Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


Chemical
Equilibrium
Just as this balance settles at a fixed
position of lowest energy, chemical
reactions “strive” toward a position of
lowest free energy—that is, chemical
equilibrium.
668
Contents and Selected Applications
16.1 The Concept of Chemical Equilibrium
Chemical Encounters: Myoglobin in the Muscles
16.2 Why Is Chemical Equilibrium a Useful Concept?
Chemical Encounters: Important Processes That Involve Equilibria
16.3 The Meaning of the Equilibrium Constant
Chemical Encounters: The Manufacture of Sulfuric Acid
16.4 Working with Equilibrium Constants
16.5 Solving Equilibrium Problems—A Different Way of Thinking
16.6 Le Châtelier’s Principle
Chemical Encounters: Catalysts in Industry
16.7 Free Energy and the Equilibrium Constant
Go to college.hmco.com/pic/kelterMEE for online learning resources.
669
“I’m running late.” We seem to hear
that expression so often in our hyper-
charged, have-to-do-it-now world. Running
late might mean being stuck in traffic or on mass
transit. There is not much you can do in a sea of cars
and trucks. But sometimes you actually do run to get
where you are going. As you struggle to make up time, you
breathe more heavily than normal, desperately sucking air into your
lungs. Your body’s efforts to get more oxygen to the muscles are greatly
enhanced by a protein in muscle cells called myoglobin.
When you are resting, the myoglobin becomes loaded up with oxygen
molecules. These oxygen molecules can be quickly released when your
muscle cells undergo activity. How does the myoglobin acquire and re-
lease oxygen when we need it?
Myoglobin (Mb), shown in Figure 16.1, is a protein with a molecular
weight of about 16,900 g/mol. A key part of the protein is the
heme group,
shown in Figure 16.2. The heme contains an Fe
2
+
ion that can form a
bond with one molecule of oxygen,creating a myoglobin–oxygen complex
as follows:
Mb + O
2
n MbO
2
When your cells need the oxygen, it is released from the complex by reversing the
direction of the reaction:
MbO
2
n Mb + O
2
The free myoglobin is then available to bind more oxygen, which it can later release
as needed, and continue in this reversible cycle of bind–release–bind–release. Let’s
examine this reaction more closely to help us understand the concepts of reversible
reactions and the essential details of reactions such as this, which are known as
chemical equilibria.
Strenuous activities such as running
require a sharp change in the amount of
oxygen your muscles require. The inter-
action of myoglobin and oxygen in the
muscles accommodates this need.
Oxygen
FIGURE 16.1
Myoglobin (Mb) is a protein with a molecular weight of about
16,900 g/mol. A key part of the protein is the heme group,
highlighted on the left, which can bind to an oxygen molecule.
CH
2
CH
2
CH
3
CH
2
COOH
CH
2
COOH
CH
3
Fe NN
N
N
C
CH
C
C
C
C
C
CC
CH
CH
3
CH
2
OO
CH
2
CH
CH
3
HC
C
CH
C
CC
CH
C
C
C
C
FIGURE 16.2
This drawing of a heme group highlights the Fe
2+
ion, which
can bind to one molecule of oxygen as shown.
