Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

608 Chapter 14 Thermodynamics: A Look at Why Reactions Happen
ADP ATP
ADP AMP
The change in the free energy of the reaction is zero. Is this reaction sponta-
neous? Is it nonspontaneous? We can look at the phase transition of water from
solid to liquid to help sort all this out.
H
2
O(s) n H
2
O(l)
At temperatures below the melting point of water, the reaction is spontaneous in
the direction of H
2
O(s); that is, water freezes. At temperatures above the melting
point of water, the reaction is spontaneous in the opposite direction; ice melts. At
0°C and 1 atm, the process is not spontaneous in either direction. At this temper-
ature, the change in free energy for the process is zero (G = 0) and the reaction
proceeds neither toward the products nor toward the reactants. We say that the
reaction has reached
equilibrium. We will focus on this important concept in
Chapter 16, but for now, we need to know that a reaction at equilibrium hasn’t
stopped; it is just at a point where moving toward either the reactants or toward
the products is not thermodynamically favored. The reaction continues to make
both products and reactants at equal rates.
Remember that G is temperature-dependent (G = H − TS), so the
value of G can be changed by adjusting the temperature. That is, we can melt ice
or freeze water, depending on the temperature we pick. Active muscles do not
change their temperature in order to drive the production of ATP from ADP, but
this information can be used to calculate the temperature at which a process be-
comes spontaneous. Back to Mt. Everest....The base camp experiences temper-
atures ranging from a high of about –3
o
C in July to a low of about –16
o
C in
January. High up on the slopes of the mountain, the temperature drops even
lower, to around –36
o
C. For trekkers huddled in a tent, it may take a lot of effort
to get a propane stove to light (especially if the temperature drops below the boil-
ing point of propane; see Figure 14.22). How cold must it get before the propane
gas liquefies? To answer this question, we need to consider the phase transition
shown below.
CH
3
CH
2
CH
3
(l) n CH
3
CH
2
CH
3
(g) H = 15.1 kJ/mol
S = 65.4 J/mol
·
K
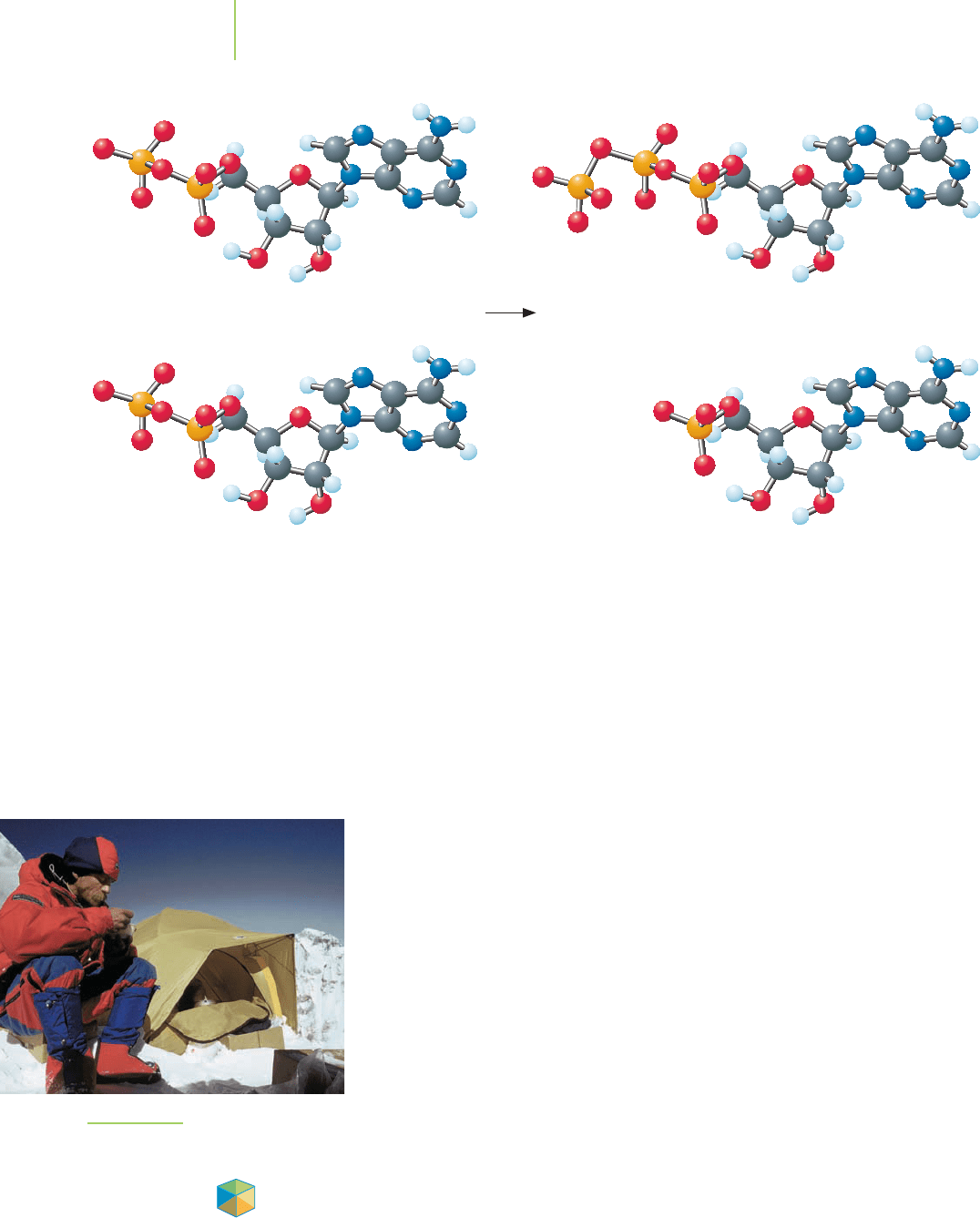
A phosphate group can be transferred from an ADP molecule to make ATP.
