Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

If you were to take a vote among chemists
as to the single most important technique
for finding out what you have, and how much of it there
is in a sample, the winner might well be chromatography,
which was the subject of the boxed feature in Chapter 12.
This technique has been used in many chemical analyses.
For instance, the analysis of steroids and other banned
substances in the urine of baseball players, football play-
ers, and Olympic athletes is done by separating out the
chemicals via chromatography. As another example,
the compounds that make up gasoline can be separated
out and identified chromatographically. The technique
has even been used to identify the gases on the planet
Venus.
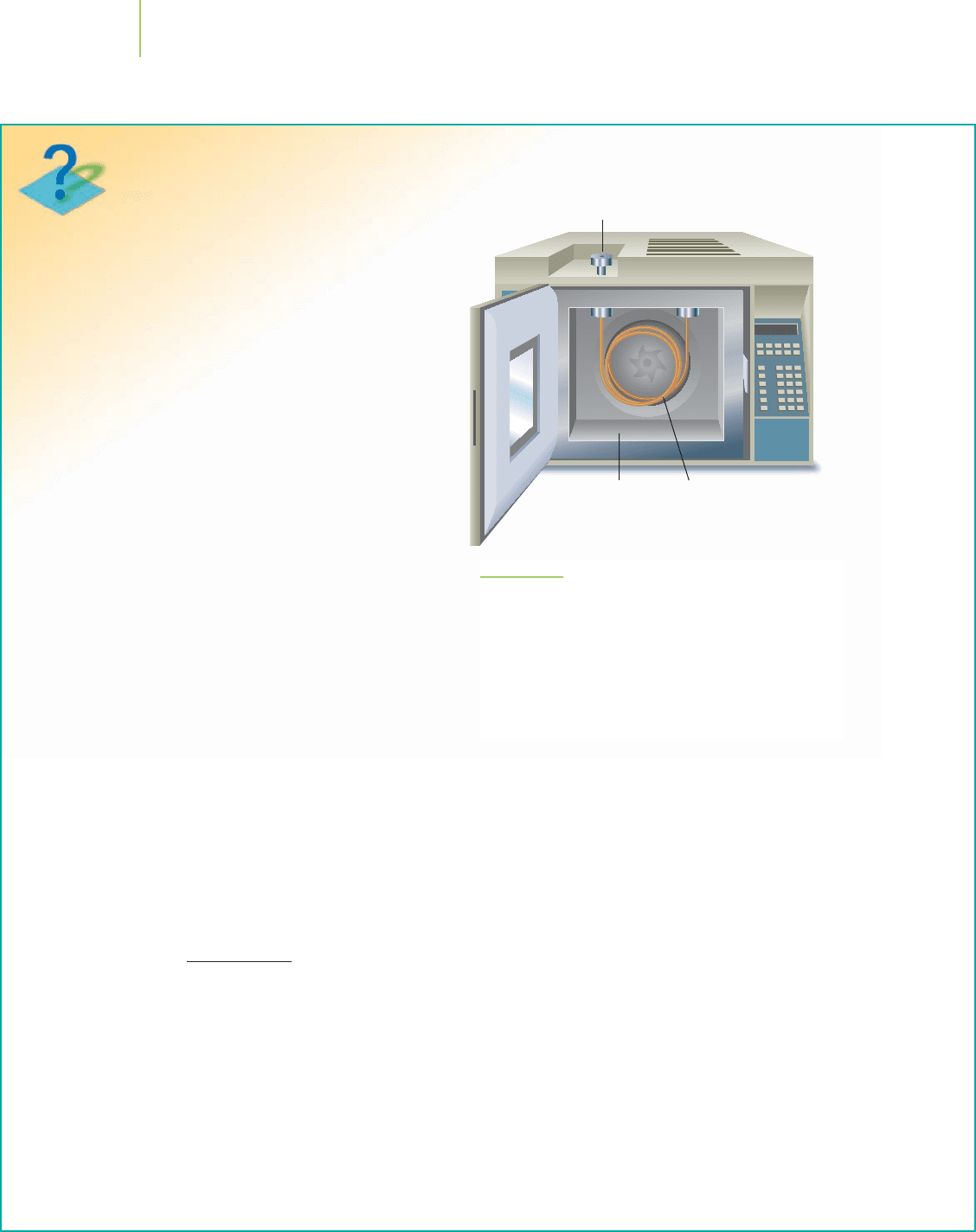
In a chromatograph, the components of a sample can
be separated on the basis of how they distribute them-
selves between two chemical or physical phases. Fig-
ure 16.6 shows the essential parts of a gas chromato-
graph. The sample to be analyzed is injected into the
instrument and pushed by an inert gas (in gas chromatog-
raphy
) or by a liquid (in liquid chromatography) into a
long tube known as a column. The gas or liquid that
pushes the sample moves, so it is called the mobile
phase
. The sample and the mobile phase pass through
the column packed with a stationary phase, so called be-
cause it stays in place on the column.
