Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

■
Reaction rates determine the speed at which a reac-
tion progresses but do not reveal anything about the
extent to which they produce a product. (Sec-
tion 15.1)
■
The average rate can be measured if you know the
initial and final concentrations over a particular time
period. (Section 15.1)
■
The instantaneous rate is the rate at a given point in
the reaction. It can be determined by measuring the
concentrations at points when the time difference
approaches zero, or it can be measured by determin-
ing the slope of a line tangent to a plot of the rate
versus time. (Section 15.1)
■
The initial rate of a reaction is the instantaneous rate
as the reaction starts. (Section 15.1)
■
The half-life of a reaction is the time required for the
concentration of a reaction to reach 50% of the ini-
tial value. (Section 15.3)
■
Complex reaction orders can often be reduced to
pseudo-first-order reactions by keeping the concen-
tration of one of the reactants large. (Section 15.3)
■
The rate law is experimentally determined using the
method of initial rates or the method of graphical
analysis. (Section 15.4)
■
Transition state theory, which is based on collision
theory, describes the energy of a reaction during the
course of a reaction. A plot of the reaction coordi-
nate versus the energy can give meaningful informa-
tion, such as the activation energy, the presence of
any intermediates, the effect of a catalyst, and the
enthalpy for the forward and reverse process. (Sec-
tion 15.6)
■
Catalysts speed up a reaction without being con-
sumed in the reaction. (Section 15.7)
The Bottom Line
Key Words
absorb To be associated, through intermolecular forces
of attraction, by being taken up or mixing into a
substance. (p. 656)
activated complex The unstable collection of atoms that
can break up either to form the products or to re-
form the reactants. (p. 651)
activation energy (E
a
) The minimum energy of collision
that reactants must have in order to successfully cre-
ate the activated complex. (p. 630)
adsorb To be associated, through intermolecular forces
of attraction, with a surface. (p. 656)
average rate The rate of a reaction measured over a
long period of time. (p. 622)
bimolecular reaction A reaction involving the collision of
two molecules in the rate-determining step. (p. 648)
catalyst A substance that participates in a reaction, is
not consumed, and modifies the mechanism of the
reaction to provide a lower activation energy. Cata-
lysts increase the rate of reaction. (p. 654)
chemical kinetics The study of the rates and mechanisms
of chemical reactions. (p. 621)
collision theory A theory that correlates the number of
properly oriented collisions with the rate of the reac-
tion. (p. 629)
desorbed Released from a surface. (p. 656)
elementary step A single step in a mechanism that indi-
cates how reactants proceed toward products. (p. 648)
environmental chemist A scientist who studies the inter-
actions of compounds in the environment. (p. 620)
658 Chapter 15 Chemical Kinetics
The principles of chemical kinetics are important not only to chemists
but to anyone concerned with the rate of a process. The rate of decay at the
landfill, the rate of ozone depletion, and the persistence of pesticides and
herbicides in the environment can easily be determined by examining the
rate laws associated with these processes. Our work in the lab can provide
the rate laws for these reactions. And, after determining the thermodynamics
of a reaction (Chapter 14), we can determine not only whether a reaction will
take place but also how long it will take to complete.

first order A reaction is first order in a particular species
when the rate of the reaction depends on the concen-
tration of that species raised to the first power.
(p. 628)
frequency factor A term in the Arrhenius equation that
indicates the rate of collision and the probability that
colliding reactants are oriented for a successful reac-
tion. (p. 652)
half-life (t
1/2
) The time required for a reaction to reach
50% completion. (p. 636)
herbicide Any compound used to kill unwanted plants.
(p. 620)
heterogeneous catalyst A specific catalyst that exists in a
different physical state than the reaction. (p. 656)
homogeneous catalyst A specific catalyst that exists in
the same physical state as the compounds in the reac-
tion. (p. 655)
initial rate The instantaneous rate of reaction when
t = 0. (p. 623)
insecticide Any compound used to kill unwanted in-
sects. (p. 620)
instantaneous rate The rate of reaction measured at a
specific instant in time. The instantaneous rate is typ-
ically measured by calculating the slope of a line that
is tangent to the curve drawn by plotting [reactant]
versus time. (p. 623)
integrated first-order rate law An expression derived
from a first-order rate law that illustrates how the
concentrations of reactants vary as a function of
time. (p. 634)
integrated rate law A form of the rate law that illustrates
how the concentrations of reactants vary as a func-
tion of time. (p. 633)
integrated second-order rate law An expression derived
from a second-order rate law that illustrates how the
concentrations of reactants vary as a function of
time. (p. 639)
integrated zero-order rate law An expression derived
from a zero-order rate law that illustrates how the
concentrations of reactants vary as a function of
time. (p. 639)
intermediate A compound that is produced in a reac-
tion and then consumed in the reaction. Intermedi-
ates are not indicated in the overall reaction equa-
tion. (p. 650)
mechanism The series of steps taken by the components
of a reaction as they progress from reactants to prod-
ucts. (p. 648)
method of graphical analysis A method of determining
the rate law for a reaction where the rate of a reaction
is plotted versus time. Also known as the method of
integrated rate laws. (p. 644)
method of initial rates A method of determining the rate
law for a reaction where the initial rates of different
trials of a reaction are compared. (p. 641)
molecularity A description of the number of molecules
that must collide in the rate-determining step of a
reaction. (p. 648)
pesticide Any compound used to kill unwanted organ-
isms (whether they be animals, insects, or plants).
(p. 620)
pseudo-first-order rate A modification of the second-
order rate that enables one to use first-order kinetics.
(p. 640)
rate The “speed” of a reaction, recorded in M/s.
(p. 621)
rate constant A constant that is characteristic of a reac-
tion at a given temperature, relating to the rate of dis-
appearance of reactants. (p. 628)
rate-determining step The slowest elementary step in a
reaction sequence. (p. 648)
rate law An equation that indicates the molecularity of
a reaction as a function of the rate of the reaction.
