1 mol of C
2
H
2
(g). Write the appropriate
mass-action equilibrium expression for this equation.
c. If the mathematical value for the equilibrium constant for
the reaction in part a were known, how would it be
mathematically converted to the equilibrium constant for
the reaction in part b?
34. One of the reactions used to produce formaldehyde (CH
2
O)
is
CH
3
OH(g) +
1
⁄2O
2
(g)
CH
2
O(g) + H
2
O(g)
a. Write the mass-action equilibrium constant for the
reaction.
b. Rewrite the reaction, keeping the same stoichiometric
ratio, but showing the reaction utilizing 1 mol of oxygen.
Write the mass-action equilibrium expression for this
reaction.
c. How are the two equilibrium expressions mathematically
related?
35. The reaction that allows many biochemical reactions to take
place involves the breakdown of ATP (adenosine triphos-
phate) into ADP (adenosine diphosphate), as discussed in
Chapter 14. The equilibrium constant of this reaction, at
37
o
C, is approximately 1.4 ×10
5
. One of the early steps in the
breakdown of glucose from food is the attachment of a phos-
phate group. The equilibrium constant for this process is ap-
proximately 4.7 ×10
−3
. In living cells these two reactions are
combined. What would be the equilibrium constant for the
resulting combined reaction?
36. The production of tin is important because it has many prac-
tical uses, from plating iron objects to helping deliver
fluoride in toothpaste as SnF
2
. Combine the first two of the
following reactions, and use the appropriate equilibrium
constants, to obtain the equilibrium constant for the third
reaction.
SnO
2
(s) + 2CO(g)
Sn(s) + 2CO
2
(g) K
1
= 14
CO(g) + H
2
O(g)
CO
2
(g) + H
2
(g) K
2
= 1.3
SnO
2
(s) + 2H
2
(g)
Sn(s) + 2H
2
O(g) K
3
= ?
37. Many compounds have more than one important use. Such
is the case with the weak acid phenol (C
6
H
5
OH). It can be
used, in dilute form, as an antiseptic and as a component in
making some plastics. In water, phenol dissociates slightly as
shown here:
C
6
H
5
OH(aq)
H
+
(aq) + C
6
H
5
O
−
(aq)
The equilibrium constant for this reaction is 1.3 × 10
−10
.To
analyze a solution containing phenol, you may carefully add
measured amounts of aqueous NaOH. Recall that in water,
the following reaction also takes place:
H
+
+ OH
−
H
2
O K = 1.0 × 10
14
Focus Your Learning 711
Adding these two equations produces a third that represents
the reaction between sodium hydroxide and phenol.
Using equilibrium constants, evaluate the feasibility of the
analysis—that is, whether the reaction will proceed toward
products enough for the analysis to be performed.
38. Procaine (C
13
H
20
N
2
O
2
) can be used to produce the anes-
thetic novocaine (C
13
H
21
N
2
O
2
Cl). Procaine is a weak base
that undergoes the following reaction in aqueous solutions:
C
13
H
20
N
2
O
2
+ H
2
O
OH
−
+ C
13
H
20
N
2
O
2
H
+
K = 7.1 × 10
−6
If a solution of procaine were to be analyzed by adding care-
fully measured amounts of hydrochloric acid (HCl), explain
how you could use the following reaction to assist in the eval-
uation of the viability of the analysis.
H
+
+ OH
−
H
2
O K = 1.0 × 10
14
39. Using information from Problems 37 and 38, determine the
equilibrium constant value for the reaction between phenol
and procaine. Would it be a reasonable analytical strategy to
employ phenol as a reactant to determine the concentration
of procaine in solution?
40. Using the information from Problems 37 and 38, determine
the equilibrium constant value for the reaction between HCl
and NaOH. Would it be a reasonable analytical strategy to
employ HCl as a reactant to determine the concentration of a
NaOH solution?
41. At temperatures near 400
o
C, the K
p
value for the synthesis of
ammonia is 2.5 ×10
−4
. At 400
o
C, what would be the approx-
imate value of K for the following reaction?
3H
2
(g) + N
2
(g)
2NH
3
(g)
42. The reaction of nitrogen monoxide and chlorine gas has a
large value for the equilibrium constant, K, at 298 K. How-
ever, it is often easier to measure the partial pressures of each
component of a gaseous system than it is to measure their
concentration. What is the value of K
p
at this temperature?
