Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

65. Consider the calculation completed in question 63. How
many grams of silver must be used to cover the area with a
thin film that is one atom thick?...three atoms thick?
66. Consider the copper-coated notecard in question 64. How
thick must the thin film be to increase the mass of the note-
card by 5.00 g?
Chemical Applications and Practice
67. A recent advance in thin films has involved the use of tita-
nium dioxide on window glass. The film helps keep dust
from depositing on the windows. The exact technique for
applying the thin film of TiO
2
is proprietary information
(meaning that the information is not available to the public).
But the process involves heating the glass and passing the film
material as a gas stream over the glass. The resulting interac-
tion forms TiO
2
as a film during cooling. Which of the three
techniques described in this chapter most resembles this
process?
68. The thin film in the application described in problem 67
is approximately 5.00 × 10
2
angstroms thick (1 ang-
strom = 1 × 10
−10
m). Describe how this measurement is
made. How much additional mass does the thin film add to
a 2.500-ft
2
piece of glass? (Assume the density of the film is
4.26 g/cm
3
.)
Section 13.6
On the Horizon—What Does the Future Hold?
Skill Review
69. Uses for zeolites, both naturally formed from clays and spe-
cially synthesized, vary greatly but typically depend on what
property of these versatile substances?
70. Recycling is an important part of the process of conserving
and reusing resources. List two common items that are typi-
cally recycled.
71. Instead being thrown away, your old TV can be recycled.
What types of materials make up the TV? Which of these can
be recycled?
72. Many used automobiles can be recycled. What are some
of the materials that could be harvested from a modern
automobile?
73. Name and briefly describe the three classes of biopolymers.
74. Describe the chemical and physical makeup of an aerogel.
Provide at least one application for an aerogel.
Chemical Applications and Practices
75. Zeolite catalysts may be employed in the production of the
hydrocarbons used in gasoline. Would this be an example of
a heterogeneous or a homogeneous catalyst? How can a cata-
lyst be used to reduce waste?
76. Chemical companies now advertise many products, such as
pharmaceuticals, as containing one chiral isomer. What is the
advantage of using a chiral catalyst rather than a nonchiral
catalyst when synthesizing a product that must be only one
type of isomer?
77. In terms of density, insulation, and resistance to cracking,
what advantages does an aerogel offer over other ceramic
materials?
578 Chapter 13 Modern Materials
78. One of the guidelines for practicing Green Chemistry is to
reduce the amount of chemical waste that occurs in manu-
facturing compounds. From where does this waste arise?
What is typically done with chemical waste in “nongreen”
processes?
Comprehensive Problems
79. How do molecular and ionic solids, in both the molten and
the dissolved state, differ in their ability to conduct an electric
current?
80. When selecting molecules that make good candidates for liq-
uid crystal displays, chemists search for (compact; long rigid)
molecules with (strong; weak) dipole moments. Explain why
each of your circled choices is appropriate for liquid crystals.
81. Metals such as silver, chromium, and platinum are known for
their shiny appearance. Most metals, in their pure state, also
have this appearance. Use the delocalized electron model to
explain this property of metals.
82. Superconductor materials are able to conduct electricity,
typically with pairs of electrons, as a consequence of the
arrangement of sheets or layers within their structure.
These ceramic materials are continually being studied to
make it possible to have superconducting ceramics at
higher temperatures. Use Internet resources to obtain an
example of the chemical composition and structure of a
ceramic superconductor.
83. Use the Internet or other sources to find out how Charles
Goodyear used crosslinking with sulfur to change the prop-
erties of some rubber substances he was working with.
84. Use the Internet to find the twelve principles of Green Chem-
istry. Which of these principles are used in your chemistry
laboratory?
85. Replacing hazardous pesticides with less persistent (but
just as effective) compounds is a common goal. What are
the hazards associated with pesticides if they persist in the
environment?