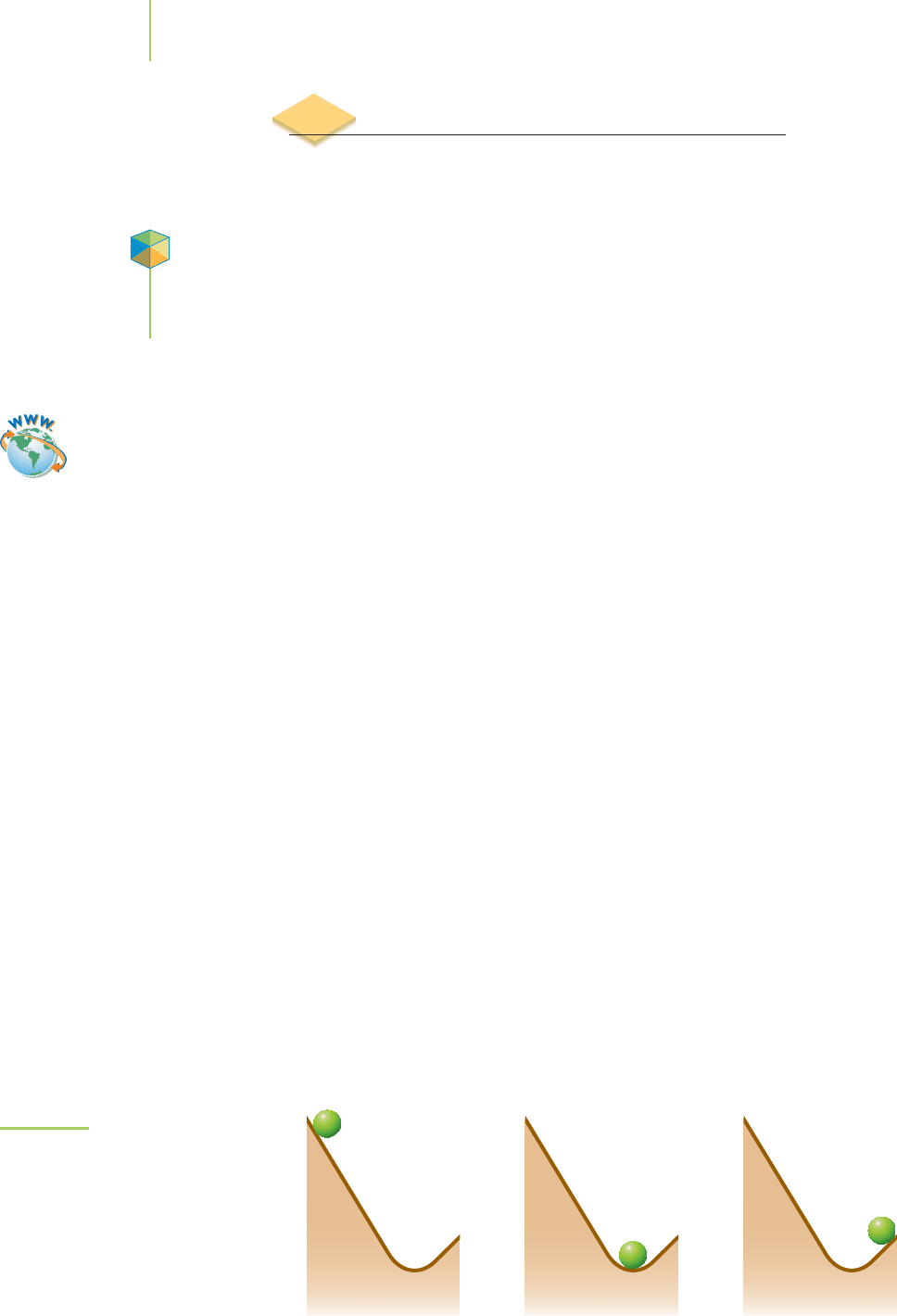
16.1 The Concept of Chemical Equilibrium
Why does myoglobin’s reversible cycle of binding and releasing oxygen take
place? We know from our understanding of thermodynamics (Chapter 14) that
spontaneous reactions lower the free energy, G, of the chemicals involved. When
we consider myoglobin and oxygen in living systems, we find that the two com-
peting reactions exist. In one, myoglobin combines with oxygen. In the other, the
MbO
2
complex releases oxygen. The end result is that the molecules in each reac-
tion will never be completely converted into products, because these products are
always being converted back into reactants. In a closed system, these reversible re-
actions will settle into a state in which the forward and reverse reactions occur at
equal rates, where no net change in the concentrations occurs, even though both re-
actions continue. We describe this state as the position of chemical equilibrium,
or just
equilibrium (the plural is equilibria). What thermodynamic parameter
determines when an equilibrium exists? The concentrations will no longer change
when the free energy of the system is at a minimum for the process. At that point, the
change in free energy of the system, ∆G, is 0 kJ/mol.
We symbolize a system that can settle into equilibrium by using double ar-
rows, to represent the fact that both the forward and reverse reactions occur. For
example, the myoglobin and oxygen reaction equilibrium is written
Mb + O
2
MbO
2
As discussed in Chapter 15, we express the molar concentration of a substance by
placing its chemical symbol in brackets, [ ], such as [Mb] or [MbO
2
].
Let’s examine what happens to the concentrations of the reactants and prod-
ucts as the minimum free energy is approached (see Figure 16.3.) To make things
as straightforward as possible, we will consider the reaction occurring in a beaker,
rather than in the human body where all kinds of complications (such as “run-
ning late”) affect the chemistry.
Let’s assume that we place oxygen in our beaker so that its concentration is
3.0 ×10
−4
M.Wewouldsay[O
2
]
0
=3.0 ×10
−4
M,where the subscript0 means the
concentration at “time =0,” which is another way of saying the initial concentra-
tion. In fact, the amount of myoglobin normally present in muscle cells varies a
great deal among, and within, biological species. In humans, a mid-range value
is [Mb]
0
=2.0 ×10
−4
M. We will assume for this discussion that we have a closed
system (not the human body) and that there is no MbO
2
complex initially, so
[MbO
2
]
0
= 0 M. As the reaction proceeds, oxygen binds to myoglobin. The
forward reaction proceeds with a rate constant of k
1
,known in this particular reac-
tion as the
binding rate constant.
k
1
Mb + O
2
n MbO
2
670 Chapter 16 Chemical Equilibrium
Application
C
HEMICAL
ENCOUNTERS:
Myoglobin in
the Muscles
A
C
B
A
C
B
A
C
B
FIGURE 16.3
Like a ball finding its lowest energy point
in a valley, reactions find their lowest free
energy position. This is the equilibrium
position (B).
