Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

628 Chapter 15 Chemical Kinetics
0
0 50 100 150 200 250 300
Days
10
20
30
40
50
60
70
80
90
100
Percentage of original concentration
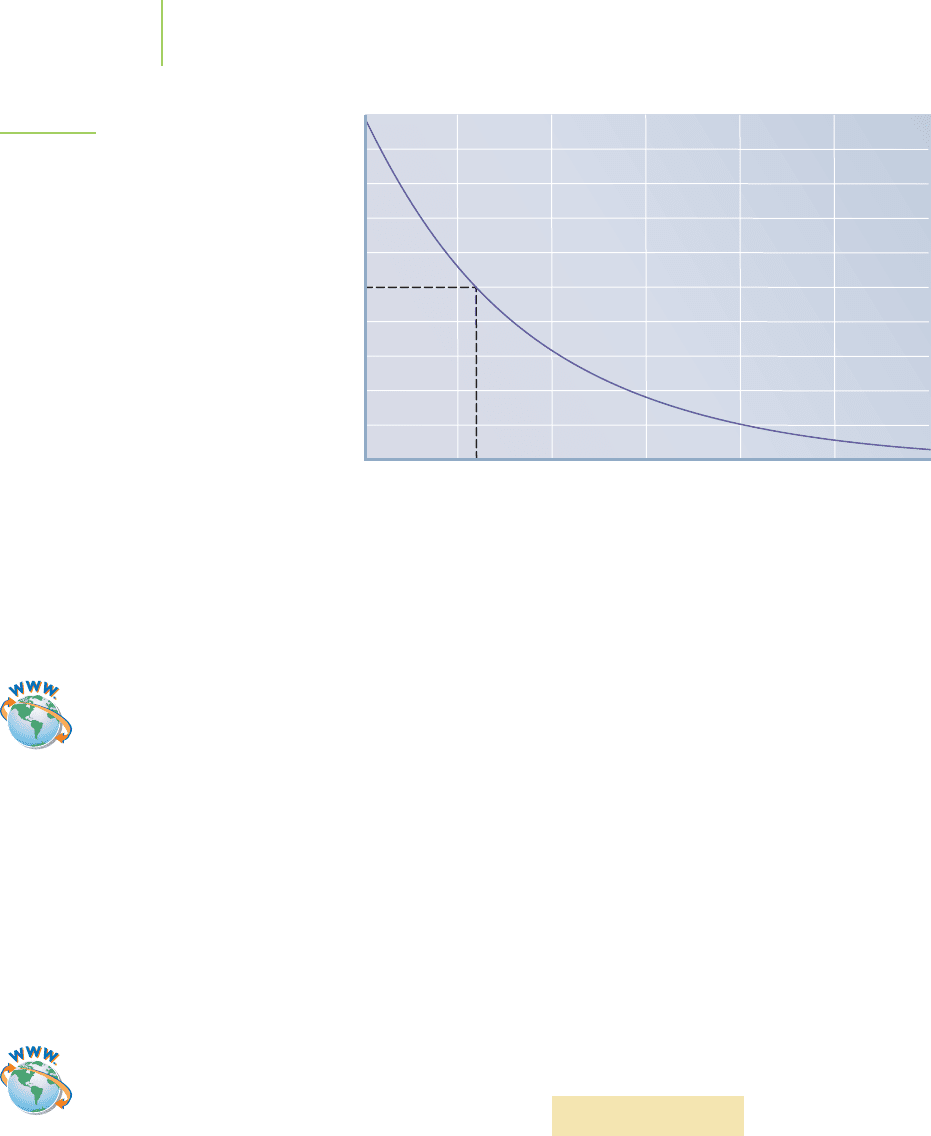
FIGURE 15.6
The environmental decomposition of
atrazine in groundwater. The half-life is
the time it takes for a given concentra-
tion to be halved.
because of its ability to oxidize microbes. We observe by experiment that the rate
of the reaction depends on the concentration of hydrogen peroxide. As the con-
centration decreases, the rate of the reaction decreases.
Rate ∝ [H
2
O
2
]
When we examine the relationship more closely, the following mathematical
equation, called a
rate law, emerges. This rate law states that the rate of the reac-
tion of hydrogen peroxide is equal to the product of a constant (which we call the
rate constant) times the concentration of H
2
O
2
.
2H
2
O
2
(aq) n 2H
2
O(l) + O
2
(g)
Rate = k[H
2
O
2
]
From the rate law, we determine that the reaction is first order in hydrogen
peroxide. That is, the rate depends on the concentration of H
2
O
2
raised to the first
power. The reaction is first order overall because the rate equation for the reaction
is dependent only on the concentration of H
2
O
2
to the first power—that is, it is
linearly related. It is important to remember that the value of the rate constant,
the compounds included in the rate law, and the orders of the compounds in the
equation can be found only experimentally.