(p. 628)
reaction coordinate A measure that describes the
progress of the reaction. (p. 651)
reaction profile A plot of the progress of a reaction ver-
sus the energy of the components. (p. 651)
second-order rate law The rate of a reaction is directly
related either to the square of the concentration of a
single reactant or to the product of the concentra-
tions of two reactants. (p. 639)
termolecular (trimolecular) reaction A reaction in which
three molecules collide in the rate-determining step
of the reaction. (p. 648)
transition state The point along the reaction coordinate
where the reactants have collided to form the acti-
vated complex. (p. 651)
transition state theory The theory that describes how the
energy of activation is related to the rate of reaction.
(p. 651)
unimolecular reaction A reaction in which one molecule
alone is involved in the rate-determining step of the
reaction. (p. 648)
zero-order reaction A reaction in which the rate is not
related to the concentrations of any species in the
reaction. (p. 639)
Key Words 659
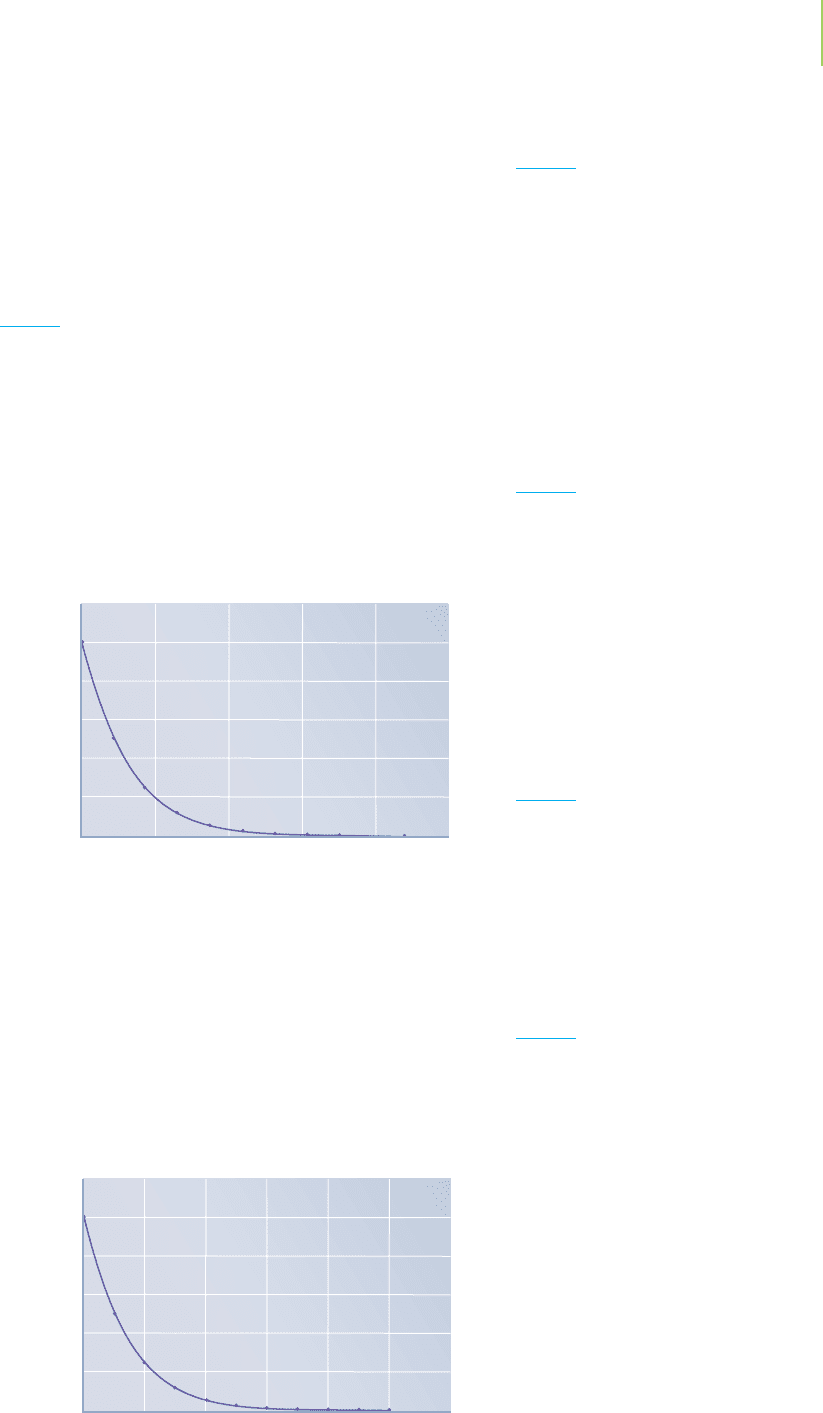
12. Plot the accompanying data on a piece of graph paper or
using a computer. Then determine the following:
a. The initial rate, in M/min, of the reaction
b. The instantaneous rate, in M/min, at t = 15 min
c. The instantaneous rate, in M/min, at t = 45 min
d. The average rate, in M/min, of the reaction
Chemical Applications and Practice
13. The common disinfectant hydrogen peroxide (H
2
O
2
) can de-
compose according to the following balanced equation:
2H
2
O
2
(aq) → 2H
2
O(l) + O
2
(g)
a. How much faster is the appearance of H
2
O than the ap-
pearance of O
2
?
b. Write the expression that defines the rate of the reaction
based on the rate of disappearance of H
2
O
2
and the rates
of appearance of H
2
O and O
2
.
660 Chapter 15 Chemical Kinetics
Distance (mi) Time (s)
00
0.055 5
0.193 10
0.611 20
1.120 30
[Pesticide] Time (min)
0.10000 M 0
0.055294 M 15
0.030575 M 30
0.016906 M 45
0.009348 M 60
Focus Your Learning
The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
Section 15.1 Reaction Rates
Skill Review
1. Use the analogy of a runner in a 10-km road race to define
each of these pertinent reaction kinetics terms:
a. Extent of reaction c. Instantaneous rate
b. Average rate d. Initial rate
2. Use the analogy of firing a bullet from a gun to define each of
these kinetics terms:
a. Extent of reaction c. Instantaneous rate
b. Average rate d. Initial rate
3. For the following reaction in a 4.00-L container, it was found
that 1.00 × 10
−4
mol of C
4
H
8
reacted over a time period
from 10:58
A.
