Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

First Thoughts
For us to answer this question, we must think about how much space a face-
centered cubic unit cell occupies. Then, if we know how much space is actually
taken up by atoms, we can determine the amount of free space in the unit cell.
Solution
Because we haven’t specified any particular example, let’s consider a generic fcc unit
cell. The volume of a fcc unit cell is
(r
√
8)
3
. We know that a face-centered cubic
unit cell has four complete atoms (each with a volume of
4
3
πr
3
), so the total vol-
ume of the spheres is
4 ×
4
3
πr
3
or
16πr
3
3
. The ratio of the two volumes will tell us
the percentage of the unit cell that is taken up by atoms:
% occupied
=
16πr
3
3
(r
√
8)
3
=
16π
3
r
3
(
√
8)
3
r
3
=
16π
3
(
√
8)
3
=
16.755
22.627
= 0.7405 ×100% = 74.05%
The percent of occupied space in the unit cell is 74.05%, which leaves 25.95% of the
unit cell vacant. That’s a lot of empty space. Note that the value of r cancels out of
the equation, so our answer is valid for any ccp crystal.
Further Insights
Is there really this much empty space in a solid? Yes, according to our calculations,
there are a lot of holes in a solid. Is the space really empty? As we’ll see later in this
chapter, it isn’t completely empty in a metal. Electrons occupying large molecular
orbitals occupy a lot of this empty space.
PRACTICE 13.2
Polonium forms the simple cubic unit cell. How much of a primitive unit cell is
empty space?
See Problems 7, 8, 11, 12, and 17.
The ease of calculating the volume of the unit cell enables us to determine the
density of a crystal without measuring it. Mathematically, the density of a solid is
the mass divided by the volume. Because we’ve already determined the volume of
the unit cell, we need to determine its mass. How do we do this calculation? If we
know how many atoms make up the unit cell and we know the mass of one atom,
then we can calculate the mass of a unit cell. For example, gold crystallizes in
the cubic closest packed structure (fcc unit cell). The atomic mass of gold is
197.0 g/mol, or 197 amu per atom. As we’ve done before, we’ll keep a couple of
extra figures in each calculation, rounding off only at the end.
197.0gAu
mol
×
1 mol Au
6.022 ×10
23
atoms
=
3.2713 ×10
−22
g
atom Au
Then
3.2713 ×10
−22
g
atom Au
×
4atomsAu
fcc unit cell
= 1.3085 ×10
−21
g/unit cell
What is the density of a block of gold? Because we can calculate the volume and
the mass of any unit cell, we can determine the density of a crystalline solid if we
know the unit cell. For instance, gold has a mass of 1.3085 × 10
–21
g / unit cell
and a unit cell volume of 6.756 × 10
7
pm
3
. The density (mass/volume) can be
calculated:
6.756 ×10
7
pm
3
×
1.0 ×10
−12
m
pm
3
×
100 cm
m
3
= 6.756 ×10
−23
cm
3
1.3085 ×10
−21
g
6.756 ×10
−23
cm
3
= 19.4g/cm
3
548 Chapter 13 Modern Materials
The value we’ve calculated for gold (19.4 g/cm
3
, based solely
on physical measurements of the unit cell) agrees quite
well with the experimental value for the density of gold
(19.3 g/cm
3
). Table 13.2 lists the important calculations for
the face-centered cubic unit cell.
Other information about the crystalline solid can be deter-
mined by examining the unit cell. For example, the number
of species that surround a particular ion can be determined
from the packing arrangement.
EXERCISE 13.3 He Isn’t Heavy, He’s Dense
Some metals are much denser than others. What makes them so dense? The
arrangement of the atoms in the solid, coupled with the mass of the element, ac-
counts for the different densities we observe. Let’s calculate the density of an iron
bar. Iron, whose atomic radius is 124 pm, forms bcc unit cells.