The rate law, as we will see, can be used to determine the rate of a reaction at
any reactant concentration. It will also tell you which species are the most impor-
tant contributors to the rate of a reaction. The typical rate law has the following
form:
Rate = k[A]
n
[B]
m
where k is the rate constant, [A] and [B] are the concentrations of substances
involved in the reaction, and n and m are the orders of the corresponding com-
pounds. The values of n and m are measures of how dependent the rate is on the
concentration of a particular reactant, and they must be experimentally deter-
mined. The reaction also can be described in terms of the overall order, which is
calculated by adding n and m (and the exponents of any other reactants in the
rate law). The order of a compound or the overall order of a reaction can cer-
tainly be negative or even a noninteger number. Again, it is important to remem-
ber that the order of a compound in a reaction cannot be determined just by looking
at the reaction.
The reaction of nitrogen monoxide gas with hydrogen gas is
2NO(g) + 2H
2
(g) n 2H
2
O(l) + N
2
(g)
Video Lesson: Rate Laws: How
the Reaction Rate Depends on
Concentration
Video Lesson: Determining the
Form of a Rate Law
The experimentally determined rate law is
Rate = k[NO]
2
[H
2
]
The reaction is said to be second order in nitrogen monoxide, because the rate
depends on [NO] to the second power. The rate is first order with respect to hy-
drogen gas, because the exponent is 1, which means there is a linear relationship
between the rate and [H
2
]. The reaction is third order (the sum of the individual
orders, 2
+ 1) overall. A reaction can have fractional orders, though we will not
deal with that here.
EXERCISE 15.3 The Rate Law
Environmental chemists are concerned with the damaging effects of compounds on
the ozone layer. One such class of compounds is the nitrogen oxides, such as NO(g)
and NO
2
(g). Under certain conditions, the reaction of NO(g), an air pollutant re-
leased in automobile exhaust, with oxygen can produce N
2
O
4
(g). The rate law for
this reaction was determined experimentally and is shown below. What is the reac-
tion order of each of the compounds in the rate law? What is the overall order of the
reaction?
Solution
The experimentally determined rate law says that the reaction is first order in NO(g)
and first order in O
2
(g). Overall, then, the reaction is second order.
PRACTICE 15.3
The following reaction proceeds at 300°C. What is the reaction order of the com-
pound in the following reaction with the given rate law? What is the order of the
reaction?
See Problems 19 and 20.
Collision Theory
In Exercise 15.3 and Practice 15.3, the rate law is expressed as the concentration
of the reactants raised to a power. This is not uncommon, but as we’ve mentioned
before, it isn’t true for reactions in general. Why do the orders of the rate law and
the stoichiometric factors of a reaction often differ? Reactions are typically more
complex than they appear when written on paper. To understand how this can
cause the rate law to differ from what we may expect, we need to consult
collision
theory
. This theory describes how the rate of a reaction is related to the number of
properly oriented collisions of the molecules involved. Collision theory is heavily
based on the kinetic molecular theory we discussed in Chapter 11.
Kinetic molecular theory says that the thermal motion of particles (the ki-
netic energy) can be used to explain how a gas behaves. For instance, the pressure
15.2 An Introduction to Rate Laws 629
+
O
2
(g)
Rate = k[NO][O
2
]
+2NO(g)N
2
O
4
(g)
H
2
(g)
Rate = k[HI]
2
+2HI(g)I
2
(g)
+
Visualization: Gas Phase
Reaction of NO and Cl
2
Video Lesson: The Collision
Model
630 Chapter 15 Chemical Kinetics
of a gaseous system is related to the number of collisions of the molecules with
the sides of their container in a given time. If we increase the number of mole-
cules per unit volume, the number of collisions per second also increases, assum-
ing the temperature is constant. The pressure of the system can be increased if we
raise the temperature of the gas and leave the volume constant. This is because
the molecules are moving faster (more kinetic energy) and engage in more colli-
sions per second. In short, higher kinetic energy equals more collisions.
