Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

EXERCISE 15.5 How Long Will It Take?
Diazinon crystals are sprinkled around a home’s foundation to kill and repel ants
from the home. How long will it take for the diazinon to decompose, assuming first-
order kinetics, to 25% of its original concentration in the soil? (From Table 15.2, the
half-life of diazinon is 4.0 × 10
1
days.)
First Thoughts
The problem doesn’t state the original concentration of the diazinon in the soil.
However, we don’t need to know the concentration to determine the answer, be-
cause we have the ratio of initial concentration to final concentration (that is,
[diazinon]
t
=0.25[diazinon]
0
).
Solution
Using the half-life, we first calculate the rate constant for the reaction:
t
1/2
=
0.693
k
4.0 ×10
1
days =
0.693
k
k = 0.0173 day
−1
Note that the unit of the rate constant is reciprocal days and that we retain an extra
figure in the value because we will use the data in the next part of the calculation. If
25% of the diazinon remains, then
ln
0.25[diazinon]
0
[diazinon]
0
=−(0.0173 day
−1
)t
ln 0.25 =−0.0173 day
−1
t
−1.386 =−0.0173 day
−1
t
t = 8.0 × 10
1
days
Further Insight
There is an alternative method by which we can calculate the answer. One half-life
reduces the original concentration by 50% (50% = 0.5 =
1
⁄2). Two half-lives reduce
the concentration to 25% (
1
⁄2
×
1
⁄2 =
1
⁄4). Three reduce the concentration to 12.5%
(
1
⁄2
×
1
⁄2
×
1
⁄2 =
1
⁄8). Because we want to know the time required for the reaction to
reduce the concentration of starting material to 25% of the initial concentra-
tion, we need two half-lives (4.0
×
10
1
days + 4.0
×
10
1
days =8.0
×
10
1
days).
Therefore,for a first-order process, the fraction remaining, [A]
t
/[A]
0
equals 2
−n
,
where n
= number of half-lives. For example, for n = 3 half-lives, [A]
t
/[A]
0
=
2
−3
or 0.125.
PRACTICE 15.5
What is the length of time you would have to wait for alachlor to decompose to 25%
of its original value? ...to 6.25%? ...to 0.001%?
See Problems 27 and 28.
Other Rate Laws
Not all reactions follow first-order kinetics. Other orders do exist, even noninte-
ger orders. Reactions that take place on metal surfaces typically follow zero-order
kinetics. Hydrogenation of vegetable oils, the decomposition of ammonia on a
tungsten wire, and the reaction of N
2
O with oxygen in your car’s catalytic con-
verter are reactions on metal surfaces. They follow zero-order kinetics.
638 Chapter 15 Chemical Kinetics
Diazinon
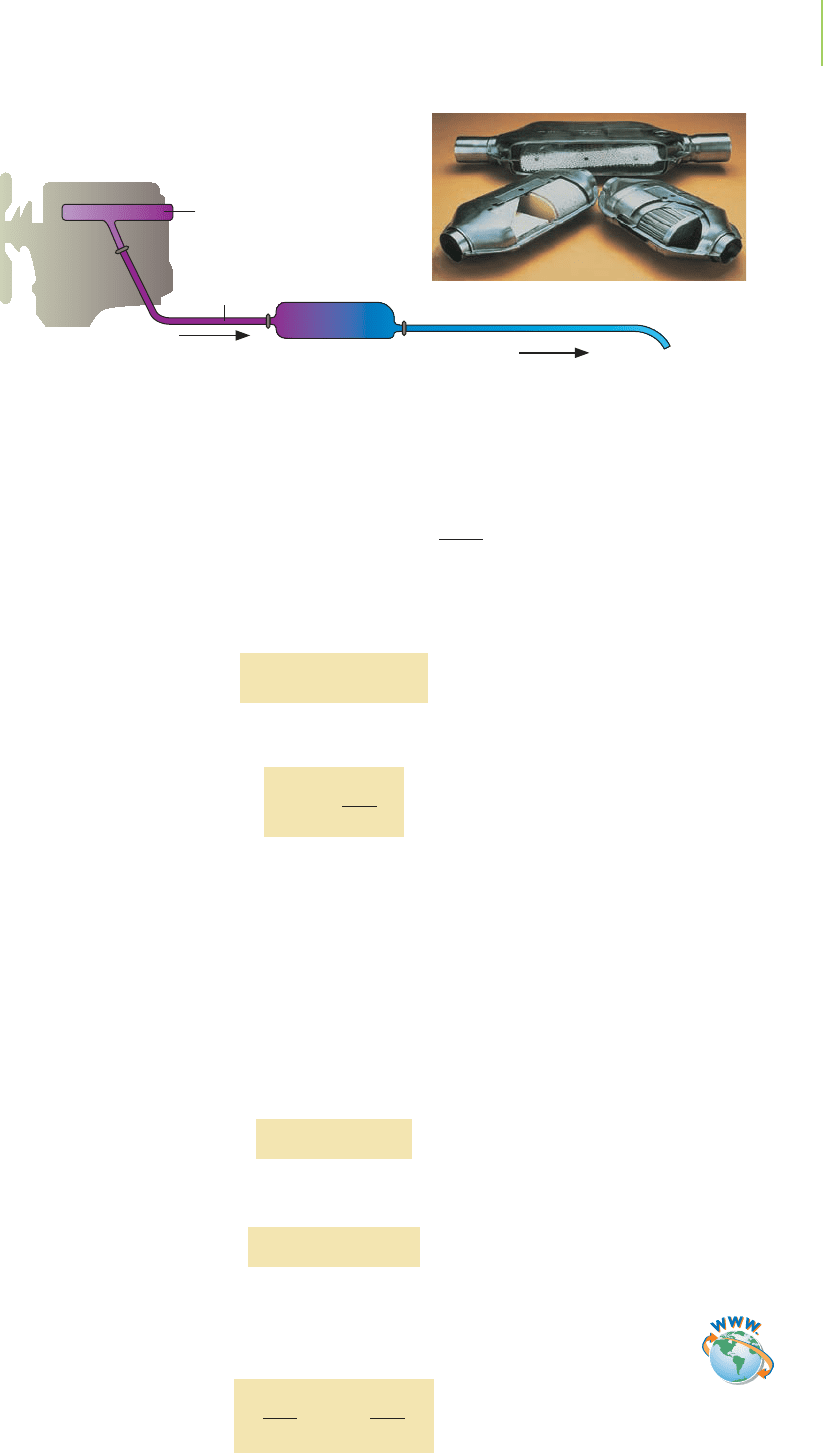
In a zero-order reaction, the rate law does not depend on the concentration of
any of the compounds in the reaction. The rate of the zero-order reaction is con-
stant. No matter what the original concentration happens to be, the reaction
always proceeds at the same rate.
