Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

Fast
Step 2: Turn off light
Why is it useful to know the individual steps that make up the overall reaction?
Chemists often are interested in more detailed descriptions of chemical reactions,
including how the reactions occur at the molecular level. We know from our pre-
vious discussions that chemical equations contain a wealth of information about
a reaction. We can determine the spontaneity of the process, the enthalpy of the
process, and the stoichiometry of the reactants by examining the chemical equa-
tion. However, the overall chemical equation doesn’t show how the reactants
collide to become products. To address this concern, investigators study a single
reaction to determine exactly how each molecule moves during the course of the
process. The knowledge of exactly how a reaction proceeds is useful in predicting new
reactions, determining the rates of those reactions, discovering new applications of
chemistry, and learning how substances interact with humans and our environment.
Their study is part of the field of mechanistic chemistry.
A
mechanism for a reaction is the set of steps that compounds take as they
proceed from reactant to product. The mechanism of a reaction accounts for the
experimentally determined rate of the reaction and is consistent with the overall
stoichiometry of the reaction. In some cases, a mechanism is only one step. In oth-
ers, there are a multitude of steps. However, we can’t tell this by looking at the
overall chemical equation.
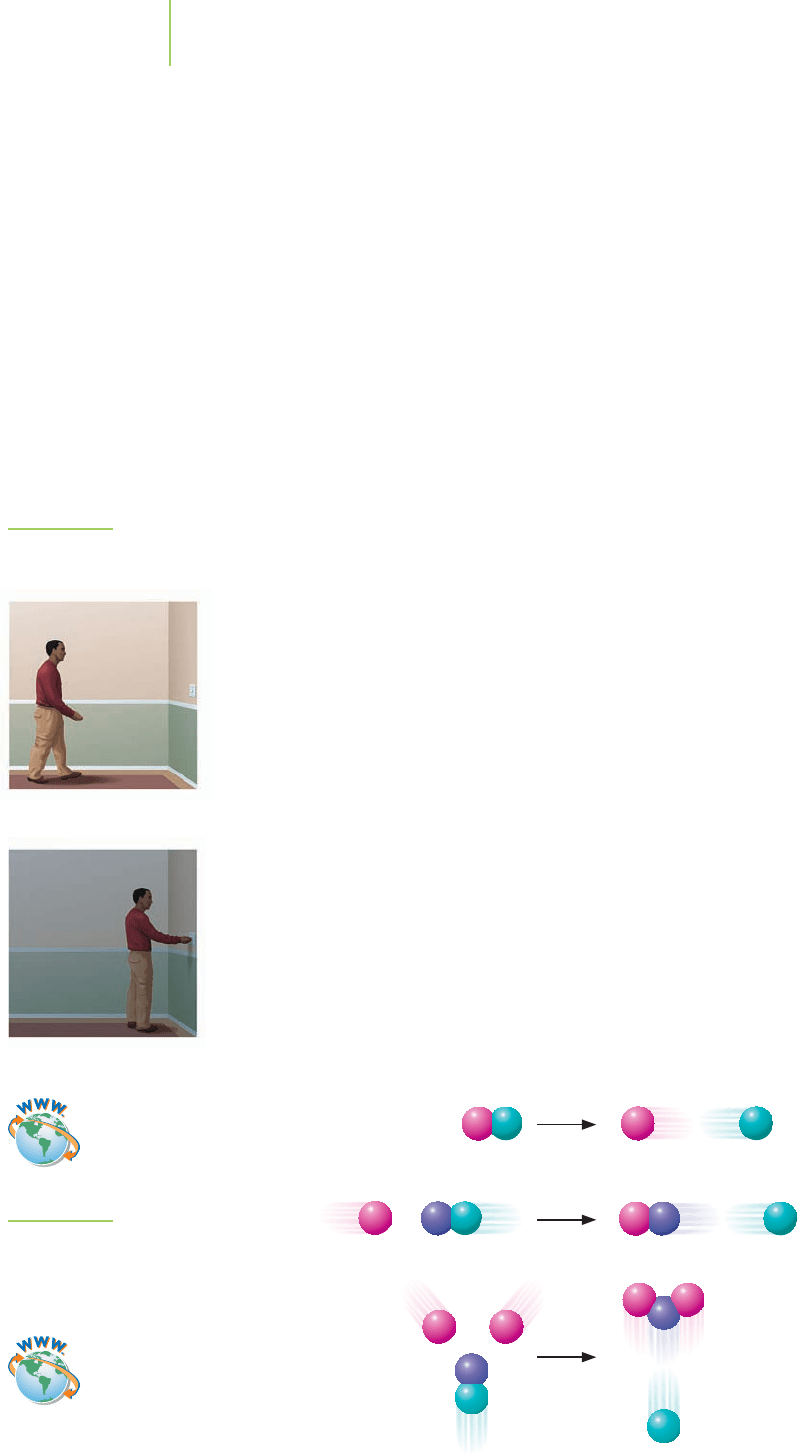
Each of the single steps in a chemical reaction is called an
elementary step.For
example, as shown in Figure 15.15, the overall process of turning off the lights in
a room is made up of two elementary steps: (1) walking to the light switch and
(2) flipping the light switch. One of these steps, walking to the light switch, is
slower. The other, flipping the switch, is so fast that its contribution to the
time required to complete the overall process is negligible. Any calculations of
the time required to turn off the lights, then, can be reduced to the time it takes
to walk to the light switch. In other words, the time it takes to turn off the lights
in the room is nearly equal to the length of time it takes to walk to the light
switch. This slow step is known as the
rate-determining step.