Solution
We can determine the density of a block of iron by dividing the mass of the appro-
priate number of atoms by the volume of the unit cell. The body-centered cubic
unit cell has the volume
r
4
√
3
3
. For an iron body-centered cubic unit cell,
the volume is
124 pm ×
4
√
3
3
= (286.4)
3
= 2.348 ×10
7
pm
3
or
2.348 ×10
7
pm
3
×
1m
10
12
pm
3
×
100 cm
1m
3
= 2.348 ×10
−23
cm
3
In a bcc unit cell containing two atoms of iron, the total mass is
2atoms×
55.847 g
mol
×
1 mol
6.022 ×10
23
atoms
= 1.855 ×10
−22
g
The density of iron is the mass divided by the volume:
1.855 ×10
−22
g
2.348 ×10
−23
cm
3
= 7.90 g/cm
3
The experimentally measured density of iron is 7.86 g/cm
3
.
PRACTICE 13.3
Lead is used as fishing weights because of its malleability and density. What is the
density of lead (Pb)? Lead has an atomic radius of 175 pm and crystallizes in a fcc
arrangement.
See Problems 16, 18, 21, and 22.
Calculating the positions of the atoms in a unit cell based on an X-ray dif-
fraction pattern used to be a tedious task that could take months or years to com-
plete. Today, scientists use the X-ray diffractometer to generate X-rays and direct
them at a single crystal at a variety of different angles. Powerful computers collect
the data over a period of hours and store them until the diffraction pattern is
complete, at which time the computers go to work deciphering the pattern into a
picture of the crystal. Depending on the quality of the crystal, the location of each
of the atoms in the solid can be accurately determined overnight.
13.1 The Structure of Crystals 549
Face-Centered Cubic Unit Cell Calculations
Length of side
(r
√
8)
Volume of unit cell
(r
√
8)
3
Length of diagonal 4r
Mass of unit cell 4 × mass of one atom
TABLE 13.2
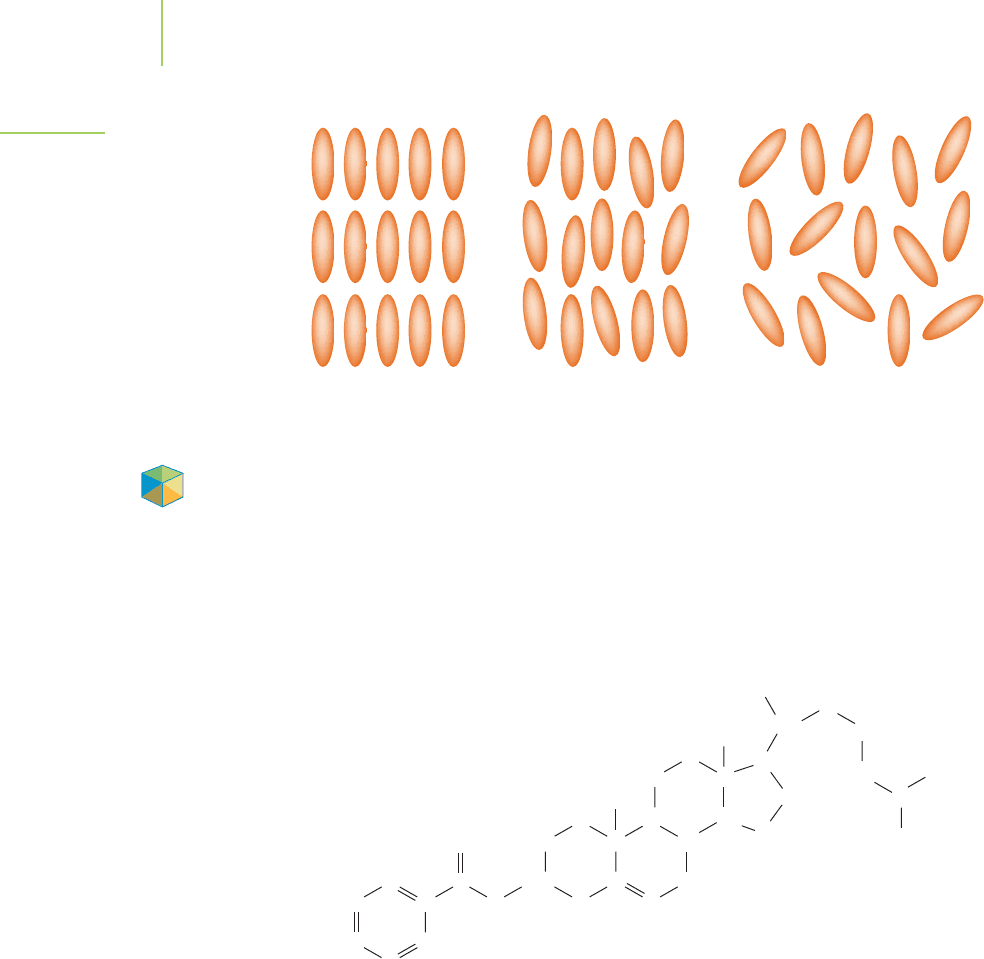
Liquid Crystals
We use liquid crystals in the displays of watches, pocket calculators, computers,
and privacy windows. What is a liquid crystal and how does it work? The liquid
crystal is a transitional phase between a solid and a liquid. There are some excep-
tions, but the molecules of the liquid crystal generally have long, rigid structures
with a strong dipole moment that point along a common axis (Figure 13.12) to
align into highly ordered crystalline solids. An example of a solid that forms a liq-
uid crystal is cholesteryl benzoate.