Thinking Beyond the Calculation
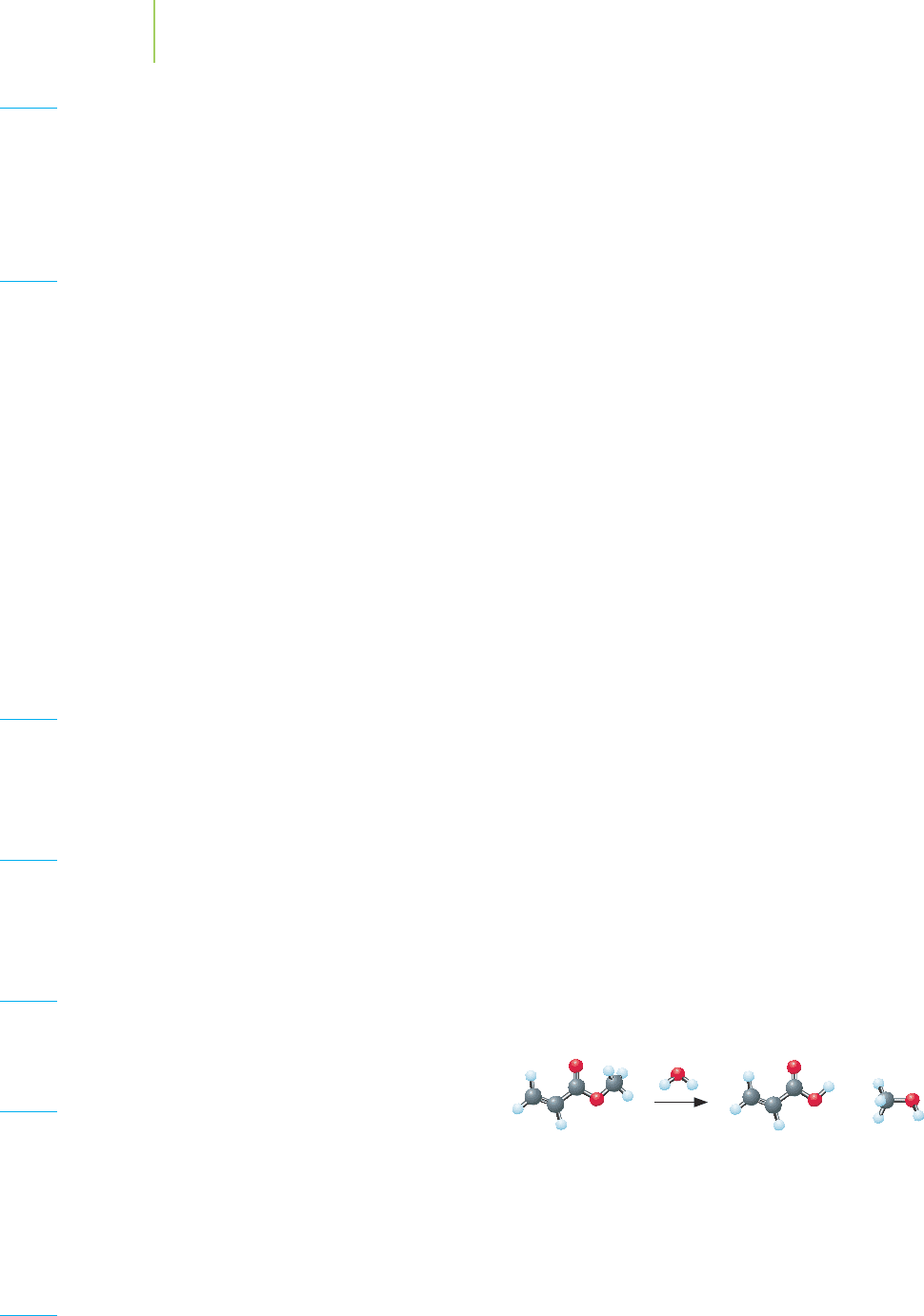
86. A chemist prepares a polymer from methyl acrylate (also
known as methyl propenoate). The resulting polymer was
made by dissolving a small amount of the monomer in solu-
tion and then forming the polymer. The polymer was very
gel-like in nature, with a spongy appearance.
Acrylic acid MethanolMethyl acrylate
+
a. Draw any potential resonance structures for the monomer.
b. Draw the polymer that would result from the poly-
merization of methyl acrylate.
c. What uses would such a polymer find?
d. If the monomer is treated with water and acid, it reacts
to form acrylic acid and methanol. How many grams of
methanol would be obtained from the reaction of 1.50 g
of methyl acrylate with excess water?
e. What properties that are not found in a polymer of methyl
acrylate would you expect a polymer of acrylic acid to
possess?

579
Thermodynamics:
A Look at
Why Reactions
Happen
Sugary between-meal snacks are a good
source of energy. Each contains sucrose,
which is broken down in the body into
glucose and fructose. Both of these mol-
ecules undergo complete oxidation and
provide energy for the body.
Contents and Selected Applications
Chemical Encounters: Bioenergetics
14.1 Probability as a Predictor of Chemical Behavior
14.2 Why Do Chemical Reactions Happen? Entropy and the Second
Law of Thermodynamics
Chemical Encounters: Glycolysis
14.3 Temperature and Spontaneous Processes
Chemical Encounters: Psychrotrophs and Psychrophiles
14.4 Calculating Entropy Changes in Chemical Reactions
Chemical Encounters: More Glycolysis
14.5 Free Energy
Chemical Encounters: Pyruvate and Lactate
14.6 When G = 0; A Taste of Equilibrium
Chemical Encounters: ATP Formation
Go to college.hmco.com/pic/kelterMEE for online learning resources.

After a hearty breakfast, we leave for work full
of energy and ready to conquer the day. However,
the midmorning hours can be difficult to get through
because our energy level drops a couple of hours after we
eat. This is especially true if we had a big bowl of sugar-coated,
sugar-injected cereal for breakfast. To make matters worse, the mid-
afternoon hours are no easier—they seem to be the longest of the day.
Why does this happen and what can we do to give ourselves that “burst of
energy” we need when we feel so tired? Enter the snack. Whether it takes the form
of a chocolate bar, a donut, or a bottle of juice, it has the effect of raising our energy
level. Just like breakfast, lunch, and dinner, the snack provides our bodies with a
source of glucose. How does the consumption of glucose give us energy?