On the ride through the column, all of the compo-
nents of the sample (called the analytes) interact physi-
cally or chemically with the stationary phase. Here is
where our study of equilibria comes in. The interaction
of each analyte,“A,” with the mobile and stationary
phases can be described by the reversible reaction
A
mobile phase
A
stati
onary phase
The equilibrium constant (called a distribution constant,
K
D
) has the mass-action expression
K
D
=
[A
stationary phase
]
[A
mobile phase
]
If an analyte interacts considerably with the stationary
phase, the concentration of the analyte in the station-
ary phase will be greater than its concentration in the
mobile phase. Therefore, the value of K
D
will be a num-
ber greater than 1. In such cases, the analytes will slow
down and take more time to travel through the column.
If the analytes do not interact well with the stationary
phase, the value of K
D
will be small, and the analytes will
move quickly through the column. For a given set of
conditions in the chromatograph, each analyte will have
How do we know?
Equilibrium and chromatographic analysis
678 Chapter 16 Chemical Equilibrium
Injection port
Oven Column
(coiled tube)
Detector
(not visible)
FIGURE 16.6
The essential parts of a gas chromatograph. There is an inlet
connected to a column into which the sample is fed. The
sample is then pushed through the column by a carrier gas
such as helium (in gas chromatography) or by a liquid, often
an aqueous solution (in liquid chromatography). This phase
moves, so it is called the mobile phase. It passes through the
column containing a stationary phase, so called because it
stays in place on the column.
its own distribution constant (K
D
) and will exit the col-
umn at a different time.
Figure 16.7 shows chromatograms of the compounds
in refinery gas processed by a petroleum company, as
well as the important compounds in a sample of caffeine
and some “street drugs.” The components in a sample
from an athlete or a refinery can be identified by using
chromatography. However, the method has even more
uses. For instance, chromatography is often one of the
first steps in many very sophisticated analyses. Once the
analytes are separated in a chromatography instrument,
they can immediately be fed into other instruments, such
as a mass spectrometer or infrared spectrometer, to con-
firm their identity. In some cases, the information from
the other instruments is used to determine the identity
of an unknown component in a mixture. (Figure 16.8
shows two of the more important multistep analyses,
which are commonly referred to as hyphenated tech-
niques.) For this reason, chemical equilibrium, the basis
of chromatography, is often the most important process
in a multistep instrumental analysis.
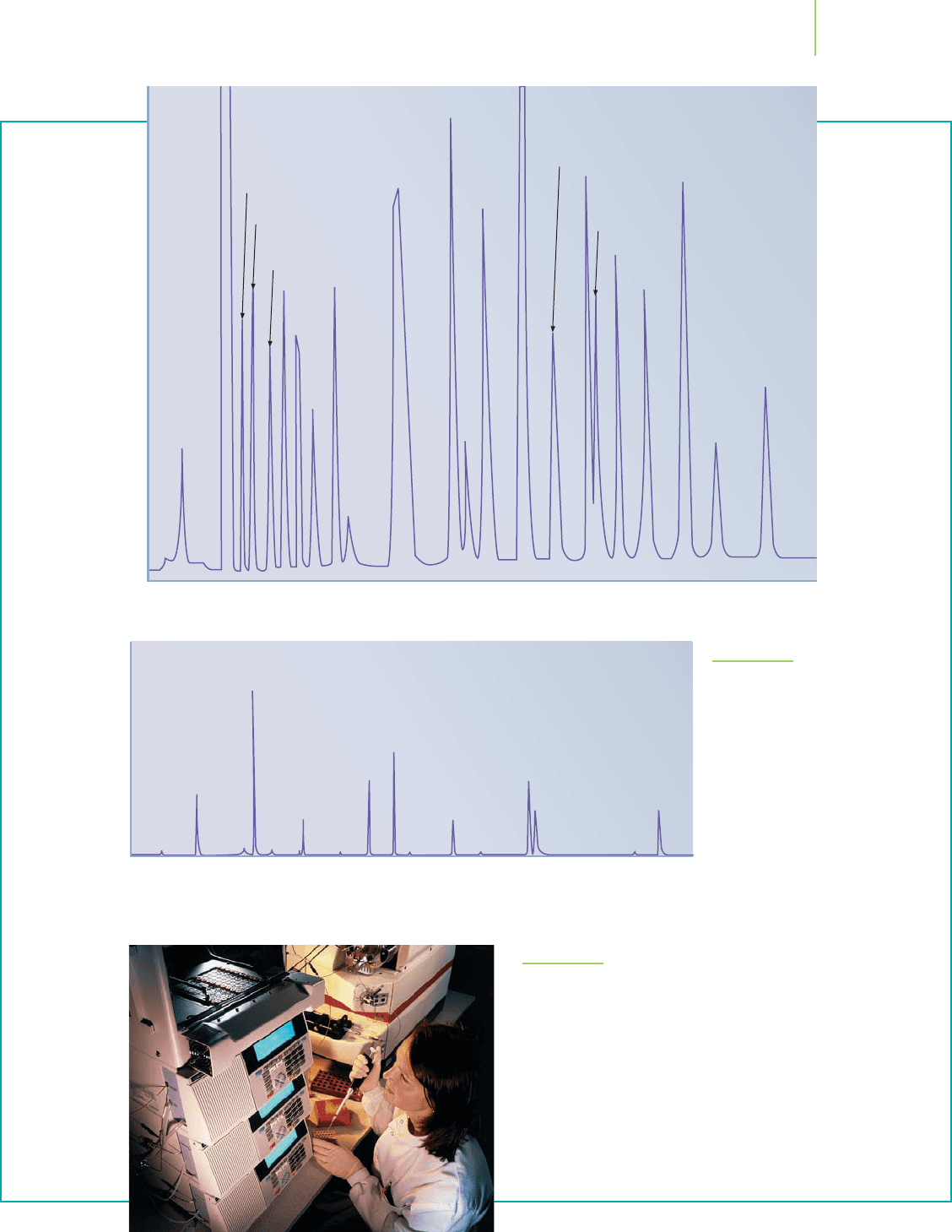
16.3 The Meaning of the Equilibrium Constant 679
1 Methamphetamine 3 Amobarbital 5 Procaine 7 Nortriptyline
2 Nicotine 4 Caffeine 6 Cocaine 8 Heroin
1
2
3
4
5
6
7
8
FIGURE 16.7
Chromatograms of compounds in refin-
ery gas (top) and common drugs (left)
show the importance of chromatogra-
phy as a separation technique.