If the temperature of a solid with these characteristics is increased, the long,
rigid structures of the molecules and the strong dipole moment continue to
maintain a high degree of order within the structure. In fact, even well into
the liquid state, order is maintained despite the increase in translational freedom
(the ability to move in many directions). When the temperature gets hot enough,
the degree of order in the structure is eliminated. In other words, in the transi-
tional state between completely solid and completely liquid, the molecules are
highly ordered and yet quite fluid (the
liquid crystal state).
The strong dipole moment is the key to the behavior of liquid crystals. From
Chapter 9, we know that dipole moments align themselves within an electric
field. When such a field is applied to the liquid crystal, its molecules orient them-
selves with the electric field. This highly ordered structure is transparent to light
directed along the axis of orientation. If the electric field is removed, the transla-
tional freedom of the molecules scrambles their alignment. Visually, a liquid
crystal in this state is opaque because the slight randomness scatters light. The net
effect is a material that can be made transparent or opaque with the flick of a
switch.
H
C
HC
HC
O
CH
3
CH
3
H
3
C
CH
C
C
H
CH
C
O
C
H
CH
2
CH
CH
CH
CH
2
CH
2
CH
2
C
C
CH
2
CH
2
CH
C
CH
2
CH
2
CH
2
CH
3
CH
3
CH
2
CH
2
CH
CH
Cholesteryl benzoate
550 Chapter 13 Modern Materials
Solid Li
q
uid cr
y
stal Li
q
uid
FIGURE 13.12
Liquid crystals. The liquid crystal has
properties that resemble the liquid and
properties that resemble the solid. It is
a fluid, yet highly organized phase.
Application
σ
2s
σ
2s
*
n
2s
n
1s
σ
1s
σ
1s
*
HERE’S WHAT WE KNOW SO FAR
■
The dimensions and the density of a solid can be determined if the mass and
the radii of the components (be they ions, molecules, or atoms) are known.
■
X-ray crystallography is a useful technique that can also determine the
arrangements of atoms in the unit cell.
■
The liquid crystalline state is a transitional phase that exists between the solid
and liquid phase for some molecules. Typically, these molecules have long,
rigid structures with strong dipole moments.
13.2 Metals
To fill a cavity in a tooth, a dentist cleans out the hole and packs it with a com-
posite material made of glass and plastic resin, or with a “silver” filling, to arrest
the growth of the enamel-eating bacteria. The composite filling is popular, but
because the silver filling is much harder, more durable, and relatively inexpensive,
there is still a need for it. Why did we put quotation marks around the word
silver? The filling is actually is a mixture of about 50% mercury and 50% other
metals (including silver, tin, and copper). This is one of countless applications
of metals in our lives. In this section, we will examine the properties of metals
and learn why these elements are so useful.
Using a cast iron skillet to cook your eggs and bacon used to be the rule, not
the exception. You had to have a mitten handy to pick the skillet up off the stove,
or your hand would be badly burned. Metals, such as the iron in the skillet, have
long been known to conduct heat and electricity (see Chapter 5).
Why do metals
conduct heat?