Antoine Lavoisier (the scientist we met in Chapter 3, whose measurements led
to the formulation of the law of conservation of mass before he was guillotined in the
French Revolution) noticed that living things consume foods and transform them
into the energy that maintains life. Lavoisier’s views on the process seem rudimen-
tary given our modern understanding, but they were quite revolutionary in his day.
The addition of glucose to living cells is an example of this food-to-energy transfor-
mation process. To the biochemist, this is part of the broader field of
bioenergetics,
the study of the energy changes that occur within a living cell.
In this chapter we will discuss some of the chemical reactions in the field of
bioenergetics. Although we’ll soon introduce terms such as entropy, spontaneity, free
energy, and equilibrium, the underlying concept of probability demands our imme-
diate attention. Chemists benefit from understanding these topics. They use proba-
bility in many ways: to locate electrons, to determine the macroscopic properties of
compounds and mixtures, and to predict the outcome of chemical reactions. We will
use probabilities to discover why chemical processes occur—including the chemical
transformations of the body and their relationship to our ability to live.
Application
C
HEMICAL
E
NCOUNTERS:
Bioenergetics
14.1 Probability as a Predictor
of Chemical Behavior
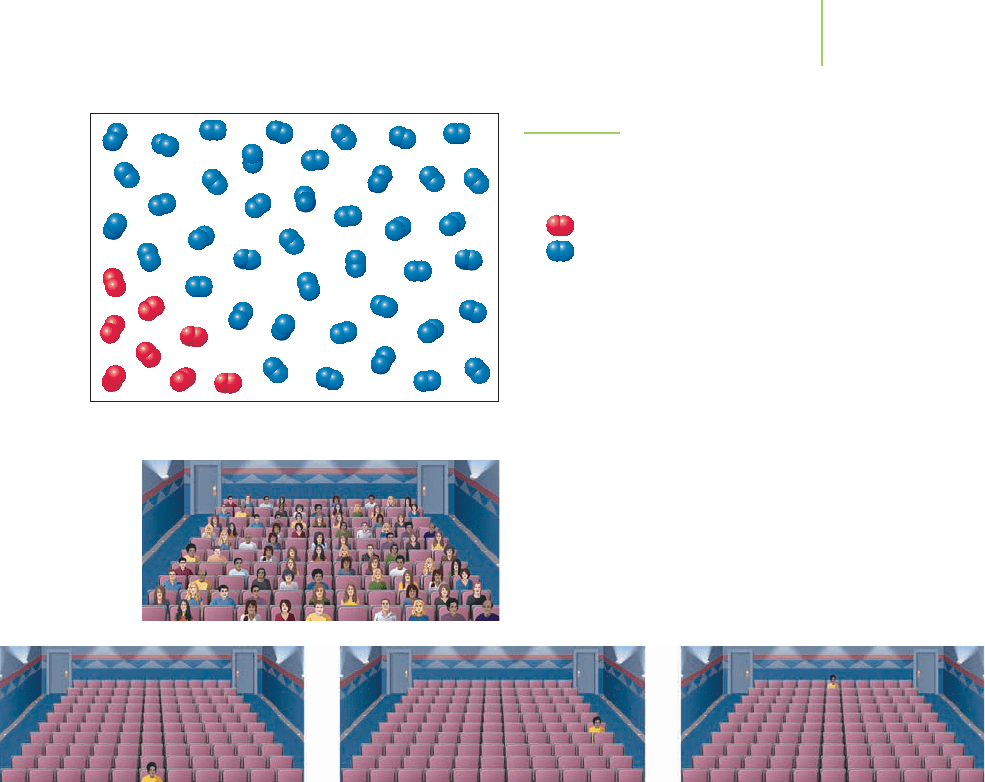
If you entered a classroom or movie theater and noticed all the males seated on
one side and all the females on the other, as shown in Figure 14.1, would you
think that some announcement, rule, or social convention had dictated that
arrangement? Perhaps. But this seating pattern could also have emerged from
purely random choices. In fact, there are numerous ways in which a room full of
people could be sitting, and “separate seating” is just one of many possibilities.
Let’s shift our focus to the room in which you are now sitting as you read this.
As we know, the air in the room is a mixture of primarily oxygen and nitrogen
gases. Oxygen and nitrogen molecules do not chemically interact with each other,
so they can occupy essentially any unfurnished position in the room. Is it possible
that these gases could be arranged such that all of the oxygen molecules were in
one corner of the room and all of the nitrogen molecules in another, as shown in
Figure 14.2? Possible? Yes. Likely? No. Is there something that these two seemingly
unrelated situations—the room of people and the room of molecules—share?
The short answer is yes, the two situations have a lot in common. To under-
stand how this could be so, let’s introduce some common terminology to help in
our discussion. We refer to the
macrostate of a system (whether seated people or
FIGURE 14.1
An interesting arrangement of the students
in a classroom.
580
molecular positions in a room) when we want to take a snapshot of the overall sit-
uation. The macrostate of the theater seating is that it is sorted by gender. The dis-
persed oxygen and nitrogen molecules in the room exert a certain pressure and
have a certain composition; this is all part of the macrostate of the gases in the
room.