Video Lesson: The Concept of
Equilibrium
Tutorial: The Equilibrium
Condition and Equilibrium
Constant
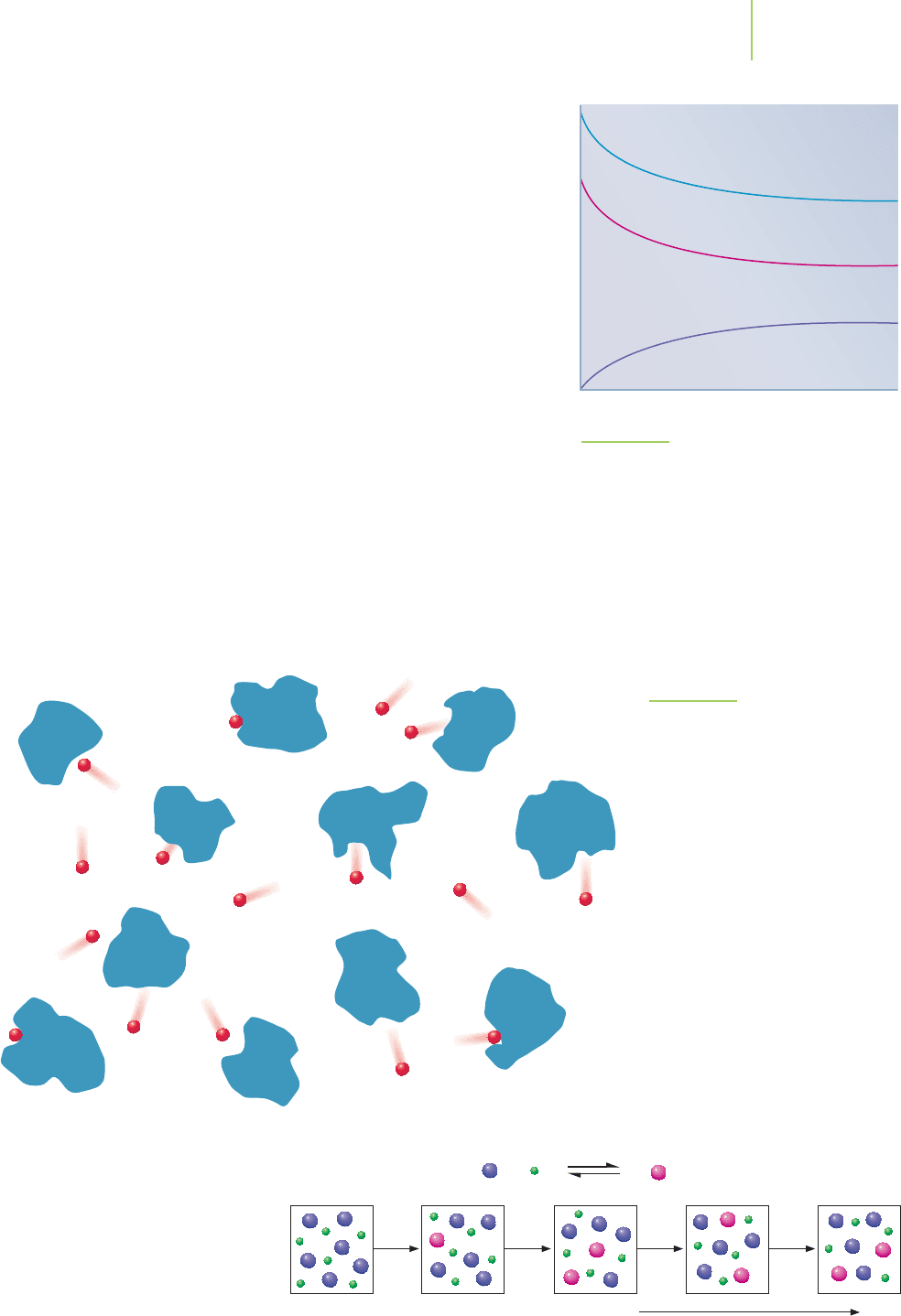
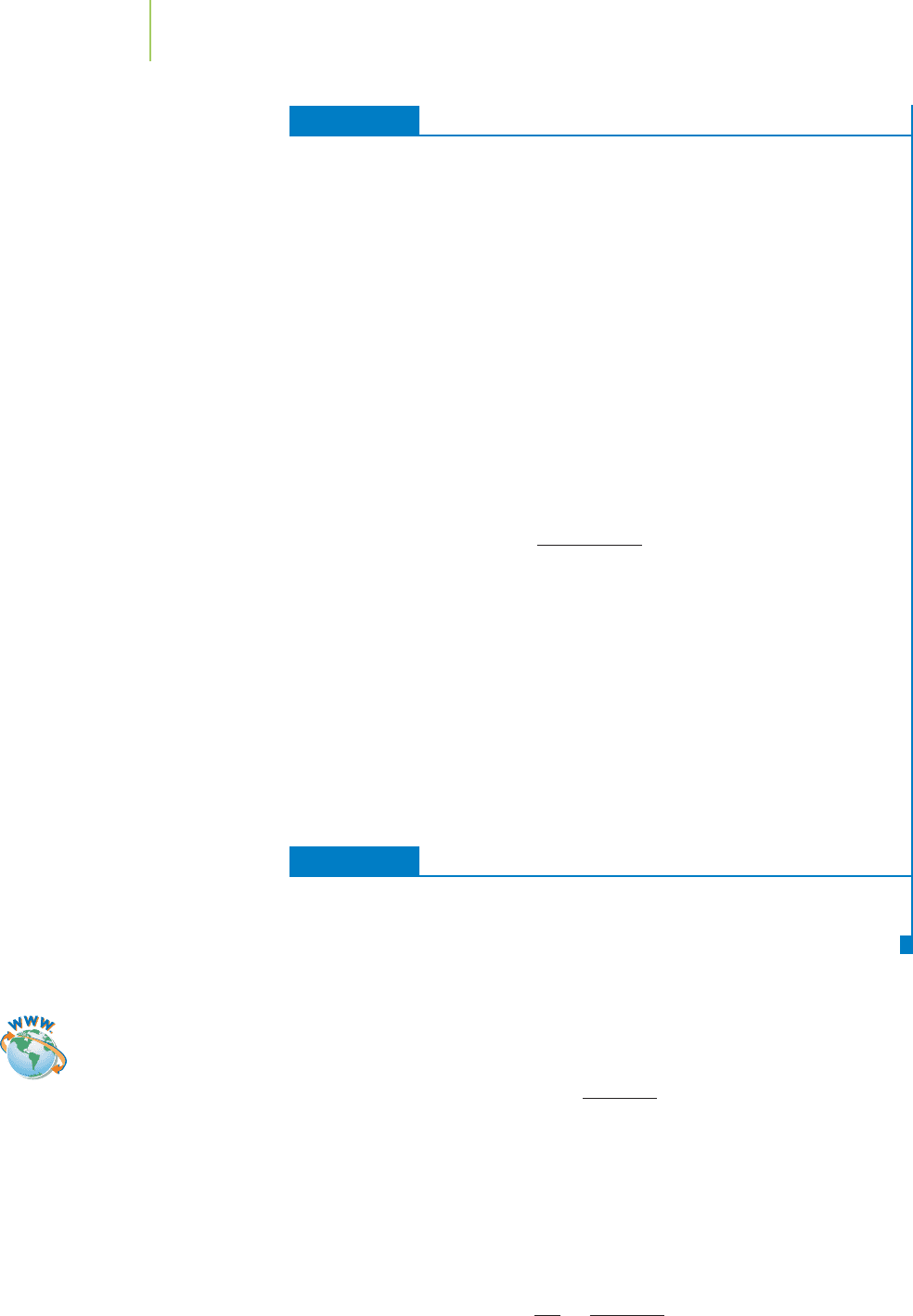
FIGURE 16.5
Equilibrium is a dynamic process. The
forward and reverse reactions are occur-
ring at equal rates. The result is no net
change in concentration of Mb (the blue
shapes), O
2
(the red dots), or MbO
2
at
equilibrium.
The concentration of oxygen decreases, as does that of free myoglobin,
as shown in Figure 16.4. The concentration of the MbO
2
complex in-
creases from zero as the reaction proceeds. As soon as the MbO
2
com-
plex forms, however, it begins to dissociate back into Mb and O
2
with
a rate constant of k
–1
, known in this particular reaction as the release
rate constant
.
k
–1
MbO
2
n Mb + O
2
Both reactions proceed until the free energy of the system is at a min-
imum, which occurs when the rates of the forward and reverse reactions
are equal. That statement embodies the two key ways to define chem-
ical equilibrium:
Definitions of Equilibrium
1. The free energy change of both forward and reverse reactions is
zero (∆G =0), and the free energy of the system is at its minimum.
2. The rates of the forward and reverse reactions are equal.
rate
f
= rate
r
k
1
[Mb][O
2
] = k
−1
[MbO
2
]
This is shown in Figure 16.5. The forward and reverse reactions that
bind and release oxygen are still occurring. In fact, they are occurring at the same
rate. Therefore, equilibrium is a dynamic process. And there is no overall change in
the concentration of Mb, O
2
, or MbO
2
at equilibrium.
16.1 The Concept of Chemical Equilibrium 671
Concentration
Time
[Mb]
[O
2
]
[MbO
2
]
FIGURE 16.4
When myoglobin and oxygen are mixed, the con-
centration of oxygen decreases, as does that of
free myoglobin, as shown.The concentration of the
MbO
2
complex increases from 0 M. As soon as
the MbO
2
complex forms, however, it begins to
dissociate back into Mb and O
2
.
+
Time Time Time Time
Reactants Product
Equilibrium
The initial concentrations of reactants and
products change over time until equilibrium
is reached. At this point, the forward and
reverse reactions continue to produce their
respective products, but the overall concen-
trations of each component in the reaction
remain constant.