One of the theories that explains these properties (and others) is
called
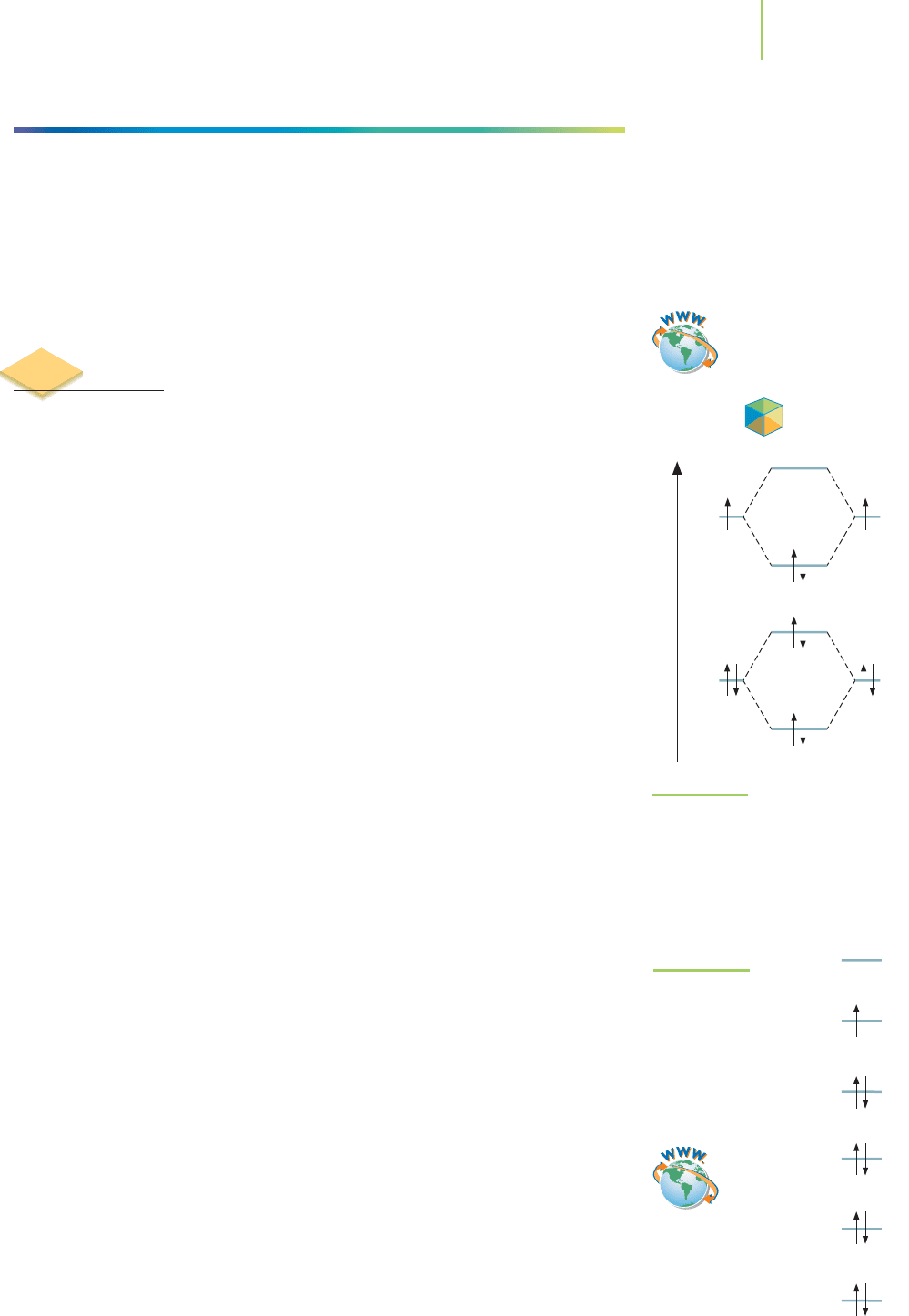
band theory. This theory addresses how adjacent metal atoms interact. For
example, two lithium atoms can interact with each other by sharing their elec-
trons. As we discussed in Chapter 9, this gives rise to our postulate that dilithium
can exist. A closer look at the molecular orbitals of dilithium reveals that the va-
lence electrons come from the 2s orbital. A bonding orbital lies below the energy
of the 2s orbital, and an antibonding orbital lies above the energy of the 2s orbital
(Figure 13.13). The valence electrons are placed in the molecular orbitals in such
a way as to fill the bonding orbital and leave the antibonding orbital empty.