FIGURE 14.22
Trying to boil water at a camp on the
Lhotse Face of Mt. Everest (23,500 ft).
Application
When this reaction is at equilibrium, neither the forward nor the backward
reaction will be spontaneous (∆G = 0). The temperature to which this corre-
sponds is the boiling point. Because we know the equation that relates ∆G to the
temperature, we can calculate the boiling point of this reaction.
G = H − T(S)
0 = 15,100 J/mol − T(65.4 J/mol·K)
T(65.4 J/mol·K) = 15,100 J/mol
T = 231 K
T =−42
◦
C
Our calculations suggest that if the temperature falls to −42°C (−44°F), the
change in the free energy of the process will be zero. The reaction will be at equi-
librium, and propane will be at its boiling point. If the temperature gets any lower
than −42°C, the propane will be liquid, and the portable stove will become very
difficult to light.
EXERCISE 14.9 Temperature Dependence of G
In one of the final reactions to produce copper metal from its ore (chalcocite), cop-
per(I) oxide reacts with carbon to produce gaseous carbon monoxide and copper
metal. Above what temperature does the reaction become spontaneous?
Cu
2
O(s) + C(s) n 2Cu(s) + CO(g) H = 59 kJ/mol
S = 165 J/mol
·
K
Solution
G = H − T(S)
0 = 59,000 J/mol − T(165 J/mol·K)
T(171 J/mol·K) = 59,000 J/mol
T = 358 K
T = 85
◦
C
Above 85°C, this reaction becomes spontaneous (G < 0). So, at room tempera-
ture, the reaction doesn’t proceed without outside assistance. In practice, this reac-
tion is often run at very high temperatures to produce copper metal. The copper can
be further purified by electrolytic deposition (see Chapter 19).
PRACTICE 14.9
What is the boiling point of methanol? ...ofwater?
CH
3
OH(l) n CH
3
OH(g)H
2
O(l) n H
2
O(g)
vap
H = 35.27 kJ/mol
vap
H = 40.66 kJ/mol
vap
S = 104.6 J/mol
·
K
vap
S = 109.0 J/mol
·
K
See Problems 67 and 68.
Changes in Pressure Affect Spontaneity
As the temperature changes, the value of G for a reaction changes. In fact, a
nonspontaneous reaction can often be made spontaneous if the temperature is
adjusted enough, such that H – TS becomes negative (see Table 14.5). For a
muscle cell in need of a quick energy fix, changing the temperature in order to
make the conversion of ADP into ATP and AMP (adenosine monophosphate)
spontaneous is not an option. Thankfully, temperature isn’t the only variable that
14.6 When ∆G = 0; A Taste of Equilibrium 609
can change the spontaneity of a reaction. Pressure and concentration also play a
major role in determining reaction spontaneity.
The entropy change of a reaction depends on its pressure. This occurs because
there are fewer microstates in a compressed gas than there are if the pressure of
the same sample is reduced by expanding the volume of the container. Mathe-
matically, it has been shown that the pressure causes a change in the standard free
energy of a reaction in accordance with the following equation:
∆G = ∆G° + RT lnQ
p
where G is the change in the nonstandard state free energy
G° is change in the standard state free energy
R is the universal gas constant, 8.3145 J/mol
·
K
T is the temperature in kelvins
Q
p
is a term called the pressure reaction quotient
We’ll discuss the pressure reaction quotient in much greater detail in Chapter 16.
However, for purposes of our current discussion, you may consider the pressure
reaction quotient, Q
p
,to bethe ratio of the pressures of all of the gaseous products
to the pressures of the gaseous reactants, raised to their respective stoichiometric
coefficients. For example, here is the equation for the Haber process for the
formation of ammonia (part 2 of Exercise 14.5), along with the expression for
its Q
p
:
3H
2
(g) + N
2
(g) n 2NH
3
(g)
Q
p
=
P
2
NH
3
P
N
2
P
3
H
2
For a reaction such as the fermentation of glucose, Q
p
is equal to the pressure of
carbon dioxide squared, because neither glucose nor ethanol is represented as a
gas in the equation.
C
6
H
12
O
6
(s) n 2C
2
H
6
O(l) + 2CO
2
(g)
Glucose Ethanol
Q
p
=
P
2
CO
2
1
The brewmaster at a local brewpub knows about the effect of pressure on
the fermentation of glucose. If the vat is left open to the atmosphere (1 atm), the
yeast carries out the fermentation at 25
o
C. The reaction, as we saw earlier in this
chapter, is spontaneous (–229 kJ/mol). If the brewmeister shuts the hatch on
the vat and seals it, the formation of carbon dioxide begins to build up pressure
as the glucose is catabolized. At some point, the pressure could reach 52.0 atm
(assuming the vat is strong enough to hold that pressure). Is the reaction sponta-
neous at that pressure?
G = G° + RT lnQ
p
G =−229,000 J/mol + (8.3145 J/mol
·
K)(298 K) ln
(52.0)
2
1
G =−229,000 J/mol + 19,580 J/mol
G =−209,420 J/mol
G =−209 kJ/mol
The reaction is still spontaneous, as you might expect for a reaction that produces
energy for a living organism. However, the reaction has a lower free energy than
it had at lower pressure.