672 Chapter 16 Chemical Equilibrium
EXERCISE 16.1 Equilibrium Concentrations
Hydrogen and iodine gases can be combined to form hydrogen iodide,
H
2
(g) + I
2
(g)
2HI(g)
Given the following initial and final concentrations, calculate the equilibrium con-
centration of HI.
[I
2
]
0
= 0.037 M [H
2
]
0
= 0.121 M [HI]
0
= 0.099 M
[I
2
] = 0.022 M [H
2
] = 0.106 M [HI] = ??? M
First Thoughts
The mole ratios of reactants to product are 1-to-1-to-2, so 1 mol each of H
2
and I
2
react to produce 2 mol of HI in a constant volume. Because the volume remains
constant, we can think of the reaction stoichiometry this way: 1 M H
2
and 1 M I
2
react to produce 2 M HI.
Solution
The concentrations of I
2
and H
2
both decrease by 0.015 M.
0.015 M H
2
(or I
2
) ×
2M HI
1M H
2
(or I
2
)
= 0.030 M HI produced
[HI] = [HI]
0
+ 0.030 = 0.13 M
Further Insights
Much of the discussion in this chapter and in those that follow will consider how we
can predict equilibrium concentrations when we know only about the system and
the initial conditions. We will spend considerable time later in this chapter dis-
cussing how the ability to predict what will happen in a system is important in the
chemical industry. For example, in the development of a manufacturing process,
understanding how changes in conditions affect the equilibrium process enables
the manufacturer to produce the largest possible yield of product, as well as save the
company (and ultimately the consumer) money.
PRACTICE 16.1
Calculate the equilibrium concentration of HI if [I
2
]
0
= 0.050 M,[H
2
]
0
= 0.044 M,
[HI]
0
= 0.206 M, and [I
2
] = 0.041 M.
See Problem 21.
It has been shown by experiment that the equilibrium concentrations of myoglo-
bin, oxygen, and the MbO
2
complex are related by the ratio of the product to
reactant concentrations known as the
mass-action expression or, often, the
equilibrium expression, of the reaction.
K =
[MbO
2
]
[Mb][O
2
]
The value K is called the equilibrium constant for the reaction at a given tempera-
ture. We can get the equivalent result by separating the rate constants from the
concentrations in the rate expressions as follows. At equilibrium, the rate of
the forward and reverse reaction are equal.
k
1
[Mb][O
2
] = k
−1
[MbO
2
]
Rearranging the equation to put the rate constants on one side gives
k
1
k
−1
=
[MbO
2
]
[Mb][O
2
]
Video Lesson: The Law of Mass
Action and Types of Equilibrium
Therefore, the equilibrium constant, K, is the ratio of the forward rate constant to
the reverse rate constant.
K =
k
1
k
−1
The value for k
1
in the myoglobin–oxygen reaction at 20°C is 1.9 ×10
7
M
−1
·s
−1
,
and k
−1
is 22 M
−1
·s
−1
, so we can calculate the value of K:
K =
k
1
k
−1
=
1.9 ×10
7
22
= 8.6 ×10
5
We typically would express a calculation such as this by including the units.
However, the equilibrium constant does not have units. This is true because the
actual thermodynamic definition of an equilibrium constant is based on the ratio
of a substance’s concentration to a standard state concentration. Even though
the reasoning is subtle, the bottom line is that the equilibrium constant is typi-
cally given without units. In the reaction of myoglobin and oxygen, K is simply
8.6
×
10
5
.
These last two results—that there exists a mass-action expression to find
the equilibrium concentrations of Mb, O
2
, and MbO
2
, and that there exists an
equilibrium constant for this expression at a particular temperature—can be
extended to any reversible reaction. For example, in a general equilibrium (reac-
tants A and B yielding products C and D) represented with stoichiometric coeffi-
cients m, n, p, and q,
mA + nB
pC + qD
The mass-action expression is
K =
[C]
p
[D]
q
[A]
m
[B]
n
Exercise 16.2 explains why each equilibrium concentration is raised to the power
of its coefficient in the mass-action expression.
EXERCISE 16.2 The Mass-Action Expression
The production of ammonia for use in fertilizers, via the Haber process, can be
depicted as
H
2
(g) + H
2
(g) + H
2
(g) + N
2
(g)
NH
3
(g) + NH
3
(g)
Write the mass-action expression for the formation of ammonia.
First Thoughts
We did something unusual by breaking the equation down to list each individual
molecule without the coefficients, a rather cumbersome way of displaying an equa-
tion. Our goal in doing this is to demonstrate how the product of the coefficients in
the mass-action is displayed in exponential form.