2NO(g) + Cl
2
(g)
2NOCl(g) K = 6.3 × 10
4
(at 298 K)
Section 16.5 Solving Equilibrium Problems—
A Different Way of Thinking
Skill Review
43. Consider the acetic acid system described earlier in the text:
CH
3
COOH(aq)
CH
3
COO
−
(aq) + H
+
(aq) K = 1.8 × 10
−5
What is the equilibrium hydrogen ion concentration, [H
+
],
given each of these initial concentrations?
a. [CH
3
COOH]
0
= 0.500 M;[CH
3
COO
−
]
0
= 0.0 M
b. [CH
3
COOH]
0
= 0.100 M;[CH
3
COO
−
]
0
= 0.100 M
c. [CH
3
COOH]
0
= 0.010 M;[CH
3
COO
−
]
0
= 0.0 M
44. Consider the chemical system:
2NOCl(g)
2NO(g) + Cl
2
(g) K = 1.6 × 10
−5
What is the equilibrium concentration of nitrogen monoxide,
[NO], given each of these initial concentrations?
a. [NOCl]
0
= 0.500 M;[Cl
2
]
0
= 0.0 M
b. [NOCl]
0
= 1.500 M;[Cl
2
]
0
= 2.00 M
c. [NOCl]
0
= 2.25 M;[Cl
2
]
0
= 1.20 M
45. At some temperature, the reaction H
2
+ I
2
2HI has
K = 617. Predict in which direction, forward or reverse, the
reaction would proceed when:
a. [H
2
]
0
= 0.240 M;[I
2
]
0
= 0.080 M; [HI]
0
= 0.20 M
b. [H
2
]
0
= 0.030 M;[I
2
]
0
= 0.100 M; [HI]
0
= 1.50 M
46. At some temperature, the reaction H
2
+ I
2
2HI has
K = 617. Predict in which direction, forward or reverse, the
reaction would proceed when:
a. [H
2
]
0
= 0.990 M;[I
2
]
0
= 0.280 M; [HI]
0
= 0.500 M
b. [H
2
]
0
= 0.250 M;[I
2
]
0
= 1.000 M; [HI]
0
= 0.500 M
47. Using the value of K = 8.6 × 10
−5
for the myoglobin and
oxygen reaction described in the text, Mb +O
2
MbO
2
,in-
dicate which of the systems described in the following table
are at equilibrium. If an example is not at equilibrium, pre-
dict the direction of change (forward or reverse) that would
take place to attain the equilibrium condition.
[Mb] (M)[O
2
] (M) [MbO
2
] (M)
a. 3.5 × 10
−4
2.5 × 10
−4
0
b. 1.0 × 10
−4
1.0 × 10
−4
8.6 × 10
−13
c. 2.0 × 10
−4
1.5 × 10
−4
2.6 × 10
−11
d. 0 2.5 × 10
−4
1.0 × 10
−10
48. The decomposition of 2 moles of carbon dioxide into carbon
monoxide and oxygen gas can occur under certain condi-
tions. If, at a particular temperature, K = 4.5 × 10
−5
, predict
the direction of change (forward or reverse) that would take
place to attain the equilibrium condition. (Hint: Start by
writing the balanced equation.)
[CO
2
] (M) [CO] (M)[O
2
] (M)
a. 0.44 1.0 × 10
−5
1.0 × 10
−5
b. 1.0 × 10
−4
0.22 1.5 × 10
−8
c. 6.3 × 10
−8
3.9 × 10
−2
4.7 × 10
−12
49. Barium sulfate (BaSO
4
) is a slightly soluble compound that
has some medical applications when used to diagnose gas-
trointestinal problems. Because barium can be toxic, it is
important to keep the concentration of barium ions at a
minimum. Given the following reaction and equilibrium
value, determine the maximum equilibrium concentration
of Ba
2+
(aq) when in the presence of solid BaSO
4.
BaSO
4
(s)
Ba
2+
(aq) + SO
4
2−
(aq) K = 1.1 × 10
−10
50. Many municipal water supplies are treated with fluoride ions
(F
−
) in an attempt to strengthen dental enamel. If the water
contains significant amounts of calcium ions, a precipitation
712 Chapter 16 Chemical Equilibrium
+
reaction can take place. When solid calcium fluoride is pre-
sent in water, the following equilibrium is established:
CaF
2
(s)
Ca
2+
(aq) + 2F
−
(aq) K = 4.0 × 10
−11
What would be the equilibrium concentration of fluoride ion
present in this equilibrium?