610 Chapter 14 Thermodynamics: A Look at Why Reactions Happen
Application
Changes in Concentrations Affect Spontaneity
In a manner similar to that for gaseous reactions, the free energy of a reaction
changes as the concentrations of the reactants and products change.
Does this
make sense?
The equation relating the concentrations of reactants and products
to the observed free energy change (G) is
G = G° + RT lnQ
Note that this equation is mathematically similar to the equation for determining
the effect of changing pressure.
The greatest difference between this equation and the one presented in the
previous section is the value of the
reaction quotient (Q). For reactions in solution,
this reaction quotient is calculated by multiplying the initial molar concentra-
tions of all of the products, raised to the power of their respective stoichiometric
coefficients, divided by the initial molar concentrations of all of the reactants,
raised to the power of their stoichiometric coefficients. Mathematically, for the
reaction
rA + sB n tC + uD
the reaction quotient is
Q =
[C]
t
0
[D]
u
0
[A]
r
0
[B]
s
0
where [ ]
0
= the initial molar concentration of the substance.
What does this mean? Reactions with large concentrations of reactants have
a Q value less than 1. This means that RTlnQ is negative and that the observed
free energy (∆G) is less than the standard free energy (G°). In other words, large
concentrations of reactants and small concentrations of products increase
the spontaneity of the forward reaction (this situation decreases the value of the
observed ∆G). When the concentration of reactants is small and the concentra-
tion of products is large (Q > 1.0), the value of RT lnQ is greater than zero and the
observed ∆G is more positive than G°. In this case, the reaction is less sponta-
neous. Again, we will examine a similar relationship in much greater detail in
Chapter 16 and will explore more deeply what happens when G
= G°, the
equilibrium condition.
Coupled Reactions
Many reactions, such as the formation of glucose-6-phosphate from glucose, are
nonspontaneous. Metabolic sequences have developed to account for this. As
we’ve seen in this chapter, biological reactions are often coupled with other reac-
tions to produce a spontaneous process. Organisms typically couple a reaction
that has a very large negative ∆G with reactions that have positive free energy
changes, as shown in Figure 14.23. The result is a spontaneous reaction.
In glycolysis, there are four coupled reactions. Two of these reactions use the
negative free energy change of ATP
n ADP (∆G = –30.5 kJ/mol) to place phos-
phate groups on a glucose molecule before it is broken down. Then, as the resulting
carbohydrate is catabolized, the organism uses the stored energy of the carbohy-
drate to drive the reverse of this reaction (ADP
n ATP; ∆G = +30.5 kJ/mol). In
doing so, the glycolytic pathway consumes two molecules of ATP but produces
four molecules of ATP using the stored energy of the carbohydrate. The net result
is the accumulation of two molecules of ATP for each glucose molecule.
In this chapter we have examined why chemical reactions proceed and have
shown that it is directly related to an increase in the entropy of the universe. We
14.6 When ∆G = 0; A Taste of Equilibrium 611
ADP
Nonspontaneous
reaction
AB
ATP
Spontaneous
reaction
AB
FIGURE 14.23
Biochemical reactions typically involve
the coupling of a spontaneous reaction
with a nonspontaneous reaction. Coup-
ling the reactions makes the nonsponta-
neous reaction spontaneous.
Application

Motor
Elevator
Counterweight
612 Chapter 14 Thermodynamics: A Look at Why Reactions Happen
The Bottom Line
■
The multiplicity of a system can be used to deter-
mine the behavior of the system. The most probable
macrostate is the one that has the most contributing
microstates. (Section 14.1)
■
Spontaneous processes occur without outside assis-
tance. The reverse of the spontaneous process is non-
spontaneous. (Section 14.2)
■
Entropy is a measure of how energy and matter can
be distributed in a chemical system. Entropy is not
disorder. (Section 14.2)
■
The second law of thermodynamics says that the en-
tropy of the universe (as we defined the term uni-
verse in Chapter 5) continues to increase. Any process
that is spontaneous must correspond to an increase
in the entropy of the universe (∆S
universe
> 0).
(Section 14.2)
■
The third law of thermodynamics says that the en-
tropy of a pure perfect crystalline material is zero.
This law enables us to calculate the entropy of any
compound in any state. (Section 14.4)
■
The free energy, ∆G, can be calculated via the Gibbs
equation (∆G
= ∆H – T∆S) to determine the spon-
taneity of a process. (Section 14.5)
■
When ∆G = 0, the system is at equilibrium. The for-
ward and reverse reactions still proceed, but their
rates are equal. (Section 14.6)
■
Coupled reactions are used by the body to make non-
spontaneous processes become spontaneous. The
use of ATP in this manner assists in the overall pro-
duction of energy from glucose. (Section 14.6)
routinely consider the spontaneity of a reaction as a function of the values of ∆H,
∆S, and reaction conditions such as temperature, pressure, and concentration.
We’ve noted that the free energy change (∆G) depends on all of these things.
Although we now know whether a reaction will proceed, we haven’t discussed
how fast reactions proceed. We know that the production of energy from glucose
is a spontaneous process, as is the rusting of a nail. But the rates of these two re-
actions are quite different, with important effects on us and our surroundings. In
the next chapter, we’ll discover that the degree of spontaneity implies nothing
about the speed of a reaction.
Elevators are raised by a motor. To
facilitate the motion of the elevator, a
counterweight helps pull the elevator up.
The motor and the counterweight couple
their action to produce a working
elevator.