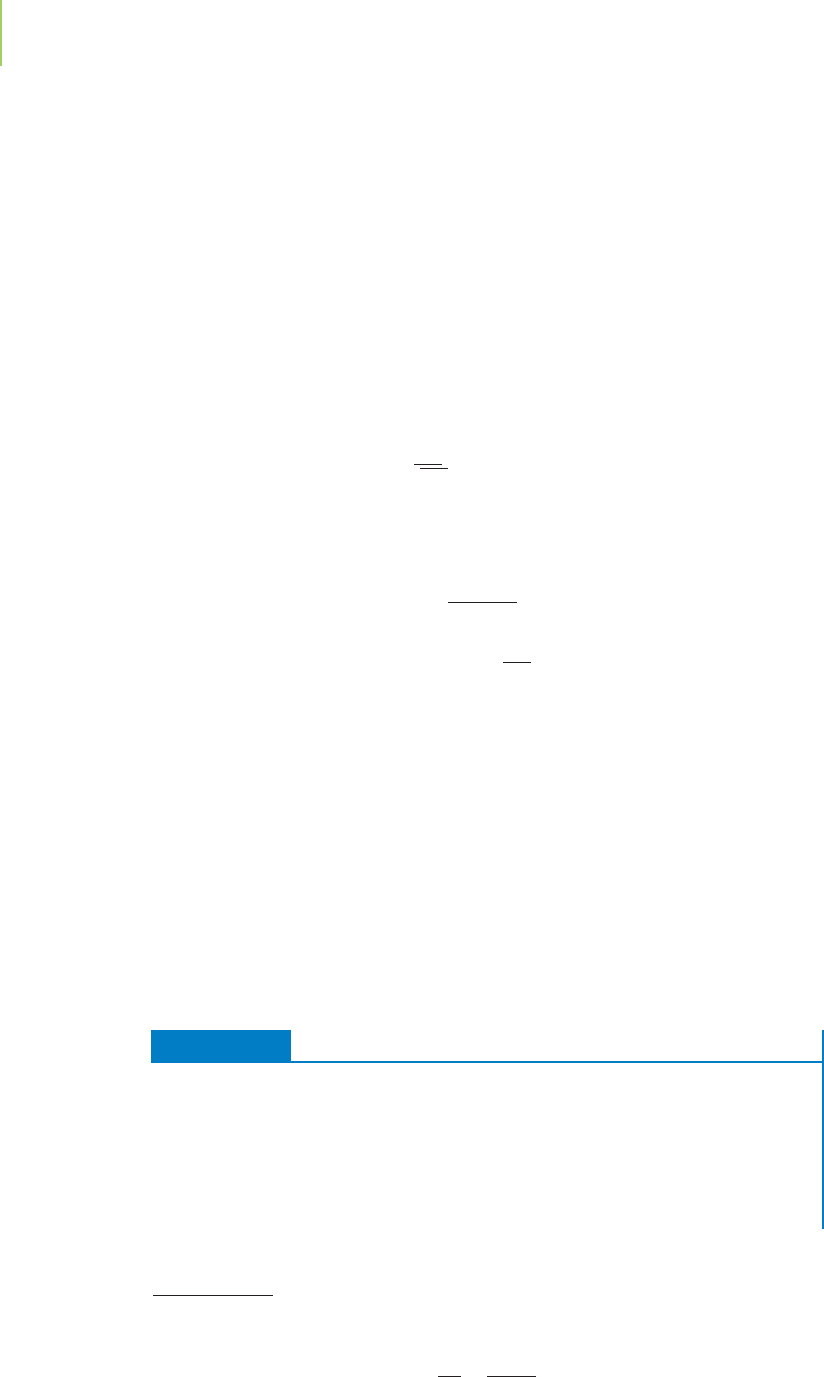
Within each elementary step, reactants come together and undergo successful
collisions to make products. The number of molecules that collide in this process
defines the
molecularity of the step (Figure 15.16). A unimolecular reaction involves
one molecule as the only reactant. When two molecules collide, the reaction is
said to be a
bimolecular reaction. Reactions that involve the collision of three mol-
ecules simultaneously (which are called
termolecular reactions,or trimolecular
reactions
) are also known, but they are rare because they require that three mole-
cules collide at the same time and in the proper orientation. In such cases, the
decrease in the entropy associated with three molecules coming together at
one time is often prohibitive (see Chapter 14). In general, an elementary step has
only a single bond breakage or formation.
648 Chapter 15 Chemical Kinetics
Slow
Step 1: Walk to light
Unimolecular reaction
Bimolecular reaction
Termolecular reaction
FIGURE 15.16
Molecularity of reactions. The number of
molecules that must collide at one time
to produce the reaction determines the
molecularity.
FIGURE 15.15
The elementary steps completed
in turning off the lights.
Visualization: Decomposition of
N
2
O
5
Video Lesson: Defining the
Molecularity of a Reaction

If we know the elementary steps in a reaction, we can obtain a wealth of in-
formation about the rate of the reaction. Most important, rate laws can be written
directly from elementary steps. Specifically, the rate orders for the reactants in an
elementary step are given by the stoichiometric coefficients of the reactants in
that step. Moreover, the rates of the elementary steps in a mechanism need not be
the same—indeed, in most cases they are not. A mechanism with two elementary
steps typically has a fast step and a slow step. The slow step in a mechanism is the
one that will determine the rate of the overall reaction.
The reaction of NO
2
and F
2
gas is a useful case study:
2NO
2
(g ) + F
2
(g ) → 2NO
2
F(g)
After careful experimentation, a mechanism involving two elementary steps has
been suggested for the reaction. This first step was determined to be slow; the
second was determined to be relatively fast:
NO
2
(g ) + F
2
(g ) → NO
2
F(g ) + F(g) (slow)
F(g) + NO
2
(g ) → NO
2
F(g ) (fast)
2NO
2
(g ) + F
2
(g ) → 2NO
2
F(g)
Note that the sum of the elementary steps gives the balanced equation. From
these steps, we can determine the rate law for the overall reaction in a way that is
similar to our discussion of the length of time required to turn off the lights. The
slow elementary step will determine the rate of the reaction, so we can use this
rate-determining step to write the rate law. Judging on the basis of the stoichio-
metric coefficients of the reactants in the slow step, the overall reaction is first
order in NO
2
, first order in F
2
, and second order overall.
Rate = k[NO
2
][F
2
]
Not all mechanisms are so easy to work with. For instance, the reaction of NO
with O
2
to make NO
2
is much more complicated than our first glance suggests.
From our previous discussion in Section 15.4, we know the overall equation for
the reaction:
2NO(g ) + O
2
(g ) → 2NO
2
(g )
We saw that the experimentally determined rate equation for the reaction is
Rate = k[NO]
2
[O
2
]
This implies that the reaction is a termolecular process, which is unlikely because
it would require three molecules to collide simultaneously. The mechanism of
this reaction is known, and it has the elementary steps shown below.