Rate of disappearance of A =−
[A]
t
Rate = k[A]
0
= k
The integrated zero-order rate law, determined by integrating the rate law over
time, for a zero-order reaction is different from the integrated first-order rate law:
[A]
t
=−kt + [A]
0
Substituting 0.5[A]
0
for the concentration of A at time t, we can rearrange the
equation to get the formula for the half-life of the zero-order reaction:
t
1/2
=
[A]
0
2k
The half-life of a zero-order reaction directly depends on the initial concentra-
tion of the reactant. Larger concentrations of the reactant will mean a larger half-
life for the reaction. Note that the rate of a zero-order reaction is constant but that
the half-life depends on the initial concentration of reactant. For example, con-
sider a zero-order reaction where the rate constant k = 2.5
×
10
−4
M/s. When
[A]
0
=1.00 M, the half-life of the reaction is 2000 s. If [A]
0
=0.25 M, the half-life
of the reaction is 500 s. The rate of the reaction (rate = k) is a constant, but the
half-life changes as the concentration changes.
The
second-order rate law can be more complicated, because there are two
cases that fit the definition of a second-order reaction. In one of those cases, the
reaction is second order in only one reactant:
Rate = k[A]
2
In the other case involving a second-order rate law, the reaction could be first
order in two different species:
Rate = k[A][B]
In second-order reactions with only one reactant, the integrated rate law is
determined using the method we have explored for the zero-order and first-order
reactions. After integration and rearrangement, the
integrated second-order rate
law
takes the form
1
[A]
t
= kt +
1
[A]
0
15.3 Changes in Time—The Integrated Rate Law 639
Exhaust
manifold
Exhaust
pipe
Tailpipe
Catalytic
converter
CO, NO, O
2,
N
2
O
,
NO
2
CO
2
, N
2
Catalytic converters
catalyze the reaction
of N
2
O into N
2
.The
reaction is zero order.
Video Lesson: Second-Order
Reactions

The half-life of this type of second-order reaction can be determined by assum-
ing that the concentration of A at some time, t, is equal to one-half the initial con-
centration ([A]
t
= 0.5[A]
0
). Simplifying the equation gives
t
1/2
=
1
k[A]
0
The half-life for a second-order reaction (involving only one compound in the rate
law) is inversely dependent on the initial concentration of the reactant. Smaller ini-
tial concentrations of the reactant will mean a longer half-life for the reaction.
If the second-order reaction contains two species in the rate law, the inte-
grated rate law becomes much more complicated. In fact, the math gets so com-
plicated that chemists typically manipulate the experimental conditions to reduce
the amount of calculation. Consider the reaction of one of the components of
smog with ozone:
NO(g) + O
3
(g) n NO
2
(g) + O
2
(g)
Rate = k[NO][O
3
]
The reaction is first order in NO, first order in O
3
, and second order overall.
To calculate the concentration of a reactant, determine the rate constant, or
find the time required to reach a certain concentration with this type of rate law,
we conduct the reaction with a relatively small concentration of one of the reac-
tants and a very large concentration of the other.
What effect does this have on the
rate law?
Because one of the concentrations is very large, any change in its con-
centration is negligible, and we can assume that its concentration remains constant
through the course of the reaction. An analogy is having someone who is rich spend
$1 in a day (decreasing to 50 cents after one half-life!) from a fortune of $1 bil-
lion. The change is hardly noticeable. The fortune is essentially constant.
Someone who had only $10, however, would notice the effect of spending $1 im-
mediately. Having the concentration of one component much larger than that of
the other reduces the rate law to a much simpler form. For example, if we per-
form the reaction with 0.100 M NO and 0.001 M O
3
, the concentrations of the
species after the reaction is complete can be determined:
[O
3
]
f
= 0.001 M − 0.001 M = 0.000 M
[NO]
f
= 0.100 M − 0.001 M = 0.099 M ≈ 0.100 M
The O
3
is consumed in the reaction, but the concentration of NO is only slightly
affected. We can make the assumption that the concentration doesn’t change.
