Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

83. In the chapter, we note that metabolism of glucose results
initially in the formation of two molecules of pyruvate for
every molecule of glucose.
a. According to Figure 14.8, what is the net production of
ATP molecules for every molecule of glucose consumed?
b. How many grams of pyruvate would result from the
consumption of 0.057 g of glucose? (Assuming all of the
glucose is converted into pyruvate.)
c. This process, known as glycolysis, is spontaneous. How
can these reactions be spontaneous if they also result in the
generation of molecules with high-potential energy?
84. We know from Chapter 2 that about 76% of all chlorine
atoms are chlorine-35 and about 24% are chlorine-37. Let’s
say that you were able to separate the two isotopes and form
two samples of Cl
2
, one that is pure
35
Cl–
35
Cl and the other,
37
Cl–
37
Cl. You then took each sample and reacted it with
hydrogen gas to form HCl.
Cl
2
H
2
n 2HCl
Based on your understanding of thermodynamics, discuss
which reaction—the one with the Cl-35 isotope or the one
with the Cl-37 isotope—would have the greater enthalpy of
reaction, entropy, and free energy.
85. “Is there life on other worlds?” has been asked for centuries,
though we have made the greatest strides in obtaining data
via NASA space problems within the past 40 years. How
would the biochemical reactions we’ve studied in this chapter
be affected on Venus with its surface temperature of over
400°C? What about the effect on Enceladus, a moon of Sat-
urn which has a surface temperature of –330°C?
Thinking Beyond the Calculation
86. The oxidation of glucose was discussed in detail in this chap-
ter. A similar compound, fructose, also undergoes oxidation
in biological organisms to give (eventually) CO
2
and water:
___ C
6
H
12
O
6
(s) + ___ O
2
(g) → ___ CO
2
(g) + ___ H
2
O(l)
a. Balance the equation and predict the numerical value of
the change in entropy for this process.
b. Use the appendix to calculate the values of H°, S°, and
G° for this reaction. Compare your calculated value for
the entropy change to the predicted value. Assume that the
thermodynamic values for fructose are equivalent to those
for glucose.
c. If the combustion reaction is done in the laboratory, the
water is isolated as vapor. Recalculate the values of H°,
S°, and G° for the reaction where both products are
gases.
d. If 5.0 g of fructose is consumed, how many liters of CO
2
(g)
would be produced? Where does this CO
2
go in a living
organism? (Assume 25°C and 1 atm.)
e. How much heat is liberated from the combustion of 5.0 g
of fructose in the laboratory?
f. In the first step of the catabolism of fructose, a phosphate
is attached to the sugar unit:
C
6
H
12
O
6
(aq) +ATP(aq) →ADP(aq) + C
6
H
11
O
9
P
2−
(aq)
G =−16.7 kJ/mol
We also know that ATP can be made from ADP and
inorganic phosphate in the body:
ATP(aq) + H
2
O(l) → ADP(aq) + HPO
4
2−
(aq)
G =−30.5 kJ/mol
What is the free energy change for the reaction of fructose
with phosphate?
C
6
H
12
O
6
(aq) + HPO
4
2−
(aq) → C
6
H
11
O
9
P
2−
(aq) + H
2
O(l)
G = ?
g. Why would an organism have a need to utilize fructose in
the same manner as glucose? What is a typical source of
fructose?
Fructose
618 Chapter 14 Thermodynamics: A Look at Why Reactions Happen

619
Contents and Selected Applications
Chemical Encounters: Atrazine and the Environment
15.1 Reaction Rates
15.
2 An Introduction to Rate Laws
15.3 Changes in Time—The Integrated Rate Law
Chemical Encounters: Decomposition of DDT
Chemical Encounters: Persistent Pesticides
15.4 Methods of Determining Rate Laws
15.5 Looking Back at Rate Laws
15.6 Reaction Mechanisms
Chemical Encounters: Metabolism of Methoxychlor
15.7 Applications of Catalysts
Chemical Encounters: Destruction of Ozone
Chemical
Kinetics
Aerial spraying. No, this plane isn’t parked
in that field. It’s flying inches above it,
spraying to control pests and weeds.
Pesticides and herbicides enhance crop
production, result in greener lawns, and
eliminate nasty pests from our homes. But
once they’re added to the environment,
how long do they stick around?
Go to college.hmco.com/pic/kelterMEE for online learning resources.