If we wanted to describe the individuals in the theater, we might discuss which
seat they are in, which way they are facing, and whether their arms or ankles are
crossed. Each of these individual descriptions is referred to as a
microstate. For in-
stance, each arrangement of people in the theater indicates a different microstate.
How does this apply to molecules? The total of the microstates in a system defines,
or describes, the macrostate of the system. For individual molecules, the microstate
can include their translational motion (changing from one location to another),
their vibrational–rotational state (this involves the atomic movements around a
chemical bond), and their electron configuration (ground state versus the excited
state, even the oxidized state).
How might you describe the macrostate of the gases in your room? You could
measure their temperature and pressure. You could also assess the number of
molecules of each component in the room. Given the huge number of molecules
of gas in the room, we would predict an even larger number of microstates for the
gas. We mentioned the hypothetical condition of all the oxygen molecules being
in one corner. To bring about that unlikely macrostate of air, the oxygen mole-
cules (being restricted to the corner) would have to assume a limited number of
the multitude of possible microstates in the room.
Why have you never encountered the macrostate that has all the oxygen mol-
ecules stuck in one corner of the room? One answer can be found in looking at
14.1 Probability as a Predictor of Chemical Behavior 581
= O
2
= N
2
FIGURE 14.2
Is this arrangement of oxygen molecules
within a room possible?
A macrostate is a snapshot description of
an overall situation.
A microstate is a snapshot description of a particular representation
of individual particles. Each of these three pictures includes a differ-
ent microstate for a single person in a movie theater.

Two individual macrostates.
the probability that such an event could occur. Out of all the possible macrostates,
what are the chances that “all O
2
on one side, all N
2
on the other side” would occur?
In short, there isn’t a very good probability that this situation would happen.
(Read on for a more detailed answer!)
Think back to the seating choices for a person entering a room. Which situa-
tion has more choices, the strict division by gender or “open seating”? Which
situation would you consider to have the greater number of microstates that sat-
isfy the conditions for a particular macrostate? Ludwig Boltzmann (1844–1906),
an Austrian mathematician/physicist, dealt with this idea, along with its powerful
implications, mathematically. We can get a sense of what he did by looking inside
the tanks of oxygen that scuba divers and mountain climbers carry with them as
they set off on their quests. Look closely at Figure 14.3. This photo, taken of
mountain climbers George Leigh Mallory and Andrew Irvine before their fateful
June 1924 attempt to scale the summit of Mt. Everest, shows the climbers each
with two tanks of oxygen strapped to their backs. Let’s assume that these tanks are
connected by a valve and that one of the tanks is empty and the other full. What
will happen to the distribution of the gas if the valve is opened? The final volume
of the two tanks, which we’ll call V
f
, is double the initial volume of just
one tank, V
i
. We can also express this by saying that the ratio
V
f
V
i
= 2. How many
ways can the individual molecules be distributed within the two tanks? (That is,
how many “microstates” are there?) To make things a little simpler, let’s assume
that the tanks contain only two molecules of a gas. If we call the molecules A and
B, we can have a total of four microstates, as shown in Figure 14.4. We will call the
final number of microstates W
f
(for the German word Wahrscheinlichkeit, “prob-
ability”), and W
f
= 4 in this case. The initial number of microstates, W
i
, is equal
to 1, representing A and B both present in one tank, before the valve was opened
allowing the gases to spread apart. This means that for two molecules in two
tanks, the ratio
W
f
W
i
=
4
1
.
How is the number of microstates,
W
f
W
i
, related to the volume,
V
f
V
i
? For N
molecules,
W
f
W
i
=
V
f
V
i
N
582 Chapter 14 Thermodynamics: A Look at Why Reactions Happen
FIGURE 14.4
The possible arrangements of two
molecules into two tanks.
Microstate Tank 1 Tank 2
1AB
2AB
3AB
4BA
FIGURE 14.3
George Mallory and Andrew Irvine on Mt.
Everest pack heavy cylinders of oxygen
on their backs. This photo, taken in 1924
during their ascent to the summit, is the
last known image of the pair.
14.1 Probability as a Predictor of Chemical Behavior 583
That is the equation that Boltzmann derived. Using our numbers,
4 = 2
2
What if our tanks contained three molecules (N =3) instead of two?
W
f
W
i
=
V
f
V
i
N
W
f
W
i
= 2
3
= 8
In this case, there would be eight possible microstates. More molecules in the
tanks make available more microstates—more ways in which the molecules can
distribute themselves among the two tanks. This means that the probability of
all of the molecules being in only one tank—a single microstate among all
other possibilities—becomes smaller. This is the key point: As the number of
molecules increases, the likelihood of their all being in one tank decreases sharply,
and the probability of the gases tending toward an equal distribution increases.
Let’s look further into this point and discuss its vital implications.