Because the final concentration of NO has essentially remained constant, it can
be combined with the rate constant.And the rate equation for the reaction can be
reduced to
Rate = k [NO][O
3
] = k [NO]
0
[O
3
]
and, because k = k [NO],
Rate = k [O
3
]
where k is the new rate constant. What is the order of our new rate law? Because
of our choice of the initial concentrations, the kinetics for this reaction has taken
on the form of a first-order rate law. Because of our modification, we say that this
is a
pseudo-first-order rate equation.
We can do this manipulation with any reaction. By increasing the concentra-
tion of a particular reactant to a very large value, any order of a rate law can be
simplified to a pseudo-first order rate equation. This enables us to study reactions
no matter what order they appear to be. We must remember, however, that any
modification of the rate equation means that the new rate constant is not the
same rate constant as in the original reaction.
640 Chapter 15 Chemical Kinetics
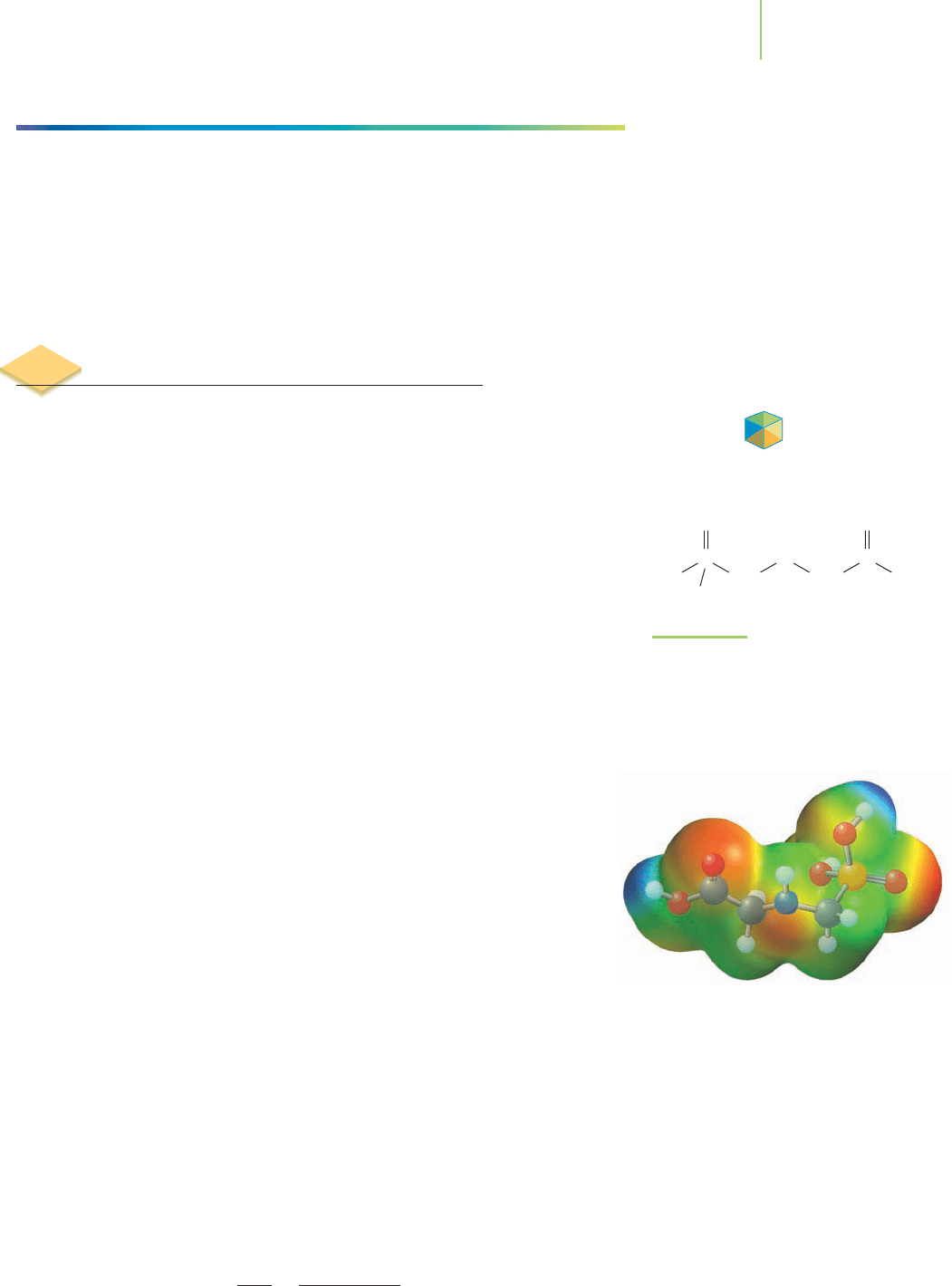
15.4 Methods of Determining Rate Laws 641
H
N
CH
2
P
CH
2
O
HO
C
OH
O
HO
FIGURE 15.13
Roundup herbicide contains glyphosate.