2NO(g )
k
1
k
−1
N
2
O
2
(g )
(
fast)
N
2
O
2
(g ) + O
2
(g )
k
2
−→ 2NO
2
(g )
(slow)
What is the meaning of the double arrows in the fast equation? These arrows in-
dicate that the reaction can proceed in both the forward direction and the reverse
direction at the same time. Moreover, each of these directions has a rate constant
associated with it (k
1
and k
−1
). The slow step in our mechanism is the reaction of
N
2
O
2
(g) with oxygen, and we could write the rate equation on the basis of this in-
formation. Note that we’ve specified the rate constants for both reactions:
Rate = k
1
[NO]
2
for the fast step (k
1
>>
k
2
)
Rate = k
2
[N
2
O
2
][O
2
]
for the slow step (k
2
<<
k
1
)
It follows that the rate law for the overall reaction should be
Rate = k
2
[N
2
O
2
][O
2
]
for the overall reaction
15.6 Reaction Mechanisms 649
Video Lesson: Determining the
Rate Laws of Elementary
Reactions
Video Lesson: Calculating the
Rate Laws of Multistep
Reactions
However, this doesn’t agree with the experimentally determined rate law that we
noted above:
Rate = k[NO]
2
[O
2
]
This difference arises because N
2
O
2
(g) is an intermediate in the reaction. An
intermediate is a compound that is formed and consumed during the course of a
reaction. If we examine the overall reaction, we don’t see the intermediate N
2
O
2
as one of the reactants. Measuring the concentration of this species, then, could
be difficult. We need to rewrite the rate equation so that the rate reflects only the
compounds in the overall reaction. To deal with this, we will assume that the fast
reaction reaches equilibrium, an assumption that greatly simplifies our determi-
nation of the overall rate law for the reaction. What does this assumption mean?
Within a reaction that is at equilibrium, the rate of the forward reaction is equal
to the rate of the reverse reaction.
2NO(g )
k
1
k
−1
N
2
O
2
(g ) (fast)
k
1
[NO]
2
= k
−1
[N
2
O
2
]
Then we can rearrange our equation to solve for [N
2
O
2
]:
[N
2
O
2
] =
k
1
[NO]
2
k
−1
= k
[NO]
2
where the new rate constant
k
is equal to
k
1
k
−1
.*
We can now substitute for the concentration of the intermediate, [N
2
O
2
], in
our original rate equation and simplify:
Rate = k
2
[N
2
O
2
][O
2
]
[N
2
O
2
] = k
[NO]
2
Rate = k
2
k
[NO]
2
[O
2
] = k
[NO]
2
[O
2
]
where the new rate constant
k
is equal to
k
2
k
.
This agrees with our experimentally determined rate equation. The mathe-
matics used to convert our rate law containing the intermediate into the experi-
mentally observed rate law are based on the assumption that the fast reaction has
reached equilibrium. This assumption requires that the rate constant for the re-
verse of the fast reaction be much larger than the rate constant for the slow step.
EXERCISE 15.8 Rate Laws
Write the overall reaction, identify any intermediates, and write the rate law for the
following proposed mechanism for the decomposition of IBr(g) to I
2
(g) and Br
2
(g).
IBr(g) n I(g) + Br(g) (slow)
IBr(g) + Br(g) n I(g) + Br
2
(g) (fast)
I(g) + I(g) n I
2
(g) (fast)
650 Chapter 15 Chemical Kinetics
* Note: Alternatively, we could rearrange this equation to place the rate constants on one side and the con-
centrations of compounds on the other side:
k
1
k
−1
=
[N
2
O
2
]
[NO]
2
This gives rise to something that looks remarkably similar to Q, the reaction quotient from Chapter 14.
We’ll explore this in much greater detail in Chapter 16.