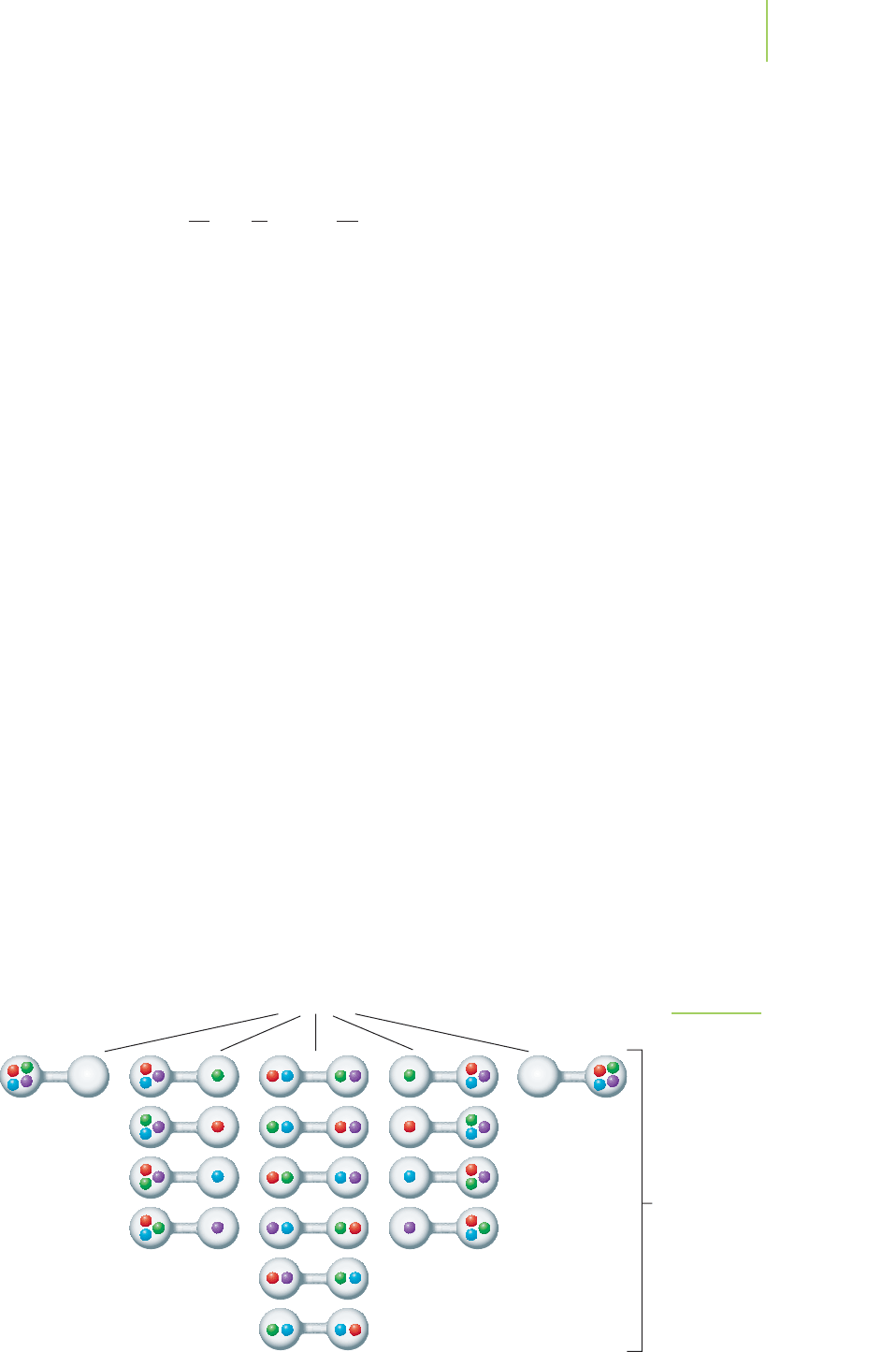
Let’s put four oxygen molecules in the full tank. Here Boltzmann’s equa-
tion indicates that there should be 16 (2
N
= 2
4
= 16) different microstates of
the oxygen molecules in the two-tank system. This is exactly what we predict
by drawing each of the arrangements as in Figure 14.5. But if we look closely,
we see that some of the microstates would give the same macrostate for the
system. Figure 14.5 shows that only 5 unique macrostates could arise from the
16 different microstates. Only 6 of these 16 microstates describe the equal dis-
tribution of the gases. The probability is therefore 6/16, or 37.5%, that the
four molecules will arrange themselves into the two tanks equally. The proba-
bility of exactly equal distribution has diminished. However, there are many
more microstates at or near equal distribution than microstates that are all on
one side of either tank. Only 2 of the 16 microstates, or 12.5%, describe all of
the molecules being on one side or the other.
As illustrated in Figure 14.6, greatly increasing the number of molecules in
our experiment (from 8 to 32 to 128) also greatly increases the number of mi-
crostates that are available. As a result, the probability of equal or nearly equal
distribution of the molecules between the two tanks increases. At the same time,
the probability of all of the molecules being in one tank or the other is ap-
proaching zero. For even small real-world-sized samples of molecules (such as
0.01 mol or 0.1 mol), equal or nearly equal distribution of the gases becomes the
most likely outcome. The bottom line is that probability—a mathematical
construct—governs physical behavior, and in this case, probability says that
the gases will spread from one tank to occupy both tanks evenly.