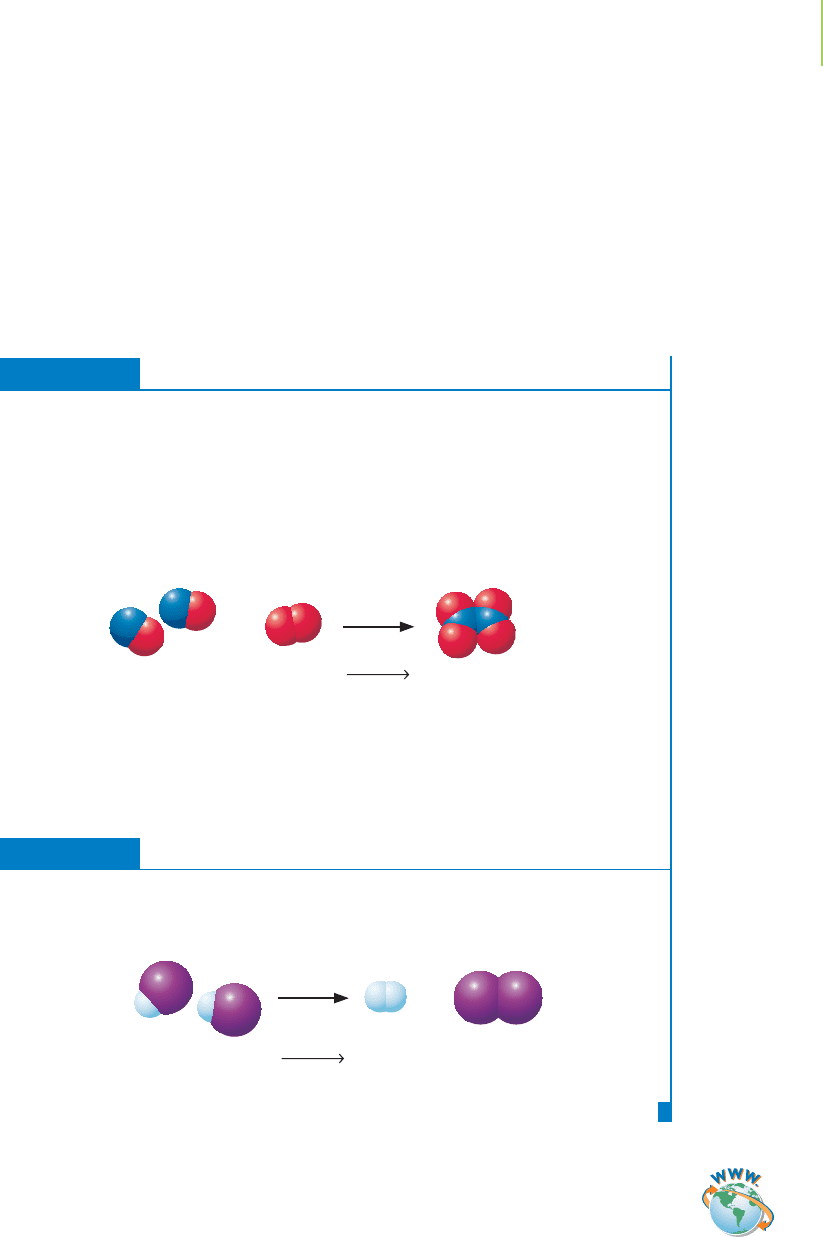
Collision theory, which is summarized in Table 15.1, requires that molecules
collide in order to react. One of the more important statements in collision the-
ory, as shown in Figure 15.7(a), says that the collisions
must be energetic enough to make a product. The
minimum energy required, the
activation energy (E
a
),
is specific to a particular reaction. However, an ener-
getic collision alone is not enough to cause a reaction
to occur. The collision must also occur between prop-
erly oriented molecules; see Figure 15.7(b). Because
the equation we write to describe a reaction doesn’t
address all of these issues, rate laws are difficult to
derive by simply examining the overall equation.
We’ll revisit this statement in Section 15.6.
Cold = low T
Low kinetic energy
Hot = high T
High kinetic energy
Increasing the temperature of a reaction
increases the kinetic energy of the com-
ponents of the reaction. This means that
the particles move faster and, as a re-
sult, have many more collisions than
they do in the colder reaction. More col-
lisions mean a faster reaction.
(a)
(
b
)
Low KE
No reaction
Reaction
No reactionNot proper orientation
ReactionProper orientation
High KE
FIGURE 15.7
Collision theory. (a) Collisions must be
energetic enough to be considered suc-
cessful. (b) Successful collisions only
occur between properly oriented
molecules.
Collision Theory
A reaction occurs when the following conditions have been met:
• Molecules collide.
• Molecules have enough kinetic energy.
• Molecules are oriented properly.
Implications:
• Larger concentrations have faster reaction rates.
• Reactions with higher temperatures have faster rates.
• Rates depend on the number of properly oriented collisions.
• Predicting the rate of a reaction is difficult.
TABLE 15.1
(a)
(b)

HERE’S WHAT WE KNOW SO FAR
■
The rate of a reaction is the change in concentration (M) per unit time. Rates
are always positive numbers, often reported in M/s.
■
Average rates and instantaneous rates differ only in the time measurement.
Instantaneous rates have ∆t ≈ 0.
■
The rate law (Rate = k[A]
n
[B]
m
) indicates the relationship between the rate
and the concentrations of reactants.
■
The order of a reactant can be used to indicate quickly the relationship be-
tween the reactant concentration and the rate.
■
Collision theory explains why the rate of a reaction typically decreases as time
passes.
15.3 Changes in Time—The Integrated Rate Law
After the discovery in 1939 that DDT (1,1,1-trichloro-2,2-bis-(4-chlorophenyl)
ethane, or dichlorodiphenyltrichloroethane) can be used to control mosquito-
borne malaria, its use soared. Especially important was its use to protect soldiers
who were fighting in the Pacific Rim countries in World War II. Since its discov-
ery, DDT has been sprayed to eliminate insects from cotton crops, spiders from
residences, and mosquitoes from towns all across the globe (Figure 15.8). Initial
testing showed that the compound wasn’t very toxic to mammals. However, be-
cause the metabolism of DDT is very slow, small amounts of DDT in the envi-
ronment tended to accumulate in animals (including humans) until toxic levels
were present. Evidence of this caused Sweden in 1970 and the United States in
1972 to ban the use of DDT as a pesticide, although it is still used in some other
countries, such as Ethiopia and South Africa. Despite the 30-year ban on DDT
use in the United States, the insecticide can still be found in the environment,
mostly in waterways like that shown in Figure 15.9. The hazard of DDT accumu-
lation in the food chain versus the benefit of saving human lives by preventing
malaria in the populations of, for example, East African countries is still a topic of
intense debate.