620
In 2004, American farmers continued a
decades-long increase in food production, har-
vesting over 700 million metric tons of wheat, corn,
rice, and other grains. According to the U.S. Department
of Agriculture, corn production in Iowa alone more than
quadrupled, from 40 bushels per acre to 180 bushels per acre, in
the 75 years between 1930 and 2004. Although the introduction of the
tractor and other automated farm machinery has played a large role in this
increase in production, the use of
insecticides and herbicides (compounds used to
kill unwanted plants) has had a substantial impact. No longer do insects and weeds
run rampant through cornfields and destroy crop yields. Atrazine, a herbicide, is
one of the agents most commonly sprayed onto the soil from which corn crops
grow in order to control weeds; approximately 15 million pounds are used annually
in Nebraska alone.
When it is introduced into a farmer’s field, atrazine works well to control broadleaf
weeds, such as pigweed, cocklebur, velvetleaf, and certain grass weeds, without harm-
ing the corn plants. What happens to the atrazine that doesn’t land on weeds? Some
of it travels into the soil, where microbes and water can degrade it into by-products.
This degradation process has been studied extensively by scientists. One particular
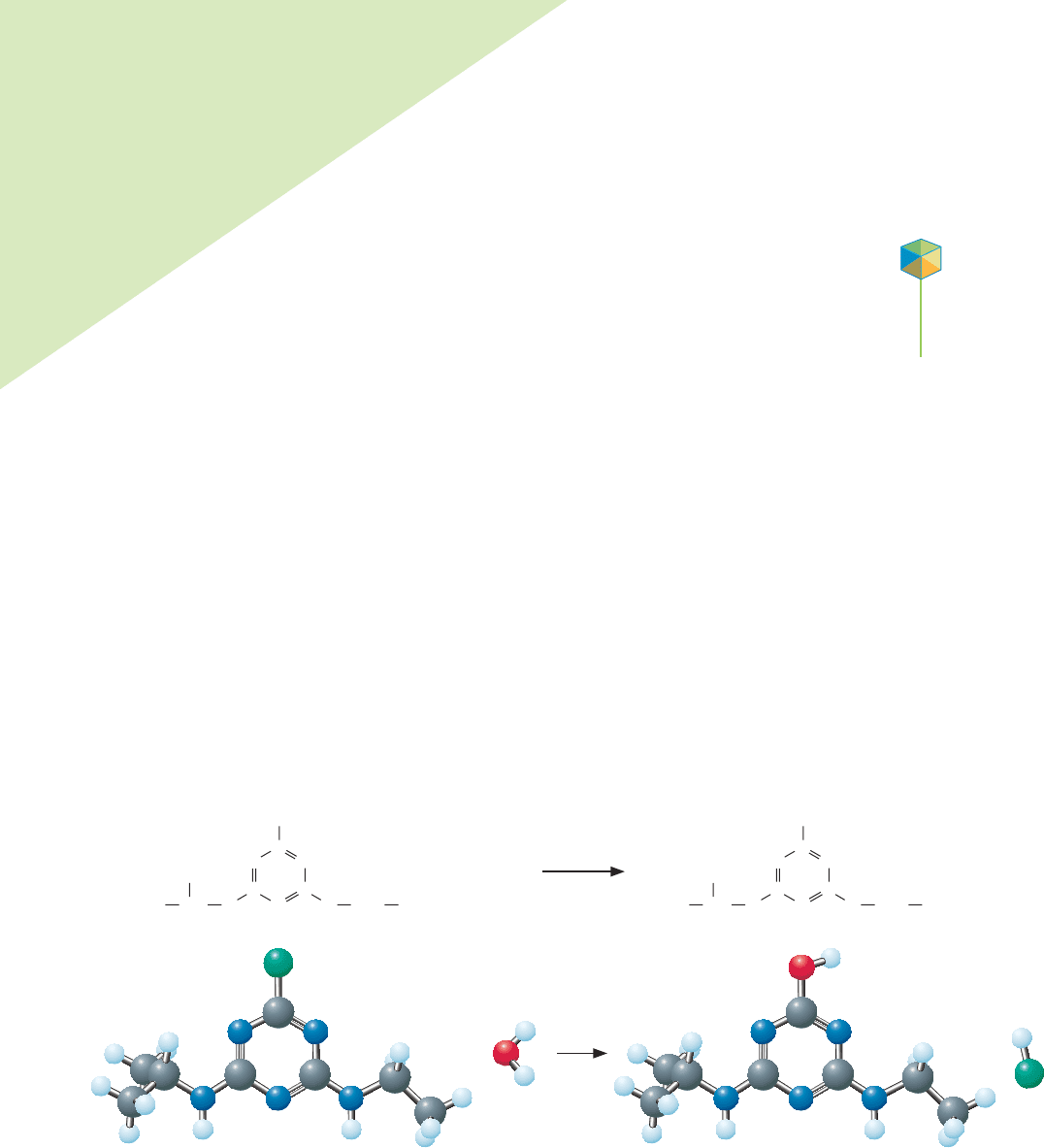
reaction, the hydrolysis of atrazine, in which the chlorine atom on atrazine is re-
placed with a hydroxy (—OH) group, is of particular interest to researchers. The
product, hydroxyatrazine, is rapidly metabolized by microbes living in the soil and
groundwater and is viewed by the U.S. Environmental Protection Agency as not
harmful to humans. The length of time it takes atrazine to metabolize is largely
determined by this initial hydrolysis reaction.