5 different macrostates
16 different
microstates
FIGURE 14.5
Microstates describing the possible
arrangements of four molecules
between two tanks.
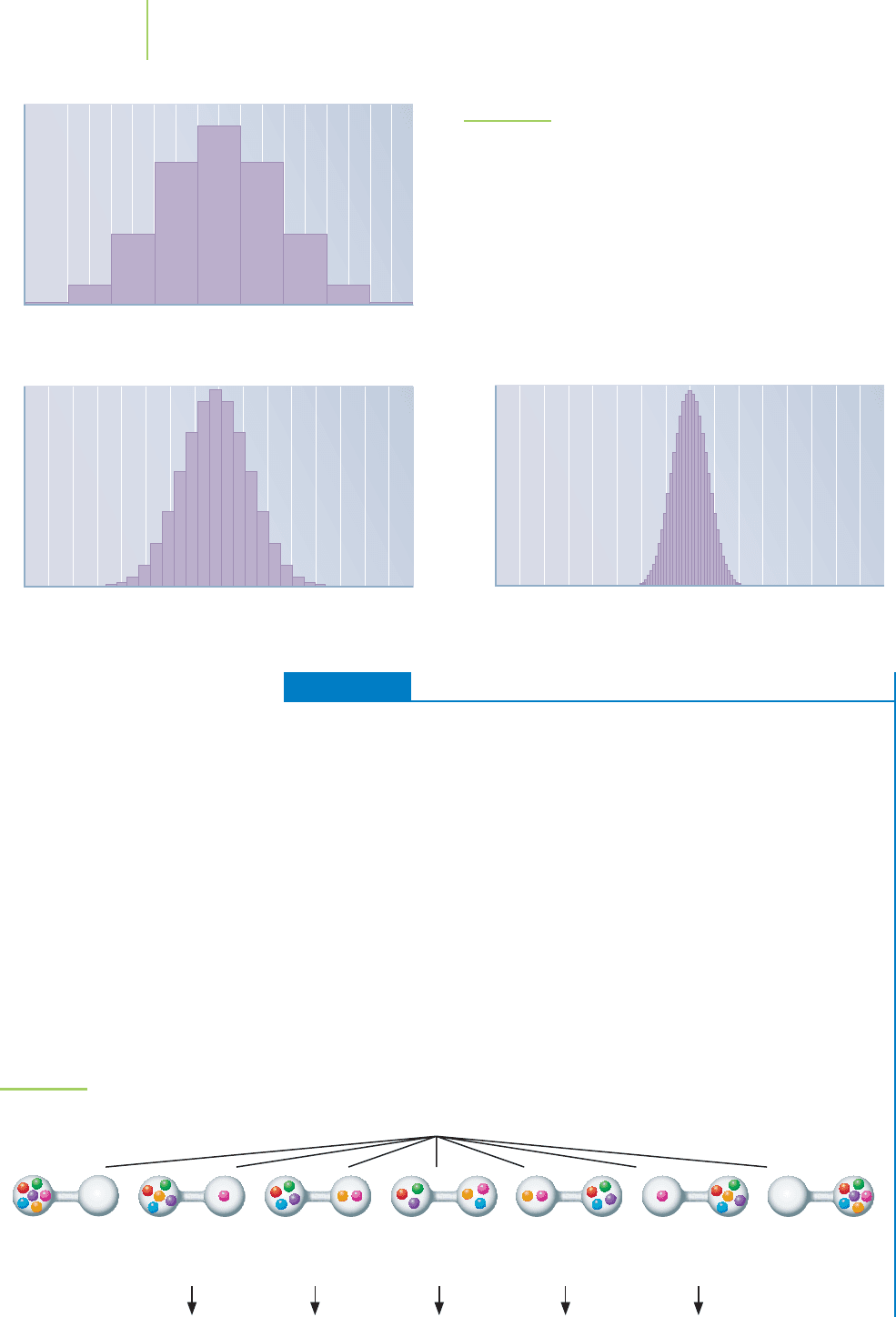
EXERCISE 14.1 Probability
A valve connects two glass jars. One of the jars contains six atoms of gaseous he-
lium, and the other is empty. Determine the probability of the macrostate in which
each jar will fill equally (that is, each jar will contain three atoms of helium) after the
valve is opened. For the same system, what is the probability of finding four mole-
cules in the left-hand jar and two in the right? What about the probability of find-
ing all six molecules in the left-hand jar?
Solution
Because there are six molecules in this example, there are 2
6
= 64 possible mi-
crostates. Drawing each of them out and grouping them into similar macrostates
leads us to the conclusion that there are only 7 macrostates (see Figure 14.7). There
are 20 microstates that indicate equal distribution, so the probability of equal dis-
tribution of the six molecules of gas is 20/64, or 31%. The probability of finding two
molecules of gas in the right-hand jar and four in the jar on the left is 15/64, or 23%.