In organisms that are resistant to DDT, an enzyme known as dehydrochlori-
nase converts DDT into dichlorodiphenyldiethene (DDE). Unfortunately, DDE
can accumulate within birds and weaken their eggshells by interfering with the
15.3 Changes in Time—The Integrated Rate Law 631
Application
C
HEMICAL
ENCOUNTERS:
Decomposition
of DDT
FIGURE 15.8
DDT and the mosquito. DDT is still one of the most cost-
effective methods of controlling mosquito-borne malaria.
Although many countries have banned its use because of
DDT’s persistence in the environment, many homes in
central Africa are still sprayed inside and out.
DDT
+
DDT DDE
CCH
CCl
3
H
H
C
H
CCl Cl
C
HC
CH
C
C
H
HC C
CHC
CC
CCl
2
H
H
C
H
CCl Cl
C
HC
CH
C
C
H
HC C
CHC
+
HCl
DDT
Dehydrochlorinase
632 Chapter 15 Chemical Kinetics
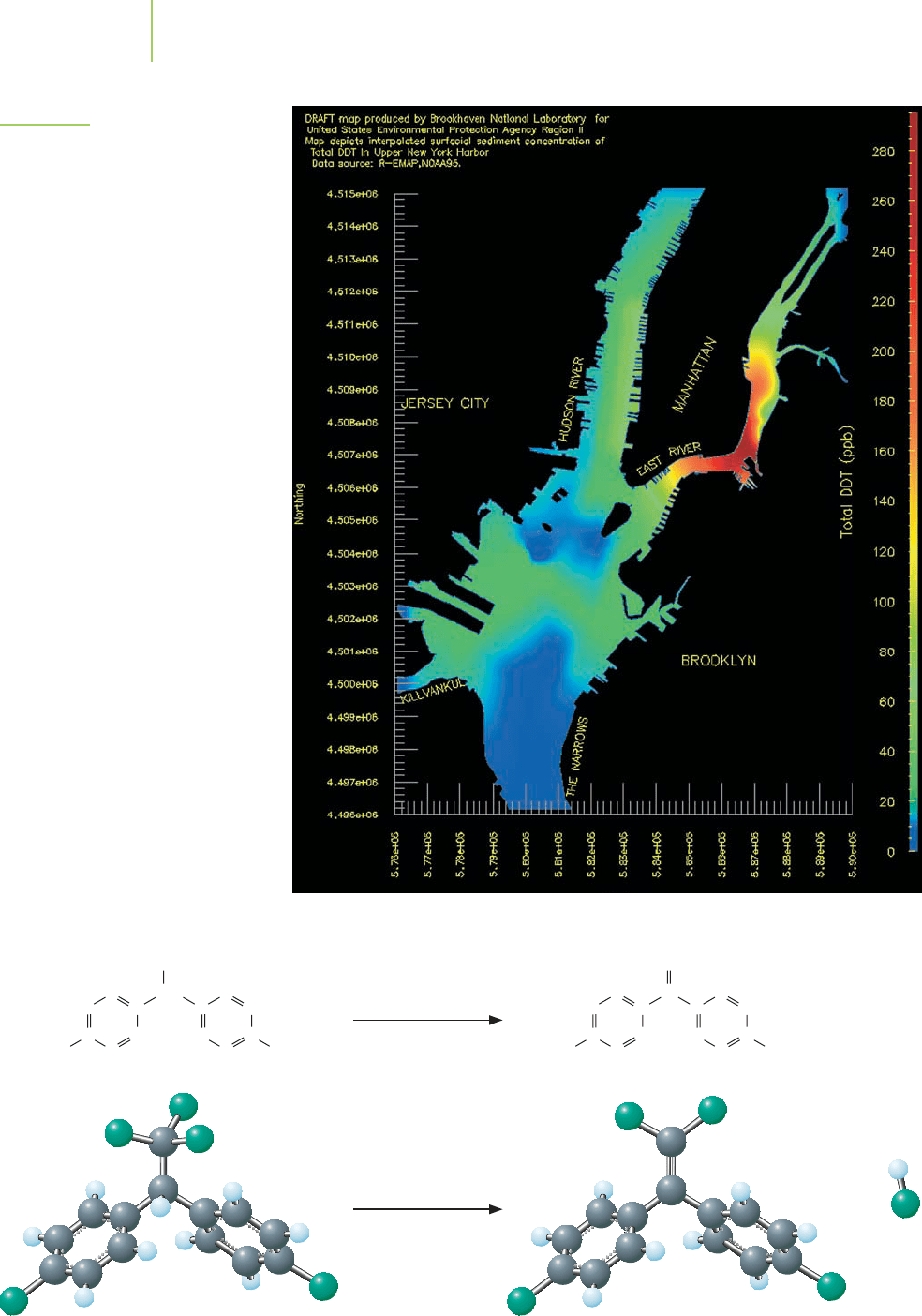
FIGURE 15.9
Even though the use of DDT has been
banned in the United States since the
1970s, DDT is still present in the environ-
ment. This plot of New York harbor shows
that the sediments in the East River harbor
still contain large quantities of DDT.