Time (minutes)
Detector response
H
2
CO
2
C
2
H
6
CH
4
H
2
S
Propane
Propylene
Isobutane
n-Butane
1-Butene
Isobutene
t-2-Butene
c-2-Butene
i-C
5
n-C
5
1, 3-Butadiene
CO
C
2
H
4
C
2
H
2
O
2
N
2
0.0 40.0
FIGURE 16.8
Multistep analyses are commonly referred to as hyphenated techniques.
They include techniques such as GC-MS (gas chromatography–mass
spectrometry) and LC-NMR (liquid chromatography–nuclear magnetic
resonance) (see photo). These techniques are used to separate, identify,
and quantitate the solutes within a solution.
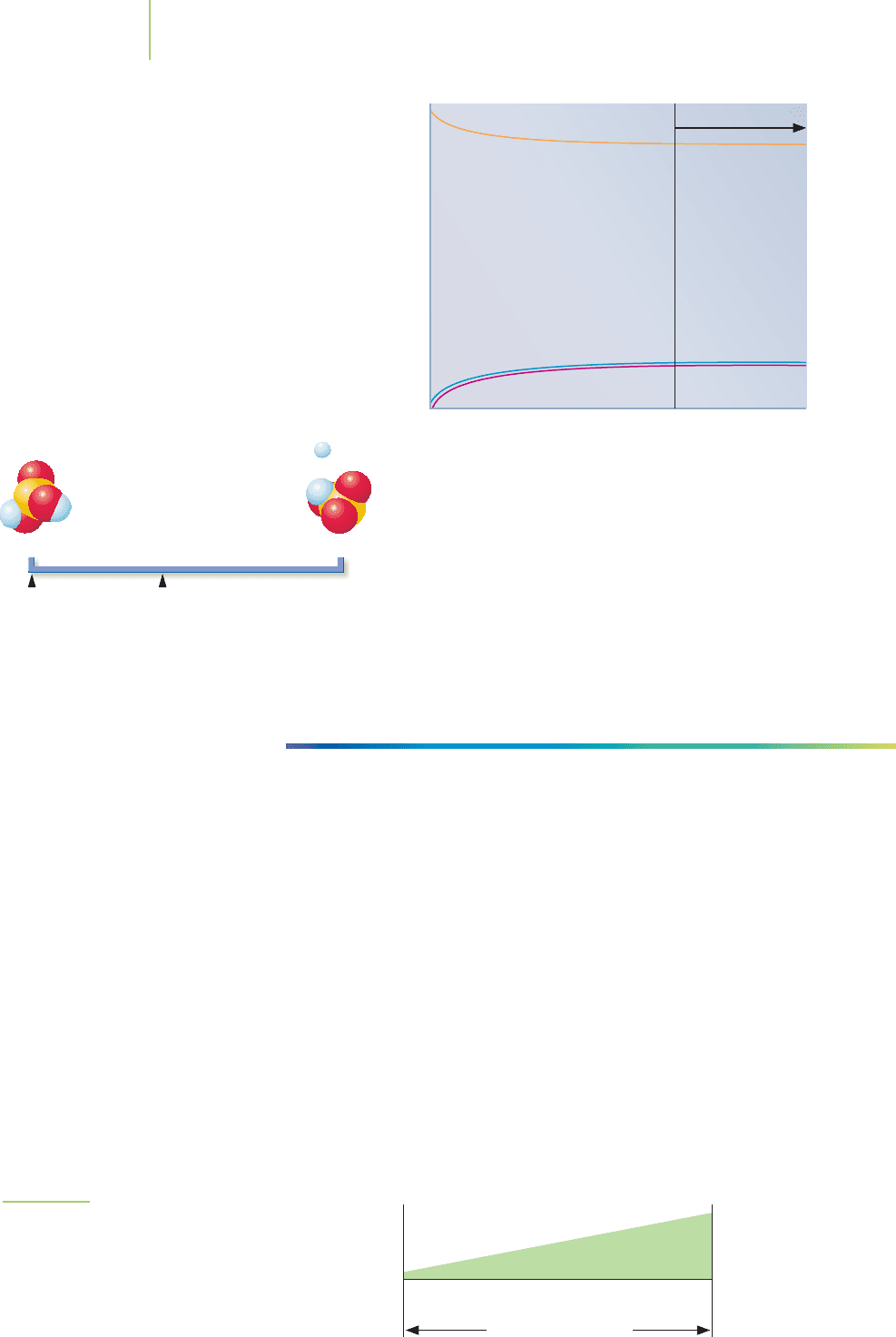
Reactants Products
Small K Large KIntermediate K
Equilibrium position
Concentration
Time
Equilibrium
[H
+
]
[H
2
SO
3
]
[HSO
3
–
]
Let’s examine another example of an equilibrium constant.