Environmental chemists study the interaction of compounds and the environment,
including the chemistry of the soil, water, and air. Their work proves that pesticides
(a
pesticide is any compound used to kill unwanted organisms) do not always rapidly
disappear from the environment. For example, their analyses of lakes, rivers, and
streams in states that use atrazine show that it persists in the environment for quite
a long time. Depending on certain environmental and biological factors (including
soil depth, temperature, and the presence of microorganisms, especially fungi), the
Application
C
HEMICAL
ENCOUNTERS:
Atrazine and the
Environment
++
+
Atrazine
Cl
C
CC
N
NN
H
N
H
N
CH
2
H
3
CCH CH
3
CH
3
OH
C
CC
N
NN
H
N
H
N
CH
2
H
3
CCH CH
3
CH
3
Hydroxyatrazine
H
2
O
+
HCl
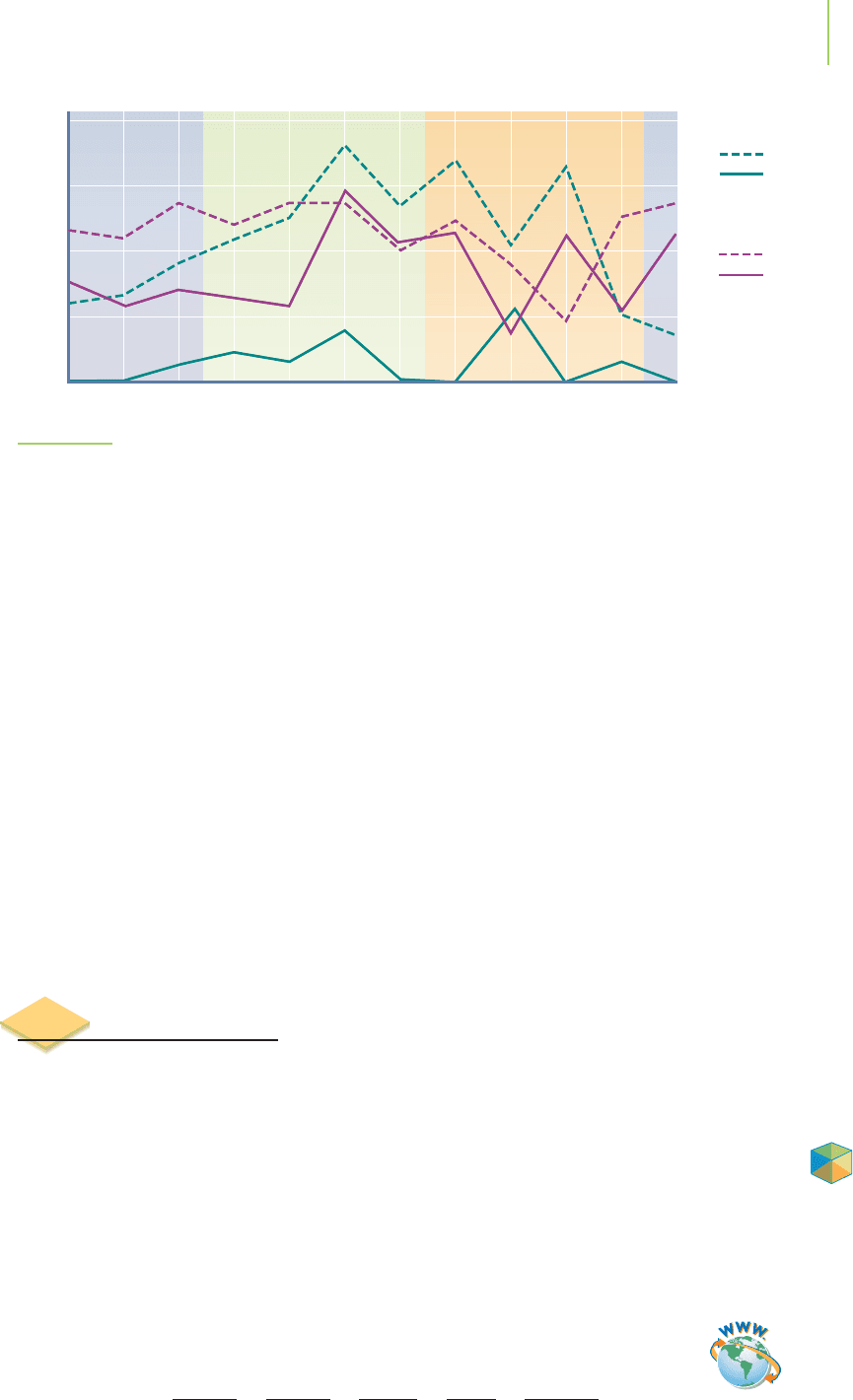
15.1 Reaction Rates 621
concentration of atrazine in soil can decline by half in less than a month, though
about 60 days is typical. However, in natural water samples, atrazine typically de-
grades to one-half of its original concentration more slowly, in about 400 days. Be-
cause its degradation is relatively slow, atrazine is present in many water samples
throughout the year, as shown in Figure 15.1. Laboratory studies, however, indicate
that the hydrolysis of atrazine is spontaneous (∆G < 0 kJ/mol) and exothermic
(∆H =−35 kJ/mol).