M. to 11:15 A.M.
C
4
H
8
(g) → 2C
2
H
4
(g)
What is the average rate, in M/s, for this reaction? Is it possi-
ble to have an instantaneous rate faster than the average rate?
Explain.
4. The reaction described in Problem 3 was performed a second
time. Into a 1.5-L flask was placed 3.25 mol of C
4
H
8
(g). After
56 min, 1.00 mol of C
2
H
4
(g) was obtained. What is the aver-
age rate, in M/s, for this reaction?
5. Using the same reaction as in Problem 3 (C
4
H
8
→ 2C
2
H
4
),
compare the rate of appearance of C
2
H
4
to the rate of disap-
pearance of C
4
H
8
. Write out this relationship using the style
presented in the chapter that depicts the rate, as it would be
expressed based on either of the components.
6. The following reaction shows PH
3
decomposing into two
products.
4PH
3
(g) + 3O
2
(g) → 4P(g) + 6H
2
O(g)
Write the rate expression that depicts the rate of disappear-
ance of the reactant when compared to the appearance of
each product.
7. Determine the rate of reaction, in M/s, for each of these sys-
tems over the time period indicated. Assume that the reac-
tion is
A → B
a. [A]
0
= 0.350 M;[A]
t
= 0.300 M; t
0
= 0 s; t = 100 s
b. [A]
0
= 1.522 M;[A]
t
= 0.350 M; t
0
= 0 min; t = 15 min
c. [A]
0
= 0.050 M;[A]
t
= 0.010 M; t
0
= 0 days; t = 399 days
d. [A]
0
= 0.280 M;[A]
t
= 0.140 M; t
0
= 35 min; t = 2.5 h
8. Determine the rate of reaction, in M/s, for each of these
systems over the time period indicated. Assume that the reac-
tion is
A → 2B
a. [A]
0
= 0.350 M;[A]
t
= 0.280 M; t
0
= 0 s; t = 100 s
b. [A]
0
= 2.50 M;[A]
t
= 0.250 M; t
0
= 0 s; t = 15 min
c. [A]
0
= 1.750 M;[A]
t
= 0.010 M; t
0
= 0 days; t = 250 days
d. [A]
0
= 0.125 M;[A]
t
= 0.105 M; t
0
= 15 s; t = 2.5 min
9. In the general reaction shown below, the rate of disappear-
ance of A is 4.5 × 10
−2
M/s.
2A + 3B → C + 2D
a. What is the rate of disappearance of B?
b. What is the rate of appearance of C?
c. What is the rate of appearance of D?
10. In the general reaction shown below, the rate of disappear-
ance of A is 1.85 × 10
−4
M/s.
A + 2B → 2C + 3D
a. What is the rate of disappearance of B?
b. What is the rate of appearance of C?
c. What is the rate of appearance of D?
11. Either on a piece of graph paper or using a computer, plot the
data given in the accompanying table, which are from a drag
race. Then determine:
a. The initial speed
b. The instantaneous speed at t = 8 s
c. The instantaneous speed at t = 25 s
d. The average speed of the dragster
e. In your own words, how does this compare to the typical
plot of a reaction?
Focus Your Learning 661
14. Reducing the level of nitrogen monoxide compounds emit-
ted in automobile exhaust is a priority of environmentally
concerned citizens. One response to this has been the devel-
opment of catalytic converters. The NO in the exhaust passes
over a rhodium-containing converter and is changed to the
components already present in clean air.
2NO(g) → N
2
(g) + O
2
(g)
If the rate of appearance of N
2
were 1.5 × 10
−6
mol/s, what
would you calculate as the rate of disappearance of NO?
15. The relationship between ozone and humans has a rather
unique feature. Whether ozone benefits humans depends
largely on proximity. Ozone is considered beneficial when it
occurs in the upper atmosphere, but it can cause serious
respiratory problems if it is in the atmosphere layer closest to
us. Ozone can be formed by the following reaction:
O
2
(g) + O(g) → O
3
(g)
This graphical representation shows the decomposition of
oxygen over time. Redraw the graph and sketch a line that
would depict the appearance of ozone over the same time
period.
15.2 An Introduction to Rate Laws
Skill Review
17. Judging on the basis of collision theory, indicate whether
each of these modifications would increase the rate of a reac-
tion. Explain your answers.
a. increasing the temperature
b. decreasing the temperature
c. increasing the initial concentration of reactant
d. diluting the reaction with more solvent
18. Judging on the basis of collision theory, indicate whether
each of these modifications would have an effect on the rate
of a reaction. Explain your answers.
a. increasing the volume of the reaction
b. decreasing the number of reactant molecules
c. adding some product molecules to the reaction
d. removing the product molecules as they form
19. The following equation represents the rate law for the de-
composition of an important pesticide (symbolized here as
Pest).
a. What is the order of the reaction?
b. What is the meaning of k?
c. What effect would doubling the concentration of pesticide
have on the rate of the reaction?
d. What effect would doubling the concentration of the
pesticide have on the value of k?
Rate = k [Pest]
2
[H
+
]
20. Report the overall order of each of these reactions:
a. Rate = k[NO][O
3
] c. Rate = k[H
2
][Cl
2
]
1/2
b. Rate =k[NO][H
2
][H
2
O]
−1
21. Calculate the rate of the following reaction, if the rate con-
stant is 3.95 × 10
−4
s
−1
and [A] = 0.509 M. The reaction is
first order in A and zero order in B.
A + B → 2C + D
22. Calculate the rate constant for a reaction if, when [A] =
0.672 M, the rate of the reaction is 2.99 × 10
−3
M/s. The re-
action is second order in A.
A → B
Chemical Applications and Practices
23. The hypochlorite ion (ClO
−
) is present in commercial
bleach. One aqueous reaction in which this ion participates is
3ClO
−
→ ClO
3
−
+ 2Cl
−
Without any further information, give two reasons why we
could not claim that the rate law is Rate = k[ClO
−
].