What happens if three lithium atoms participate in bonding? The result of
mixing three atomic valence orbitals gives rise to three valence molecular or-
bitals (Figure 13.14). One of these orbitals is bonding, one is antibonding, and
one is nonbonding. A
nonbonding molecular orbital can be thought of as one that
neither enhances nor diminishes the molecular bonding. The nonbonding mole-
cular orbital arises when there is an odd number of atomic orbitals that mix in a
given energy level. The result of this mixing is an odd number of MOs in the en-
ergy level (one of the orbitals is the nonbonding MO). Three lithium atoms con-
tribute a total of three valence electrons to the valence molecular orbital diagram.
Placing them in the diagram results in a full bonding MO and a half-filled non-
bonding MO.
The overlap of four lithium atoms results in two bonding orbitals (completely
full of electrons) and two antibonding orbitals (empty), as shown in Figure 13.15.
An equal number of bonding molecular orbitals and antibonding molecular or-
bitals are formed. And the bonding orbitals reside at an energy level below that of
the antibonding orbitals.
If we increase the number of atoms that participate in forming molecular
orbitals to eight, we find that half of the orbitals (the bonding orbitals) fill with
electrons. The other half of the molecular orbitals is comprised of the empty an-
tibonding orbitals. In addition, the orbitals within the bonding and antibonding
13.2 Metals 551
σ
2s
σ
2s
2s 2s
Energy
*
σ
1s
σ
1s
1s 1s
*
FIGURE 13.13
Molecular orbital diagram of dilithium.
Two bonding and two antibonding MOs
make up the diagram for dilithium. Plac-
ing electrons in this MO diagram pro-
vides a full bonding MO and an empty
antibonding MO.
FIGURE 13.14
Molecular orbital dia-
gram of trilithium. Note
that the energy of the
MOs derived from 2s
orbitals is approaching
the energy of the MOs
derived from 1s orbitals.
Application
Video Lesson: Metallurgical
Processes
Video Lesson:
Band Theory of
Conductivity

levels begin to have noticeably different energies. This difference arises due to the
requirements of the symmetry for molecular orbitals; as the size of the molecular
orbital increases, the number of nodes within the molecular orbitals increases
and results in a decrease in the magnitude of the molecular orbital’s energy.
Overall, the energy difference between the antibonding and the bonding molec-
ular orbitals becomes smaller (bonding MOs increase in energy and antibonding
MOs decrease in energy).
As more and more atoms combine to make a metallic crystal, the difference in
energy between bonding and antibonding orbitals becomes negligible. The result
is a band of molecular orbitals constructed from the overlap of s orbitals. In met-
als, the range of energies in this band is often wide enough to overlap with simi-
larly constructed bands of p and d orbitals. Many of these bands are empty, or
only partially filled, with electrons. For any metal not at absolute zero, many
of the electrons occupy the unfilled portions of the higher energy bands. Because
the molecular orbitals encompass the entire metallic crystal, the electrons are
free to roam from one end of the metal crystal to the other.
Crystals made from nonmetal elements, however, have a slightly different
arrangement. As we see from Figure 13.15, the lower energy bands are accompa-
nied by an empty higher energy band that doesn’t overlap. For instance, carbon
has a band of molecular orbitals that results from the overlap of the 2s and 2p
atomic orbitals. Higher in energy lies a band of molecular orbitals that result
from the overlap of the 3s and 3p atomic orbitals. The lower-energy, partially
filled bonding molecular orbital band is known as the
valence band. This band
contains the valence electrons. The empty higher-energy molecular orbital band
is the
conduction band. Some nonmetal elements have conduction bands that are
much greater in energy than their valence band. Still others have conduction
bands that overlap or nearly overlap with the valence bands.
Band theory describes a metal as a lattice of metal cations spaced throughout
a sea of delocalized electrons. How does this theory explain why metals are duc-
tile, malleable, conductive, and shiny? Imagine, for example, deforming a metal
bar with a hammer and displacing some of the metal ions. The sea of delocalized
electrons can immediately adjust to the change, so the overall structure doesn’t
become significantly weaker as it is bent. Metals, then, are
ductile (able to be
pulled) and
malleable (able to be pounded) to different degrees. Additionally, be-
cause electrons can easily move from one end of the molecular orbital to the
other, metals can conduct electricity.