The general herbicide is sold for use on
farms and in removing unwanted weeds
around the yard.
HERE’S WHAT WE KNOW SO FAR
■
The integrated rate laws enable researchers to calculate one of four things
about a reaction (rate, rate constant, initial concentration, or final concentra-
tion) if three of these are known.
■
The half-life of a reaction indicates the time required for the reaction to reach
50% completion.
■
Modifying a second-order reaction by using a large concentration of one of
the reactants reduces the integrated rate law to a pseudo-first-order rate law.
15.4 Methods of Determining Rate Laws
Glyphosate herbicides are absorbed through the foliage of weeds, inhibiting a
plant’s ability to make new amino acids. Because these herbicides react relatively
quickly with water (
t
1/2
< 7
days), the rate of their biological reaction in plants is
important. Weed scientists study the rates of amino acid inhibition and environ-
mental degradation in order to suggest modifications to improve glyphosate
effectiveness (Figure 15.13). The greatest benefit of the glyphosate herbicides is
attributable to their rapid degradation in the environment. The rapid degrada-
tion means this class of herbicide causes less harm to the environment than other
herbicides. The rates of the degradation of these compounds have been measured
by experiment, and the overall rate laws have been determined from those
experiments.
We, too, can use the information from a chemical reaction to obtain the final
rate law for a reaction. The first of two commonly used procedures that we will
consider is the
method of initial rates. In this appraoch, we measure and compare
initial rates rather than comparing instantaneous rates later in the reaction. We
use initial rates to determine the rate law because we can precisely measure and
control the starting concentrations of the reactants and precisely identify the
time for the reaction to reach a given point. In addition, limited side reactions
(reactions that make products other than what we’re interested in), rapid deter-
mination of the rate, and a well-defined time period make the comparison of
multiple reactions experimentally straightforward.
We’ll use as our example the reaction of nitrogen monoxide, NO(g), with
oxygen to make nitrogen dioxide, NO
2
(g), a component of smog. We can
measure the initial rate of this reaction as we change the concentrations of
both NO(g) and O
2
(g). We conduct three separate reactions with different
concentrations of reactants, measure the initial rate, and complete the table
on the next page. Note that we have carefully chosen our starting concentra-
tions to keep one reactant the same in two experiments while doubling the
other reactant.
2NO(g) + O
2
(g) n 2NO
2
(g)
We can determine the rate law for this reaction by assuming that the rate is
based solely on the two reactants in the equation. We write
Rate = k[NO]
n
[O
2
]
m
where n and m are the orders of the two reactants. If we compare the rate of the
third reaction to the rate of the second reaction, in which [NO] is constant and
[O
2
] changes, the equation simplifies dramatically. To do this, let’s divide the rate
law for the third experiment into the rate law for the second experiment.
rate
2
rate
3
=
k[NO]
n
[O
2
]
m
k[NO]
n
[O
2
]
m
Application
Glyphosate

642 Chapter 15 Chemical Kinetics
Next, we add the values from the table to the equation. Then we simplify the
equation. Although the value of the rate constant, k, is not known, it should be
the same in both reactions:
1.13 ×10
−1
M·s
−1
5.64 ×10
−2
M·s
−1
=
k(0.0252)
n
(0.0250)
m
k(0.0252)
n
(0.0125)
m
2 =
(0.0250)
m
(0.0125)
m
2 = 2
m
m = 1
Why did we divide the second equation by the third? When one of the reactant
concentrations is held constant ([NO], in this case) and the other concentration
([O
2
]) is doubled, the effect on the rate is due only to the change in [O
2
]. This
effect is related to a power of 2 and reveals the order of the second reactant
(m = 1). To continue, we divide the third rate law by the first because [O
2
] is con-
stant while [NO] changes, so the effect on the rate is due only to the change in [NO].
We substitute concentrations and solve:
5.64 ×10
−2
M·s
−1
1.41 ×10
−2
M·s
−1
=
k(0.0252)
n
(0.0125)
1
k(0.0126)
n
(0.0125)
1
4 =
(0.0252)
n
(0.0126)
n
4 = 2
n
n = 2
We are nearly ready to write the rate law for the reaction because we know the
order of the reactants. We are missing only the rate constant. We can solve for k
by choosing one of the experiments we’ve completed and substituting the con-
centrations into the rate law. Solving for the rate constant, we obtain
Rate = k[NO]
2
[O
2
]
1.41 ×10
−2
= k(0.0126)
2
(0.0125)
k = 7.11 × 10
3
M
−2
·s
−1
The method of initial rates requires that we can obtain and compare the ini-
tial rates of reactions. We need at least three reactions to solve for two unknown
orders. And in every case, we’ve used experimentally determined data to calculate
the rate law for the reaction.
Note that the units for the rate constant are different depending on the order
of the rate law. Specifically, the units are M
−(order −1)
·s
−1
.
Automobile exhaust produces nitrogen compounds
that react with oxygen.
[NO] [O
2
] Initial Rate
Experiment (M)(M)(M/s)
1 0.0126 0.0125 1.41 × 10
−2
2 0.0252 0.0250 1.13 × 10
−1
3 0.0252 0.0125 5.64 × 10
−2
EXERCISE 15.6 Initial Rates
The reaction of Cl
2
with NO occurs at a very rapid pace. Use the data in the table
below to determine the rate law for the reaction. Then calculate the rate constant.