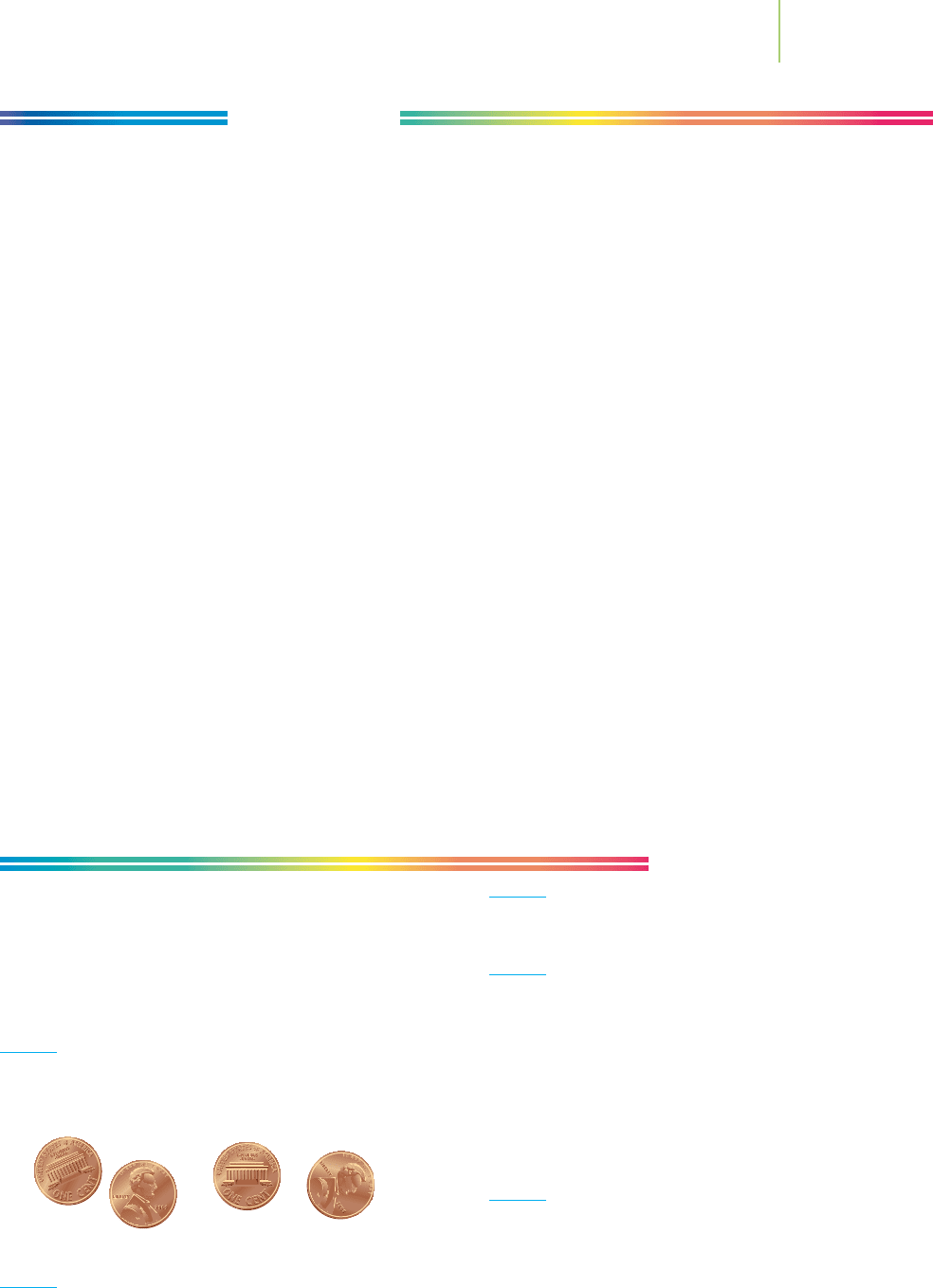
Focus Your Learning 613
Key Words
bioenergetics The study of the energy changes that
occur within a living cell. (p. 580)
catabolism The biological degradation of molecules to
provide an organism with smaller molecules and
energy. (p. 587)
entropy A measure of how the energy and matter of a
system are distributed throughout the system.
(p. 587)
equilibrium The state of a reaction when G = 0. The
reaction hasn’t stopped; rather, the rates of the for-
ward and reverse reactions are equal. (p. 608)
free energy (G) The state function that is equal to the
enthalpy minus the temperature multiplied by the
entropy. Used to determine the spontaneity of a
process. (p. 601)
Gibbs equation G = H − TS (p. 601)
glycolysis A series of biochemical reactions that convert
glucose into two molecules of pyruvate. The process
results in the formation of two molecules of ATP.
(p. 585)
macrostate The macroscopic state of a system that indi-
cates the properties of the entire system. (p. 580)
metabolism The biochemical reactions of an organism.
(p. 595)
microstate The state of the individual components within
a system. (p. 581)
multiplicity The number of microstates within a system.
(p. 587)
nonspontaneous process A process that occurs only with
continuous outside intervention. (p. 585)
pressure reaction quotient (Q
p
) The ratio of the pressures
of all of the gaseous products raised to their respec-
tive stoichiometric coefficients, divided by the pres-
sures of all of the gaseous reactants raised to their
stoichiometric coefficients. (p. 610)
reaction quotient (Q) The ratio of the initial molar con-
centrations of all of the products raised to the power
of their respective stoichiometric coefficients, divided
by the initial molar concentrations of all of the reac-
tants raised to the power of their stoichiometric
coefficients. (p. 611)
reversible conditions The conditions that occur when a
process proceeds in a series of infinitesimally small
steps. (p. 593)
second law of thermodynamics A spontaneous process is
accompanied by an increase in the entropy of the
universe; ∆S
universe
> 0. (p. 588)
spontaneous process A process that occurs without con-
tinuous outside intervention. (p. 585)
standard molar free energy of formation (
f
G°) The free
energy of a compound formed from its elements in
their standard states. (p. 605)
thermodynamics The study of the changes in energy in a
reaction. (p. 586)
third law of thermodynamics The entropy of a pure per-
fect crystal at 0 K is zero. (p. 598)
The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
Section 14.1
Probability as a Predictor of Chemical Behavior
Skill Review
1. Suppose that four pennies fall from your pocket to the floor.
How many microstates for the four pennies would exist?