Why can metals conduct heat? Kinetic energy can be conveyed easily across a
crystalline lattice that consists of positive ions within a sea of electrons; metals
552 Chapter 13 Modern Materials
2s
Increasing energy
Antibonding orbitals
Bonding orbitals
FIGURE 13.15
Molecular orbital diagram of lithium metal.
As the number of lithium atoms participating
in bonding increases, the regions of anti-
bonding and bonding molecular orbitals
become continuous.
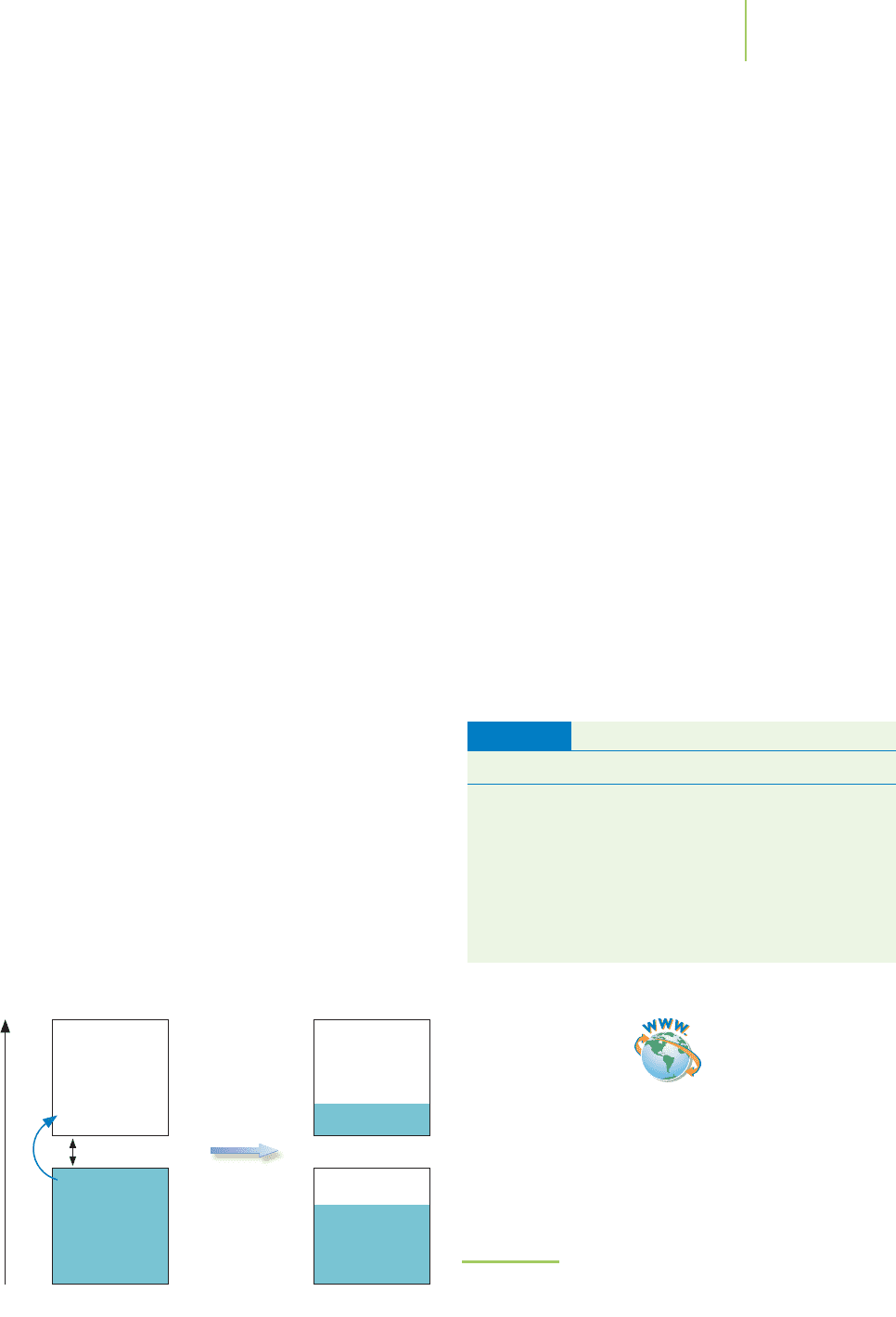
Energy
Small band gap
e
–
Conduction
band
(unfilled)
Valence
band
(filled)
Electrons
excited
Conduction
band
(partially filled)
Valence
band
(partially filled)
conduct heat easily. In other words, kinetic energy can be transferred from posi-
tive ion to positive ion with ease. This stands in contrast to the difficulty involved
in transferring kinetic energy across an ionic or molecular solid, where the ki-
netic energy must be transferred from cation to anion to cation (or from mole-
cule to molecule). This explains why metal ice cube trays feel colder than plastic
ice cube trays. The metal trays, as thermal conductors, are better able to pull the
heat from your hand. The plastic ice cube trays are made of a thermal insulator
(there isn’t enough thermal energy available to promote electrons into the con-
duction band in the plastic). Or, to say it differently, the valence electrons in plas-
tic are tied to covalent bonds specifically joined to a given pair of atoms and are not
free to move around.