2NO(g) + Cl
2
(g) n 2NOCl(g)
15.4 Methods of Determining Rate Laws 643
Initial Rate
Experiment [NO]
(M) [Cl
2
]
(M)(M/min)
1 0.10 M 0.10 M 0.18
2 0.10 M 0.20 M 0.36
3 0.20 M 0.20 M 1.45
Solution
The rate law for the reaction can be determined by using the method of initial rates.
The overall rate law, based on the reaction, is
Rate = k[NO]
m
[Cl
2
]
n
The order of the reaction with respect to NO is
Rate 3
Rate 2
=
1.45 M·min
−1
0.36 M·min
−1
=
k(0.20)
m
(0.20)
n
k(0.10)
m
(0.20)
n
1.45
0.36
=
(0.20)
m
(0.10)
m
4 = 2
m
m = 2
The order of the reaction with respect to Cl
2
is
Rate 2
Rate 1
=
0.36 M·min
−1
0.18 M·min
−1
=
k(0.10)
m
(0.20)
n
k(0.10)
m
(0.10)
n
0.36
0.18
=
(0.20)
n
(0.10)
n
2 = 2
n
n = 1
The overall rate law and the value for the rate constant can then be calculated. Note
that the rate is still expressed in concentration per unit time. Also note that while the
orders in this rate law equal the coefficients in the equation for this example, this is
not always true. We will deal with this in Section 15.6.
Rate = k[NO]
2
[Cl
2
]
1
k =
0.18
(0.10)
2
(0.10)
= 1.8 ×10
2
M
−2
·min
−1
PRACTICE 15.6
Use the initial rates for the reaction of carbon monoxide (CO) with hemoglobin (Hb)
to determine the rate law and the rate constant.What is the overall order of the reaction?
See Problems 57–60, 66, and 67.
Experiment [Hb] (M) [CO] (M) Initial Rate (M/s)
1 2.21 × 10
−6
M 1.00 × 10
−6
M 0.619 × 10
−6
2 4.42 × 10
−6
M 1.00 × 10
−6
M 1.24 ×10
−6
3 4.42 × 10
−6
M 3.00 × 10
−6
M 3.71 ×10
−6
644 Chapter 15 Chemical Kinetics
The second method for determining the rate law of a reaction has us examine
only one reaction instead of a series of reactions. In this
method of graphical
analysis
(also known as the method of integrated rate laws), we plot how the con-
centration of a reactant changes with time. Although this method does require us
to measure the rate of the reaction as it proceeds over a long time period, little
mathematical manipulation of the data is needed to determine the rate law. Var-
ious versions of the plot can be quickly constructed in order to establish a linear
relationship between time and a measure of concentration that confirms one of
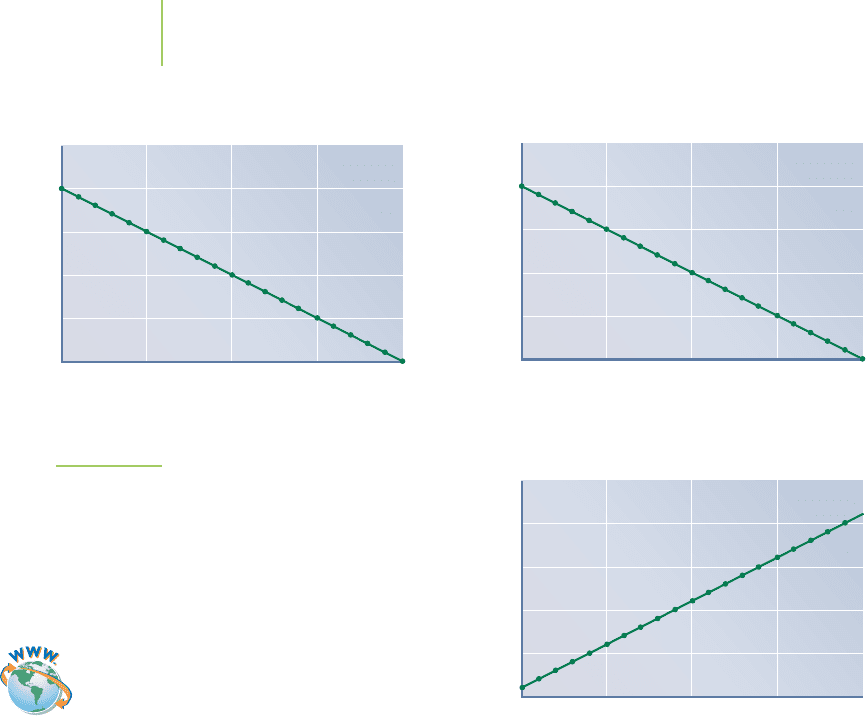
our common rate laws. Figure 15.14 displays plots of zero-order, first-order, and
second-order reactions. Each is a linear relationship that is derived from the inte-
grated rate law. If our data are linear when plotted in one of these ways, it suggests
that the data fit that model. Computer-based data acquisition can also help us to
do statistical analyses to determine the model that best fits the data.
Here are the possible outcomes that we will consider:
■
The zero-order reaction produces a linear plot when the concentration of re-
actant is plotted against time. The slope of the line is equal to –k.