(Consider only heads up versus tails up in your explanation.)
5. Contrast the meanings of the terms macrostate and
microstate.
6. Define the term multiplicity.
7. One hundred chemistry students are about to take an exam.
There are two equal 200-seat classrooms for the students.
Knowing that students must have vacant seats next to them
during the exam, describe at least two macrostate arrange-
ments you could predict for the two rooms.
8. In the situation defined in Problem 7, how could the posi-
tions of the students allow you to use microstates to explain
your answer?
Chemical Applications and Practice
9. Even a sample of only 1,000 atoms is too small to weigh ac-
curately, and yet we can calculate a probable distribution of
their numbers between two equal containers. How many mi-
crostates are possible if 1,000 atoms of He become distrib-
uted between two containers of equal size? Explain why an
equal distribution becomes more probable as the number of
He atoms increases.
Focus Your Learning
2. What is the probability that all four coins from Problem 1
would land heads up?
3. What is the probability that the four coins from Problem 1
would land two heads up and two tails up?
4. What is the most probable outcome for the four coins in
Problem 1?

10. A certain lecture hall is 25 m long, 12 m wide, and 15 m tall.
The oxygen molecules necessary to sustain life in the room
are, of course, free to circulate throughout the room. Explain,
using probability and microstates, why a student sitting in
the back should not be concerned that all the freely moving
oxygen molecules might concentrate at the front row of the
classroom.
11. Some powdered creamer is added to a cup of hot coffee.
Compare the initial macrostate to the final macrostate as
the creamer dissolves. Has the number of microstates for the
creamer and coffee increased from the initial state to the final
state? Explain the evidence you would use to explain your
answer.
12. A glass of tea is cooled by placing ice cubes in it. However,
after a few minutes, the ice has melted. Compare the initial
macrostate to the final macrostate. Has the number of mi-
crostates for the ice and tea increased from the initial state to
the final state? Explain the evidence you would use to explain
your answer.
13. Which of these would you classify as increasing the number
of microstates?
a. Water in a glass evaporates.
b. A precipitate of AgCl forms from the addition of a drop of
a 0.10 M solution of AgNO
3
into a glass of chlorinated tap
water.
c. An icicle forms from a downspout.
14. Which of these would you classify as increasing the number
of microstates?
a. A chunk of ice melts when placed in a glass of tap water at
room temperature.
b. A glass of tap water freezes when placed in a refrigerator
freezer compartment.
c. The combustion of gasoline produces carbon dioxide and
water vapor.
Section 14.2 Why Do Chemical Reactions Happen?
Entropy and the Second Law of Thermodynamics
Skill Review
15. Which of these, if any, need outside intervention to take
place? Describe the necessary intervention.
a. Combustion (combination with oxygen) of a piece of
paper in the air
b. Making a hard-boiled egg
c. Increasing muscle mass
d. Formation of calcium carbonate (bathtub ring) from
evaporating hard water
16. Which of these, if any, need outside intervention to take
place? Describe the necessary intervention.
a. The melting of butter on a warm pancake
b. Opening this book to this page
c. Paddling a canoe upstream
d. Cooking eggs for breakfast
17. What is the relationship between entropy and a nonsponta-
neous process?
18. What is the relationship between entropy and rate of change?
19. What is the relationship between entropy and number of
microstates?
20. What is the relationship between a nonspontaneous process
and the number of microstates?
21. Suppose you were studying in a residence hall. Down the hall
someone is preparing popcorn. The irresistible aroma even-
tually reaches your room. To take your mind off this, explain
how the second law of thermodynamics enables you to detect
this tasty temptation from so far away.
22. A scientist on the planet Zoltan expresses the third law of
thermodynamics by saying that the entropy of any com-
pound under standard state conditions is zero. Would this
change the ∆S values for the reaction of pyruvate to lactate
mentioned in the chapter? Explain.
Chemical Application and Practice
23. While pumping gasoline into your car, you might spill some
gasoline on your hands. However, the gasoline quickly evap-
orates with a cooling sensation on your skin. How can this
process be spontaneous if it requires the input of the energy
from your skin?
24. In a previous problem you were asked about the freezing of
water in a refrigerator. Water poured in a tray will sponta-
neously freeze in a freezer. Explain why this process arranges
the water molecules in a more orderly crystal form and yet is
still spontaneous.
25. Solid carbon dioxide is called dry ice. At room temperature,
this material spontaneously changes from an orderly solid to
a gas, bypassing the liquid phase entirely. When undergoing
this change, the molecules absorb heat from the nearby
surroundings. This, in turn, slows down the molecules
of the surroundings.
What can be said about
the change in entropy
for the carbon dioxide
molecules relative to
nearby air molecules?
What can be said about
the change in the en-
tropy of the universe
for this process?
614 Chapter 14 Thermodynamics: A Look at Why Reactions Happen
Dry ice.