Solution
The overall reaction is the sum of the elementary steps in the mechanism.
2IBr(g) n I
2
(g) + Br
2
(g)
The intermediates are produced and consumed in the reaction. They are I(g) and
Br(g). Because the first step in the mechanism is the slow step, it is rate determining.
Therefore, the rate law for the reaction can be written directly from this step:
rate =k [IBr]
It is first order in IBr(g) and first order overall.
PRACTICE 15.8
Write the overall reaction, identify any intermediates, and write the rate law for the
following proposed mechanism for the production of nitrogen dioxide (NO
2
).
NO(g) + O
2
(g) NO
3
(g) (fast)
NO
3
(g) + NO(g) n 2NO
2
(g) (slow)
See Problems 85 and 86.
Transition State Theory
The destruction of ozone by atomic oxygen is one of the ways in which stratos-
pheric ozone can be depleted:
O
3
(g ) + O(g) → 2O
2
(g )
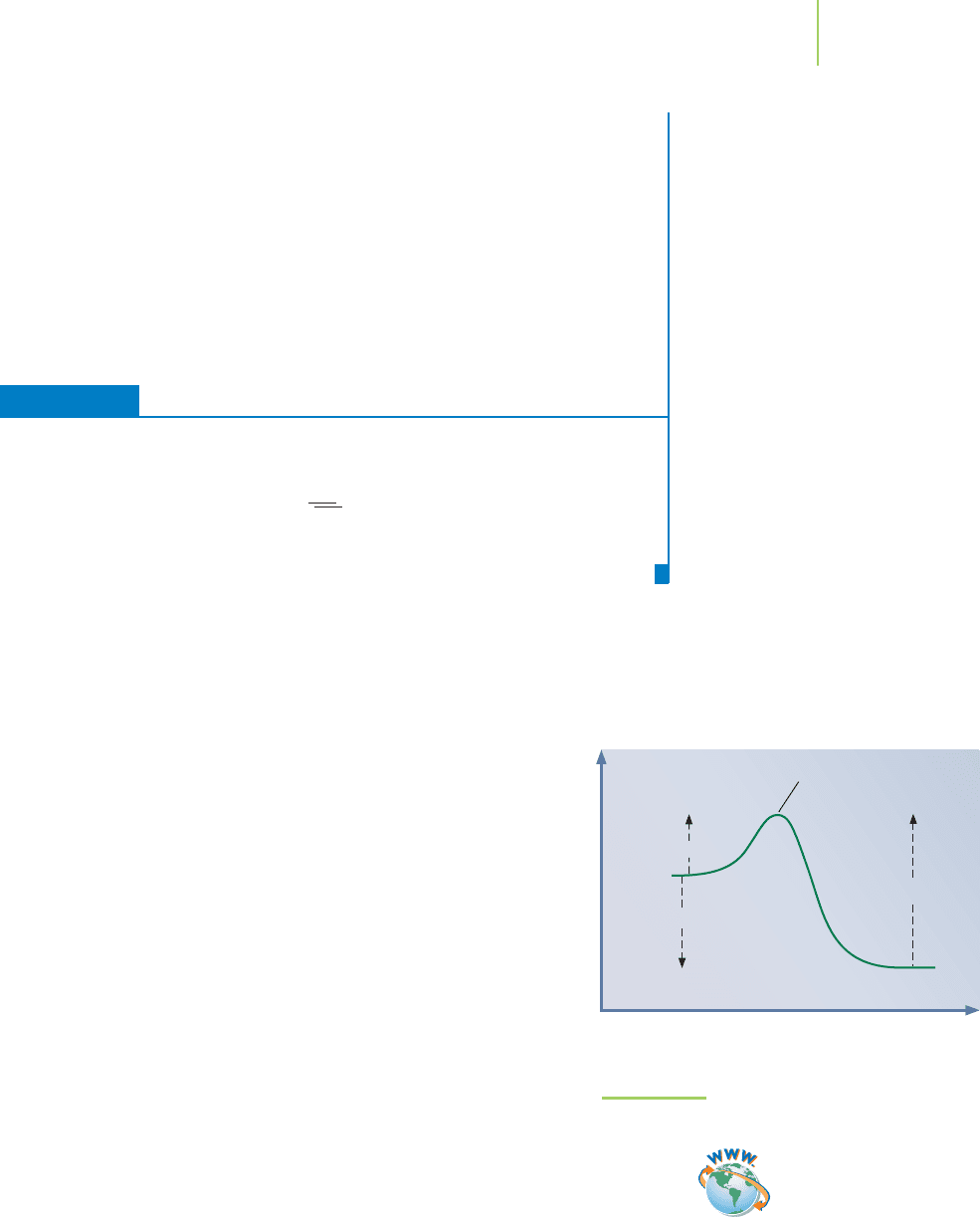
By examining this reaction in the laboratory, we can determine the
change in enthalpy (∆H =−392 kJ/mol) and measure the rate of
the reaction. However, it is often helpful to examine a reaction by
consulting a plot of the energies for the reactants, products, and
any intermediates. If we make a graph of the energies as the reac-
tants proceed along a
reaction coordinate (the pathway describing
the changes in each molecule in the reaction, even though they are
happening at different times) to become product, we obtain a
reaction profile like that shown in Figure 15.17. On the basis of our
earlier discussion of collision theory, we know that the reactants
must have enough energy to overcome a barrier known as the
activation energy (E
a
)—assuming that the reactants collide in the
proper orientation. For the reaction of atomic oxygen and ozone,
the barrier is rather small (E
a(forward)
=19 kJ/mol) compared to the
reverse reaction. The reaction profile illustrates this.
The reaction profile is used to explore
transition state theory
and how it applies to a reaction. This theory describes how the
bonds in the reacting molecules reorganize to represent the bonds in the prod-
ucts. At some point on the reaction coordinate the collision occurs, and the atoms
in the reactants occupy a
transition state. The collection of atoms at the transition
state, called the
activated complex, is very energetic at this point in the reaction—
more so than the reactants, products, or intermediates. The activated complex is
not a separate isolable compound. Rather, it is a snapshot of the reaction at the
point in time when the molecules have collided. From the activated complex, the re-
action could proceed to products, or the complex could dissociate back into the
reactants.
The reaction profile shows the activation energy for the forward reaction, the
activation energy for the backward reaction, and the overall change in the energy
of the reaction (∆E) in a graphical way. This change in energy (∆E) is equal to ∆H
15.6 Reaction Mechanisms 651
Progress of reaction
19 kJ/mol
–392 kJ/mol
411 kJ/mol
Transition state
∆H
(reaction)
E
a(forward)
E
a(reverse)
Potential energy
O
3
+ O
O
3
+ O
2O
2
2O
2
→
FIGURE 15.17
Reaction profile.
Visualization: Transition States
and Activation Energy
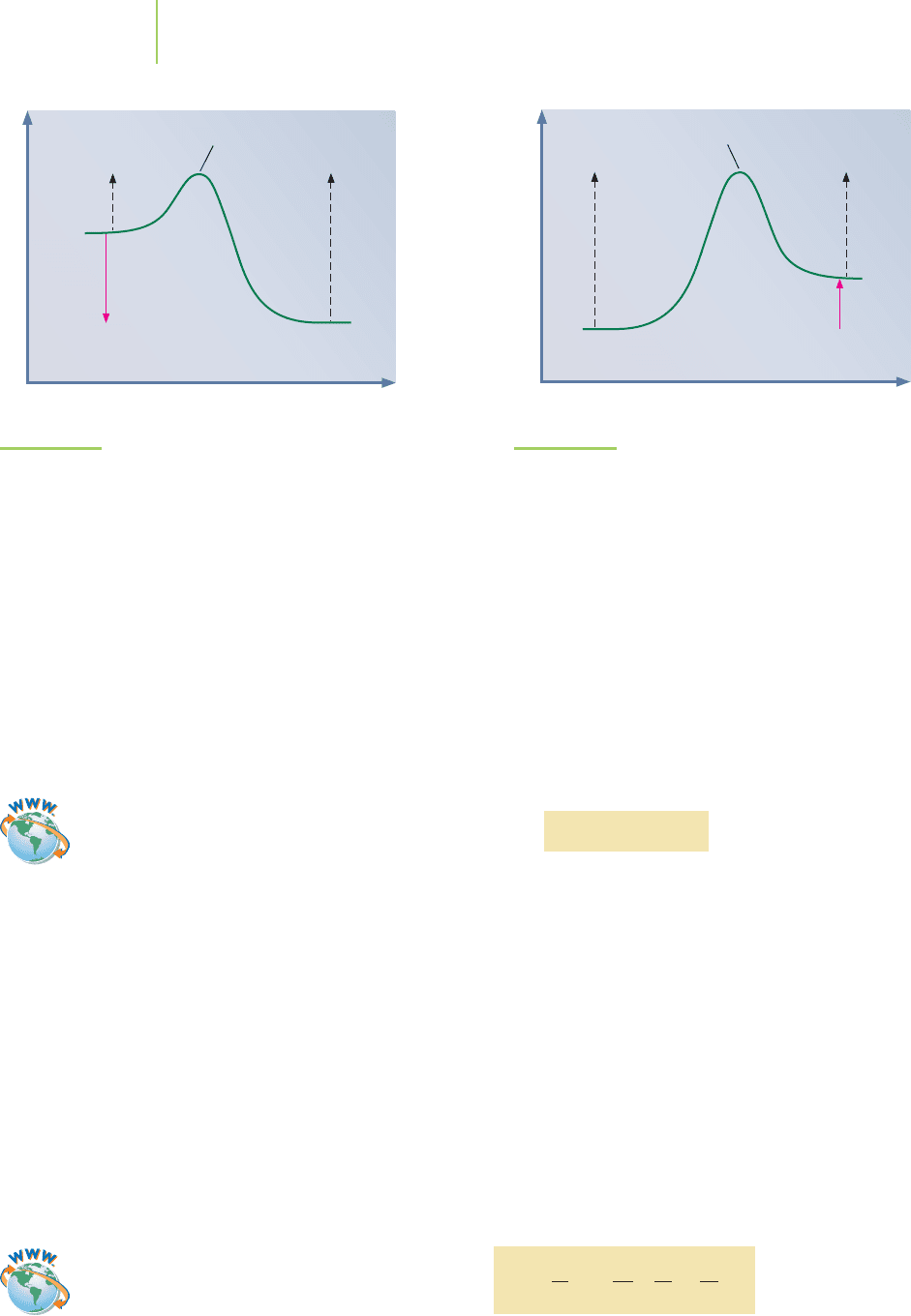
for the reaction at constant pressure and volume. Even for cases where ∆E and ∆H
are not equal (unequal volumes or pressures), the difference is usually very small.