51. One of the least soluble compounds known is antimony sul-
fide (Sb
2
S
3
). If the concentration of antimony ion in a solu-
tion in which Sb
2
S
3
was present were 2.2 × 10
−19
, and no
other solute was present, what would you calculate as the
equilibrium constant for the following reaction?
Sb
2
S
3
(s)
2Sb
3+
(aq) +3S
2−
(aq)
52. The equilibrium constant for a reaction is very temperature
dependent. Assume 0.060 is the equilibrium constant for the
Haber process at a given temperature.
N
2
(g) + 3H
2
(g)
2NH
3
(g)
What is the equilibrium concentration of H
2
if the equilib-
rium concentrations of N
2
and NH
3
are found both to be
0.0010 M?
53. Using the same value of K for the ammonia synthesis reac-
tion in Problem 52, solve for:
a. The equilibrium concentration of NH
3
when [N
2
] =
0.0010 M and [H
2
] = 0.010 M
b. The equilibrium concentration of H
2
when [NH
3
] =
0.020 M and [N
2
] = 0.015 M
54. Calculate the [NO
2
] given the equilibrium concentrations
based on the reaction
2NO(g) + O
2
(g)
2NO
2
(g) K = 1.71 × 10
12
a. [NO] = 0.00020 M;[O
2
] = 0.000050 M; [NO
2
] = ?
b. [NO] = 0.00010 M;[O
2
] = 0.000010 M; [NO
2
] = ?
Chemical Applications and Practices
55. Codeine (C
18
H
21
NO
3
), an analgesic drug obtained by pre-
scription, produces the following reaction when added to
water.
C
18
H
21
NO
3
(aq) + H
2
O(l)
OH
−
(aq) + C
18
H
21
NO
3
H
+
(aq)
K =1.6 × 10
−6
a. What reactions are taking place in the solution?
b. Which among these are important? Which are unim-
portant?
c. If a solution had the following concentrations, in which
direction would it proceed?
[C
18
H
21
NO
3
]
0
=0.10 M; [OH
−
]
0
=0 M;
[C
18
H
21
NO
3
H
+
]
0
=0 M
d. Once equilibrium was achieved, what would be the
concentrations of the species mentioned in part c?
56. Acetylsalicylic acid (C
9
H
8
O
4
, also known as aspirin) dissoci-
ates in water with an equilibrium constant, at 25
o
C, of
3.0 × 10
−4
.
C
9
H
8
O
4
(aq)
H
+
(aq)+ C
9
H
7
O
4
−
(aq)
a. If the initial concentration of C
9
H
8
O
4
were 0.10 M, what
would you calculate as the amount of C
9
H
8
O
4
remaining
at equilibrium?
b. What percentage of C
9
H
8
O
4
reacted?
c. Draw an equilibrium line chart that illustrates this system.
57. One method to obtain silver metal from impure lead samples
is named after the work of Samuel Parkes (1761–1825). A
silver-containing lead sample is melted, and zinc is added to
the molten sample. The molten zinc makes a coating on the
surface. The molten silver is approximately 300 times more
soluble in the molten zinc than in the impure molten lead.
(The zinc–silver mixture is later removed, and pure silver is
obtained by distilling away the zinc.) An equilibrium
constant can be written for the concentration of silver in the
lead mixture versus the amount in the zinc: K
D
=
[Ag
(Zn)
]/[Ag
(Pb)
]. If the [Ag
(Zn)
] was 0.0010 M and the
[Ag
(Pb)
] was 0.000011 M, would it be wise to wait to see
whether more Ag could be extracted? Or has the extraction
for this system reached a maximum? Explain your answer.
58. The solubility of a solute in one solvent compared to another
can be used to our advantage. The ratio of the dissolved
amounts of solute to solvent, called a partition coefficient,
may be used in a similar way as the distribution constant de-
scribed earlier. The K
D
value for a compound between a
water layer and an ether layer is 0.024. (Note: This represents
the amount dissolved in ether divided by the amount dis-
solved in water.) Suppose that the compound was ether-
extracted from a plant and is now 0.015 M in ether. What will
be the molarity of the compound that will form, in water,
when water is placed in contact with the ether?
59. At relatively high temperatures, the following reaction can be
used to produce methyl alcohol:
CO(g) + 2H
2
(g)
CH
3
OH(l) K = 13.5
a. If the concentration of CO, at equilibrium, were found to
be 0.010 M, what would be the equilibrium concentration
of hydrogen gas?
b. Draw an equilibrium line chart that illustrates this system.