Solution
If we base the mass-action expression on the reaction exactly as it is written, we get
the following mass-action expression:
K =
[NH
3
][NH
3
]
[H
2
][H
2
][H
2
][N
2
]
=
[NH
3
]
2
[H
2
]
3
[N
2
]
Further Insights
The exponential form on the right-hand side is what we would have obtained had
we written the Haber process in the usual way:
3H
2
(g) + N
2
(g)
2NH
3
(g)
16.1 The Concept of Chemical Equilibrium 673
This shows why the mass-action expression includes the equilibrium concentration
of each substance raised to the power of its coefficient. If the coefficient is 1, as with
N
2
, it is not shown as an exponent, because raising anything to the power of 1 does
not change its value.
PRACTICE 16.2
One of the ways to produce hydrogen gas industrially involves the reaction of
methane with high-temperature steam. The reaction can be written as
CH
4
(g) + H
2
O(g)
CO(g) + H
2
(g) + H
2
(g) + H
2
(g)
Write the mass-action expression for this reaction.
See Problems 5 and 6.
EXERCISE 16.3 Practice with Mass-Action Expressions
Write the mass-action expression for each of these reactions:
a. PCl
5
(g)
PCl
3
(g) + Cl
2
(g)
b. S
8
(g)
8S(g)
c. Cl
2
O
7
(g) + 8H
2
(g)
2HCl(g) + 7H
2
O(g)
Solution
In each case, the mass-action expression for the general reaction
mA + nB
pC + qD
is of the form in which the equilibrium concentration of each reactant or product is
raised to the power of its coefficient.
K =
[C]
p
[D]
q
[A]
m
[B]
n
Products Coefficient of S
hh
a.
K =
[Cl
2
][PCl
3
]
[PCl
5
]
b.
K =
[S]
8
[S
8
]
g
Reactant
Coefficient Coefficient
of H
2
OofHCl
hh
c.
K =
[H
2
O]
7
[HCl]
2
[Cl
2
O
7
][H
2
]
8
g
Coefficient of H
2
Note: Mathematically [C]
p
[D]
q
=[D]
q
[C]
p
, so the order in which we write these
terms doesn’t matter, as long as we keep each exponent with its term. For in-
stance, the exponent p must remain with [C].
PRACTICE 16.3
Write the equilibrium expression for each of these reactions:
a. NO(g) + O
3
(g)
NO
2
(g) + O
2
(g)
b. HCl(aq) + NH
3
(aq)
NH
4
Cl(aq)
c. C
2
H
2
(g) + 2H
2
(g)
C
2
H
6
(g)
See Problems 9 and 10.
674 Chapter 16 Chemical Equilibrium
Concentration-based mass-action expressions are generally suitable repre-
sentations for the equilibria that occur in chemical reactions. However, in some
aqueous solutions, especially where the solute concentrations are high, chemists
often make a correction using activities instead of molarities, to get the most
meaningful results. For example, if we prepare a solution that contains 3.0 mol of
hydrochloric acid (HCl) in a liter of water, we say that the HCl concentration is
3.0 molar. We expect this strong acid to dissociate into H
+
and Cl
−
ions, and we
therefore assert that in the solution, the concentration of each ion, H
+
and Cl
−
,is
3.0 M. However, this is not completely true. Some of the hydrogen cations do in-
teract with the chloride anions in solution, and the ions interact with the water
solvent through ion–dipole interactions, as we discussed in Chapter 10. This
means that the effective concentration is likely to be somewhat different from the
intended concentration, especially in relatively concentrated solutions. This
effective concentration of the solute is called its
activity. We will generally not
consider the impact of activity in our discussions, but it is important for you to
know that such an idea exists and can be dealt with quantitatively.
HERE’S WHAT WE KNOW SO FAR
■
Most reactions do not go to completion. Rather, they reach a point of mini-
mum free energy. We call this the point the position of chemical equilibrium.
■
At chemical equilibrium, the rates of the forward and reverse reactions are
equal.
■
Chemical equilibrium is a dynamic, not static, condition.
■
A reaction at chemical equilibrium can be described by a mass-action
expression for which there is an equilibrium constant, K, that depends on
temperature.
16.2 Why Is Chemical Equilibrium
a Useful Concept?
The reaction of myoglobin with oxygen is just one of countless examples of
equilibrium processes in living systems. In fact, the chemistry of blood is filled
with equilibria. The chemistry of the environment also involves equilibrium
chemistry. As we will explore in Section 16.6, we can control the position of
equilibrium—that is, we can make it possible for reactions to proceed almost all
the way toward products or to reach a point at which mostly reactants exist. For
example, we can select the conditions so that the greatest possible amount of am-
monia is formed from hydrogen and nitrogen in the Haber process. We can also
maximize the amount of sulfur trioxide that is formed from the reaction of sul-
fur dioxide with oxygen as part of the industrial-scale preparation of sulfuric
acid. We can attempt to reduce the concentrations of acid in lakes and streams.
We can learn about the impact of chlorofluorocarbons (CFCs) on stratospheric
ozone levels. We can prepare pharmaceuticals to work in the most effective ways.