By looking at the reaction profile, we can tell whether a reaction is exothermic
(Figure 15.18) or endothermic (Figure 15.19).
H
reaction
∼
=
E = E
a(forward)
− E
a(reverse)
To what is the activation energy related? In 1888, Svante Arrhenius (1859–1927),
a Swedish chemist, studied how temperature affected the rate of a reaction. What
came out of this work is a relationship between the activation energy and the rate
of a reaction. His mathematical equation is
k = Ae
−E
a
/RT
where k is the rate constant, A is the frequency factor that relates how many
successfully oriented collisions occur in a particular reaction, E
a
is the activation
energy, T is the temperature in kelvins, and R is the universal gas constant
(8.314 J/mol·K). This equation says that the rate constant of a reaction is related
to the size of E
a
.As E
a
gets larger, the rate constant gets smaller and the rate of the
reaction decreases.
This equation can be used to determine the rate of a reaction on the basis of
the temperature of the reaction and the amount of energy the reactants require to
make the activated complex. To use it, however, we must know the activation
energy (E
a
) and the frequency factor (A) for the reaction. Fortunately, by per-
forming the reaction at two different temperatures, we can utilize this equation to
calculate the activation energy without knowing the frequency factor. Alterna-
tively, if we know the activation energy and the rate of reaction at a particular
temperature, we can determine the rate of reaction at any other temperature. The
equation that relates this calculation can be derived by taking the natural loga-
rithm of the Arrhenius equation above, at two temperatures:
ln
k
2
k
1
=
E
a
R
1
T
1
−
1
T
2
where k
1
and k
2
are the rate constants for the reaction obtained at two different
temperatures, T
1
and T
2
.
652 Chapter 15 Chemical Kinetics
Progress of reaction
Transition state
∆H
(reaction)
endothermic
E
a(forward)
E
a(reverse)
Potential energy
FIGURE 15.19
Endothermic reaction profile. The products are more energetic
than the reactants in the endothermic reaction.
E
a(forward)
is larger than E
a(reverse)
.
Progress of reaction
Transition state
∆H
(reaction)
exothermic
E
a(forward)
E
a(reverse)
Potential energy
FIGURE 15.18
Exothermic reaction profile. The reactants are more energetic
than the products in the endothermic reaction.
E
a(reverse)
is larger than E
a(forward)
.
Video Lesson: The Arrhenius
Equation
Video Lesson: Using the
Arrhenius Equation
This equation provides a quick method to determine the energy of activation
for a reaction, but the resulting answer can contain significant error, because its
value is based on only two reactions. Alternatively, we can determine the value of
E
a
graphically by using a series of rate constants calculated at different tempera-
tures. To see how this is done, let’s examine the original equation produced by
Arrhenius:
k = Ae
−E
a
/RT
If we take the natural log of both sides of the equation, we get
ln k = lnA −
E
a
RT
Rearranging this equation as shown enables us to determine the activation en-
ergy by plotting the rate constant of a reaction at several temperatures.
ln k =−
E
a
R
1
T
+ ln A
y = mx + b
If we construct an “Arrhenius plot” of ln k versus 1/T for a series of reactions,
we obtain a straight line whose slope is equal to –E
a
/R and whose intercept is ln A,
as shown in Figure 15.20. This method not only enables us to determine the en-
ergy of activation quite accurately but also provides a convenient way to deter-
mine the frequency factor A.
EXERCISE 15.9 Energy Barrier
The reaction of NO, a component in smog, with ozone has been extensively studied.
Data for the temperature dependence are tabulated below. What is the activation
energy for this reaction?