26. The entropy of molecules can also depend on their relative
atomic motions. The atoms in molecules can twist, rotate,
and vibrate around a chemical bond. For which molecule
would you be able to predict more microstates: a molecule of
nitrogen or a molecule of ammonia (NH
3
)? Explain the basis
of your choice.
27. The following equation illustrates a gaseous reaction that is
possible in the atmosphere. Which direction in the reaction
shows the greater entropy for the system? Explain your
choice.

28. Another reaction common in the lower atmosphere is the
formation of ozone from NO
2
. Which direction in the reac-
tion shows the greater entropy for the system? Explain your
choice.
O
2
(g) + NO
2
(g)
O
3
(g) + NO(g)
Section 14.3 Temperature and Spontaneous Processes
Skill Review
29. Classify each of these as representing either a positive change
in entropy for the universe or a negative change in entropy
for the universe, or indicate that you would need more infor-
mation to determine this.
a. S
sys
=+; S
surr
=+
b. S
sys
=+; S
surr
=−
c. S
sys
=−; S
surr
=+
30. Classify each of these as representing either a positive change
in entropy for the universe or a negative change in entropy
for the universe, or indicate that you would need more infor-
mation to determine this.
a. S
sys
=−; S
surr
=−
b. S
sys
=+; S
surr
= 0
c. S
sys
=−; S
surr
= 0
31. How does the multiplicity of a system change as the molecu-
lar motions increase in the system?
32. How does the molecular motion of the surroundings change
as energy from the surroundings flows into a system?
33. How does the entropy of the surroundings change as energy
flows out of a system?
34. Can we predict the change in entropy of the universe as en-
ergy flows into the system? Explain.
Chemical Applications and Practice
35. Mercury remains a liquid over a fairly wide temperature
range, which is one reason why it has been used in ther-
mometers. Classify each of these changes as a positive or a
negative change in the entropy of a sample of mercury.
a. Liquid mercury changes to a solid.
b. Liquid mercury expands as it is heated.
c. A small amount of mercury evaporates from the surface
of a column of mercury in a sealed thermometer.
36. Chlorine gas (Cl
2
) is a very toxic compound that can form as
a result of the improper use of bleach and ammonia cleaning
solutions. Classify each of these changes as a positive or a
negative change in the entropy of a sample of this green gas.
a. Chlorine gas condenses to a liquid.
b. Chlorine gas is heated in a container.
c. Chlorine gas deposits on a cold surface.
37. If you are enjoying an iced carbonated soft drink while study-
ing, you may have noticed that the carbon dioxide used to
carbonate the beverage bubbles slowly out of the beverage as
it warms. Is this a positive or a negative entropy change for
the CO
2
? Explain.
38. In the bubbling of carbon dioxide mentioned in Problem 37,
did the surroundings gain or lose heat? Is the sign for
S
universe
, at this temperature, positive or negative?
Section 14.4
Calculating Entropy Changes in Chemical Reactions
Skill Review
39. Propane stoves make use of the following combustion
reaction:
___ C
3
H
8
(g) + ___ O
2
(g) → ___ CO
2
(g) + ___ H
2
O(g)
Balance the equation, report the number of moles of gas
molecules on each side of the equation, and predict the
change in entropy of the system.
40. Methane can be combusted by the following reaction:
___ CH
4
(g) + ___ O
2
(g) → ___ CO
2
(g) + ___ H
2
O(g)
Balance the equation, report the number of moles of gas
molecules on each side of the equation, and predict the
change in entropy of the system.
41. Repeat the prediction for Problem 39, but assume that the
reaction is performed under temperatures that cause
the propane and water to be liquid, yet the oxygen and car-
bon dioxide remain as gases.
42. Repeat the prediction for Problem 40, but assume that
the reaction is performed under temperatures that cause the
methane and water to be liquid, yet the oxygen and carbon
dioxide remain as gases.
43. Explain why the change in the number of moles of gases,
from reactant to product, is typically more influential in de-
termining the sign on entropy changes than the change in
moles of liquids and solids.
44. The third law of thermodynamics specifies a particular con-
dition for a perfect crystal. Why must the temperature be 0 K
instead of 0°C?
45. Predict the sign on the entropy change for each of these
reactions.
a. NH
3
(g) + HCl(g) → NH
4
Cl(s)
b. 2HgO(s) → 2Hg(l) + O
2
(g)
c. Cd(s) +
1
⁄2O
2
(g) →CdO(s)
46. Using the values for S
o
found in the appendix, determine
the actual values for the standard change in entropy for the
reactions in Problem 45.
47. Predict the sign on the entropy change for each of these
reactions.
a. 2SO
2
(g) + O
2
(g) → 2SO
3
(g)
b. 2NH
3
(g) → N
2
(g) + 3H
2
(g)
c. CO(g) + 2H
2
(g) → CH
3
OH(l)
48. Using the values for S° found in the appendix, determine
the actual values for the standard change in entropy for the
reactions in Problem 47.
Chemical Applications and Practices
49. Several states have implemented regulations for fuel alterna-
tives in automobiles. One such gasoline alternative is ethanol
(C
2
H
5
OH). Balance the following combustion reaction and
determine the change in entropy for the reaction.
___ C
2
H
5
OH(l) + ___ O
2
(g) →____CO
2
(g) + ____ H
2
O(g)
Focus Your Learning
615

50. One important source of salt (NaCl) is the evaporation of
ocean water. Calculate the change in the standard molar en-
tropy as the dissolved ions precipitate the solid.
Na
+
(aq) + Cl
−
(aq) →NaCl(s)
51. Graphite and diamond are both made only of carbon. How-
ever, pencils (incorporating the graphite form) sell for a few
cents, whereas engagement rings (displaying diamonds)
often sell for thousands of dollars. Using the appendix, deter-
mine the change in entropy for the conversion of diamond to
graphite.