Does it make sense for a process to be so slow, compared to (for exam-
ple) the combustion of propane in a propane torch, even if it is spontaneous?
As we noted in
Chapter 14, the answer is a resounding yes. Values for free energy, enthalpy, and en-
tropy are useful only in determining the thermodynamic properties of a reaction
(that is, whether the reaction is spontaneous). The spontaneity of a reaction does not
indicate anything about its rate. In order for us to determine how quickly the reac-
tion occurs, we have to examine
chemical kinetics—the study of the rates and mecha-
nisms of chemical reactions, including the factors that influence these properties. To
begin our study of chemical kinetics, we will briefly leave the farm fields and travel to
the Olympic Games.
Frequency of detection (%)
Jan.
0
10
20
30
40
Feb. Mar. Apr. May June July Aug. Sept. Oct. Nov. Dec.
Lafayette Creek—Urban basin
Herbicides
Pesticides
Little River —Agricultural basin
Herbicides
Pesticides
The frequency of pesticide detections in
a stream draining an agricultural basin
was related to the agricultural cycle.
FIGURE 15.1
Atrazine and other herbicides persist in the environment long after they are first applied.
15.1 Reaction Rates
The Olympic Games rely heavily on the use of accurate timekeeping. In the bob-
sled and luge events, such as that shown in Figure 15.2, the accuracy of the time-
keeping determines whether an athlete or team wins a gold or no medal at all. For
example, at the 1998 Winter Olympic Games in Nagano, Japan, Silke Kraushaar
of Germany placed first in the women’s luge with a time of 50.617 s for her best
run. The silver medal went to Barbara Niedernhuber, also from Germany, with a
time of 50.625 s. The difference between these times is very small.
Let’s examine the luge event more closely to help us introduce some new
terms related to kinetics, the main topic of this chapter. The women’s luge course
at Nagano in 1998 was 1194 m long. Judging on the basis of her winning time,
how fast did Silke Kraushaar travel? Speed is calculated by dividing the distance
traveled by the change in time. Kraushaar traveled 1194 m in 50.617 s, so her
rate
of travel (speed) was 84.92 km/h (52.77 mi/h).
Speed =
distance
time
=
1194 m
50.617 s
×
1km
1000 m
×
3600 s
h
=
84.92 km
h
Application
Tutorial: Reaction Rate and
Concentration

But top speeds for luge and bobsled events routinely hit
135 km/h (84 mi/h)! What we’ve calculated is the
average
rate
of her travel.
Chemical reactions also have rates. Whereas the speed
of a bobsledder can be listed in kilometers per hour (or
miles per hour), the speed of a reaction is often described
in units of concentration, often molarity, per second. For
instance, under certain laboratory conditions, a 0.50 M
solution of the herbicide atrazine can be completely hy-
drolyzed in 24 h. We can calculate the rate of the reaction
by dividing the concentration that is consumed in the reaction by the time it took
to complete the hydrolysis.
Average rate =
0.50 M
24 h
×
1h
3600 s
= 5.8 ×10
−6
M/s
We can say that the rate of this reaction for the 24 h period is 5.8
×
10
–6
M/s.
Determining the average rate of a reaction is one of the tasks accomplished in
chemical kinetics. However, when we study kinetics, we have to be careful to
note the difference between the extent of a reaction and the rate of the reaction.