■
The first-order reaction produces a linear plot when the natural logarithm (ln)
of the concentration of reactant is plotted against time. The slope in this case
is also equal to the negative of the rate constant, –k.
■
The second-order reaction produces a linear relationship when the reciprocal
concentration is plotted against time. The slope, which is equal to the rate con-
stant, is positive in this case.
If the concentration of a reactant is followed as a function of time, this is the best
method to use in determining the rate law. By graphing the data in three different
ways, we can determine the overall order of the reaction (zero order, first order,
or second order).
Time (seconds)
Zero-Order Reaction
[A] (M)
0
0.05
0.1
0.15
0.2
0.25
1 6 11 16 21
Slope = –k
Time (seconds)
Slope = –k
Slope = k
Second-Order Reaction
ln[A]
0
0.05
0.1
0.15
0.2
0.25
1 6 11 16 2
1
Time (seconds)
First-Order Reaction
1/[A]
0
0.05
0.1
0.15
0.2
0.25
1 6 11 16 2
1
FIGURE 15.14
A linear relationship exists for the data if time is
plotted versus [A] for zero-order reactions, versus
ln[A] for first-order reactions, and versus 1/[A] for
second-order reactions. Note that the slope of the
line is equal to the negative of the rate constant for
zero-order and first-order plots. The slope of the
second-order plot is equal to the rate constant.
Visualization: Rate Laws
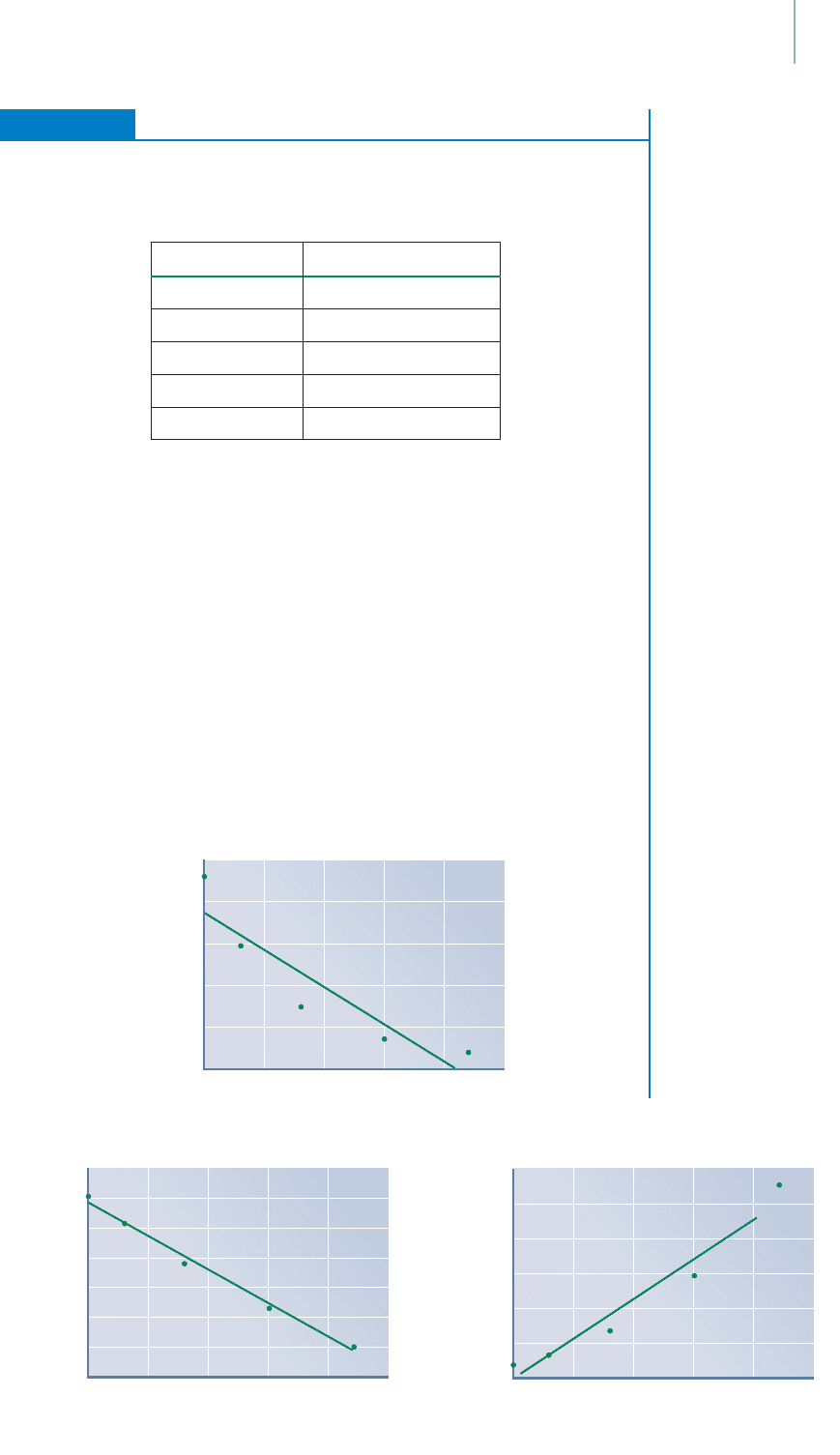
First Thoughts
We must plot the data for each of the three models we have discussed: zero order,
first order, and second order. If might be useful to add two columns to your table for
ln[atrazine] and 1/[atrazine], respectively.