The ionization of sulfurous acid (H
2
SO
3
) to form hydrogen ions
and bisulfite ions (HSO
3
−
) has a value for K of 0.0120 at 25
o
C.
H
2
SO
3
(aq)
H
+
(aq) + HSO
3
−
(aq) K = 0.0120
This is an intermediate value; that is, K is relatively close to 1. At
equilibrium, there remain significant amounts of both reactants
and products. When the value for K is close to 1, the equilibrium lies somewhere
between the reactants and products sides. Again, our line chart helps us see the
equilibrium position.
HERE’S WHAT WE KNOW SO FAR
■
Processes that have very large values of K are those in which mostly products
are present at equilibrium.
■
Processes that have very small values of K have mostly reactants present at
equilibrium.
■
Processes with K values not too far from 1 have significant amounts of both
reactants and products at equilibrium.
The meaning of the equilibrium constant is shown graphically in Figure 16.9. As
we continue to build our understanding of equilibria, we will learn that the equi-
librium position for a reaction is related to the equilibrium constant as well as to
the temperature, the pressure, and changes in the concentration of the reactants
and products. As we explore these relationships, our goal is not only to interpret
the meaning of K but also to understand how we can manipulate our reaction
conditions to produce what we want (products or reactants) under the best pos-
sible conditions. In industrial-scale manufacturing, such as the production of
sulfuric acid, these “best possible conditions” can include chemical, environmen-
tal, and economic factors.
680 Chapter 16 Chemical Equilibrium
Reactants
SE
Products
The value of the equilibrium constant for the ionization of
sulfurous acid (H
2
SO
3
) indicates that a significant amount of
un-ionized reactant remains at equilibrium. Note, as well,
that when equilibrium is finally reached, the concentrations
of each component in the reaction become constant.
FIGURE 16.9
The relative equilibrium points of typical reactions
based on their equilibrium constants. High values of K
favor product formation. Small values of K favor the
reactants side. Intermediate values favor a middle-of-
the-road position.
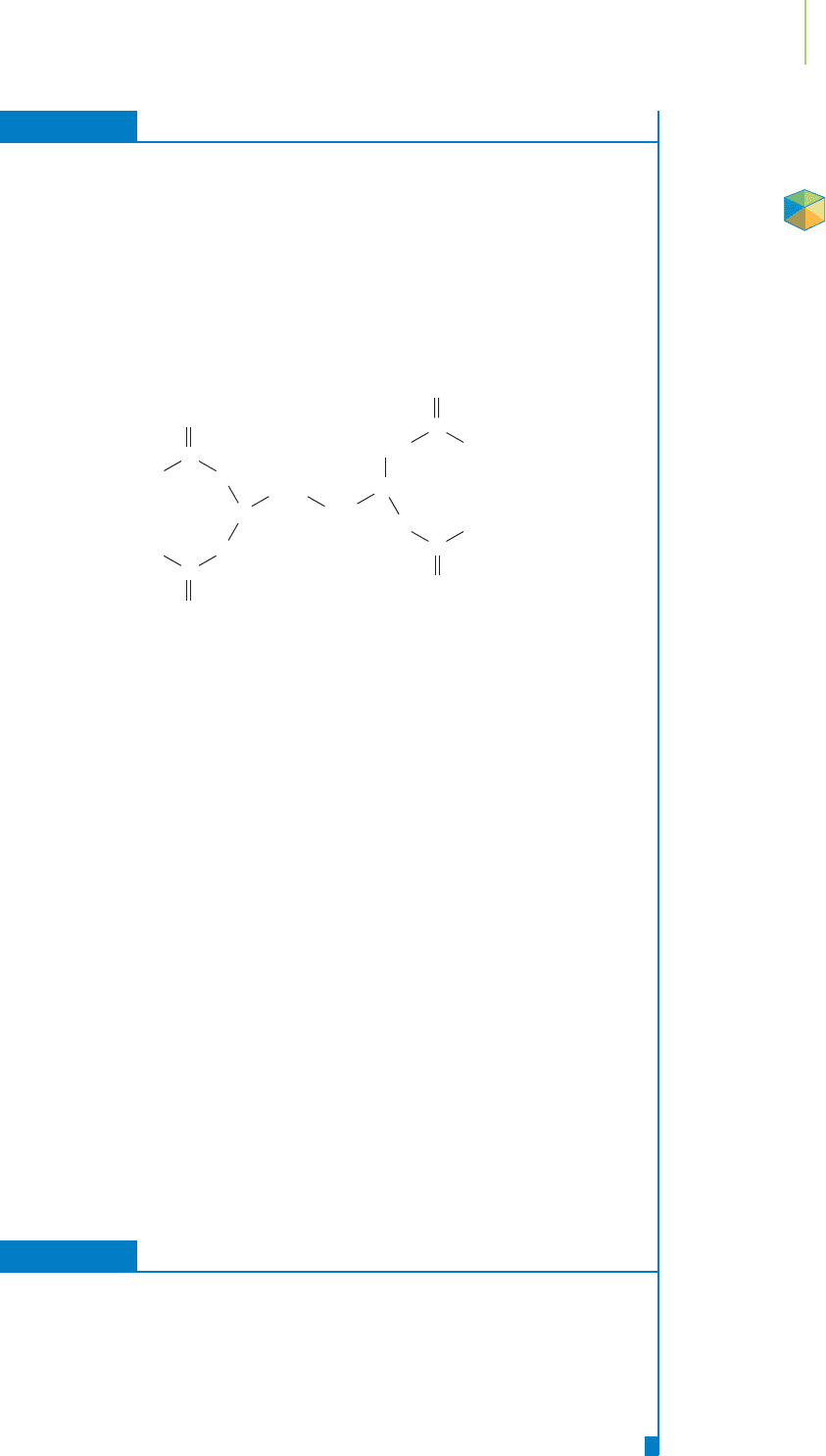
EXERCISE 16.4 Interpreting K
One of the most important chemicals in the toolbox of analytical chemists has the
chemical name ethylenediaminetetraacetic acid, or, more simply, EDTA. A chemist
would like to determine the concentration of several metal ions, including
Co
2+
,Zn
2+
,Ni
2+
, and Ca
2+
, in a solution by titrating them (recall titrations
from Section 4.3) with the ionic form of EDTA known as EDTA
4−
(shown
below). The values of K for the reaction of the metal ion with EDTA
4−
are listed