The extent of a reaction (which we’ll cover in Chapter 16) is a measure of the
completeness of a reaction. The rate of a reaction describes how quickly it gets to
that point. For example, the combustion of methane to make the flame on your
cooking stove is an essentially complete reaction that is also relatively fast. The
oxidation of iron on a suspension bridge is also complete, but it is quite slow.
Kinetics deals only with “how fast or slow and by what route.” Kinetics tells us noth-
ing about the extent of a reaction.
Instantaneous Rate, Initial Rate, and Average Rate
At the 2001 Grand Nationals in Chicago, Whit Bazemore in his Matco Tools
Pontiac Firebird “funny car” finished the quarter-mile drag strip in 4.750 s (Fig-
ure 15.3). Using this information (0.2500 mi in 4.750 s), we can calculate the av-
erage speed of the funny car as 0.05263 mi/s, or 189.5 mi/h. However, the speed
of the funny car at the start was much less than this (0 mi/h), and the speed at the
finish line was a lot faster (323.3 mi/h) than the average speed. The rate of a
chemical reaction changes throughout, much like the rate of travel of a funny car
at a drag race. However, chemical reactions differ because they typically start
622 Chapter 15 Chemical Kinetics
FIGURE 15.2
In many Olympic events, fractions of a second
determine the winners. Here, Armin Zoeggeler of
Italy races at top speed on the luge track in the
2002 Winter Olympics in Salt Lake City, Utah.
Atrazine
rapidly and then slow down with time. In other words, at the start
of a reaction, the rate of chemical change is typically fast. As the re-
action nears its end, the rate is typically slow. Over a given time
period (∆t), though, it has an average rate that describes how long
it took to reach that certain point in the reaction.
Average rate =
concentration
t
In short, just as the speed of a dragster changes during a race, the
rate of the reaction changes as the reaction proceeds.
What if we consider a change in time that is negligible (∆t ≈0)?
When this happens, we are examining the
instantaneous rate of a re-
action. This is what we determine at the finish line in the funny car
race (323.3 mi/h). It is also what we measure as the lights turn green
at the start of the race (0 mi/h). At these points, and at any other
point we pick, we are measuring the instantaneous rate. In a chem-
ical reaction, the rate at the start of a reaction, when the reactant
concentrations are greatest, is important. The instantaneous rate of
the reaction measured at the start is referred to as the
initial rate of
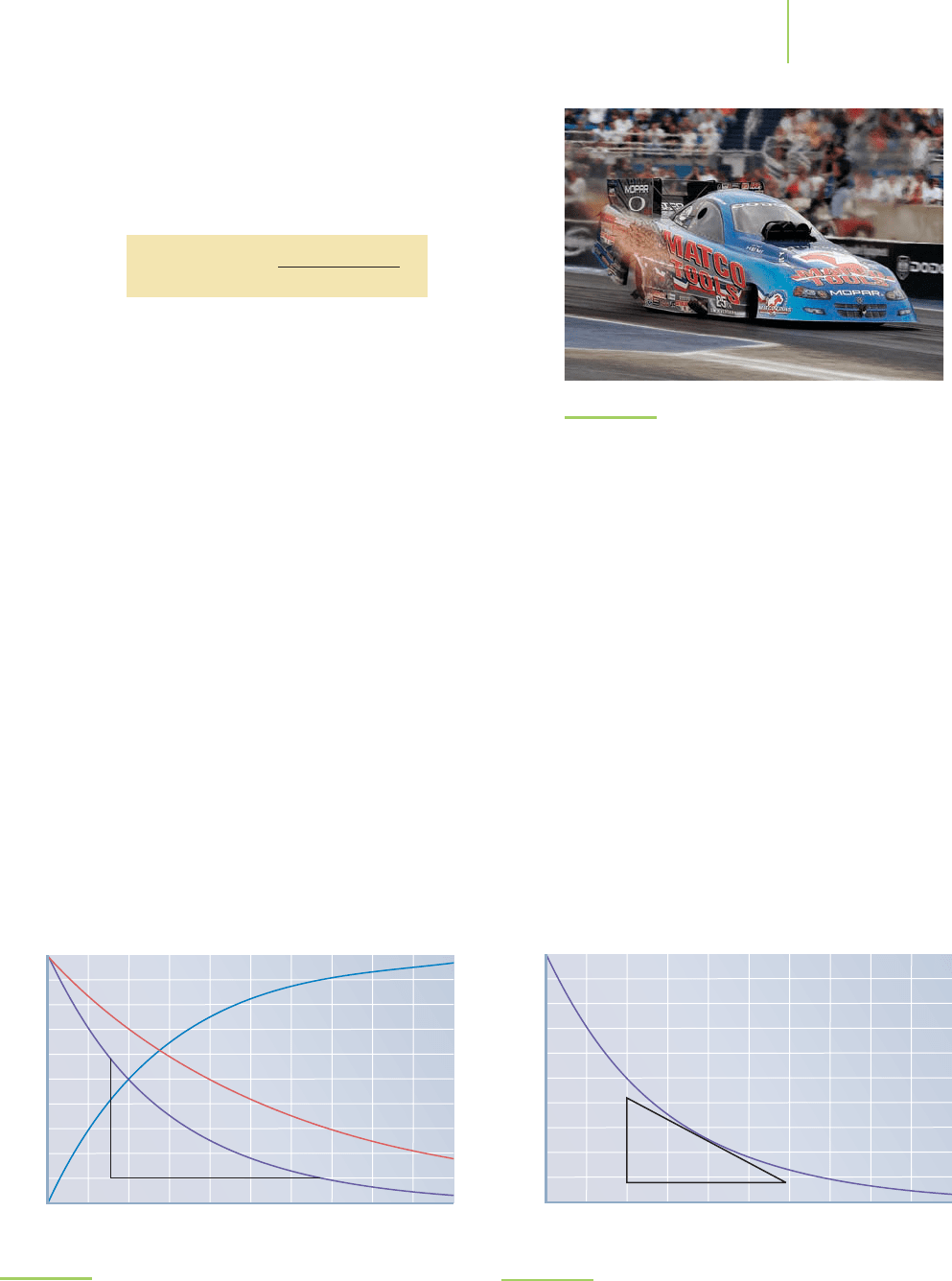
reaction. Instantaneous rates can be measured if we have a plot of the reaction
such as that shown in Figure 15.4. If, on the plot of our reaction, we draw a line