52. Using the appendix to note the standard S° value for a
metal, explain why the value for the metal alone is lower than
the value for any of the listed compounds of that metal.
Section 14.5 Free Energy
Skill Review
53. Predict the relative magnitude of the temperature that would
result in a spontaneous process in each of these combinations.
a. H =+; S =+
b. H =+; S =−
54. Predict the relative magnitude of the temperature that would
result in a spontaneous process in each of these combinations.
a. H =−; S =+
b. H =−; S =−
55. Determine the value of H in each of these.
a. G =−24.5 kJ/mol; S = 287 J/mol·K; T = 298 K
b. G = 1.38 kJ/mol; S = 24 J/mol·K; T = 298 K
c. G = 500.0 kJ/mol; S =−6439 J/mol·K; T = 325 K
d. G =−24.5 kJ/mol; S = 187 J/mol·K; T = 39.9°C
56. Determine the value of G in each of these cases.
a. H =−20.5 kJ/mol; S = 259 J/mol·K; T = 298 K
b. H = 350 kJ/mol; S = 73 J/mol·K; T = 157 K
c. H = 299 kJ/mol; S
=−639 J/mol·K; T = 325 K
d. H =−4505 kJ/mol; S = 107 J/mol·K; T = 19.3°C
57. Determine the value of S in each of these cases.
a. G =−20.5 kJ/mol; H = 259 kJ/mol; T = 298 K
b. G = 150 kJ/mol; H =−73 kJ/mol; T = 157 K
c. G = 209 kJ/mol; H =−639 kJ/mol; T = 35.0 K
d. G =−4505 kJ/mol; H = 107 kJ/mol; T = 135.8°C
58. Determine the value of T in each of these cases.
a. G =−20.5 kJ/mol; H = 259 kJ/mol;
S = 260 J/mol·K
b. G = 150 kJ/mol; H =−73 kJ/mol;
S =−290 J/mol·K
c. G = 209 kJ/mol; H = 639 kJ/mol; S = 560 J/mol·
K
d. G =−4505 kJ/mol; H = 107 kJ/mol;
S = 1160 J/mol·K
59. Determine the spontaneity of each of these reactions at
298 K.
a. NH
3
(g) + HCl(g) → NH
4
Cl(s)
b. 2HgO(s) → 2Hg(l) + O
2
(g)
c. Cd(s) +
1
⁄2O
2
(g) →CdO(s)
60. Determine the spontaneity of each of these reactions at
298 K.
a. 2SO
2
(g) + O
2
(g) → 2SO
3
(g)
b. 2NH
3
(g) → N
2
(g) + 3H
2
(g)
c. CO(g) + 2H
2
(g) → CH
3
OH(l)
Chemical Application and Practices
61. One of the authors of this text formerly drove an automobile
that could best be described as blue and rust. Consider that the
rust was formed, at least partially, by the reaction illustrated
by this equation:
3O
2
(g) + 4Fe(s) → 2Fe
2
O
3
(s)
Assuming standard conditions, what would you calculate as
the G° for this reaction?
62. The impressive white cliffs of Dover in England have stood
without significant change from their basic structure of cal-
cium carbonate for eons. One possible reaction for the de-
composition of calcium carbonate is
CaCO
3
(s) → CaO(s) + CO
2
(g)
Use the
f
G° values of these compounds to determine the
value of G° for the reaction. Comment on how thermody-
namics helps explain the relative stability of this beautiful
landmark.
63. Sulfur dioxide and sulfur trioxide are important anhydrides
for making sulfurous and sulfuric acid, respectively. When
sulfur-containing coal is burned, sulfur gases pose environ-
mental threats to air quality. Given the following two com-
bustion reactions, determine the G° value for the change
from SO
2
to SO
3
.
S(s) +
3
⁄2O
2
(g) → SO
3
(g) G° =−371 kJ/mol
S(s) + O
2
(g) → SO
2
(g) G° =−300 kJ/mol
SO
2
(g) +
1
⁄
2O
2
(g) → SO
3
(g) G° = ?
64. As we note earlier, graphite and diamond are both composed
entirely of carbon atoms. Using the following reactions at
298 K, determine the value of G° for the conversion of
graphite into diamond.
C
diamond
(s) + O
2
(g) → CO
2
(g) G° =−397 kJ/mol
C
graphite
(s) + O
2
(g) → CO
2
(g) G° =−394 kJ/mol
C
graphite
(s) → C
diamond
(s) G° = ??
65. As we saw in earlier in this chapter, the production of glucose
requires the input of energy. This is typically shown as green
plants using sunlight to help combine carbon dioxide with
water to form glucose through photosynthesis. However, in
some deep areas of the ocean, sunlight does not reach bacte-
ria that may grow around thermal vents. These organisms
have been shown to produce energy through oxidation of a
sulfur compound, H
2
S. Determine H°, S°, and G° for
this biochemically important reaction. Assume sulfur is in
the standard form—rhombic allotrope.
H
2
S(aq) + O
2
(g) → 2H
2
O(l) + 2S(s)
66. Smog produced over a city is formed from trace amounts of
automobile exhaust in a reaction with atmospheric oxygen.
One of the steps in the formation of ozone is the oxidation of
oxygen gas by nitrogen dioxide (a by-product of the combus-
tion of gasoline). Determine H°, S°, and G° for this
reaction.