Band theory also explains why metals are shiny. Because the molecular or-
bitals within the valence bond are so plentiful and close together, electrons can
absorb and release almost any wavelength of light from the infrared to the ultra-
violet. The absorption promotes the electron into an unfilled molecular orbital
within the band; the release of a photon returns the electron to its original loca-
tion in the band. The result is that metals reflect light, giving rise to the luster that
we associate with them. White paper also reflects light but does so diffusely (in all
directions). Some metals have a distinctive color. For example, gold has a yellow
hue. This is due to the inefficiency of the metal when it reflects certain frequen-
cies of absorbed light. These metals are still shiny; they just don’t reflect all wave-
lengths of light equally.
Band theory is used in determining the color of paints, identifying the insu-
lating value of materials in your home, and (remembering the opening scenario
of the chapter) in the hospital as well, in the form of heart monitors, IV pumps,
calculators, and many other instruments.
What do such instruments have in
common?
They are all controlled by small computers, and at the heart of all
computers is the element silicon. Why silicon? Its
band gap
is the key. A band gap is a small energy gap that exists be-
tween the valence band and the conduction band, as illus-
trated in Figure 13.16. The existence of a band gap in silicon
suggests that a certain amount of energy is needed in order
to promote an electron from the valence band into the con-
duction band. If the gap is relatively small, as it is in silicon,
the metal is a
semiconductor. In these cases, the amount of
energy needed to promote an electron into the conduction
band is relatively small. (See Table 13.3 for a list of some
compounds and their band gaps.) When the temperature
13.2 Metals 553
FIGURE 13.16
The band gap in silicon.