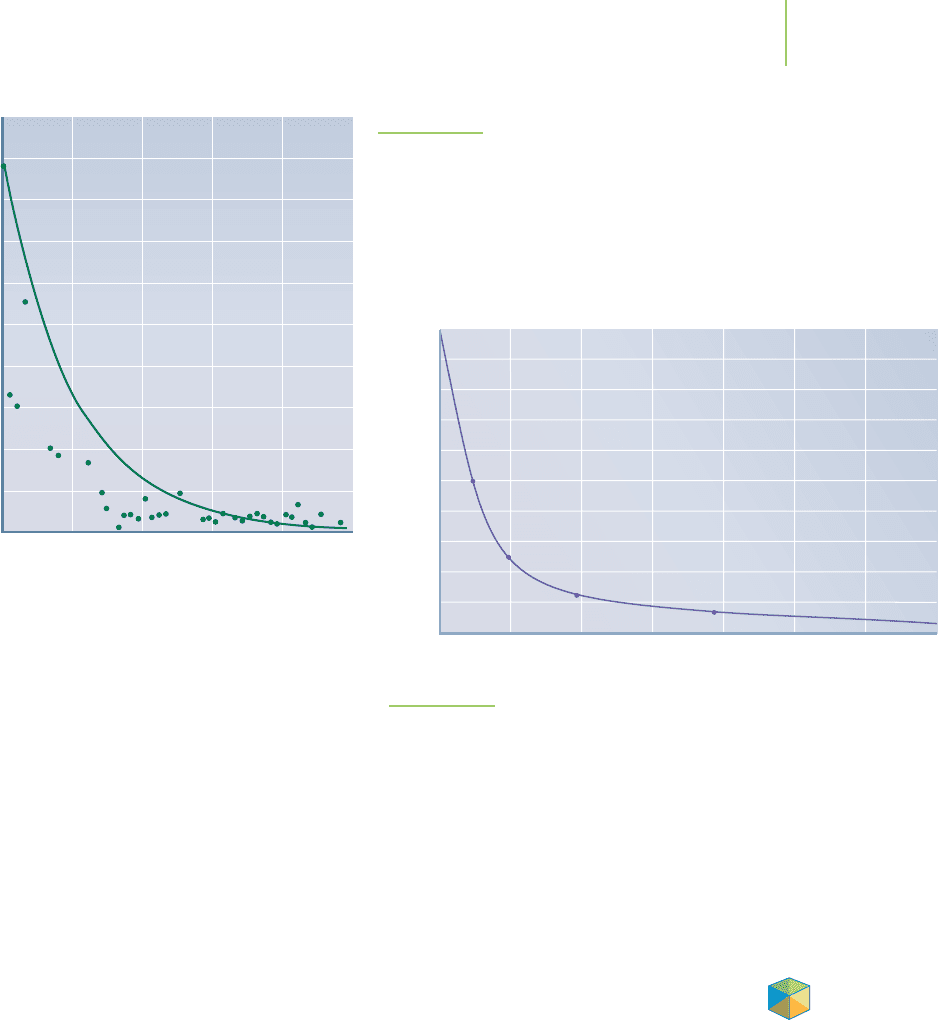
FIGURE 15.11
Plot of the radioactive decay of plutonium-239.
complex process of constructing the shells. This leads to shells that break under
the normal pressures naturally associated with nesting. For this reason, several
species of birds, such as the peregrine falcon, were nearly wiped out in the United
States.
The rate of decomposition of DDT by soil microbes is plotted against time in
Figure 15.10. Initially the rate is relatively high; then, as time passes, the rate be-
gins to decline.
Does this make sense according to what we know about collision
theory?
Yes. Reducing the number of reactant molecules reduces the number of
collisions that would result in the formation of the product.
What if we’re interested in determining what concentration of a chemical will
exist after a certain amount of time has passed? For example, the Hanford
Nuclear Reservation on the banks of the Columbia River just north of Richland,
Washington, has produced 54 million gallons of radioactive plutonium waste
over the past half-century. The waste is currently being stored in 177 under-
ground tanks as a watery sludge. How long will it take for the radioactive waste
stored at the Hanford Nuclear Reservation to decay to 1% of its current concen-
tration? We could examine the plot shown in Figure 15.11 to figure this out, but
we would need either to have a plot of the radioactive decay reaction or to know
the rate law, the rate, and rate constant of the reaction. Can we determine the
concentration at a specific time without knowing the rate or even having a plot of
the reaction? Calculus comes to our rescue. With some manipulation of the rate
law, we can make a more useful description of the rate of a reaction that will en-
able us to perform these calculations. These equations are referred to as the
inte-
grated rate laws
.