24. If the rate law for the hypochlorite reaction from the equation
in Problem 23 (3ClO
−
→ClO
3
−
+2Cl
−
), is Rate =k[ClO
−
]
2
,
what would you report as the order of the reaction? If the con-
centration of hypochlorite were tripled, what effect would this
have on the rate of the reaction (assuming no change in tem-
perature)? The order of the reaction does not match the stoi-
chiometry of the equation. What does this indicate?
0
0.5
1.0
1.5
2.0
2.5
3.0
0 50 100 150 200 250
Time (minutes)
Concentration (M)
0
0.2
0.4
0.6
0.8
1.0
1.2
0 20406080100120
Time (minutes)
Concentration (M)
16. As we have seen, the reaction that is used to produce the agri-
culturally critical fertilizer ammonia combines nitrogen and
hydrogen gas in the following manner:
3H
2
(g) + N
2
(g) → 2NH
3
(g)
a. Using the following graph of the disappearance of N
2
as a
guide, sketch two additional lines that factor in the ratios
of the different species for the disappearance of H
2
and
appearance of NH
3
, respectively.
b. If the disappearance of N
2
in a particular reaction were
1.2 × 10
−3
mol/min, what would be the rate of appearance
of NH
3
?
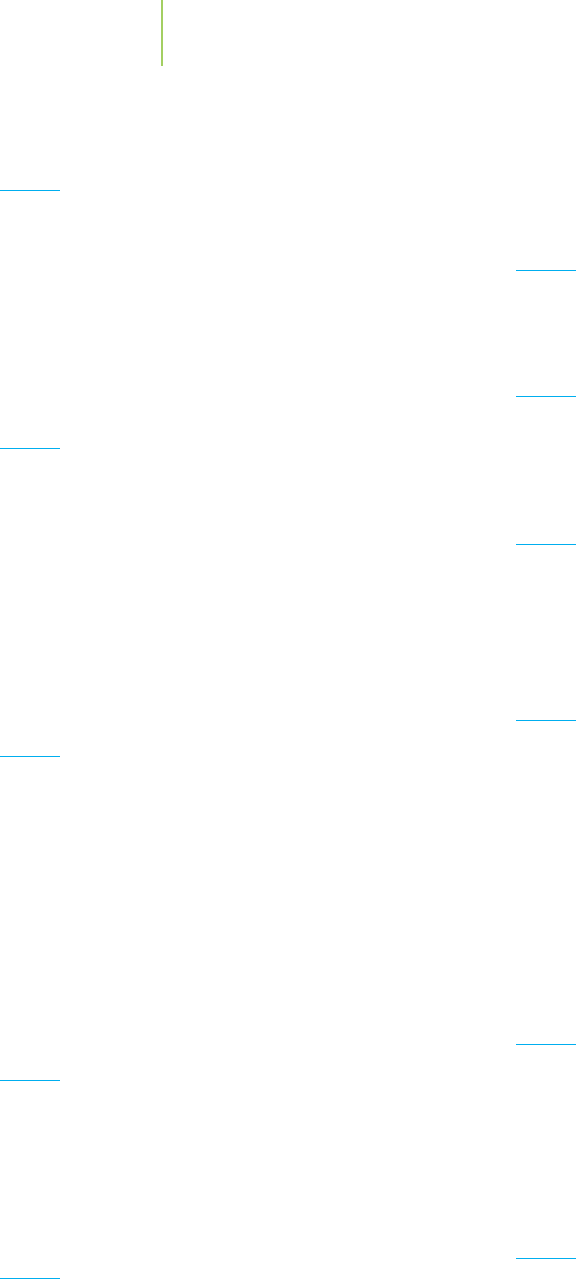
662 Chapter 15 Chemical Kinetics
Section 15.3
Changes in Time—The Integrated Rate Law
Skill Review
25. Determine the rate constant for each of these first-order
reactions:
a. decomposition of peroxyacetyl nitrate; t
1/2
= 1920 s
b. decomposition of sulfuryl chloride; t
1/2
= 525 min
c. radioactive decay of
40
K; t
1/2
= 1.25 × 10
9
years
d. radioactive decay of
14
C; t
1/2
= 5730 years
26. Determine the half-life for each of these first-order reactions:
a. radioactive decay of
131
I; k = 0.08619 day
−1
b. radioactive decay of
24
Na; k = 0.0473 h
−1
c. decomposition of DDT; k = 2.31 × 10
−4
day
−1
d. metabolism of malathion; k = 0.693 day
−1
27. In the following first-order reaction, the half-life was deter-
mined to be 43 min.
a. How long would it take for the concentration of A to drop
to 50% of the original amount?
b. To 25% of the original amount?
c. To 10% of the original amount?
A → B
28. The radioactive decay of
14
C is a first-order reaction.
a. If a sample originally contains 1.59 × 10
−5
M
14
C, how
long will it take before the concentration is 0.795 ×
10
−5
M? The half-life of
14
C is 5730 years.
b. How long will it take before the concentration is 1.00 ×
10
−6
M?
29. For each case below, calculate the concentration of A at the
time indicated. The reaction is first order with a half-life of
3.95 × 10
2
s.
a. [A]
0
= 0.100 M; t = 50.0 s
b. [A]
0
= 0.100 M; t = 100.0 s
c. [A]
0
= 0.200 M; t = 50.0 s
d. [A]
0
= 0.200 M; t = 20.0 s
30. For each case below, calculate the time required to reach the
concentration shown. The reaction is first order with a half-
life of 1.25 × 10
−3
s.
a. [A]
0
= 0.100 M;[A]=0.010 M
b. [A]
0
= 0.350 M;[A]=0.095 M
c. [A]
0
= 0.200 M;[A]=0.010 M
d. [A]
0
= 0.250 M;[A]=0.100 M
31. What was the initial concentration of A if [A] = 0.0388 M
after 2.75 days? Assume that the half-life of the first-order
reaction is 1.18 days.
A → B
32. Determine the rate constant (in s
−1
) for the first-order
decomposition of the pesticide 2,4-D, if the initial concentra-
tion was 2.73 ×10
−3
M and the concentration after 60.0 days
was 8.44 × 10
−4
M.