60. Pyruvic acid is produced as an intermediate during the me-
tabolism of carbohydrates in cells; see Chapter 14. In water it
undergoes the following reaction:
CH
3
COCOOH(aq)
H
+
(aq) + CH
3
COCOO
−
(aq)
K = 6.6 × 10
−3
a. If the equilibrium concentration of CH
3
COCOOH were
found to be 0.0010 M, what would be the equilibrium
concentration of CH
3
COCOO
−
?
b. Draw an equilibrium line chart that illustrates this system.
61. At high temperatures, methane (CH
4
) can be reacted with
steam to produce carbon monoxide and hydrogen gas, as
shown in the following reaction:
CH
4
(g) + H
2
O(g)
CO(g) + 3H
2
(g)
If the equilibrium constant is 0.25, at a specific temperature
and the equilibrium concentrations of [CH
4
] = 0.11 M,
[H
2
O] = 0.28 M, and [CO] =0.75 M, what would you calcu-
late as the [H
2
]?
62. The aroma of rotten eggs can be partially attributable to the
foul-smelling sulfur compound H
2
S. Hydrogen sulfide de-
composes according to the following reaction:
2H
2
S(g)
2H
2
(g) + 2S(s)
The equilibrium constant for the process at a specific tem-
perature is 0.020. If the initial concentration of H
2
S were
0.0010 M, what would you determine to be the equilibrium
concentrations of H
2
S and H
2
?
Focus Your Learning
713
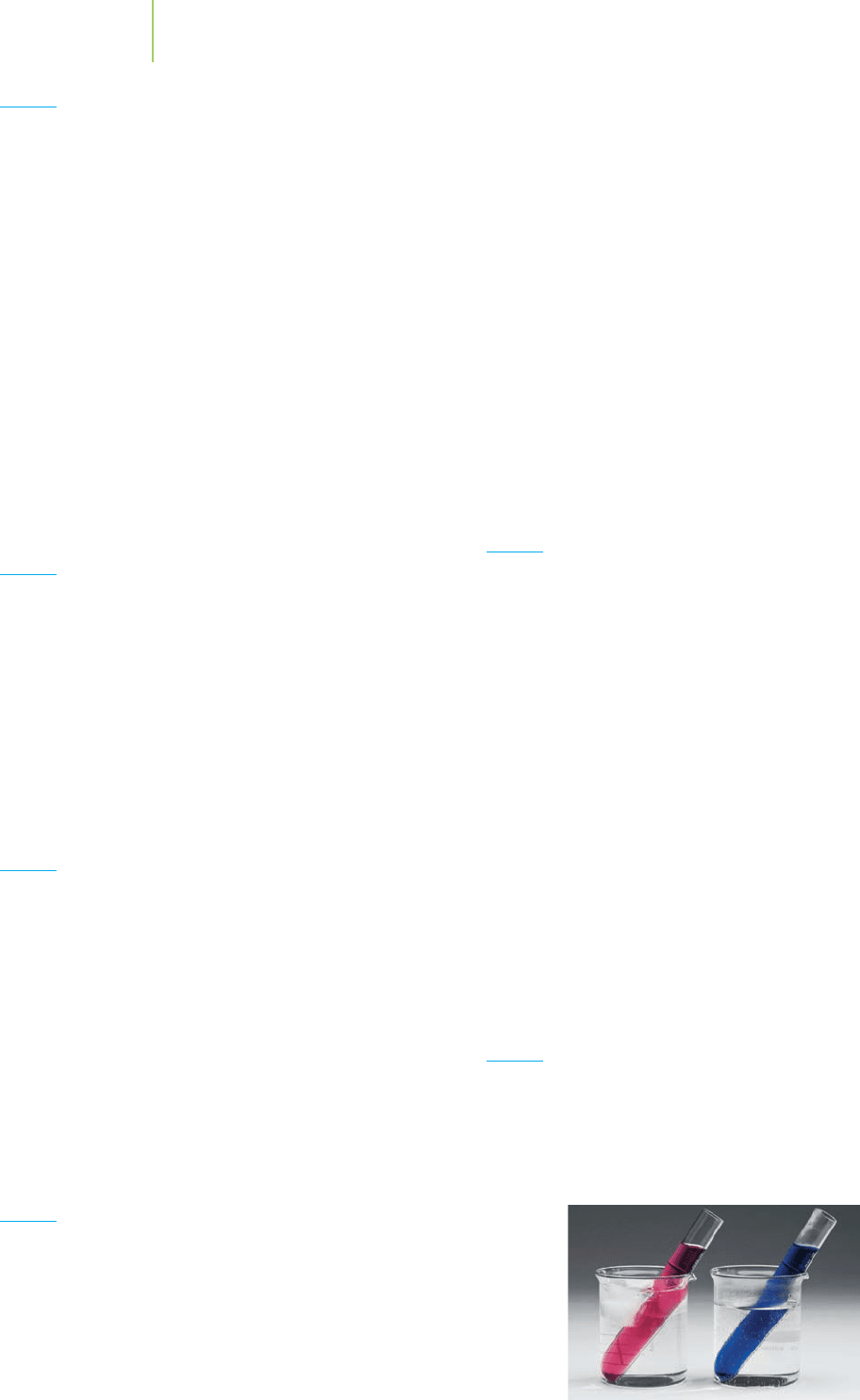
63. We discussed hydrogenation, the addition of hydrogen atoms
to a carbon–carbon double or triple bond, in Chapter 12.