NO(g ) + O
3
(g ) → NO
2
(g ) + O
2
(g )
Temperature (K) k (1/M
·
s)
195 1.08 × 10
9
298 12.0 × 10
9
15.6 Reaction Mechanisms 653
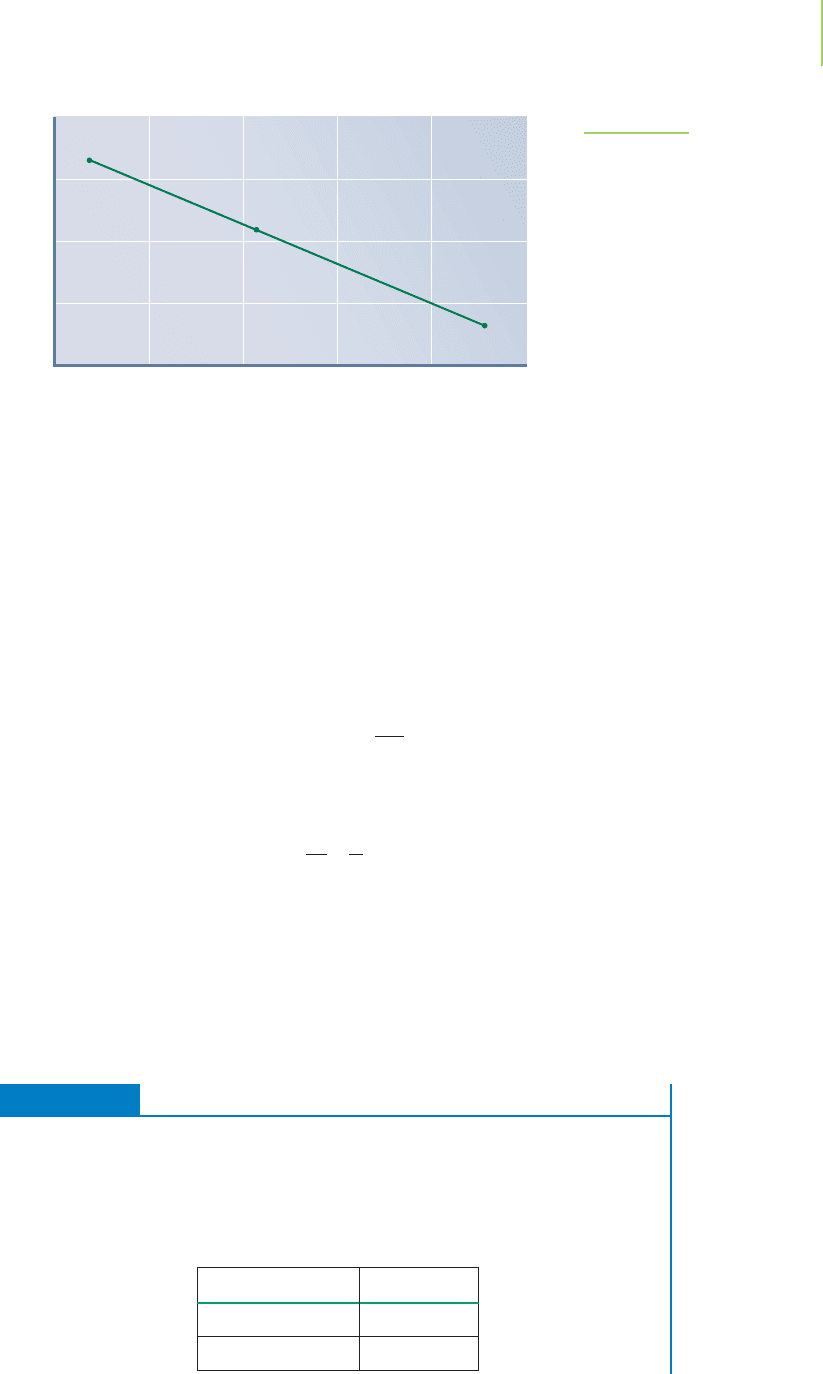
ln k
1/T
–8
–7
–6
–5
–4
0.00315 0.00325 0.00335 0.00345 0.00355 0.00365
y = –6517.3x + 16.089
FIGURE 15.20
A plot of ln k versus 1/T gives a straight line whose slope
is −E
a
/R. Each data point on this plot corresponds to the
measurement of the temperature and rate constant in
separate reactions. The slope of the line of best fit
(−6517.3) is equal to −E
a
/R. Therefore, E
a
= 54.2 kJ/mol
for this reaction. If the axes went to zero, the intercept
(16.089) corresponding to ln A would be found. The
frequency factor is 9,710,000.
Solution
The activation energy can be calculated by substituting the values from the table
into the equation:
ln
k
2
k
1
=
E
a
R
1
T
1
−
1
T
2
ln
12.0 ×10
9
1.08 ×10
9
=
E
a
8.314 J·mol
−1
·K
−1
1
195 K
−
1
298 K
2.408 =
E
a
8.314 J·mol
−1
·K
−1
1.773 ×10
−3
K
−1
20.02 J·mol
−1
= E
a
1.773 ×10
−3
K
E
a
= 11292 J·mol
−1
= 11.3kJ·mol
−1
PRACTICE 15.9
More data on the reaction of NO and O
3
are shown in the table below. Calculate E
a
for each pair of reactions. Are the values the same? Explain.
See Problems 83, 84, 87, 88, and 101.
15.7 Applications of Catalysts
Environmental chemists have comprehensively explored the rate of decomposi-
tion of atrazine in aqueous solutions. Because the rate in water is so slow
(
t
1/2
= 400
days),they’ve spent time considering how the decomposition could be
accelerated to clean up the environment more quickly. The exercise in the previ-
ous section showed that the rate of a reaction increases with an increase in
temperature. That’s one way to speed up a reaction. However, raising the temper-
ature of groundwater, rivers, and lakes is not a feasible way to increase the rate
of decomposition of herbicides and pesticides. Researchers at the University of
Wisconsin have found a better way to enhance the rate of the decomposition.They
have discovered that filtering atrazine-contaminated water through a container
full of titanium(IV) oxide in the presence of ultraviolet light greatly increases the
rate of decomposition (to t
1/2
=15 min). What does the titanium(IV) oxide do?
The titanium(IV) oxide is used as a
catalyst in the decomposition of atrazine.
A catalyst is a compound that, when added to a reaction mixture, changes the
mechanism of the reaction to a new pathway with a lower activation energy.
Rather than lowering the activation energy of the existing mechanism, it creates
a new set of elementary steps whose rate-determining step has a lower energy of
activation than the reaction would have without the catalyst. Because E
a
is lower,
the new mechanism is faster, so we often say that a catalyst increases the rate of the
reaction. Another useful aspect of catalysts is that they can be recovered from
the reaction; catalysts are not consumed in a reaction. Because of this, they don’t
appear in the net chemical equation. However, because they are involved in the
reaction, they do appear in the mechanism. To show that a particular reaction in-
volves a catalyst, we typically place it over or under the arrow in the overall equa-
tion. There are two types of catalysts, homogeneous catalysts and heterogeneous
654 Chapter 15 Chemical Kinetics
Temperature (K) k (1/M·s)
230 2.95 × 10
9
260 5.42 × 10
9
369 35.5 × 10
9
Video Lesson: Catalysts and
Types of Catalysts
catalysts. The type of catalyst, as well as the reaction profile that results, depends
on how the catalyst is mixed with the reaction.