We can analyze for the presence of an extraordinary variety of substances, from
silver to steroids. We can do these things because we understand the fundamental
ideas of equilibrium. Our ability to use equilibrium concepts to control the extent
of chemical processes has the following vital implications:
1. Economic implications via the trillions of dollars of manufactured products
prepared and sold by the chemical industry.
2. Environmental implications for the quality of air, water, and land in the closed
system that is Earth.
3. Personal implications for our health.
16.2 Why Is Chemical Equilibrium a Useful Concept? 675
Application
C
HEMICAL ENCOUNTERS:
Important Processes
That Involve Equilibria

Application
C
HEMICAL
E
NCOUNTERS:
The Manufacture
of Sulfuric Acid
676 Chapter 16 Chemical Equilibrium
Table 16.1 lists some significant processes that involve chemical equilibria.
Among them is chromatography, the subject of the accompanying boxed feature
on equilibrium and chromatographic analysis.
16.3 The Meaning of the Equilibrium Constant
More sulfuric acid (H
2
SO
4
) is produced in the United States than any other
chemical; 37.5 billion kg were produced in 2004. Sulfuric acid has a wide range of
uses, the most important being the production of agricultural fertilizers. Its in-
dustrial and agricultural importance makes examining the manufacture of sulfu-
ric acid an excellent way to illustrate the meaning of the equilibrium constant.
Most of the sulfuric acid produced in the United States is made via the
Contact
process
, which began to be used on an industrial scale in 1880. The process is a set
of steps performed on readily available materials.
Step 1: The process begins with the sulfur provided by minerals such as iron
pyrite (FeS
2
) or hydrogen sulfide gas (H
2
S). The sulfur combines with oxygen
from the air.
S(s) + O
2
(g)
SO
2
(g)
Step 2: In this step, sulfur trioxide (SO
3
) is generated by the oxidation of SO
2
produced in step 1.
2SO
2
(g) + O
2
(g)
2SO
3
(g)
Sulfur dioxide gas is passed over a catalyst bed containing 6–10% vanadium(V)
oxide (V
2
O
5
) at 600°C and then at 400°C. Temperature has a major effect on the
equilibrium of this reaction, as we will see.
Achievement of chemical equilib-
rium occurs in living systems,
chemical manufacturing, and the
environment. Equilibrium figures in
widely disparate processes related
to the chemistry of blood, ammonia
manufacture, steroid analysis, and
lakes and waterways.
Chemical Equilibrium Processes
Category Process Important Reaction
Industrial Contact process 2SO
2
(g) + O
2
(g)
2SO
3
(g)
Industrial Haber process 3H
2
(g) + N
2
(g)
2NH
3
(g)
Biological Myoglobin uptake of oxygen Mb(aq) + O
2
(aq)
MbO
2
(aq)
Analytical Chromatographic analysis A
mobile phase
A
stationary phase
Analytical Metal analysis with EDTA Ca
2+
+ EDTA
4−
CaEDTA
2−
Environmental Leaching of lead into water PbCO
3
(s) + 2H
+
(aq)
Pb
2+
(aq) +CO
2
(g) +H
2
O(l)
TABLE 16.1
Step 3: Sulfur trioxide (SO
3
) is then combined with concentrated sulfuric acid
and water to give more sulfuric acid in a net process that can be written as
SO
3
(g) + H
2
O(l)
H
2
SO
4
(aq)