Integrated First-Order Rate Law
We discussed the decomposition of hydrogen peroxide to make oxygen and water
near the start of Section 15.2. Experimentally, it has been determined that the rate
15.3 Changes in Time—The Integrated Rate Law 633
0 102030 4050
Time (weeks)
Concentration (parts per million)
0
10
20
30
40
50
60
70
80
90
100
FIGURE 15.10
An experiment to illustrate the bioremediation (biological recycling) of DDT-
contaminated ground by soil microbes and nutrients. The decomposition of DDT
occurs rapidly at first and then slows. Note that the concentration of DDT is
reported in parts per million (1 ppm of DDT = 1 µg of DDT per gram of soil).
Although the curve appears to be placed incorrectly, it is not. Computer analysis
of this set of data, and additional data not shown, produced this curve.
0
0 50,000 100,000 150,000 200,000 250,000 300,000 350,000
Ye a rs
10
20
30
40
50
60
70
80
90
100
Percentage of original concentration
Application

law for this process describes a first-order reaction. Unfortunately, this doesn’t tell
us the concentration of H
2
O
2
at any point during the reaction, unless we know
the rate of the reaction at that time and the rate constant (k). In order to calculate
the concentration of H
2
O
2
at any point during the reaction, we can mathemati-
cally convert the rate law into an
integrated first-order rate law.
2H
2
O
2
(aq) n 2H
2
O(l) + O
2
(g)
Rate = k[H
2
O
2
]
The integrated rate law gets its name from the mathematical process, known as
integration, that we follow to generate the relationship between concentration and
time. When we integrate the rate law from t
= 0 to some future time, the inte-
grated rate law for the decomposition of hydrogen peroxide becomes
ln
[H
2
O
2
]
t
[H
2
O
2
]
0
=−kt
where [H
2
O
2
]
0
is the concentration of hydrogen peroxide initially, [H
2
O
2
]
t
is the
concentration of hydrogen peroxide at a particular time t, and k is the rate con-
stant. In practice, we need know only three of the four values in the equation
(time, rate constant, initial concentration, and concentration at time t) in order
to find the fourth.
This equation is general for all first-order reactions, which we will designate
using the general form
A n Products
where A is any single reactant. Note that the stoichiometric coefficient for A is 1.
The natural logarithm (ln) of the quotient of the final reactant concentration,
[A]
t
, divided by the initial concentration [A]
0
is equal to the negative of the rate
constant, k, times the time, t, shown in the left-hand side of the box below. We can
use this equation to calculate the time required to reach a given concentration.
Alternatively, we can rearrange the equation to that shown on the right-hand side
of the box if we wish to calculate the concentration at a particular time:
ln
[A]
t
[A]
0
=−kt
[A]
t
= [A]
0
e
−kt
We can transpose this into an equation in the form
y = mx + b
by recognizing
that
ln
[A]
t
[A]
0
= ln[A]
t
− ln[A]
0
Substituting for
ln
[A]
t
[A]
0
yields
ln[A]
t
− ln[A]
0
=−kt
which enables us to come up with the final y = mx + b form:
ln[A]
t
= −kt + ln[A]
0
y = mx + b
where the y axis = ln[A]
t
the x axis = t
the slope
m =−k
the intercept b = ln[A]
0
How do we use the first-order equation to find the concentration of hydrogen
peroxide after 5.00 min if we have a solution in which the initial concentration,
634 Chapter 15 Chemical Kinetics
Video Lesson: First-Order
Reactions
[H
2
O
2
]
0
, is equal to 0.100 M? The rate constant for this reaction was determined
by experiment to be 3.10
×
10
–3
s
–1
. Using this equation, we can calculate the
amount of peroxide still remaining after 5.00 min. We first convert our time to
match the units for the rate constant (5.00 min
= 3.00
×
10
2
s) and then insert our
known values into the integrated first-order rate law.
ln[H
2
O
2
]
t
− ln[H
2
O
2
]
0
=−kt
ln[H
2
O
2
]
t
− ln(0.100 M) =−(3.10 × 10
−3
s
−1
)(3.00 ×10
2
s)
ln[H
2
O
2
]
t
− ln(0.100 M) =−0.930
ln[H
2
O
2
]
t
+ 2.3026 =−0.930
ln[H
2
O
2
]
t
=−3.2326
[H
2
O
2
]
t
= e
−3.2326
= 0.0395 M
The concentration of hydrogen peroxide remaining after 5.00 min is 0.0395 M.
EXERCISE 15.4 Working with the Integrated First-Order Rate Law
Archaeologists near the Dead Sea in 1998 reported the discovery of a substance that
they believe ancient peoples used as glue. A sample of newly prepared collagen
exhibits 15.2 disintegrations per minute per gram (15.2 dis/min/g) of carbon. (A
disintegration is the decomposition of a radioactive nucleus such as
14
C; see Chap-
ter 21.) The
14
C decay rate for a sample of the ancient glue (made from collagen) was
found to be 5.60 disintegrations per minute per gram (5.60 dis/min/g) of carbon.
What is the age of the glue? The decomposition of
14
C is a first-order process with a
rate constant of 1.209
×
10
–4
year
–1
.