33. In the following cases of first-order reactions, something is
wrong with the data. Determine which value is incorrect and
indicate why.
a. [A]
0
= 0.100 M;[A]
t
= 0.900 M; t
0
= 0 s; t = 10 s
b. [A]
0
= 0.090 M;[A]
t
= 0.010 M; t
0
= 10 s; t = 0 s
34. In the following cases of first-order reactions, something is
wrong with the data. Determine which value is incorrect and
indicate why.
a. [A]
0
= 0.100 M;[A]=0.050 M; t
0
= 0 s; t = 10 s;
t
1/2
= 24 min
b. [A]
0
= 0.900 M;[A]=0.450 M; t
0
= 0 s; t = 10 s;
k = 0.085 s
−1
35. What is the half-life of the decomposition of ammonia on a
metal surface, a zero-order reaction, if [NH
3
] = 0.0333 M
initially and [NH
3
] = 0.0150 M at t = 450.0 s?
36. Determine the concentration of ammonia from Problem 35
when t = 800.0 s.
37. What is the value of the rate constant for a zero-order reac-
tion with t
1/2
= 3.55 h? Assume the original concentration
[A]
0
= 0.100 M.
38. What is the value of the rate constant for a second-order re-
action with t
1/2
=300.0 days? Assume the original concentra-
tion [A]
0
= 1.50 M.
39. What is the half-life of a second-order reaction if the concen-
tration of A drops to 10% of its original value of 2.00 ×
10
−3
M in 520 min?
2A → B
40. What is the concentration of A after 3.5 h in the second-order
reaction illustrated in Problem 39, if [A]
0
= 0.100 M and
k = 1.2 ×
10
−4
M
−1
·s
−1
?
41. Determine the concentration of the reactant in each of these
cases. Assume that the reaction is second order with
k = 0.0312 M
−1
·min
−1
.
a. [A]
0
= 0.100 M; t = 10.0 s
b. [A]
0
= 0.500 M; t = 10.0 s
c. [A]
0
= 0.339 M; t = 200.0 s
d. [A]
0
= 0.0050 M; t = 24 days
42. Determine the concentration of the reactant in each of these
cases. Assume that the reaction is second order with
k = 0.410 M
−1
·h
−1
.
a. [A]
0
= 0.100 M; t = 10.0 h
b. [A]
0
= 0.500 M; t = 10.0 h
c. [A]
0
= 0.222 M; t = 1.75 h
d. [A]
0
= 0.0010 M; t = 14 days
43. A graphical plot of concentration of a reactant versus time
during a reaction will reveal, for reactions above zero order, a
changing rate. Use collision theory to explain why the rate
slows over time.
44. When you examine the three integrated rate expressions
mentioned in the chapter, you can easily note that all three
contain the symbol k. What would be the units for k in each
of the zero-order, first-order, and second-order rate expres-
sions? (If necessary, use any time unit merely as “time.”)
45. Suppose two students are discussing their recent chemistry
lab experiment. The first student, Pablo, remarks that his
first-order reaction has a half-life of 25 min. The other stu-
dent, Peter, replies that coincidently, his second-order reac-
tion also has a half-life of 25 min. Then both go back to
repeat their experiments, but with different amounts of
Focus Your Learning 663
starting materials. Neglecting experimental error, explain any
differences, or lack of, that they might find this time.
46. a. What advantage does changing a reaction to pseudo-first-
order kinetics give to an experimenter?
b. Describe how to alter a kinetics experiment in order to
study it in the pseudo-first-order kinetic model.
c. Explain why the concentration of one component in the
pseudo-first-order model can be made to be part of the
specific rate constant of a reaction.
Chemical Applications and Practices
47. An agricultural chemist is attempting to detect the decompo-
sition of a new herbicide. The chemist notes that after appli-
cation, the compound decays from 100.0% potency to 75.0%
potency over a time period of 1 week (168 h). Assume that
the original concentration [A]
0
= 1.00 × 10
−3
M.
a. What would be the specific rate constant if the reaction
were first order with respect to the herbicide?
b. What would be the specific rate constant if the reaction
were second order with respect to the herbicide?
c. What would be the specific rate constant if the reaction
were zero order with respect to the herbicide?
48. Use the values obtained in Problem 47 to determine the half-
life of the herbicide, assuming:
a. first-order kinetics
b. second-order kinetics
c. zero-order kinetics
49. Assume that the fermentation of glucose by yeast, to produce
ethanol, is a first-order process. Under certain conditions the
value of the specific rate constant is 0.00205 h
−1
. If the initial
concentration of glucose were 0.980 M, what would be the
concentration after 244 h of fermentation?
50. The precipitation of metal ions with sulfide is often used as
an identifying technique for the metal ions. The production
of hydrogen sulfide to be used in the precipitation, however,
can be dangerous. Consequently, H
2
S can be generated by
placing thioacetamide (CH
3
CSNH
2
) in an aqueous acid so-
lution. If the first-order decay constant for thioacetamide
under those conditions were 0.46 min
−1
, what would you
calculate as the time required for 0.100 M thioacetamide to
reach a concentration of 0.0100 M?
a smoke detector to decay (into neptunium) from its initial
radiation level to 66.6% of its original value?
52. Explain why nuclear decay processes can be considered
always to follow first-order kinetics?
15.4 Methods of Determining Rate Laws
Skill Review
53. Plot the following data and determine whether they follow
zero-order, first-order, or second-order kinetics.
54. Plot the data in Problem 12 and determine whether they fol-
low zero-order, first-order, or second-order kinetics.
55. Explain the change in the rate of the reaction in each case
below.
Rate = k[A][B]
a. We double [A].
b. We double [B].
c. We double [A] and [B].
56. Explain the change in the rate of the reaction in each case
below.
Rate = k[A]
2
[B]
a. We double [A].
b. We double [B].
c. We triple [A].
57. Use the method of initial rates to determine the order of each
component, along with the general rate law and the value
of the rate constant, in the following hypothetical reaction.
What is the overall order of the reaction?
A + B
→
2C
58. Use the method of initial rates to determine the order of each
component, along with the general rate law and the value of
the rate constant, in the following hypothetical reaction.
What is the overall order of the reaction?