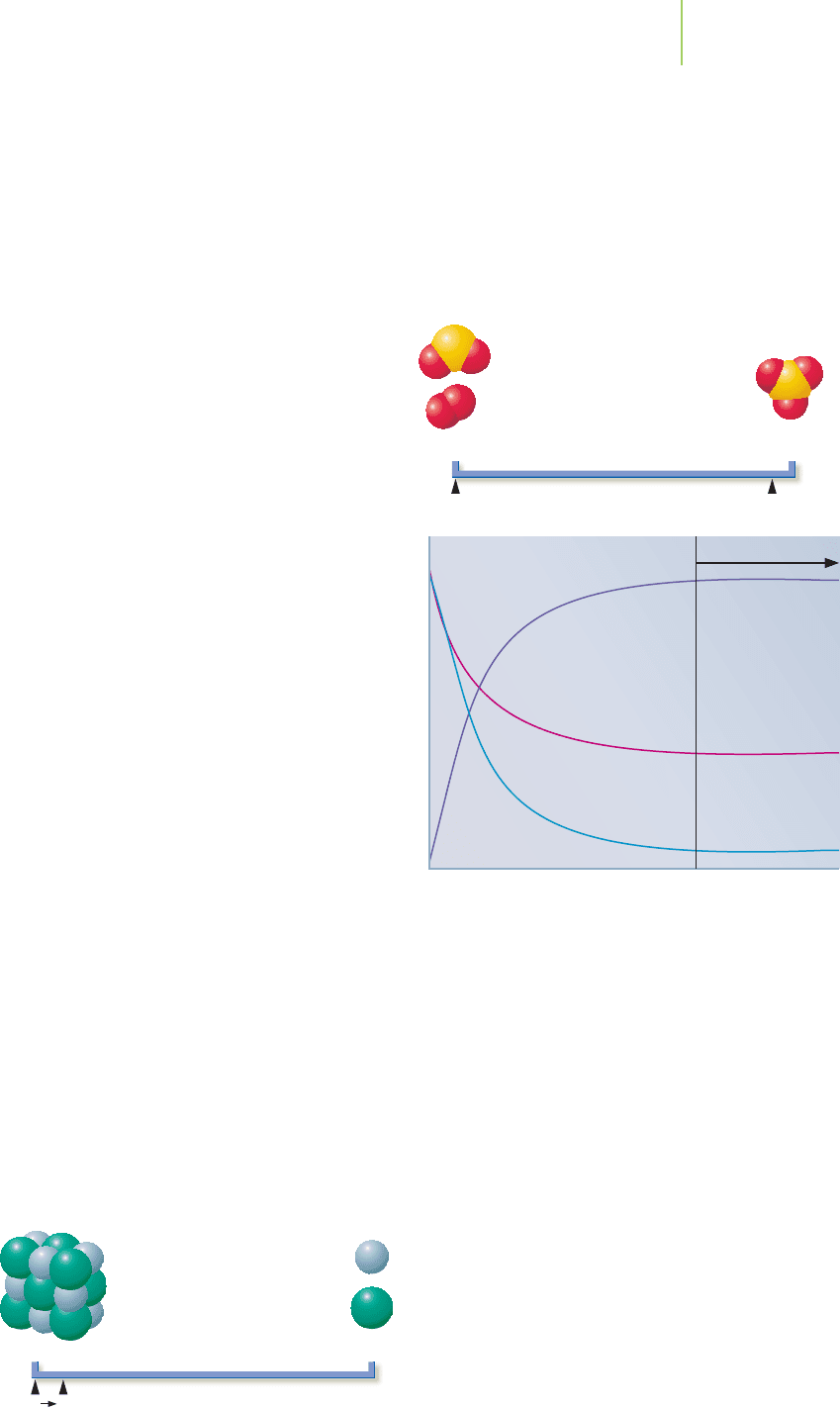
Let’s revisit step 2 of the contact process, the reaction of SO
2
and oxygen to
make SO
3
. The equilibrium constant, K, for this reaction at 27°C is 4.0 × 10
24
.
What does this value tell us about the relative amounts of reactants and products
when the reaction reaches equilibrium? When the value for K is very large, the
equilibrium lies essentially all the way to the products side.
(When we say,“reactants and products side,”we are always re-
ferring to these in terms of the forward reaction, written from
left to right.) The reaction goes almost, but not fully, to
completion. We can use a line chart, as shown to the right,
to visualize the meaning of the equilibrium constant for this
reaction by comparing the equilibrium position (E) to the
starting position (S). We start with only reactants (R) and
have no products (P). In practice, the contact process is not
carried out at 27°C because it takes too long for this reaction
to reach equilibrium at that temperature. In the chemical in-
dustry, time is money. To speed up the process, the tempera-
ture is raised, which lowers the equilibrium constant for this
reaction. Unfortunately, for this and many other equilibria,
the extent of the reaction indicated by the equilibrium constant
and the kinetics of the reaction (how fast it gets there), can force
us into a compromise between reaction speed and reaction yield.
We will discuss the interplay of these variables and their effect
on selecting reaction conditions in Section 16.6.
Every reaction has its own set of temperature-dependent
values of K (which is also known as K
c
when only concentra-
tions are used to determine its value), the equilibrium con-
stant. Our chapter-opening reaction of myoglobin with
oxygen has K = 8.6 × 10
5
at 20°C, which is fairly large. The
opposite, a small equilibrium constant, is also possible. For
example, an equation can be written that illustrates the for-
mation of silver and chloride ions in a saturated solution of silver chloride (AgCl)
from solid AgCl. Recall from Chapter 11 that a saturated solution is one in which
no more solute will dissolve. The small equilibrium constant of 1.7 ×10
−10
indi-
catesthatthe product concentrationsarequitesmall,[Ag
+
]=[Cl
−
]=1.3×10
−5
M.
This implies that very little silver chloride dissolves at 25°C before equilibrium
(the saturated solution) is reached.
AgCl(s)
Ag
+
(aq) + Cl
−
(aq) K = 1.7 × 10
−10
When the value for K is very small, the equilibrium lies nearly all the way to the
reactants side. We can show this using our line chart. In this case, the equilibrium
position is quite close to the silver chloride reactant, and we would say that silver
chloride isn’t very soluble in water.
16.3 The Meaning of the Equilibrium Constant 677
Reactants
SE
Products
Concentration
Time
Equilibrium
[SO
2
]
[O
2
]
[SO
3
]
As the reaction proceeds, the concentra-
tion of product increases and the con-
centrations of reactants decrease. At
some point, the concentrations of each
component reach and maintain a con-
stant value. This is the point when equi-
librium is reached.
Reactants
SE
Products