A
homogeneous catalyst is part of a reaction that is catalyzed in a homogeneous
mixture (that is, the catalyst is intimately mixed with the reactants). For example,
the destruction of ozone by chlorine radicals (a homogeneous catalyst) is illus-
trated by the net reaction of ozone with oxygen atoms:
O
3
+ O−−−−−−−→2O
2
Cl
The mechanism of this reaction consists of two elementary steps:
Step 1: The ozone molecule reacts with elemental chlorine (the cata-
lyst) to make a molecule of ClO and a molecule of oxygen.
Step 2: The ClO reacts with an oxygen atom (the other reactant in the
net equation) to make another molecule of oxygen and regenerate
elemental chlorine.
The chlorine atom appears first as a reactant and later as a product; chlorine be-
gins and escapes the reaction without being changed and therefore is not con-
sumed. This is exactly what catalysts do. Is ClO a catalyst too? No. It is formed in
the course of the reaction and then consumed before products are made. The ClO
never escapes the reaction. As we saw earlier, this is exactly what happens to an
intermediate.
The presence of chlorine atoms causes a tremendous increase in the rate of
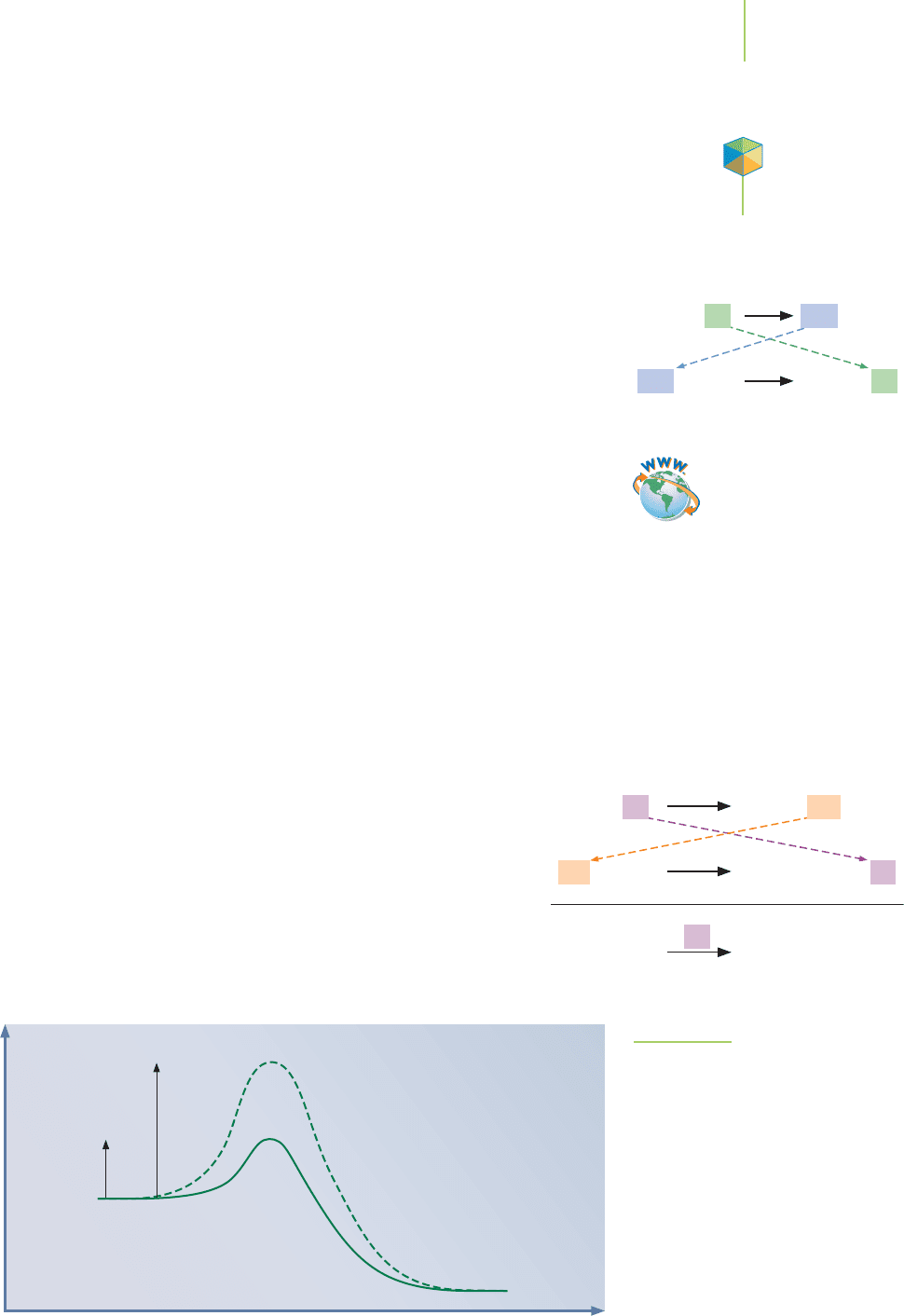
the decomposition of ozone. How does a catalyst cause a reaction’s rate to in-
crease? Consider the reaction profile, shown in Figure 15.21, for the first-order
decomposition of hydrogen peroxide by a homogeneous catalyst. In the noncat-
alyzed reaction, the reaction follows a unimolecular mechanism with a clearly
defined high-energy barrier (the activation energy) separating the reactants from
the products. If a catalyst such as iodide is added to this reaction, the mechanism
of the reaction changes. The iodide is the catalyst, and it
is shown over the reaction arrow to identify it as such.
What is the role of OI
–
in the reaction? The reaction makes
an intermediate, which is more stable than the activated
complex. The solid line in the reaction profile in Figure
15.21 illustrates the new reaction.
What is the outcome of adding a catalyst? Remember
that our catalyst has produced a new mechanism for
the reaction. If we plot the reaction profile for this new
mechanism, we can see that the activation energy is
lower. This means that more molecules will have the
15.7 Applications of Catalysts 655
Application
C
HEMICAL ENCOUNTERS:
Destruction of Ozone
Step 1
Step 2
O
3
O
2
Cl
Intermediate Catalyst
ClO++
OO
2
ClClO + +
Step 1
Step 2
Net reaction
H
2
O
2
H
2
OOI
–
I
–
++
H
2
O
2
O
2
H
2
OOI
–
I
–
I
–
++
2H
2
O
2
O
2
2H
2
O+
+
Progress of reaction
Reactants
Products
Catalyzed E
a
Uncatalyzed E
a
Potential energy
FIGURE 15.21
Decomposition of H
2
O
2
with and without
a homogeneous catalyst. The dotted line
represents the reaction profile without
the catalyst; the solid line indicates the
effect of a homogeneous catalyst.