Solution
Using a graphical analysis software package (or three sheets of graph paper), we plot
the data on three different graphs. In the first, the concentration is plotted versus
time, the second relates the ln[atrazine] versus time, and the third illustrates
1/[atrazine] versus time. Examination of the results leads us to the conclusion that
the middle plot—the one that relates first-order kinetics—is the appropriate graph.
We choose this one because the data points seem to lie closest to the line of best fit,
without being systematically curved. The reaction must be first order overall and
first order in atrazine (the reactant).
EXERCISE 15.7 Determining the Reaction Order
The decomposition of atrazine in the presence of titanium dioxide has been studied
and the rate of the reaction measured. Plot the data shown in the table, and deter-
mine whether the reaction follows zero-order, first-order, or second-order kinetics.
15.4 Methods of Determining Rate Laws 645
Time (hours)
First-Order Reaction
ln[n]
–13.0
–12.5
–12.0
–11.5
–11.0
–10.5
–10.0
–9.5
0 5 10 15 20 25
y = –0.1151x – 10.075
R
2
= 0.9913
[n]
Time (hours)
Zero-Order Reaction
5.0 × 10
–5
4.0 × 10
–5
3.0 × 10
–5
2.0 × 10
–5
1.0 × 10
–5
0
0 5 10 15 20 25
y = –2 × 10
–6
x + 4 × 10
–5
R
2
= 0.842
Time (hours)
Second-Order Reaction
0 5 10 15 20 25
1/[n]
3.0 × 10
5
2.5 × 10
5
2.0 × 10
5
1.5 × 10
5
1.0 × 10
5
5.0 × 10
4
0
y = 11261x – 522.33
R
2
= 0.9475
Time (h) [Atrazine] (M)
0 4.65 × 10
−5
3 2.98 × 10
−5
8 1.49 × 10
−5
15 6.98 × 10
−6
22 3.67 × 10
−6
Further Insights
There are a number of mathematical tools we can use to compute the best fit of the
data to one of our reaction orders. One of these tools is the correlation coefficient,
r, which tells how well any two measures are mathematically related to each other. A
perfect directly linear relationship would yield a correlation coefficient, r,of1.As
infants grow, their height and weight give correlation coefficients tending toward 1.
A perfect inverse relationship gives r values of −1. As we increase in age past about
45 years old, we inevitably run more slowly. Age and running speed in older runners
are inversely related and give an r value that is closer to −1. If there is no relation-
ship between variables, such as between human hair color and the number of straw-
berries people eat in July, we get an r value of 0. If a first-order model were to fit our
data, it would have the highest correlation coefficient of the three possible models
that we have introduced.
PRACTICE 15.7
Plot the data for the following reaction as was done in Exercise 15.7. Determine the
overall order of this reaction.You may wish to use a graphing program to determine
which graph gives the straightest line.
2NO
2
(g) n 2NO(g) + O
2
(g)
646 Chapter 15 Chemical Kinetics
Time (s) [NO
2
] (M)
0 0.0100
50 0.0079
100 0.0065
150 0.0055
200 0.0048
300 0.0038
400 0.0031
See Problems 53, 54, 61, 62, 65, and 68.
15.5 Looking Back at Rate Laws
A summary of the data that we have discussed thus far is appropriate. In short,
here’s what we know so far:
■
Reaction rates determine the speed at which a reaction progresses but do not
reveal anything about the extent to which they produce a product.
■
We can measure the average rate if we know the initial and final concentra-
tions over a particular time period.
■
The instantaneous rate is the rate at a given point in the reaction. It can be de-
termined by measuring the concentrations at points when the time difference
approaches zero, or it can be measured by determining the slope of a line
tangent to a plot of the rate versus time.
■
The initial rate is the instantaneous rate as the reaction starts.
■
The rate law is experimentally determined using the method of initial rates or
the method of graphical analysis.
+ +
+ +
HC C
CCH
C
H
CH
3
O
H
2
O
Step 1
Step 2
H
C
CCH
Cytochrome P450
HC C
C
H
H
C
CH
Methoxychlor
CCl
3
OCH
3
HC C
CCH
C
H
HO
CH
3
O
H
H
C
CCH
HC C
C
H
H
C
CH
CCl
3
OCH
3
HC C
CCH
C
H
HO
H
2
O
H
C
CCH
Cytochrome P450
HC C
C
H
H
C
CH
bis-Hydroxy
methox
y
chlor
CCl
3
OCH
3
HC C
CCH
C
H
HO
CH
3
O
H
H
C
CCH
HC C
C
H
H
C
CH
CCl
3
OH
■
The half-life of a reaction is the time required for the concentration of a reac-
tion to reach 50% of the initial value.
■
Complex reaction orders can often be reduced to pseudo-first-order reactions
by keeping the concentration of one of the reactants relatively large.
Specific information for the three rate orders that we have discussed is included
in Table 15.3. There are many more overall orders for reactions than those listed
here.