that is tangent to the curve (that is, a line that just touches the curve and is going
in the same direction at that point), as is done in Figure 15.5, and we measure the
slope of that line, we get the instantaneous rate of the reaction at that specific
time. This is a useful way to measure instantaneous rates.
Environmental chemists often measure the rate of a reaction. For example,
the environmental hydrolysis of alachlor (also known as Lasso™, a common her-
bicide) occurs more rapidly than the hydrolysis of atrazine. However, the rate is
still slow enough that new ways to decrease the time that the herbicide spends in
the environment before chemically degrading are being sought. One such way in-
cludes the reduction of alachlor to an acetanilide via electrochemical techniques
such as those that we will discuss in Chapter 19.
15.1 Reaction Rates 623
FIGURE 15.3
“Funny cars,” with their characteristically powerful en-
gines and oversized rear wheels, get ready to race the
quarter-mile. Bazemore powered his Matco Tools Pontiac
Firebird to a national record time of 4.750 s at a track
record speed of 323.27 mph to lead the 16-car field.
Average rate = rise/run
Rise
Run
0 200 400 600 800 1000
Time (seconds)
1200 1400 1600 1800 2000
0
0.01
0.02
0.03
0.04
0.05
0.06
0.07
0.08
0.09
0.1
Concentration (M)
FIGURE 15.4
Calculating the average rate of a reaction from a sample plot of the re-
actant concentration versus time. Average rates and instantaneous rates
differ in the length of time used to calculate them. The average rate of a
reaction is calculated by dividing the rise by the run.
0
0 200 400 600 800 1000
Time (seconds)
1200 1400 1600 1800 2000
0.01
0.02
0.03
0.04
0.05
0.06
0.07
0.08
0.09
0.1
Concentration (M)
Instantaneous rate = rise/run
Rise
Run
FIGURE 15.5
Calculating the instantaneous rate of a reaction from the same
sample plot used to calculate the average rate in Figure 15.5. The
slope of a tangent line can be used to find an instantaneous rate.
Alachlor
The rate of this reaction can be determined in the laboratory by measuring
the change in the concentration of one of the compounds over a period of time.
Mathematically, the rate of the reaction is equal to the rate of the disappearance
of alachlor, the rate of appearance of the acetanilide, or the rate of the ap-
pearance of Cl
–
, because they are all in a 1-to-1 mole ratio. The sign placed
in the equation is used to indicate whether the compound being measured
is disappearing (minus sign) or appearing (plus sign). Remember that the
∆ symbol represents a change, measured by taking the final value minus
the initial value. Even though the sign of the rate for the disappearance
would appear to generate a negative value for the rate, it does not. In fact, reaction
rates are always positive.