One source of ethylene (C
2
H
4
) is the hydrogenation of acety-
lene (C
2
H
2
). The equilibrium constant for the reaction varies
greatly with temperature. If the equilibrium constant for the
following reaction is 4.2 × 10
15
, what would be the equilib-
rium concentration of hydrogen gas in a system when the
equilibrium concentrations of C
2
H
2
and C
2
H
4
were, respec-
tively, 1.2 × 10
−5
M and 0.025 M?
C
2
H
2
(g) +H
2
(g)
C
2
H
4
(g)
64. Butanoic acid (CH
3
CH
2
CH
2
COOH) has the aroma of
spoiled butter. A better-smelling compound, an ester, can be
made when butanoic acid is treated with methanol and an
acid catalyst.
CH
3
CH
2
CH
2
COOH + CH
3
OH
CH
3
CH
2
CH
2
COOCH
3
+ H
2
O
Under certain conditions, the equilibrium constant for the
reaction is 1.5 × 10
−2
. What would be the equilibrium con-
centration of the product ester if the initial concentrations of
each reactant were 0.500 M? Note that water is not the sol-
vent and must be included in K.
65. The element vanadium is unusually resistant to corrosion.
Alloyed with iron (approximately 5% vanadium) it produces
a useful type of steel. One reaction used to obtain vanadium
has a K value of 14 at 298 K.
VO
+
(aq) + 2H
+
(aq)
V
3+
(aq) + H
2
O(l)
Starting with [VO
+
]
0
= 0.15 M and [H
+
] = 0.100 M, what
would you calculate as the equilibrium concentrations of
VO
+
(aq), H
+
(aq), and V
3+
(aq)?
66. Using the reaction in Problem 65, decide in which direction
the reaction would proceed when the following concentra-
tions were known. (Prove your answers.)
a. [VO
2+
] = 0.10 M;[H
+
] = 0.25 M;[V
3+
] = 0.85 M
b. [VO
2+
] = .0025 M;[H
+
] = 0.10 M;[V
3+
] = 0.025 M
67. Using the K value of 8.6 × 10
−5
presented in the text for the
myoglobin and oxygen reaction Mb + O
2
MbO
2
, what
would you calculate as the [Mb], [O
2
], and [MbO
2
] when the
initial concentrations were as follows: [Mb] = 0.00100 M;
[O
2
] = 0 M, and [MbO
2
] = 0.000020 M?
68. As was mentioned in Problem 32, ethyl acetate can be pre-
pared by the reaction of acetic acid and ethanol.
C
2
H
4
O
2
+C
2
H
6
O
C
4
H
8
O
2
+H
2
O K =2.2 (at 25°C)
What would you calculate as the equilibrium concentration
of each component of the mixture if the initial concentra-
tions of each component in the mixture were 0.100 M? (As-
sume that water is not the solvent.)
Section 16.6 Le Châtelier’s Principle
Skill Review
69. Using Le Châtelier’s principle, decide whether each of these
changes would cause the equilibrium of the system presented
to shift to the left, would cause it to shift to the right, or
would have no effect on the equilibrium.
CH
4
(g) + 2O
2
(g)
CO
2
(g) + 2H
2
O(g)
(This is the exothermic reaction of burning methane in a lab
burner.)
a. Removing CO
2
from the system
b. Adding heat to the system
c. Decreasing the volume of the container
d. Adding H
2
O(g) to the system
e. Adding inert He to the system
70. Calcium oxide (CaO) is also known as lime. It is one of the
leading chemicals produced worldwide, thanks to its many
uses in plant and animal foods, insecticides, paper making,
and plaster products. It is produced, at high temperatures,
from calcium carbonate.