The probability of finding all of the molecules in the left-hand jar is 1/64, or 1.6%.
584 Chapter 14 Thermodynamics: A Look at Why Reactions Happen
Number of molecules in the left cylinder
Probability
0 8 16 24 32 40 48 56 64 72 80 88
128 molecules
96 104 112 120 128
Number of molecules in the left cylinder
Probability
0246810121416182022
32 molecules
24 26 28 30 32
Number of molecules in the left cylinder
Probability
012345678
8 molecules
FIGURE 14.6
Probability of distribution of a gas between two cylinders.As
the number of molecules increases in the system, the tanks’
containing equal numbers of molecules becomes the more likely
event. Note that the probability of equal distribution is a bell-
shaped (Gaussian) curve.
6 different
microstate
arrangements
15 different
microstate
arrangements
20 different
microstate
arrangements
7 different
macrostates
15 different
microstate
arrangements
6 different
microstate
arrangements
1 microstate
arrangement
1 microstate
arrangement
FIGURE 14.7
Probability distribution for six molecules
of gas in two tanks.
ATP
ATP
ATP
ATP
ATP
NADH NADH
ATP
CH
2
OH
CH
2
O
CHO
OH
2
C
O
OH
HO
OH
OH
OH
CHOH
CH
2
O
COO
–
CO
CH
3
COO
–
CO
CH
3
HO
OH
O
P
P
CHO
CHOH
CH
2
O
P
P
PRACTICE 14.1
Let’s use the same valve and glass jar set-up, with one jar containing eight atoms
of gaseous helium. Then the valve is opened. Determine the probability of the
macrostate that shows three atoms of He in one jar and five atoms of He in the
other jar.
See Problems 1–4, 7, and 8.
14.2 Why Do Chemical Reactions Happen?
Entropy and the Second Law of
Thermodynamics
When sucrose enters the body, it is broken down into two simpler molecules,
glucose and fructose. Glucose and fructose are structural isomers, both with the
formula C
6
H
12
O
6
.
C
12
H
22
O
11
(s) + H
2
O(l) n C
6
H
12
O
6
(aq) + C
6
H
12
O
6
(aq)
Sucrose Fructose Glucose
Both molecules are used by the body to generate energy. For example, glucose in
a cell undergoes
glycolysis, the series of ten chemical transformations, shown in
Figure 14.8, that produces two molecules of pyruvate. The pyruvate is further
converted, releasing energy via a different set of transformations. In addition,
during glycolysis two new energy storage molecules known as ATP (adenosine
triphosphate, Figure 14.9) are produced. Glucose provides a major source of
energy for living cells via the glycolysis pathway, which is the sole source of meta-
bolic energy in some mammalian tissues and cell types. Many anaerobic (non-
oxygen-consuming) microorganisms depend entirely on glycolysis for energy to
carry out other biological reactions and survive.
Glycolysis is an example of a
spontaneous process. The rusting of iron nails,
the melting of ice in a glass, and the decay of wood buried in moist soil are also
spontaneous processes. That is, these processes occur without continuous outside
intervention. Say a brand new deck of cards falls off the kitchen table. The princi-
ples of probability say that there are many more ways in which the cards can land
out of order than ways in which they can land sequentially. Furthermore,
some of the cards will land face up and some face down. This disorder hap-
pens without our assistance—the process occurs spontaneously.
The reverse of a spontaneous process is known as a
nonspontaneous
process
. Nonspontaneous processes do not occur without continuous outside
14.2 Why Do Chemical Reactions Happen? Entropy and the Second Law of Thermodynamics 585
Application
C
HEMICAL
ENCOUNTERS:
Glycolysis
FIGURE 14.8
Glucose enters the glycolysis pathway on
its way to complete oxidation. The reac-
tions along the pathway produce two
molecules of pyruvate and energy.
Adenosine triphosphate (ATP)
OP
O
O
O
P
O
O
O
P
OC
CC
C
H
H
O
O H
H
OH
O
H
HH
C
H
NH
2
N
H
C
C
C
C
N
N
N
FIGURE 14.9

FIGURE 14.10
A green patina on the bronze Silent
Witness Memorial near Gander,
Newfoundland, Canada.
intervention. A rusty nail does not revert to a polished iron nail without continu-
ous help. Decayed wood buried in moist soil does not re-form freshly cut pieces
of wood, and a deck of cards spread out on the ground does not leap into nu-
merical and suit-based order. Just like these transformations, the reactions in
chemistry can be considered either spontaneous or nonspontaneous.
What happens to a copper or bronze statue when it is exposed to the
environment?
As illustrated by the Newfoundland memorial erected to the
memory of those lost in the worst plane crash in Canadian history (see Fig-
ure 14.10), a green patina forms on the surface. This patina is a complex mixture
of colored compounds, such as antlerite (Cu
3
SO
4
(OH)
4
, blue-green), brochantite
(Cu
4
(SO
4
)(OH)
6
, pale green), chalcanthite (CuSO
4
·5H
2
O, blue-green), cuprite
(Cu
2
O, dark red), and tenorite (CuO, black). All of these compounds include
either Cu
+
or Cu
+2
, so they are oxidation products of the copper metal. Although
the chemistry is much more complex, we can represent this patina by showing its
formation as the simple oxidation of copper:
2Cu(s) +O
2
(g) n 2CuO(s)
A chemical reaction causes the green patina to form without our assistance, so
this is a spontaneous process. The reverse process,
2CuO(s) n 2Cu(s) + O
2
(g)
is nonspontaneous; it does not occur without some outside assistance.The reverse
reaction of a spontaneous process is always nonspontaneous.