A + B → 2C
Thioacetamide
CH
3
CSNH
2
Time (s) Concentration (M)
0 0.500
5 0.274
10 0.189
20 0.116
30 0.084
51. All nuclear decay processes (alpha, beta, and gamma decay)
follow first-order kinetics. The isotope americium-241 is
used in many smoke detectors. It has a half-life of approxi-
mately 241 years. How long would it take the americium in
Experiment [A] (M) [B] (M) Initial Rate (M/s)
1 0.10 0.10 0.222
2 0.10 0.20 0.444
3 0.20 0.20 0.444
Experiment [A] (M) [B] (M) Initial Rate (M/s)
1 0.10 0.10 0.286
2 0.10 0.20 0.143
3 0.20 0.20 0.286
66. The gas phase hydrogenation of ethene is shown in the fol-
lowing reaction:
C
2
H
2
(g) + 2H
2
(g) → C
2
H
6
(g)
a. If the following data were collected, using the method
of initial rates, for four experiments at a fixed tempera-
ture, what would you determine as the rate law for the
reaction?
664 Chapter 15 Chemical Kinetics
59. Use the method of initial rates to determine the order of each
component, along with the general rate law and the value of
the rate constant, in the following hypothetical reaction.
What is the overall order of the reaction?
A + 2B → 2C
60. Use the method of initial rates to determine the order of each
component, along with the general rate law and the value
of the rate constant in the following hypothetical reaction.
What is the overall order of the reaction?
2A + 2B → 2C
61. Use the method of graphical analysis to determine the order
for the hypothetical reaction A → C + D. What is the rate
constant (with appropriate units) for this reaction?
Experiment [A] (M) [B] (M) Initial Rate (M/s)
1 0.10 0.10 0.105
2 0.10 0.20 0.420
3 0.20 0.20 0.840
Experiment [A] (M) [B] (M) Initial Rate (M/s)
1 0.050 0.10 0.074
2 0.10 0.20 0.888
3 0.050 0.20 0.222
62. Use the method of graphical analysis to determine the order
for the hypothetical reaction A → B. What is the rate con-
stant (with appropriate units) for this reaction?
a. If the reaction about to take place is first order with respect
to the large dark atoms and zero order with respect to the
small light atoms, which graphical plot would yield a
straight line for each component?
b. How would the overall reaction rate compare between the
first and second containers?
c. What units would you assign to the rate constant for the
reaction between the two components? (You may use
seconds for the time unit.)
64. The following diagram represents a container with two gas
components in their initial conditions. The reaction is zero
order with respect to the large atoms and first order with re-
spect to the small atoms. Prepare a similar diagram, but show
what would be needed to produce a reaction that would be
three times faster than the first (assuming no change in tem-
perature or any other reaction conditions).
[A] (M) Time (min)
0.432 0
0.385 1
0.291 3
0.197 5
0.103 7
[Fe
6+
] (M) Time (min)
0.100 0.00
0.0682 1.00
0.0517 2.00
0.0300 5.00
0.00920 10.00
[A] (M) Time (s)
0.100 0
0.050 62
0.025 124
0.013 186
0.0065 248
Chemical Applications and Practices
65. Municipal water supplies are often treated with chlorine com-
pounds in order to take advantage of the oxidizing ability of
chlorine to react with potential pollutants. In some industrial
settings, other oxidizing substances are used. One example of
such an application is use of the powerful oxidizing potential
of iron(VI) compounds. Given the information below, what
order would you report for the reaction of the iron(VI) com-
pound? What would you report as the value for k?
P
C
2
H
2
P
H
2
Initial
Experiment (atm) (atm) Rate (atm/s)
1 0.10 0.10 11
2 0.20 0.10 22
3 0.10 0.20 22
4 0.20 0.05 11
63. The following two diagrams represent two different contain-
ers. Within each container, two different compounds are
placed.
Focus Your Learning 665
Concentration (M) Time (min)
0.00128 0
0.00120 10.0
0.00113 20.0
0.00107 30.0
0.000781 100.0
0.000561 200.0
b. What would you calculate as the rate constant for the
reaction?
c. If the experiment were run once again, at the same
temperature, with initial pressures of 0.020 atm for ethene
and 0.020 atm for hydrogen, what would you determine as
the rate?
67. Suppose the following represents the kinetic study of the
oxidation of a new plastic stabilizer being proposed for use in
automobile upholstery.
Stabilizer(aq) + H
+
(aq) + O
2
(g) → oxidation product
tritium, starting with a sample producing a beta decay count
rate of 545 cpm (counts per minute), would remain after
3 years?
70. Sodium hypochlorite (NaOCl) is the active ingredient in
many aqueous commercial bleaching products. If the NaOCl
added to a particular sewage treatment process had a half-life
of 15 days, what percentage of the original concentration
would remain after 1 year? (Assume first-order kinetics.)
15.5 Looking Back at Rate Laws
Skill Review
71. What mathematical effect would there be on the three graphs
for first, second, and third order, respectively, if before plot-
ting the terms on each y axis you first subtracted the initial
concentration, ln of the initial concentration, and 1/(initial
concentration), respectively, from each reading?
72. A researcher mistakenly plots the data from a first-order re-
action using log [A] instead of ln [A]. What effect would this
have on the resulting graph?
73. What sign do you expect for the slope of the line in a plot of
[A] versus t for a zero-order reaction?
74. What sign do you expect for the slope of the line in a plot of
ln[A] versus t for a first-order reaction?
75. Which indicates a faster reaction for a first-order process:
k = 1.66 × 10
−2
s
−1
or k = 8.95 × 10
−2
s
−1
?
76. Which indicates a faster reaction for a first-order process:
t
1/2
= 24 days or t
1/2
= 15 days?
Chemical Applications and Practices
77. One method of producing yellow lead chromate pigment is
via a reaction of sodium chromate. Chromium(III) ions can
be made to undergo a three-electron oxidation to the chro-
mate ion (CrO
4
2−
) with the addition of cerium(IV) ions. The
rate law was found to be
Rate = k[Cr
3+
]
2
[Ce
4+
] [CrO
4
2−
]
x
Suppose the concentration of each component in the rate law
were doubled. If this caused the rate to quadruple, what
would you calculate as the value of x?