CaCO
3
(s)
CaO(s) + CO
2
(g) (endothermic reaction)
If the system were in a closed container, what effect (shift to
the left, shift to the right, or no effect) would each of these
changes have on the favored direction of the reaction?
a. Removing CO
2
b. Adding CaO
c. Raising the temperature
d. Enlarging the size of the container
e. Adding a suitable catalyst
Chemical Applications and Practices
71. The following equilibrium constant values are found for dis-
solving two solids in water to produce aqueous solutions of
the ions shown. Note that the stoichiometry for the process
is the same in both systems.
AgCl(s)
Ag
+
(aq) + Cl
−
(aq) K = 1.7 × 10
−10
CuCl(s)
Cu
+
(aq) + Cl
−
(aq) K = 1.9 × 10
−7
a. In which system would you find the greater number of
moles of dissolved ions?
b. Would adding more solid to the other system increase the
number of dissolved ions so that it might equal the total
found in the choice for part a? Explain your reasoning.
72. The production of ethylene is important in the manufacture
of polyethylene products. The following reaction shows how
ethylene can be made from ethane by removing hydrogen
from ethane.
CH
3
CH
3
(g)
CH
2
CH
2
(g) + H
2
(g)
a. At 298 K, the equilibrium constant is 0.96. If a 1-liter
container initially contained 0.10 M CH
3
CH
3
, what would
be the equilibrium concentration of all three species?
b. After equilibrium is reached, an additional 0.010 mol of
H
2
is injected into the container without changing its
volume. What would be the concentration of all three
species when equilibrium was once again restored?
73. A dilute solution of Na
2
Co(H
2
O)
6
Cl
4
has a faint pink color
and can be used as “invisible ink.” When some of the loosely
held water is driven off, with gentle heating, Na
2
CoCl
4
forms,
with a visible change to blue. If you stored your “invisible
ink” solution in a refrigerator, would it appear pink or blue?
Explain the basis of your answer.
714 Chapter 16 Chemical Equilibrium
74. The recognizable aromas of many fruits are due to a group of
compounds known as esters, as we learned in Chapter 12. For
example, the following reaction shows the production of
pentyl acetate. (Assume that water is not the solvent.)
Which of these methods would increase the amount of prod-
uct formed, and why?
a. Add water so that the pentyl ethanoate would dissolve
better.
b. Add magnesium sulfate so it would react with any water
formed.
75. One way to produce rubbing alcohol (CH
3
CHOHCH
3
) is
from propene:
Explain what the role of the catalyst is, in relation to the equi-
librium, in this reaction.
76. Tungsten’s unusually high melting point (over 3000°C) and
its efficiency at producing light from electrical energy led to
its use in light bulb filaments. It can be obtained via the fol-
lowing reaction:
WO
3
(s) + 3H
2
(g)
W(s) + 3H
2
O(g)
Like most reactions in which a metal is obtained from an-
other compound, this reaction is endothermic. Explain why
pressure changes are not considered significant factors when
tungsten is obtained in this manner.
Section 16.7 Free Energy and the Equilibrium Constant
Skill Review
77. Determine the equilibrium constant, K
eq
, associated with re-
actions that have these free energy changes at 25°C.
a. ∆G° =−1.05 J/mol
b. ∆G° = 0.230 J/mol
c. ∆G° = 2.55 kJ/mol
d. ∆G° =−9.80 kJ/mol
78. Determine the free energy change, ∆G°, for each of these
equilibrium constants at 25°C.
a. K
eq
= 1.8 × 10
−5
b. K
eq
= 6.67 × 10
−1
c. K
eq
= 2.30 × 10
−2
d. K
eq
= 125
79. What is the free energy change, ∆G°, in kilojoules per mole,
for the formation of methanol from carbon monoxide and
hydrogen gas at 25°C?
CO(g) + 2H
2
(g)
CH
3
OH(l) K
eq
= 13.5
CH
3
H
2
OCH
3
CH CH
3
CH CH
2
OH
Catalyst
CH
3
CH
2
CH
2
CH
2
CH
2
OOCCH
3
H
2
O
Pentyl ethanoate
(pentyl acetate)
CH
3
CH
2
CH
2
CH
2
CH
2
OH CH
3
COOH
Ethanoic acid
(acetic acid)
Pentanol
80. Use the tables of ∆G° in the Appendix to determine the value
of the equilibrium constant, K
eq
, for the following reaction at
25°C.