15.6 Reaction Mechanisms
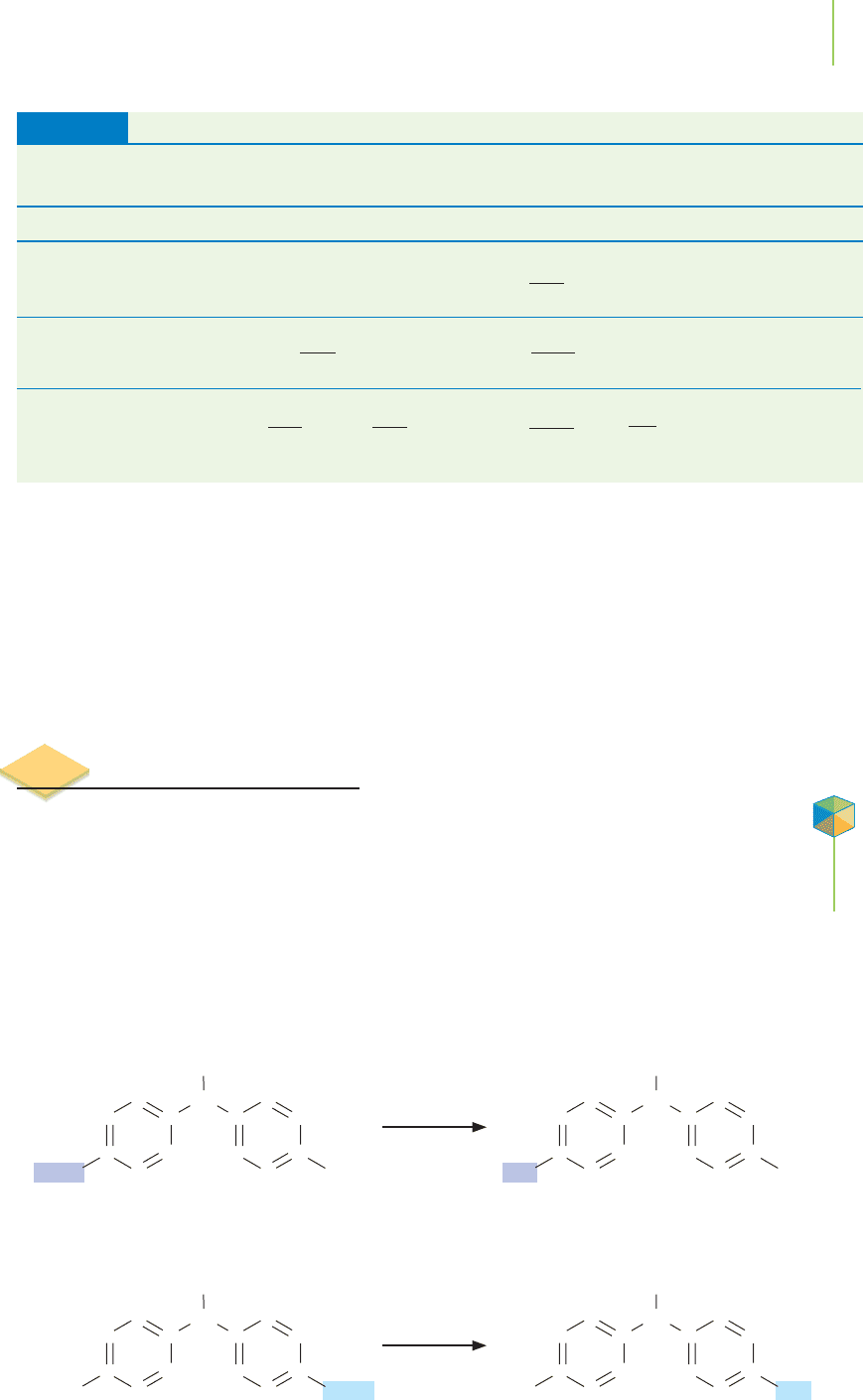
At this point, we need to do some detective work. In Exercise 15.2 we determined
the rate of metabolism of methoxychlor, an organochlorine insecticide, using ac-
tual values for the concentrations. However, upon deeper investigation, chemists
have found that the reaction proceeds in two sequential steps. In the first step, the
methoxychlor undergoes reaction with water and cytochrome P450 (an enzyme
in the liver) to make mono-hydroxymethoxychlor. In a second step, the mono-
hydroxymethoxychlor reacts with another molecule of water and cytochrome
P450 and is converted into the product bis-hydroxymethoxychlor.
15.6 Reaction Mechanisms 647
Rate Laws
The value of the rate is reported in the units of concentration per unit time. The value of the rate constant
is different for each of the three orders (zero order = M/s; first order =1/s; and second order =1/M·s).
Order Rate Law Integrated Rate Law Half-life Linear Plot
Zero Rate = k
[A]
t
=−kt +[A]
0
t
1/2
=
[
A
]
0
2k
[A] versus t slope =−k
First Rate = k[A]
ln
[A]
t
[A]
0
=−kt t
1/2
=
0.693
k
ln[A] versus t slope =−k
Second Rate = k[A]
2
1
[
A
]
t
= kt +
1
[
A
]
0
t
1/2
=
1
k[A]
0
1
[A]
versus
t slope =+k
TABLE 15.3
Application
C
HEMICAL
ENCOUNTERS:
Metabolism of
Methoxychlor