■
Rate = rate of disappearance of alachlor =
−[alachlor]
t
■
Rate = rate of disappearance of H
+
=
−[H
+
]
t
■
Rate = rate of appearance of product =
[acetanilide]
t
■
Rate = rate of appearance of Cl
−
=
[Cl
−
]
t
The rate of the decomposition of hydrogen peroxide into water and oxygen
can also be written using these rules. The rate of the reaction could be set equal to
the rate of appearance of oxygen. In this case, the rate is simply the change in its
2H
2
O
2
(aq) n 2H
2
O(l) + O
2
(g)
concentration divided by the change in time. However, if we consider the rate of
appearance of water as a measure of the rate of the reaction, we must somehow note
that two molecules of water are appearing for every molecule of O
2
(g) that is pro-
duced. This is done by dividing the rate by the mole ratio from the balanced
equation. For instance, the rate of appearance of H
2
O times
1 mol O
2
2 mol H
2
O
gives the
rate of the reaction.
624 Chapter 15 Chemical Kinetics
+++
++
Alachlor (Lasso) Acetanilide product
H
+
2e
–
2e
–
+
Cl
–
NO
O
CH
2
CH
2
CH
2
Cl
CH
2
CH
3
CH
3
CH
3
NO
O
CH
2
CH
2
CH
2
CH
3
CH
3
CH
3
CH
3
Our rate descriptions are
■
Rate based on disappearance of H
2
O
2
(g)
=
−[H
2
O
2
]
t
×
1
2
■
Rate based on appearance of O
2
(g)
=
[O
2
]
t
■
Rate based on appearance of H
2
O(l)
=
[H
2
O]
t
×
1
2
EXERCISE 15.1 Rate of the Haber Process
Farmers apply ammonia to their cornfields during spring planting by injecting
it directly into the soil. The ammonia they use is made by the Haber process;
the chemical reaction for this process is indicated by the equation shown below.
Describe the rate of the reaction in terms of the rate of appearance or disappearance
of the components of the reaction.
N
2
(g) + 3H
2
(g) n 2NH
3
(g)
Solution
The rate of the reaction can be measured by measuring the changes in concentra-
tion of each of the species. Mathematically, the rate of the reaction of N
2
is equal to
one-third the rate of disappearance of H
2
, because 1 mol of N
2
disappears for every
3 mol of H
2
that react. The rate of the reaction of N
2
is also equal to one-half the rate
of appearance of ammonia (NH
3
), because 1 mol of N
2
disappears for every 2 mol of
NH
3
that are produced. Note that the disappearance of H
2
is indicated with a minus
sign and the appearance of NH
3
a plus sign.
Rate =
−
[N
2
]
t
=−
1
3
[H
2
]
t
=
1
2
[NH
3
]
t
PRACTICE 15.1
What is the rate of the following reaction in terms of the rates of disappearance and
appearance of the components of the reaction?
2HI(aq) n H
2
(g) + I
2
(aq)
See Problems 5, 6, 9, 10, and 13.
We can examine this further by actually calculating the average rate of the
reaction. Assume that an environmental chemist begins an experiment with a
0.400 M alachlor solution. After 10 days, the concentration of alachlor is deter-
mined to be 0.350 M, and the concentration of acetanilide and that of chloride
are both 0.050 M. What is the average rate of the reaction? We know the final
value (0.350 M alachlor at 10 days) and the initial value (0.400 M alachlor at
0 days). Although time can be used with almost any unit, we often report rates as
M/s. So, we convert the time into seconds (10 days
= 864,000 s) and fill in our
equations, keeping in mind that the rate measures the change in concentration
per unit change in time. In this example, however, we don’t know the concentra-
tion of H
+
at either time, so we can’t use it to determine the rate of the reaction.
Note: It would be perfectly fine to report rates with units of M/day, M/hr, etc.
■
Rate =disappearance of alachlor
=
−
(
0.350 M − 0.400 M
)
(864,000 s −0s)
= 5.79 × 10
−8
M/s
15.1 Reaction Rates 625
0
0.5
1.0
1.5
2.0
2.5
3.0
3.5
0 1020304050
Time (minutes)
60 70 80 90 100
Concentration (M)
Instantaneous rate at 18 minutes
Instantaneous rate at 67 minutes
■
Rate =appearance of acetanilide
=
(
0.050 M − 0.000 M
)
(
864,000 s −0s
)
= 5.79 × 10
−8
M/s
■
Rate =appearance of Cl
–
=
(
0.050 M − 0.000 M
)
(
864,000 s −0s
)
= 5.79 × 10
−8
M/s
Note that no matter which calculation we complete, we should always obtain
the same rate of reaction because of the 1-to-1-to-1 mole ratio of reactants and
products.