CaCO
3
(s)
CaO(s) + CO
2
(g)
Chemical Applications and Practices
81. Hydrogen sulfide (H
2
S), which is responsible for the odor of
rotten eggs, decomposes by the reaction illustrated in Prob-
lem 62. Given the value of the equilibrium constant in Prob-
lem 62, calculate the free energy change, in kilojoules per
mole, for the decomposition reaction at 25°C.
82. The ionization of HF in water (see Problem 26) has an equi-
librium constant K
eq
= 3.5 ×10
−4
at 25°C. Calculate the free
energy change, in kilojoules per mole, for the decomposition
reaction.
Comprehensive Problems
83. Acids react by donating hydrogen ions. The strength of the
acid reaction is determined by the ability the acid has to
donate the H
+
to water. Symbolically, the reaction in water
can be represented as follows:
HA
H
+
+A
−
The following is a list of some common weak acids followed
by their water reaction equilibrium values. All are compared
at the same temperature.Which acid in the list is the weakest?
Which is the strongest?
Acetic acid, HC
2
H
3
O
2
(found in vinegar) K = 1.8 × 10
−5
Formic acid, HCHO
2
(found in ants) K = 1.8 × 10
−4
Benzoic acid, HC
7
H
5
O
2
(found in some berries)
K = 6.3 × 10
−5
84. Scientists have investigated hydrazine (N
2
H
4
) and nitrogen
monoxide (NO) in their study of rocket fuels. Use the
following reaction to write the chemical equilibrium expres-
sion for this hydrazine reaction.
N
2
H
4
(g) + 2NO(g)
2N
2
(g) + 2H
2
O(g)
85. Suppose there are two synthetic routes by which a pharma-
ceutical company can make the same prescription drug.
Method A uses expensive starting materials but has a large
value for K. Method B uses inexpensive starting materials but
has a small value for K.You have been asked to discuss briefly,
in a planning meeting, the various considerations involved in
deciding between these two methods. What would you say
about the arguments for making a profit with either method?
86. Is it possible for K
p
to equal K? Explain your answer.
87. Using only whole-number coefficients, write a balanced
chemical equation that represents the conversion of oxygen
molecules into ozone. If the equilibrium constant for that re-
action were 7 ×10
−58
, what would you calculate as the equi-
librium constant for the reaction that produces 1 mol of
ozone?
88. The electrochemical reaction powering a nickel–cadmium
rechargeable battery has an equilibrium constant value, at
298 K, of approximately 1.5 × 10
11
, as written below. What is
the value for the reverse reaction of this process? The reaction
takes place in an alkaline solution.
Cd(s) + NiO
2
(s)
Ni(OH)
2
(s) + Cd(OH)
2
(s)
Focus Your Learning
715

89. An owner of a coffee shop, who happens to be a former
chemistry student, notices that there are some similarities be-
tween customers (that is, people in the shop and those not
entering the shop) and the reactants and products at equilib-
rium in a chemical system. What type of customer move-
ment would represent equilibrium for the shop? What type
of equilibrium would the profit-minded owner prefer, one
with a large value of K or a small value of K? Explain. If Q
were less than K, would it be a good business day or a poor
business day? Explain.
K =
[customers]
[people on the street]
90. As we have noted previously, the presence of fluoride in some
municipal water systems can bring about the precipitation of
CaF
2
if the concentration of Ca
2+
is very high.
CaF
2
(s)
Ca
2+
(aq) + 2F
−
(aq) K = 4.0 × 10
−11
a. How many moles per liter of CaF
2
would dissolve in pure
water?
b. If the concentration of Ca
2+
in a water sample were
0.0050 M, as might be present in hard water samples, how
many moles per liter of CaF
2
would dissolve?
91. The following reaction has historical importance as it was
once used to produce a useful fuel called “water gas.” The
process involved using steam to convert coal into carbon
monoxide and hydrogen gas.
C(s) + H
2
O(g)
CO(g) + H
2
(g) K
p
= 21 (at 1000°C)
If the initial partial pressure of H
2
O(g) were 52 atm, what
would you calculate as the equilibrium partial pressures of
H
2
O(g); CO(g) and H
2
(g)?
92. Carbonic acid, present in carbonated beverages, can partici-
pate in two reactions that both involve removing a H
+
from
the molecule.