78. An approximation that chemists may sometimes casually use
is that increasing the temperature of a system by 10 degrees
Celsius may double the rate. Suppose you wanted to increase
a reaction rate by using only concentration changes. How
much would a concentration have to change to cause a reac-
tion to become ten times faster? (Assume the reaction is first
order.)
15.6 Reaction Mechanisms
Skill Review
79. Explain the relationship among a reaction mechanism, ele-
mentary steps and the rate-determining step.
80. Explain how it is possible for the proposed mechanism of a
reaction to be acceptable, yet perhaps not represent the actual
occurrence of events in the mechanism of that reaction.
Stabilizer H
+
(aq) O
2
(g)Rate
Experiment (aq) (M)(M)(M)(M/s)
1 0.400 0.300 0.560 7.14 × 10
−4
2 0.100 0.500 0.200 4.55 × 10
−5
3 0.100 0.100 0.200 4.55 × 10
−5
4 0.400 0.300 0.750 1.28 × 10
−3
5 0.100 0.300 0.560 3.57 × 10
−4
a. What is the rate law for the reaction?
b. What is the rate constant for the reaction at this tem-
perature?
c. What would you calculate as the rate of the reaction, at the
same temperature, if the respective initial concentrations
were 0.111, 0.200, and 1.00 for the stabilizer, hydrogen ion,
and oxygen, respectively?
d. If the rate of the same reaction, at the same conditions,
were determined to be 2.55 × 10
−4
M/s when the initial
concentration of stabilizer was 0.300 M with a hydrogen
ion concentration of 0.100, what would the initial oxygen
pressure have to be?
68. Brewed coffee left on a warming device will gradually change
flavor as a consequence of several complex reactions that
occur as the flavor oils decompose. Suppose a flavor chemist
for Dr. Beans Coffee Company collected the following data
on one such oil as it decomposed over time.
a. Using graphical techniques, determine the order of the
decay process.
b. What is the rate constant for the decay?
c. Starting with the initial concentration, how long would it
take for half the ingredient to decompose (half-life)?
69. The naturally occurring isotope of hydrogen known as tri-
tium has two neutrons and one proton. The reaction between
tritium and deuterium may provide the basis for controlled
fusion reactions. The half-life of the radioactive tritium
isotope is approximately 12 years. How much radioactive

81. You are seated at your kitchen table. Describe each of the
steps involved in answering the telephone in your home (be
very detailed). Which of these steps is the rate-determining
step for this process?
82. What is the rate-determining step in the process you use to
go to your first-period chemistry class from your bedroom?
83. What is the activation energy for a hypothetical reaction
(A n B) if k = 1.74 × 10
−2
s
−1
at 300 K and k = 4.22 ×
10
−2
s
−1
at 400 K?
84. Calculate the rate constant of a first-order reaction at 35°C,
given that k =8.5 × 10
−4
s
−1
at 25°C and E
a
= 144 kJ.
Chemical Applications and Practices
85. The aqueous reaction between hydrogen peroxide and iodide
in an acid solution is represented here:
H
2
O
2
+ 3I
−
+ 2H
+
→ 2H
2
O + I
3
−
Rate = k[H
2
O
2
][I
−
][H
+
]
One of the following mechanisms can be accepted for the
above rate law, and one cannot. Select the acceptable mecha-
nism and show proof for your selection as well as giving the
basis for rejection of the other.
Mechanism A Mechanism B
H
+
+ I
−
HI (fast) H
2
O
2
+ I
−
n H
2
O + OI
−
(slow)
H
2
O
2
+ HI n H
2
O + HOI (slow) H
+
+ I
−
HI (fast)
HOI + H
+
+ I
−
n H
2
O + I
2
(fast) H
+
+ OI
−
+ HI n H
2
O +I
2
(fast)
I
−
+ I
2
n I
3
−
(fast) I
2
+ I
−
n I
3
−
(fast)
86. When one reactant produces two products, the reaction is
said to be a disproportionation reaction. The following
shows the disproportionation of the hypochlorite ion used to
make commercial bleaching products:
3ClO
−
(aq) → 2Cl
−
(aq) + ClO
3
−
(aq)
On the basis of the following proposed mechanism, what
would you write as the rate law for this interesting reaction?
ClO
−
+ ClO
−
→ ClO
2
−
+ Cl
−
(slow)
ClO
−
+ ClO
2
−
→ ClO
3
−
+ Cl
−
(fast)
87. The solvent acetone has many industrial uses and is also a
common ingredient in nail polish remover. It does decom-
pose to a weak acid, carbonic acid. At 10.0
o
C, the rate
constant for that decomposition is approximately 6.4 ×
10
−5
L/mol·s. At 78
o
C, the rate constant for the reaction has
a value of 2.03 × 10
−1
L/mol·s.
a. What is the activation energy for this reaction?
b. What is the rate constant for this reaction at 37°C?
88. At 78
o
C, what would you calculate as the value of the Arrhe-
nius frequency factor, A, for the decomposition of acetone
(Problem 87)?
15.7 Applications of Catalysts
Skill Review
89. Provide clear and concise definitions for each term:
a. Reaction intermediate c. Homogeneous catalyst
b. Activated complex d. Heterogeneous catalyst
666 Chapter 15 Chemical Kinetics
90. a. Explain how a catalyst increases the rate of a chemical
reaction.
b. Explain why a catalyst does not appear as part of the
stoichiometry in a balanced equation.
91. Consider the following elementary steps for the decomposi-
tion of N
2
O.
N
2
O → N
2
+ O
N
2
O + O →N
2
+ O
2
a. What is the overall reaction?
b. Indicate any intermediates or catalysts involved in the re-
action.
c. Experimental evidence reveals that the rate law for this
reaction is Rate = k[N
2
O]. Which of the steps is the rate-
limiting step in the mechanism?
92. The following mechanism has been proposed for the reaction
of ICl(g) and H
2
(g).
H
2
+ ICl → HI + HCl
HI + ICl → I
2
+ HCl
a. What is the overall reaction?
b. Indicate any intermediates or catalysts involved in the
reaction.
c. The first step in the mechanism is slow compared to the
second. What is the rate law for the reaction?