NO
2
(g) + O
2
(g) → NO(g) + O
3
(g)
616 Chapter 14 Thermodynamics: A Look at Why Reactions Happen

Section 14.6
When G = 0; A Taste of Equilibrium
Skill Review
67. Determine the temperature at which the indicated system
reaches equilibrium.
a. H° = 46.4 kJ/mol; S° = 27.6 J/mol·K
b. H° = 10.6 kJ/mol; S° = 77 J/mol·K
c. H° = 124 kJ/mol; S° = 295.5 J/mol·K
68. Determine the temperature at which the indicated system
reaches equilibrium.
a. H° = 57.6 kJ/mol; S° = 17.4 J/mol·K
b. H° = 94 kJ/mol; S° = 306 J/mol·K
c. H° = 32.1 kJ/mol; S° = 552 J/mol·K
69. At equilibrium, what is the pressure for the following process
at 298 K?
CaCO
3
(s)
CaO(s) + CO
2
(g) G° = 131 kJ/mol
70. What is the free energy change for the following reaction at
298 K, given that the pressures of NH
3
and N
2
are 1.0 atm
each and the pressure of H
2
is 2.0 atm? (Hint: Use the appen-
dix to determine the free energy change under standard
conditions.)
2NH
3
(g)
N
2
(g) + 3H
2
(g)
Chemical Applications and Practices
71. At 1 atm of pressure the boiling point of pure water is
100.0
o
C. If 1000.0 g of water were brought to this point and
then completely boiled away, what would you calculate as the
entropy change for the water? (Note: The heat of vaporiza-
tion for water is 40.7 kJ/mol.)
72. There is a historical approximation known as Trouton’s rule.
This approximation states that at the normal boiling point
for nonpolar compounds, the standard molar entropy of va-
porization is 87 J/mol·K. Using Trouton’s rule, determine the
approximate heat of vaporization of water. The actual molar
entropy of vaporization for water is close to 110 J/mol·K.
Explain why the Trouton value is so much different for polar
substances such as water but is often found to be much closer
to the actual value for nonpolar substances.
73. Under proper conditions, phase changes can be classified
as reversible processes. The energy involved with melting
or freezing of a sample is called the enthalpy of fusion. The
enthalpy of fusion (
fus
H) for the rare radioactive element
actinium, Z = 89, is 10.50 kJ/mol. If the entropy of fusion is
9.6 J/mol·K, what would you calculate as the melting point
for this silvery white metal?
74. One of the reasons why you are able to read this question is
that the cellular functions necessary are supplied with energy
from the molecule ATP (adenosine triphosphate), which
couples with many biochemical reactions. The formation of
ATP can be accomplished as follows:
ADP(aq) + H
2
PO
4
−
(aq)
ATP(aq) + H
2
O(l)
G° for the reaction is 31 kJ/mol. Using that information,
what would you estimate for the reaction quotient, Q,at
25°C, for the reaction at equilibrium?
75. The gas commonly used in chemical laboratory burners is
methane (CH
4
). Shown here is the combustion reaction that
takes place when you light your lab burner.
CH
4
(g) + 2O
2
(g)
CO
2
(g) + 2H
2
O(g)
a. Use
f
G
o
data to calculate the G° value for the reaction.
b. What would the value of G be if the pressure of O
2
were
reduced to 0.20 atm from 1.00 atm, at room conditions,
with the remaining gases at standard conditions?
c. Explain why, if G has a negative value indicating a
spontaneous change, we still have to bring a flame or spark
to the system to get it to react in the lab.
76. Nitrogen gas and oxygen gas make up essentially 100% of our
air. The following reaction has G° = 174 kJ. Fortunately,
the two do not easily combine through this reaction under
the Earth’s atmospheric conditions.
N
2
(g) + O
2
(g)
2NO(g)
a. What is the value of Q
p
at 298 K for the reaction at
equilibrium?
b. What temperature would be needed for the reaction to be
spontaneous if the pressures were returned to 1.0 atm?
(Assume that enthalpy and entropy values do not change
significantly over this range.)
c. What would be the value of G at 25°C if the pressure of
both N
2
and O
2
were increased to 5.0 atm each, while the
pressure of NO
2
was decreased to 0.50 atm?
Comprehensive Problems
77. Butane, burned in small portable lighters, combusts accord-
ing to the following unbalanced equation:
___ C
4
H
10
(g) + ___ O
2
(g) → ___ CO
2
(g) + ___ H
2
O(g)
First balance the equation, and then explain your answer to
this question: “Does the combustion show an increase or a
decrease in entropy for the system?”
78. Suppose a particularly noxious compound was entering a
water supply at only one point. If it would stay at the entry
point, it might easily be removed. However, this does not
happen. Explain why environmental scientists must be aware
of the second law of thermodynamics.
79. Because it was already known that a positive change in the
entropy of the universe indicated a spontaneous reaction,
why was it important to develop the Gibbs equation, which
is also used to predict the spontaneity of a reaction? What
advantage does the Gibbs equation offer?
80. In the ongoing research to find a cure or treatment for
Alzheimer’s disease, some investigators have focused on the
appearance of plaque-like formations in brain cells. The solid
masses form from protein fibers called beta-amyloids. Use
Internet resources and journal sources to find out if the
process is based on a positive or a negative value for the S°
of the plaque-forming reaction.
81. In calculations involving Gibbs energy and equilibrium, you
must always be aware of the differences between G and
G
o
.WhenG = 0, at 25°C, what is true about Q?
82. A certain reaction is nonspontaneous at one temperature. If
raising the temperature caused the reaction to become spon-
taneous, what could you conclude about the entropy change
of the reaction? Explain your answer.
Focus Your Learning
617