next to each cation. Judging solely on the basis of these values, and assuming the
equilibrium is established very quickly, would EDTA be a reasonable choice to
react almost completely with each of these metal ions?
(sample reaction) Ca
2+
(aq) + EDTA
4−
(aq)
CaEDTA
2−
(aq)
Element Cation K
cobalt Co
2+
2 × 10
16
zinc Zn
2+
3 × 10
16
nickel Ni
2+
4 × 10
18
calcium Ca
2+
5 × 10
10
First Thoughts
What does a large value of the equilibrium constant mean? Recall our discussion in
the text, where we noted that if K is very large, the equilibrium lies essentially all the
way to the products side. This means that looking only at the value for K (and
ignoring how quickly or slowly the reactions might occur) suggests that the reac-
tions will be essentially complete.
Solution
Because each of the equilibrium constants are considerably larger than 1, EDTA
4−
will react essentially completely with these metals.
Further Insights
EDTA is a remarkable analytical reagent because of its ability to react with nearly
every metal ion. Examples of its uses include determining water “hardness” by
titrating to find the concentrations of calcium and magnesium in water, and deter-
mining the composition of metal alloys and metals in pharmaceuticals.
PRACTICE 16.4
Which of these reactions provide(s) mostly products? . . . mostly reactants?
a. NH
4
+
(aq) + H
2
O(l)
NH
3
(aq) + H
3
O
+
(aq) K = 5.6 × 10
−10
b. HF(aq) + H
2
O(l)
H
3
O
+
(aq) + F
−
(aq) K = 7.2 × 10
−4
c. HOCl(aq) + H
2
O(l)
H
3
O
+
(aq) + OCl
−
(aq) K = 3.5 × 10
−8
d. HClO
4
(aq) + H
2
O(l)
H
3
O
+
(aq) + ClO
4
−
(aq) K = 1.0 × 10
7
See Problems 25 and 26.
C
O
O
CH
2
C
O
CH
2
CH
2
N
CH
2
O
C
C
O
O
O
CH
2
CH
2
N
O
Eth
y
lenediaminetetraacetate
16.3 The Meaning of the Equilibrium Constant 681
Application

Homogeneous and Heterogeneous Equilibria
Let’s look again at the reaction of the calcium and EDTA ions in the previous
exercise. Compare that to the dissolution of silver chloride, as well as the Haber
process for the combination of H
2
and N
2
to produce ammonia (NH
3
), the focus
of Exercise 16.2.
Ca
2+
(aq) + EDTA
4−
(aq)
CaEDTA
2−
(aq)
3H
2
(g) + N
2
(g)
2NH
3
(g)
AgCl(s)
Ag
+
(aq) + Cl
−
(aq)
Note the phases of each substance. In the EDTA titration of calcium ion, the re-
actants and products are all in the aqueous phase. When all of the phases are the
same in a reaction, we say that the reaction establishes a
homogeneous equilibrium.
The formation of ammonia is another example of homogeneous equilibrium be-
cause all of the substances are gases. On the other hand, the dissolution of silver
chloride in water begins with a solid and forms ions in the aqueous phase. When
there are different phases present in the reaction, we have a
heterogeneous
equilibrium
.