Remember that average rates, instantaneous rates, and initial rates are calcu-
lated in the same manner. The only difference is that the length of the time period
is different. Long changes in time determine the average rate over those long
changes. Instantaneous rates are measured when the change in time (∆t) is very
small (that is, ∆t n 0). Experimentally, average rates, instantaneous rates, and
initial rates can be found by measuring the color, temperature, electrochemical
voltage (Chapter 19), or some other physical property of the reactants or prod-
ucts that changes over time. Then by determining the slope of a line that is tan-
gent to the curve drawn when the concentrations of reactants or products are
plotted against time, the rate can be obtained. Initial rates are measured as
instantaneous rates at the start of the reaction.
EXERCISE 15.2 The Rate of a Reaction
Methoxychlor is an important insecticide used to control parasites on livestock and
a variety of pests on vegetables and fruits. Its breakdown by soil microbes may pro-
ceed through the following reaction. Calculate the average rate of this reaction in
M/s of methoxychlor, assuming that the concentration of CH
3
OH starts at 0.000 M
and after 60.0 h climbs to 0.100 M.
2H
2
O 2CH
3
OH
H
3
CO CH
CCl
3
OCH
3
HO CH
CCl
3
OH
Methoxychlor bis-Hydroxy
methoxychlor
626 Chapter 15 Chemical Kinetics
The slope of a line that is tangent to the plot
of concentration versus time for a reaction
gives the instantaneous rate of the reaction.
Instantaneous rates can be measured at any
specific time.
First Thoughts
The equation that describes this reaction includes two starting materials and two
types of product molecules. We could use any of these compounds to determine the
average rate of the reaction, but the question asks us to work with the formation of
methanol to determine the rate. By examining the balanced equation, we see that
the rate of methanol formation is two times the rate of the reaction of methoxy-
chlor. That is, 2 mol of methanol are formed for every mole of methoxychlor that is
consumed.
Solution
The rate of the reaction can be determined on the basis of the rate of appearance of
methanol (CH
3
OH). Because methanol is forming at twice the rate of the reaction
of methoxychlor, the rate of the reaction of methoxychlor is one-half the rate of
appearance of methanol.
Rate =
1
2
[CH
3
OH]
t
=
1
2
(
0.100 M − 0.00 M
)
(
216,000 s −0.00 s
)
=
1
2
0.100 M
216,000 s
= 2.31 ×10
−7
M/s
Further Insights
The rate we have calculated in this problem is an average rate of reaction, which can
be determined by examining the length of time that was used to measure the rate
over this time period. To measure the initial rate or the instantaneous rate of the re-
action, we would need to measure the concentrations of methanol (or another of
the compounds in the equation) during a much shorter reaction time. Currently,
rates based on time changes on the order of femtoseconds (10
−15
) can be determined
with laser-based measurement techniques.
PRACTICE 15.2
Calculate the average rate of the Haber process (Exercise 15.1), assuming that at 12.5 s
the concentration of H
2
(g) was 0.355 M and at 83.3 s the concentration was 0.258 M.
See Problems 7 and 8.
15.2 An Introduction to Rate Laws
We pointed out in the introduction to this chapter that under certain conditions,
atrazine can degrade to one-half of its original concentration in 60 days. This
means that in a sample containing 5.0
×
10
–7
M atrazine, the concentration re-
duces to 2.5
×
10
–7
M after 60 days. We can calculate the rate of this reaction
using the concepts we learned in Section 15.1.
Can we predict the concentration of
atrazine after 120 days?
This question highlights a complicating factor in kinetics:
The rate of a reaction typically decreases as the reaction progresses, because fewer
reactant molecules exist after the reaction begins. The lower concentration of reac-
tant reduces the likelihood that molecules will interact to make products. As a
result, determining concentrations of reactants and products at a certain time
during a reaction requires greater understanding of how the reaction rate
changes with time. In the end, we cannot say that the concentration of atrazine
should be 0 M after 120 days (see Figure 15.6). Similarly, we cannot say that
the concentration is 3.75
×
10
–7
M after only 30 days.
The decomposition of hydrogen peroxide can be used to illustrate how the
rate of a reaction can be related to the reaction conditions. Hydrogen peroxide, a
common staple in the home medicine cabinet, is used to clean cuts and scrapes
15.2 An Introduction to Rate Laws 627
Application
Video Lesson: An Introduction
to Reaction Rates
Visualization: Reaction Rate and
Concentration