93. A schematic mechanism for the reaction of lactose and lac-
tase (an enzyme) to produce glucose and galactose is shown
below.
lactose + lactase → (lactase–lactose)
(lactase–lactose) → (lactase–glucose–galactose)
(lactase–glucose–galactose) → lactase + glucose + galactose
a. What is the overall reaction?
b. Indicate any intermediates or catalysts involved in the
reaction.
c. The first step in the mechanism is slow compared to the
rest. What is the rate law for the reaction?
94. Each of the following represents an elementary step in a
different mechanism. Classify each as unimolecular, bimole-
cular, or termolecular.
a.
40
K →
40
Ar c. 2NO → N
2
O
2
b. N
2
+ Fe → FeN
2
d. 2NO + Cl
2
→ 2NOCl
Chemical Applications and Practices
95. The industrial production of ammonia makes use of cata-
lysts. One generalized mechanism may be presented as
follows:
N
2
(g) + Fe(s) → FeN
2
(s)
3H
2
(g) + 3Fe(s) → 3FeH
2
(s)
FeN
2
(s) + 3FeH
2
(s) → 4Fe(s) + 2NH
3
(g)
a. What is the overall stoichiometry of the reaction?
b. What intermediates, if any, are present?
c. What catalyst is present?
d. Is the catalyst homogeneous or heterogeneous?

96. Diagram a reaction profile that illustrates the exothermic
reaction whose mechanism is shown here. Next use a dotted
line to show the same profile with any changes that would
reflect the role of a heterogenous catalyst.
A(aq) + B(aq) → C(aq) (slow)
C(aq) → D(g) (fast)
Comprehensive Problems
97. In the reaction shown below, the initial concentration of
H
2
O
2
is 0.250 M, and 8 s later the concentration is 0.223 M.
What is the initial rate of this reaction expressed in M/s and
M/h?
2H
2
O
2
(aq) → 2H
2
O(l) + O
2
(g)
98. What is the half-life for the first-order decomposition of
dimethyl ether at 500°C if the rate constant for the reaction
is 2.567 × 10
−2
min
−1
?
(CH
3
)
2
O n CH
4
+ H
2
+ CO
99. A first-order reaction, A
n B, has a rate of 0.0875 M/s when
[A] = 0.250 M.
a. What is the rate constant?
b. What is the half-life for this reaction?
100. If the order of a reaction component were −1, what would
be the effect of tripling the concentration of that compo-
nent?
101. If the H
o
value for a reaction were +125 kJ and the activa-
tion energy for the reverse of the reaction were +75 kJ, what
would you calculate as the value for the activation energy for
the forward reaction?
102. Consider the following reaction mechanism.
C
2
H
6
O(aq) + HCl(aq)
C
2
H
7
O
+
(aq) + Cl
−
(aq) (fast)
C
2
H
7
O
+
(aq) + Cl
−
(aq) → C
2
H
5
Cl(aq) + H
2
O(l) (slow)
a. Write the overall reaction that is indicated by the
mechanism.
b. What would you predict as the rate law for the reaction?
103. Consider the following reaction mechanism.
C
2
H
4
(aq) + HCl(aq) → C
2
H
5
+
(aq) + Cl
−
(aq) (slow)
C
2
H
5
+
(aq) + Cl
–
(aq) → C
2
H
5
Cl(aq) (fast)
a. Write the overall reaction that is indicated by the
mechanism.
b. What would you predict as the rate law for the reaction?
104. Here is the reaction of hydrogen with nitrous oxide:
2NO + 2H
2
→ 2H
2
O + N
2
The rate law was determined by experiment to be
rate = k[NO]
2
[H
2
]
Is the following mechanism consistent with the rate law?
Prove your answer.
NO + H
2
N + H
2
O (fast)
N + NO
N
2
O (fast)
N
2
O + H
2
→ N
2
+ H
2
O (slow)
Focus Your Learning
667
105. We pointed out in the text that there are several catalysts
used in motor vehicle catalytic converters, including plat-
inum, palladium, and rhodium. In diesel engines, new cat-
alytic converters include the use of cerium(IV) oxide.
a. Why do these particular metals work so well as
heterogeneous catalysts?
b. In your role as a chemical consultant for an automobile
company, you are asked to choose one of the four listed
catalysts as the most effective, based on your external
research, which would you choose and why?
106. Under most conditions, the time it takes for a sample of
methane to completely burn in oxygen is typically on the
order of a few milliseconds. A half-life of 8.0 ms is typical
for the complete combustion of one mole of methane to
produce only CO
2
(g) and H
2
O(g). How much energy is
given off after one half-life if all of the simplifying assump-
tions are valid?
107. Methane is a potent greenhouse gas that is stable in the
upper atmosphere for about 9 to 15 years.
a. Write the reaction that illustrates the complete com-
bustion of methane.
b. Why is the reaction of methane with oxygen so fast
whereas the reaction in the upper atmosphere is so slow?
108. High-temperature reactions introduce a host of intermedi-
ate species even when the simplest substances react. Investi-
gate and report on the intermediates generated at high
temperature when hydrogen and oxygen react in the space
shuttle’s main engines.
Thinking Beyond the Calculation
109. Draw a hypothetical reaction profile for the Haber process
involving 1 mol of nitrogen and 3 mol of hydrogen in a
1.0 L flask.
N
2
(g) + 3H
2
(g) → 2NH
3
(g)
a. Use information you have learned in previous chap-
ters to determine whether the reaction is exothermic or
endothermic.
b. Is this reaction spontaneous at room temperature?
At what temperature is the reaction at equilibrium?
c. On the reaction profile you drew, indicate what you’d
expect to see if the reaction were homogeneously
catalyzed.
d. Draw what you’d expect to see if the reaction were
heterogeneously catalyzed.
e. If the catalyzed reaction were accomplished at 300°C
using the quantities outlined in the start of this question,
what would be the yield, in grams, of ammonia (NH
3
(g))?
Assume the reaction produces only a 45% yield.
f. If 10.0 kg of nitrogen and 30.0 kg of hydrogen were
combined in the catalyzed reaction, what would be the
theoretical yield, in kilograms, of ammonia?