The mass-action expressions for homogeneous and heterogeneous equilibria
differ in one vital aspect, which we can examine by focusing on the formation of
a saturated solution of silver chloride. The density of solid AgCl is 5.56 g/cm
3
.We
can think of density as a measure of the concentration, because it is the amount
of substance per unit volume. Density is independent of the quantity of sub-
stance; therefore, the concentration of pure AgCl is the same whether we have 10 g
or 100 tons. Constants, such as the concentration of any pure solid or liquid, are
said to be part of the equilibrium constant and are not included in the mass-
action expression. This is a general result that is valid for pure solids and pure
liquids and is approximately correct for solvents such as water, but only when the
solutes are very dilute. This means that for the dissolution of silver chloride, our
original mass-action expression,
K =
[Ag
+
][Cl
−
]
[AgCl]
can be simplified by recognizing that AgCl is a solid:
K = [Ag
+
][Cl
−
]
We can apply this same reasoning to derive the mass-action expression for
one step in the large-scale cleanup of sulfur dioxide emitted from industrial
smokestacks. In this process, SO
2
is converted to solid CaSO
4
.
2CaO(s) + 2SO
2
(g) + O
2
(g)
2CaSO
4
(s)
K =
1
[SO
2
]
2
[O
2
]
16.4 Working with Equilibrium Constants
In this section, we begin to learn how we can work with reactions, mass-action
expressions, and equilibrium constants. This is the next step in learning how to
control experimental conditions by using equilibria to achieve desired chemical
outcomes. As with our previous section, we begin by looking at the reaction of
sulfur dioxide and oxygen.
682 Chapter 16 Chemical Equilibrium
Here are three ways of writing the balanced reaction of SO
2
with O
2
:
2SO
2
(g) + O
2
(g)
2SO
3
(g)(reaction A)
4SO
2
(g) + 2O
2
(g)
4SO
3
(g) (reaction B)
SO
2
(g) +
1
⁄2O
2
(g)
SO
3
(g) (reaction C)
We multiplied each coefficient in reaction A by 2 to obtain reaction B. We then
divided each coefficient by 4 to obtain reaction C. However, all the reactions
are the same because the mole ratios within each reaction do not change. That
is, the ratio of SO
2
to O
2
is 2 to 1 in each reaction. What about the mass-action
expressions?
K for reaction A =
[SO
3
]
2
[SO
2
]
2
[O
2
]
K for reaction B =
[SO
3
]
4
[SO
2
]
4
[O
2
]
2
K for reaction C =
[SO
3
]
[SO
2
][O
2
]
1/2
The mass-action expressions are all different! Does this change the value of K?
Let’s pick a set of typical contact process equilibrium concentration values
and substitute them into each expression: [O
2
] = 1.5 × 10
−3
M; [SO
2
] = 1.3 ×
10
−14
M; and [SO
3
] = 1.0 × 10
−3
M. To calculate the equilibrium constant, we
substitute the equilibrium concentrations of all substances into the mass-action
expression and perform the math indicated by the expression.
K
reaction A
=
[SO
3
]
2
[SO
2
]
2
[O
2
]
=
[1.0 ×10
−3
]
2
[1.3 ×10
−14
]
2
[1.5 ×10
−3
]
= 3.94 × 10
24
≈ 4 ×10
24
K
reaction B
=
[SO
3
]
4
[SO
2
]
4
[O
2
]
2
=
[1.0 ×10
−3
]
4
[1.3 ×10
−14
]
4
[1.5 ×10
−3
]
2
= 1.6 × 10
49
K
reaction C
=
[SO
3
]
[SO
2
][O
2
]
1/2
=
[1.0 ×10
−3
]
[1.3 ×10
−14
][1.5 ×10
−3
]
1/2
= 2.0 × 10
12
How do the values of K differ, and what is the relationship to the change in reac-
tion coefficients? We doubled the coefficients going from reaction A to reaction B,
and K
reaction B
= (K
reaction A
)
2
. In going from reaction B to reaction C we divided
the coefficients by 4, and K
reaction C
= (K
reaction B
)
1/4
. In general, then, when you
change the coefficients of a reaction by a factor of n,
K
new
= (K
old
)
n
EXERCISE 16.5 Reversing the Reaction
We opened the chapter by discussing the importance of the interaction of human
myoglobin with oxygen in the muscle cells.
Mb + O
2
MbO
2
K = 8.6 × 10
−7
Many biochemists and molecular biologists cite the “dissociation constant” for the
reaction written in reverse:
MbO
2
Mb +O
2
Given the relationship between the change in coefficients and the change in the
value of K just discussed, can you determine the equilibrium constant for the
reverse (dissociation) reaction?
16.4 Working with Equilibrium Constants 683
Solution
Reversing the reaction requires us to take the inverse of the original mass-action
expression. That is,
Mb + O
2
MbO
2
(original reaction)
K =
[MbO
2
]
[Mb][O
2
]
(original reaction)
MbO
2
Mb + O
2
(reverse reaction)
K =
[Mb][O
2
]
[MbO
2
]
(inverse mass-action expression)
Mathematically,
K
dissociation
= (K
original
)
−1
K
dissociation
= (8.6 × 10
−7
)
−1
= 1.2 ×10
6
PRACTICE 16.5
Given the value of K in Exercise 16.5, what is the equilibrium constant for the
following reaction?