The spontaneous oxidation of metals due to exposure to the environment is
quite common. Table 14.1 lists some of the patinas that form on other metals.
Does the spontaneity of the oxidation of copper imply anything about the speed
of the process?
The short answer is no. It takes years for the green patina on a new
copper roof to form completely. Under standard conditions, the conversion of di-
amond into graphite is also a spontaneous process, but luckily for people with
diamond jewelry, the rate of this reaction is incredibly (almost immeasurably)
slow. Rust forming on a nail and the decay of a buried log, even though they are
spontaneous, are also slow processes. Biochemical reactions of glucose, on the
other hand, are spontaneous and very rapid. The combustion of methane used to
heat a pot of soup on the stovetop is even faster.
Thermodynamics, the study of the
changes in energy in a reaction (see also Chapter 5), determines whether a process
is possible. In Chapter 15, we’ll study the chemical kinetics of these processes to
determine how fast, and by what mechanism, they occur.
586 Chapter 14 Thermodynamics: A Look at Why Reactions Happen
A disordered pile of playing cards.
Patinas That Spontaneously Form on Metals
Element Symbol
Metal or Composition Natural Color Patina Color
Aluminum Al Silvery white Light gray
Brass copper and zinc Gold Dark brown to black
Bronze copper and tin Yellow to olive brown Dark brown to black
Copper Cu Light red brown Green
Iron Fe Lustrous silvery white Reddish brown
Silver Ag White to gray Black
TABLE 14.1
Application
Video Lesson: Spontaneous
Processes
Visualization: Spontaneous
Reaction of Phosphorus
(Barking Dogs)
Glucose
EXERCISE 14.2 Spontaneity in Common Processes
Which of these processes are spontaneous?
a. Ice melting in a hot oven
b. Carbon dioxide and water reacting at STP to form methane and oxygen
c. A basketball player jumping to dunk a basketball
d. NaOH(aq) and HCl(aq) reacting when combined in a beaker
Solution
The processes described in parts (a) and (d) are spontaneous, because they happen
without continuous outside intervention. The combination of sodium hydroxide
and hydrochloric acid is often used in chemical analysis precisely because the reac-
tion is not only spontaneous but also rapid. We will have much more to say about
reaction speed in the next chapter, in which we discuss chemical kinetics. The
process described in part (b) is nonspontaneous, which suggests that the reverse
process, the combustion of methane, is spontaneous. The basketball player in part
(c) must intervene (by adding energy to oppose the force of gravity) in order to
dunk the basketball. This process is nonspontaneous.
PRACTICE 14.2
Indicate whether each of these processes is spontaneous or nonspontaneous.
a. Potassium and water reacting c. A puddle evaporating from the sidewalk
b. Leaves falling from a tree d. Photosynthesis
See Problems 15–16.
Entropy
The overall catabolism (the biological degradation of molecules to provide
smaller molecules and energy to an organism) of glucose via glycolysis is a spon-
taneous process similar to the combustion of glucose. When glucose is burned in
air, six molecules of oxygen gas and one molecule of glucose combine to produce
six molecules of water vapor and six molecules of carbon dioxide gas.
C
6
H
12
O
6
(s) + 6O
2
(g) n 6CO
2
(g) + 6H
2
O(g)
When this reaction occurs, the number of gaseous molecules increases, which
corresponds to a dramatic increase in the number of microstates that describe the
system. Because of this increase, the probability that the reaction will produce
products is greater than the probability that carbon dioxide and water will spon-
taneously form glucose and oxygen. In short, an increase in the number of micro-
states favors spontaneous reactions. We can describe this principle in more
practical terms by introducing entropy.
Entropy (S) can be thought of as a measure of how the energy and matter of
a system are distributed throughout the system. Investigations related to the
concept of entropy began in the 1820s and 1830s with Nicolas Léonard Sadi
Carnot (1796–1832) and Benoit Paul Emile Clapeyron (1799–1864). However,
the concept wasn’t mathematically developed until Rudolf Julius Emmanuel
Clausius (1822–1888) worked on it in 1865. And although Clausius properly
illustrated entropy, its relationship to the molecular level wasn’t illuminated until
Boltzmann did so several decades later.
Entropy isn’t an easy concept to master, but we can gain important insight by
considering the probabilities that have been the focus of our discussion. If the
multiplicity—the number of microstates—increases, the number of ways in
14.2 Why Do Chemical Reactions Happen? Entropy and the Second Law of Thermodynamics 587
Visualization: Entropy
Video Lesson: Entropy and the
Second Law of Thermodynamics
Tutorial: Positional Entropy