H
2
CO
3
(aq)
H
+
(aq) + HCO
3
−
(aq) K = 4.3 × 10
−7
HCO
3
−
(aq)
H
+
(aq) + CO
3
2−
(aq) K =5.6 × 10
−11
Starting with an initial concentration of H
2
CO
3
of 0.10 M,
what would you calculate as the equilibrium concentrations
of [H
2
CO
3
],[HCO
3
−
], [H
+
], and [CO
3
2−
]? Be sure to justify
any assumptions you made to solve the problem.
93. Examine the hypothetical reaction illustrated below. A snap-
shot of each reaction was taken at specific times during the
course of the reaction. Which frame represents the first frame
in which equilibrium has been reached? Explain your answer.
94. We began this chapter discussing the interaction of myo-
globin with oxygen. Hemoglobin is a much more familiar
++
Time =
0 minute
Time =
1 minute
Reactants Products
Time =
2 minutes
Time =
3 minutes
molecule, but we intentionally chose myoglobin rather than
hemoglobin for our introduction. Look at the structure
of hemoglobin in Figure 22.22 just before the start of Sec-
tion 22.4. Why is myoglobin a more reasonable molecule
than hemoglobin to choose for your introduction to
equilibrium?
95. Look up the equilibrium constants for the solubility of
lead(II) iodide and strontium oxalate in Table A5.4 in Ap-
pendix 5 at the back of the book.
a. Based on these values, draw equilibrium line charts that
include the starting and equilibrium positions of the
reaction.
b. Next, calculate the solubility of each.
c. Based on your answers, do your equilibrium line charts
require any revision? Explain your answer.
96. Essay question: At the end of Section 16.5, we introduce the
quadratic equation as a useful way to solve for concentrations
when you have an intermediate value of an equilibrium con-
stant, and your simple way of solving for the concentration
does not pass the 5% rule. Given programmable calculators,
many of which have quadratic equation solvers, is it ever
worth introducing simplifying assumptions, or should you
just solve all such equilibrium problems using the quadratic
equation?
97. We note in Exercise 16.4 that EDTA-metal ion complexes
have very high equilibrium constants. Can you surmise
why this is so based on the structure of the EDTA
4−
ion
shown in the exercise?
Thinking Beyond the Calculation
98. The industrial preparation of methanol, a potential gasoline
substitute, is accomplished by the hydrogenation of carbon
monoxide. The reaction is
CO(g) + 2H
2
(g)
CH
3
OH(g)
a. Calculate the thermodynamic parameters for this reaction
using the Appendix. Is this reaction spontaneous at 25°C?
b. Using your calculated value of ∆G°, determine the
equilibrium constant for the reaction. Does this reaction
favor products or reactants?
c. What is the value of K for the reverse reaction?
d. If the rate of the forward reaction was determined to be
3.56 × 10
−12
M/s, what is the rate of the reverse reaction at
equilibrium?
e. If a researcher began the synthesis of methanol by adding
1.5 mol of CO and 3.5 mol of H
2
to a 5.0-L flask, what
would be the equilibrium concentration of methanol?
f. What effect, if any, would the addition of more CO have
on the equilibrium concentration of methanol?
g. With added catalyst and removal of the methanol as it is
formed in the reaction, the reaction can quickly produce
100% yield. If 1.0 L of H
2
(g) at 25°C cost $0.10 and 1.0 L of
CO(g) at 25°C cost $0.30, what would it cost to produce
1.0 L of CH
3
OH(l) at 25°C? Assume the expense as-
sociated with the experimental procedure is negligible.
(Hint: Note that the problem asks for the cost to produce
liquid methanol from gaseous reactants.)
716 Chapter 16 Chemical Equilibrium
717
Contents and Selected Applications
Chemical Encounters: Common Uses of Acids and Bases
17.1 What Are Acids and Bases?
17.2 Acid Strength
Chemical Encounters: Acids in Foods
17.3 The pH Scale
17.4 Determining the pH of Acidic Solutions
17.5 Determining the pH of Basic Solutions
17.6 Polyprotic Acids
Chemical Encounters: Production and Uses of Phosphoric and Sulfuric
Acids
17.7 Assessing the Acid–Base Behavior of Salts in Aqueous Solution
Chemical Encounters: Acid–Base Properties of Amino Acids
17.8 Anhydrides in Aqueous Solution
Chemical Encounters: Acid Deposition and Acid-Neutralizing Capacity
Acids and
Bases
Acids and bases are an integral part of
our everyday life. For instance, the
acidity of the local swimming pool is
monitored daily. Adjustments to the pH
can be made by adding chemicals.
Go to college.hmco.com/pic/kelterMEE for online learning resources.