1
⁄2 MbO
2
1
⁄2 Mb +
1
⁄2 O
2
See Problems 29, 30, 33, and 34.
Combining Reactions to Describe a Process
One of the most important questions that chemists answer is “How much do I
have?” In the chemical industry, this issue is often related to quality control: “Is
my vitamin C tablet actually filled with vitamin C?”“Does my aspirin tablet con-
tain the right amount of aspirin?”“Does my potato chip have too much water in
it?”A chemical titration is the chemist’s tool often used to answer such questions.
We mentioned titrations in Exercise 16.4, and much of Chapter 18 is devoted to
the technique. However, two key features of a titration experiment are necessary
for the titration to be effective. First, the titration reaction has to be essentially com-
plete, meaning that the equilibrium constant, K, must be quite large. In addition,
a titration reaction should be fast, even when the concentrations of the reactants
are low.
In the analytical laboratory, the concentration of acetic acid (CH
3
COOH,
known technically as ethanoic acid) can be measured by titration with a solution
of sodium hydroxide of known concentration. Can we show that the equilibrium
constant, which we will call K
titration
, is very large at 25°C? The reaction is
CH
3
COOH(aq) + OH
−
(aq)
CH
3
COO
−
(aq) + H
2
O(l)
The mass-action expression is
K
titration
=
[CH
3
COO
−
]
[CH
3
COOH][OH
−
]
We can consider this reaction as the sum of two separate equations for which we
know the equilibrium constants at 25°C. Using the known equations, we can cal-
culate the value for K
titration
.
CH
3
COOH(aq) + H
2
O(l)
CH
3
COO
−
(aq) + H
3
O
+
(aq) K
1
= 1.8 ×10
−5
H
3
O
+
(aq) + OH
−
(aq)
2H
2
O(l) K
2
= 1.0 ×10
14
CH
3
COOH(aq) + OH
−
(aq)
CH
3
COO
−
(aq) + H
2
O(l) K
titration
= ?
684 Chapter 16 Chemical Equilibrium
Application
Video Lesson: Strategies for
Solving Equilibrium Problems

The mass-action expressions for the known reactions are
K
1
=
[CH
3
COO
−
][H
3
O
+
]
[CH
3
COOH]
K
2
=
1
[H
3
O
+
][OH
−
]
If we multiply the two known mass-action expressions, we get
[CH
3
COO
−
][H
3
O
+
]
[CH
3
COOH]
×
1
[H
3
O
+
][OH
−
]
=
[CH
3
COO
−
][H
3
O
+
]
[CH
3
COOH][H
3
O
+
][OH
−
]
=
K
1
K
2
=
[CH
3
COO
−
]
[CH
3
COOH][OH
−
]
This is the mass-action expression for the titration reaction! For this process,
then,
K
titration
= K
1
K
2
= (1.8 × 10
−5
)(1.0 × 10
14
) = 1.8 ×10
9
In short, when the overall reaction is the sum of other reactions, the overall
equilibrium constant is the product of the equilibrium constants for these other
reactions. In the case of the titration of acetic acid with sodium hydroxide,
K
titration
is sufficiently large (and the reaction is quite fast). Therefore, the titration
of acetic acid with sodium hydroxide should (and does!) work well.
Calculating Equilibrium Concentrations from K
and Other Concentrations
We know from our previous discussion that it is possible to calculate the equilib-
rium constant by substituting equilibrium concentrations of reactants and
products into the mass-action expression.We have one equation, the mass-action
expression, and one unknown, K. In your day-to-day work as a chemist, biologist,
medical technologist, or soil scientist, the more likely scenario is that the value of
K is already known and you will need to calculate the equilibrium concentration
of one or more of the reactants or products.
Let’s use the Contact process to show how this is done for one unknown con-
centration when the other equilibrium concentrations and K are known. At 27°C,
K = 4.0 ×10
24
for the reaction
2SO
2
(g) + O
2
(g)
2SO
3
(g)
If the equilibrium concentrations of SO
2
and O
2
are 6.4 × 10
−12
M and 1.2 ×
10
−3
M, respectively, what is the equilibrium concentration of SO
3
? We have one
equation with one unknown, so we can solve for [SO
3
].
K =
[SO
3
]
2
[SO
2
]
2
[O
2
]
4.0 × 10
24
=
[SO
3
]
2
[6.4 ×10
−12
]
2
[1.2 ×10
−3
]
[SO
3
]
2
= 4.0 × 10
24
(6.4 × 10
−12
)
2
(1.2 × 10
−3
)
[SO
3
] =
√
0.197
= 0.443 ≈ 0.44 M
Does our answer make sense? We ought to check our math, and we can do so by
substituting the values back into the mass-action expression to verify the value of
the equilibrium constant.
[SO
3
]
2
[SO
2
]
2
[O
2
]
=
[0.443]
2
[6.4 ×10
−12
]
2
[1.2 ×10
−3
]
= 4.0 × 10
24
16.4 Working with Equilibrium Constants 685

We can make such substitutions because the reaction is at equilibrium. When this
is not the case, a different way of thinking, the subject of Section 16.5, will prove
useful.