First Thoughts
This problem shows that the integrated first-order rate law can be used with ra-
dioactive compounds to determine decay rates, concentrations, or times. In these
cases, we consider the concentration of a reactant to be directly proportional to the
number of disintegrations per minute per gram. In the laboratory, this method can
be used to accurately determine the date of objects that are between 200 and 50,000
years old. Also note that we do not need to convert dis/min/g to dis/yr/g because the
units cancel in the equation.
Solution
If we assume that fresh collagen has the same activity of
14
C that the ancient glue did
when it was first made, we can calculate the length of time it would take a fresh sam-
ple of collagen to have the same activity of
14
C as the glue.
ln
5.60 dis/min/g
15.2 dis/min/g
=−(1.209 ×10
−4
year
−1
)t
ln(0.3684) =−(1.209 × 10
−4
year
−1
)t
−0.9985 =−(1.209 × 10
−4
year
−1
)t
t = 8259 year = 8260 year
The calculation reveals that it would take 8260 years for the activity in the fresh
sample to decay to the level observed in the ancient piece of wood. This implies that
the glue is 8260 years old.
Further Insight
Radiocarbon dating has been used extensively in determining the age of archaeo-
logical artifacts. The process relies on the assumption that the ratio of carbon-14 to
carbon-12 in nature has always been constant. However, when an organism dies, the
ratio begins to change as the radioactive carbon-14 decays. Controversy over the
validity of this dating method has been addressed by compensating for small
15.3 Changes in Time—The Integrated Rate Law 635
Video Lesson: Radiochemical
Dating
Application
C
HEMICAL
ENCOUNTERS:
Persistent
Pesticides
fluctuations in the original ratio. These fluctuations have been determined by dat-
ing objects with an age known by other methods. More information about radio-
carbon dating can be found in Chapter 21.
PRACTICE 15.4
Hanford’s nuclear waste contains large quantities of plutonium (mostly
239
Pu).
Researchers have determined that approximately 875 kg of solid waste plutonium
are buried there. How long will it take for the mass of plutonium-239 to drop to
10% of its original value? Assume the rate constant that describes the decay of
239
Pu
is 2.874
×
10
−5
year
−1
. How long will it take for the mass of plutonium-239 to
reach half of its original concentration?
See Problems 33 and 34.
Half-life
The persistence of pesticides and herbicides in the environment is often reported
as the amount of time that it takes for half of the original concentration to de-
compose. In general, any reaction can be reported in this manner by calculating
the amount of time it takes for the reaction to proceed to 50% completion. This
value is known as the
half-life (t
1/2
) of the reaction (Figure 15.12). Perhaps you’ve
heard this term used to express the rate of decay for a radioactive element, such
as the radioactive waste stored at the Hanford Nuclear Reservation, or in ac-
counts of the dating of ancient artifacts. The half-lives of pesticides and herbi-
cides indicated in Table 15.2 are used to judge the safety of the compounds and to
establish guidelines for the frequency of their application. How does the half-life