Video Lesson: CIA
Demonstration: Elephant Snot
Visualization: Homogeneous
Catalysis

Progress of reaction
Reactants
Products
E
a(uncatalyzed)
E
a(catalyzed)
E
a(adsorb)
E
a(desorb)
Potential energy
FIGURE 15.22
Heterogeneous catalysis of NO.
requisite energy to become activated complexes in the mechanism. Although the
overall reaction enthalpy (∆H) hasn’t changed, the activation energy has been re-
duced dramatically. A reduction in the activation energy causes the rate of the
reaction to increase.
In systems with a
heterogeneous catalyst, the catalyst and the reactants are in
different physical states. The catalyst is typically a metal in solid form, whereas the
reactants are typically in gaseous, aqueous, or liquid form. The TiO
2
decomposi-
tion of atrazine is an example of this type of catalysis. The catalytic converter
(usually made up of platinum, palladium, and/or rhodium metal) in your auto-
mobile is another. One of the reactions in the catalytic converter cuts down on
smog-forming NO by reducing it to N
2
. The general unbalanced reaction is
NO(g)
→
N
2
(g)
The heterogeneous catalyst works in a three-step process. In the first step
shown in Figure 15.22, the reactant molecules
adsorb to the catalyst—that is, they
sticks to the catalyst’s surface. Note that the word adsorb is different than the word
absorb, which means being taken up or mixed into a substance.A small activation
energy barrier must be crossed to achieve the surface binding of the reactant.
Often, there is only a very small barrier for binding of a reactant to the surface of
a catalyst. Then the reactants migrate around on the surface until they collide to
make the product. A larger, yet still low, activation energy barrier exists for this
step. In the final step, the products are
desorbed, or released from the surface of
the catalyst (the reverse of being adsorbed). The resulting reaction profile dia-
gram has a characteristic shape.
656 Chapter 15 Chemical Kinetics
All of the different-colored heteroge-
neous catalysts in this collection work in
a similar manner. The reactants must ad-
sorb to the surface of the catalyst before
the reaction can be catalyzed. After reac-
tion, the products desorb from the cata-
lyst. The reaction shown here illustrates
the catalytic hydrogenation of ethylene
to make ethane.
(a) (b) (c) (d)
Hydrogen
(H
2
)
Ethene
(C
2
H
4
)
Ethane
(C
2
H
6
)
Catalyst
surface
Hydrogen and ethene bond to the surface of the catalyst (a). The hy-
drogen atoms then migrate to the ethene molecule in steps (b) and
(c). Finally, the product molecule is released from catalyst (d).
Visualization: Heterogeneous
Catalysis
Video Lesson: CIA
Demonstration: The Cobalt(II)-
Catalyzed Reaction of
Potassium Sodium Tartrate
Video Lesson: CIA
Demonstration: The Copper-
Catalyzed Decomposition of
Acetone

15.7 Applications of Catalysts 657
The modern confectionery industry operates a booming
business, helping to satisfy that sweet tooth in most of
us. Fanciful desserts require sucrose as a sweetener. For-
tunately, the American farmer can meet the large de-
mand for sweeteners. For instance, sugar beet produc-
tion in Colorado, Montana, Nebraska, and Wyoming
yields 4.5 to 6 million tons of sucrose per year. Sugar
cane production, primarily in Hawaii, Louisiana, and
Florida, adds another 6 million tons to the total. But
even more sugar is needed. One of the ways to meet the
public’s demand for sweeteners involves corn starch and
a biological molecule known as
D-glucose isomerase,
shown in Figure 15.23. The product of these molecules is
sweeter than sucrose alone. It is known as high-fructose
corn syrup, and its use has surpassed that of sucrose in
the confectionery industry.
Like other enzymes, D-glucose isomerase acts as a
catalyst that speeds up a reaction. The enzymes work by
binding selectively to a particular molecule, forcing it
into just the right shape, and then assisting in the
NanoWorld / MacroWorld
Big effects of the very small:
Enzymes—nature’s catalysts
FIGURE 15.24
Diagram of an enzyme-catalyzed reaction. The substrate binds to the
active site on an enzyme, where the reaction is catalyzed.
reaction that makes the product. They increase the value
of A (the frequency factor from the Arrhenius equation)
and decrease the energy of activation (E
a
) at the same
time. This activity arises because the backbone of the
amino acid polymer weaves the enzyme into a structure
similar to a catcher’s mitt. Along the inside of the
catcher’s mitt (the active site of the enzyme) lie portions
of the enzyme that are polar and portions that are non-
polar. The arrangements of the polar and nonpolar
groups provide a template that exactly matches that of
the molecule they bind (the reactant or substrate). When
the substrate binds, the enzyme bends it into a confor-
mation similar to that of the product of the reaction.
Then, when the conformation is just right, the reaction
takes place. After releasing the product, the enzyme re-
turns to its original shape, ready to accept another sub-
strate (Figure 15.24). The net result is an increase in the
reaction rate without an increase in temperature, which
is particularly useful in the food industry.
For example, lactase (an enzyme that converts lactose
into glucose and galactose) is used in the dairy industry
to make digestible milk products. Because many of these
products spoil at temperatures warmer than those found
in a refrigerator, the use of enzymes to speed the reaction
without a temperature increase is quite helpful. After the
lactase has been added to milk, lactose-intolerant people
can drink all of the milk they want. And they owe their
settled stomach to one of nature’s catalysts.
FIGURE 15.23
High-fructose corn syrup is made from corn starch using
an enzyme.
D-Glucose isomerase catalyzes the reaction
that converts glucose into fructose. The reaction pro-
ceeds much more rapidly with the enzyme than without
it. The enzyme is shown here as a series of ribbons that
represent the atoms that make up the strands of protein
polymers. The strands loop and wind their way together
to make a pocket that can catalyze the reaction of glu-
cose to make fructose.
Enzyme
+
Substrate
Active site
Enzyme–substrate
complex
+
Products
Enzyme