Using Partial Pressures
Most of the equilibrium constants that chemists employ are derived using con-
centrations measured in molarity because much of the chemistry we do is in so-
lution. However, when working with gases, we can use partial pressures in units
of atmospheres.
What is the relationship between the pressure of a gas and its
concentration?
We can use the ideal gas equation, which holds well for gases in
low concentration (see Chapter 10).
PV = nRT
Concentration =
moles
volume
=
n
V
=
P
RT
The mass-action expression for the reaction of oxygen with sulfur dioxide can be
written using molarity, as usual, or by substituting the equivalent variables from
the ideal gas law
P
RT
in place of the concentration.
2SO
2
(g) + O
2
(g)
2SO
3
(g)
K =
[SO
3
]
2
[SO
2
]
2
[O
2
]
=
P
2
SO
3
(RT)
2
P
2
SO
2
(RT)
2
×
P
O
2
RT
We can combine all of the RT terms and put them on the side with the pressure-
based mass-action expression.
K =
P
2
SO
3
P
2
SO
2
P
O
2
(RT)
1
where K = the mass-action expression solved using only molar concentrations
P = the pressure in atmospheres
T = the temperature in kelvins
R = the universal gas constant
If we set K
p
equal to the pressure-based mass-action expression and rearrange the
equation, we get
K
p
=
P
2
SO
3
P
2
SO
2
P
O
2
K = K
p
(RT)
1
We have introduced a new equilibrium constant, K
p
, which is based on the
mass-action expression for the partial pressures of substances in the reaction. K
p
is often calculated with partial pressures, P, in units of atmospheres. However, re-
member that the actual definition of K involves the use of activities. Therefore, in
the end, K
p
has no units. As a matter of nomenclature, we use K
p
when dealing
with partial pressures and K (or, in some literature, K
c
) when dealing with con-
centrations. Note in this equation that the value of the exponent of RT indicates the
difference in the number of moles of gas between reactants and products. In general,
then, we can write the relationship between K and K
p
in two ways:
K
p
= K(RT)
∆n
or K = K
p
(RT)
−∆n
where n = the change in the number of moles of gas (products minus
reactants)
R = 0.08206 L·atm·mol
−1
·K
−1
T = temperature in kelvins
686 Chapter 16 Chemical Equilibrium
Video Lesson: Converting
Between
K
c
and
K
p
In the reaction of sulfur dioxide with oxygen, there is one fewer mole of
gaseous products (2SO
3
) than of reactants (2SO
2
and O
2
), so the change in the
number of moles of gas from reactant to product, n, is equal to –1. We can re-
late K to K
p
in two ways, depending on which equilibrium constant we are given.
K
p
= K(RT)
−1
or K = K
p
(RT)
1
At 673 K and 1 atm total system pressure, the value of K
p
for the oxidation of SO
2
to SO
3
is 1.58 ×10
5
, measured using the equilibrium partial pressure of each gas.
We can calculate K using this information.
K = K
p
(RT)
−n
K =(1.58 × 10
5
)(0.08206 L·atm·mol
−1
·K
−1
× 673 K)
−(−1)
= 8.73 × 10
6
EXERCISE 16.6 Converting K to K
p
Calculate K
p
for the production of ammonia via the Haber process.
3H
2
(g) + N
2
(g)
2NH
3
(g) K = 0.060 (at 500°C)
Solution
We lose 2 mol of gas going from reactants to products, so n =−2.
K
p
= K(RT)
n
K
p
= 0.060 (0.08206 L·atm·mol
−1
·K
−1
× 773 K)
−2
K
p
= 0.060 (62.43)
−2
K
p
=1.5 × 10
−5
PRACTICE 16.6
Calculate the value of K
p
at 25°C for the reaction of chlorine and carbon monoxide.
CO(g) + Cl
2
(g)
COCl
2
(g) K = 3.7 × 10
9
(at 25°C)
See Problems 41 and 42.
16.5 Solving Equilibrium Problems—
A Different Way of Thinking
The world is not at equilibrium. Its shifts are seen and felt in the massive up-
heavals of earthquakes, volcanoes, and hurricanes and in the smallest electronic
interactions of atoms. Life itself is a process in which reversible reactions within us
keep shifting their equilibrium positions, always chasing a moving target, in ways
that make our survival possible. If one aspect of being human is our ability to un-
derstand our world, then one expression of this understanding is the ability to ma-
nipulate our starting reaction conditions to control the position of equilibrium.We
cannot stop earthquakes, but we can reliably make ammonia, sulfuric acid, and a
whole host of other chemicals. We can examine the complex interactions of car-
bon with the environment. We can understand the interaction of myoglobin and
oxygen. We can pick the best conditions for chemical analyses. All these things are
based on knowing how the conditions change from the starting point to equilib-
rium. How can we do this? We must first develop a different way of thinking.
This way of thinking is based on two questions:
“Given the chemical and phys-
ical conditions, how do I judge what is likely to happen?”
and How can I use my
16.5 Solving Equilibrium Problems—A Different Way of Thinking
687