fit in with our description of the integrated first-order rate law?
Consider the first-order hydrolysis of atrazine in groundwater. The rate con-
stant for this reaction in water has been found to be 0.001733 day
−1
. Note that
we’re using a rate constant with units of day
−1
instead of s
−1
. This will be impor-
tant in the answer we generate from the integrated rate law equation.
ln
[atrazine]
t
[atrazine]
0
=−kt
636 Chapter 15 Chemical Kinetics
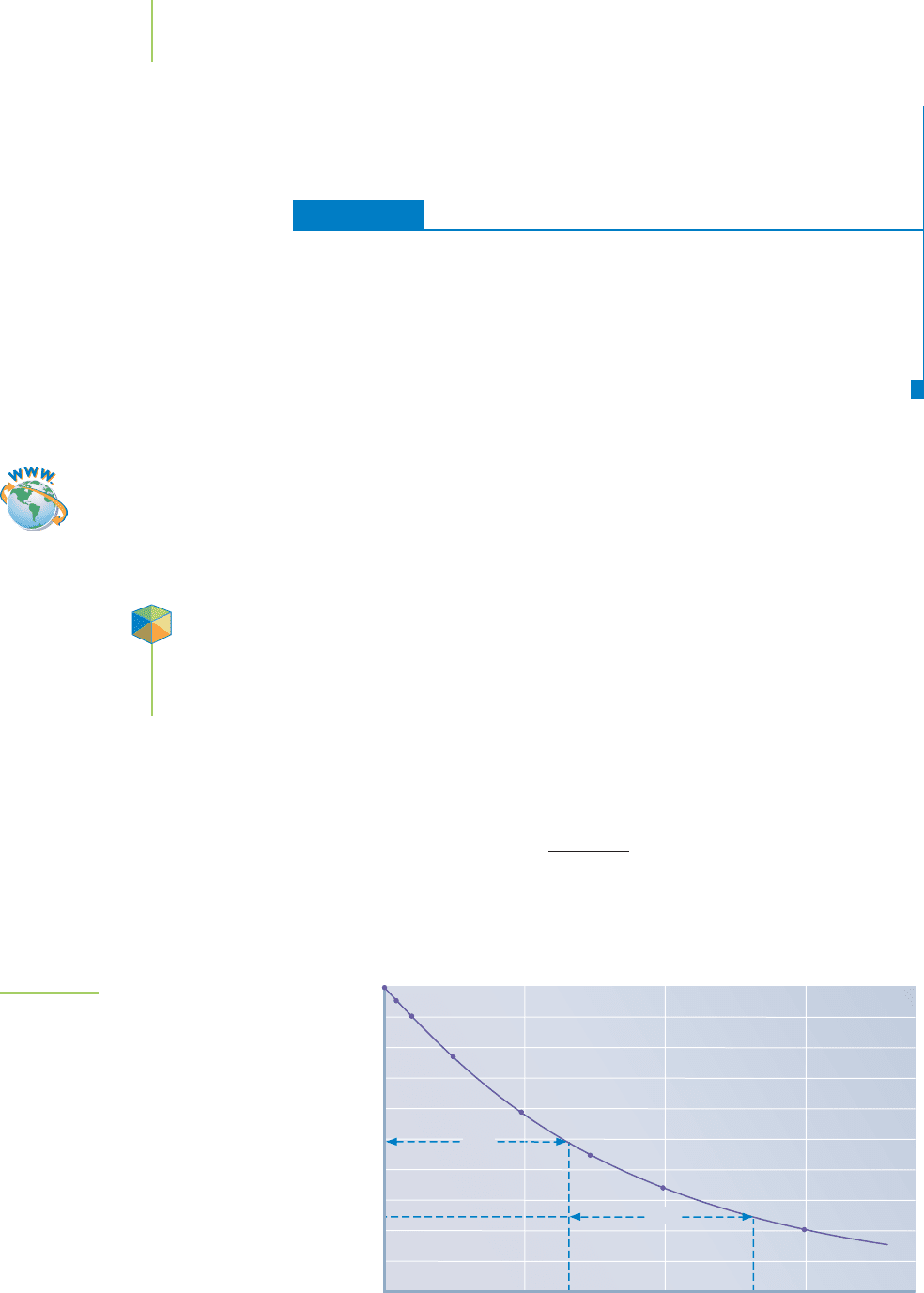
0 102030
Time (seconds)
0.025
0.050
0.100
Concentration (M)
t
1/2
t
1/2
FIGURE 15.12
The half-life of a reaction is the amount of time
required for the reaction to reach 50% of the
original concentrations. In terms of radioactive
decay or the decomposition of pesticides, the
passage of one half-life reduces the concen-
tration in half. Two half-lives reduce the
concentration to one-quarter of the original.
Visualization: Half-Life of
Reactions
Video Lesson: A Kinetics
Problem
Tutorial: Half-Life of Reactions
When the hydrolysis has consumed half of the original concentration of atrazine,
[atrazine]
t
=
1
⁄
2[atrazine]
0
= 0.5[atrazine]
0
. Substituting this into the equation
above, we find that
ln
0.5[atrazine]
0
[atrazine]
0
=−kt
1/2
The concentration of atrazine ([atrazine]
0
) cancels. Simplifying the equation fur-
ther yields
ln 0.5 =−kt
1/2
−0.693 =−kt
1/2
t
1/2
=
0.693
k
Our derivation reveals that the half-life (t
1/2
) of a first-order reaction is,
throughout the reaction, a constant that depends only on the rate constant and not
on the concentration of the reactant. This means that if we know the half-life for a
particular first-order reaction, we can calculate the rate constant, and vice versa.
Substituting the rate constant for atrazine into this equation, we find that the
half-life for the hydrolysis in water agrees with the experimental observation we
noted at the start of this chapter. Half of the atrazine added to water in the envi-
ronment disappears after 400 days.
t
1/2
=
0.693
0.001733 day
−1
= 4.00 × 10
2
days
15.3 Changes in Time—The Integrated Rate Law 637
Solubility and Half-life in Soil for Selected Pesticides
The sorption index is the ratio of pesticide concentration bound to soil particles
divided by the concentration in the aqueous phase. Pesticides with low sorption
indices are more likely to be leached into groundwater supplies, because a low
proportion of each binds to the soil. The half-life is reported for the pesticide in
sterile soil. 2,4-D, for example, is of greater environmental concern because of its
sorption index value than is DDT.
Sorption Index
Trade Name/ Water Solubility (higher = greater Soil Half-life
Brand Name (ppm) binding to the soil) (days)
2,4-D 890 20 10
Alachlor/Lasso 240 170 15
Atrazine/Aatrex 33 100 60
Dicamba/Banvel 400,000 2 14
Carbaryl/Sevin 110 300 10
Chlorsulfuron/Glean 7000 40 160
DDT 0.0055 24,000 3000
Diazinon 60 1000 40
Malathion/Cythion 145 1800 1
Metolachlor/Dual 530 200 90
Methoxychlor/Marlate 0.10 80,000 120
Pendimethalin/Prowl <1 5000 90
Pronamide/Kerb 15 200 60
Terbacil/Sinbar 710 55 120
Terbufos/Counter 4.5 500 5
Trifluralin/Treflan <1 8000 60
Source: Institute of Agriculture and Natural Resources, Univ. Nebraska Lincoln, Factors That Affect Soil-
Applied Herbicides, 2005.
TABLE 15.2