Band Gaps of Some Common Materials
Material Band Gap
Insulator Carbon (diamond) 502 kJ/mol
(>300 kJ/mol) Zinc sulfide 350 kJ/mol
Semiconductor Silicon 106 kJ/mol
(50–300 kJ/mol) Selenium 215 kJ/mol
Germanium 64 kJ/mol
Conductor Lithium 0 kJ/mol
(<50 kJ/mol) Tin (gray tin) 7.7 kJ/mol
TABLE 13.3
Video Lesson: Intrinsic
Semiconductors
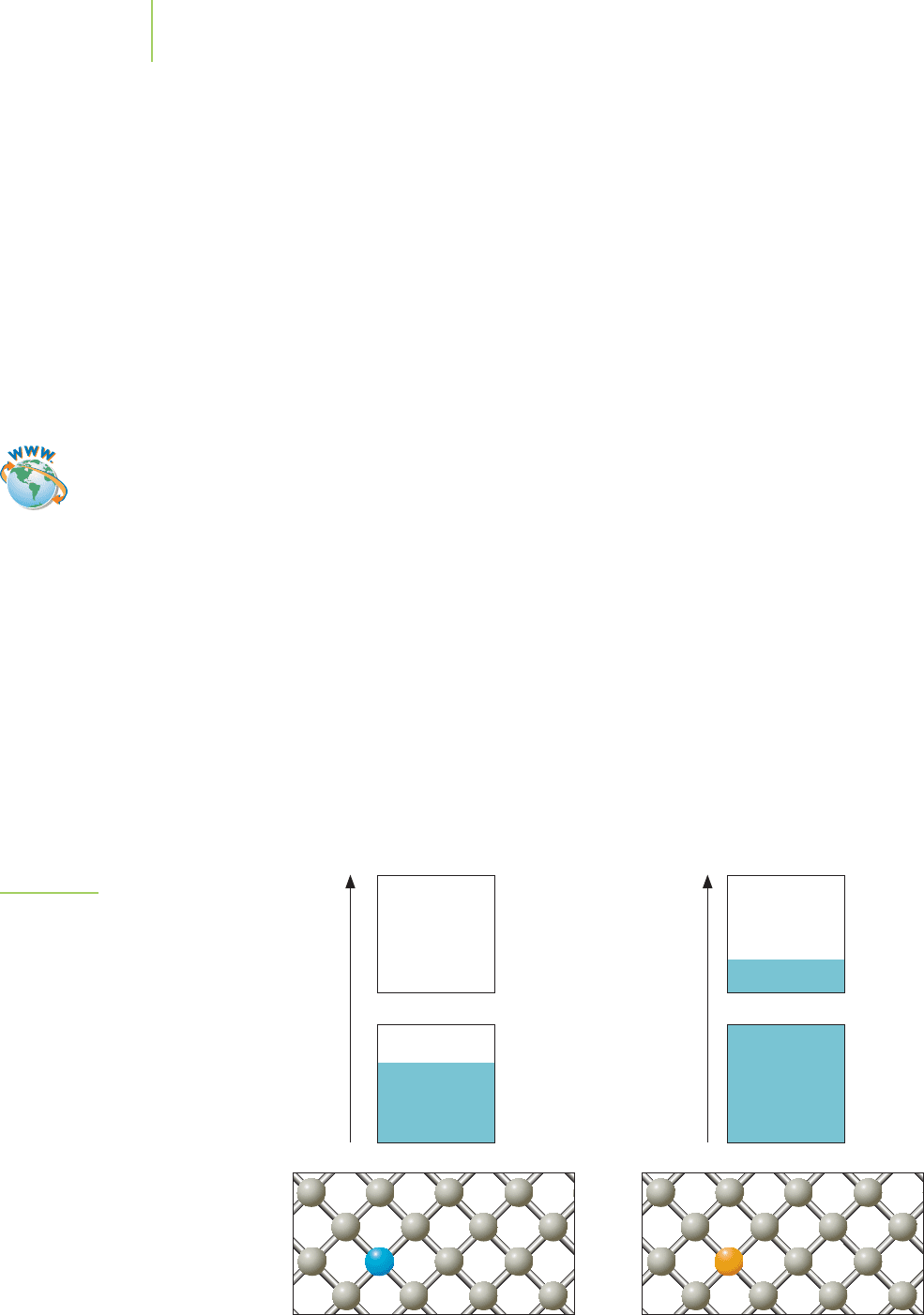
is low, there isn’t enough kinetic energy to promote many electrons into the
conduction band, and the metal doesn’t conduct much electricity. Increase
the temperature of the semiconductor, and the conductivity increases. Silicon
(band gap = 106 kJ/mol) and germanium (band gap = 64 kJ/mol) are good
examples of semiconductors.
If the band gap is sufficiently large that there are barely any electrons at room
temperature in the conduction band, the material is an
insulator. Only a tiny
amount of electrical conductivity can be found in an insulator. However, as an in-
sulator becomes hotter, its conductivity increases. Our plastic ice cube tray is a
good example of an insulator. Diamond is another good insulator (the band gap
in diamond is 502 kJ/mol). In short, when the band gap is large, the material acts
as an insulator. Remove the gap, and the material is a
conductor. For example,
compare the band gap in diamond to the amount of energy required to promote
an electron into an unfilled orbital in a conductor such as lithium, roughly
10
–45
kJ/mol.
Adding small amounts of other elements to the metal can modify the behav-
ior of a semiconductor. For example, if we
dope (add an atom or other compound
to) silicon (Group IVA) with an element that has more valence electrons than sil-
icon (such as phosphorus, Group VA), the extra valence electrons must be placed
into the conduction band (Figure 13.17). As a consequence, the semiconductor
becomes capable of conducting current. The result is called an
n-type semicon-
ductor
because the addition of the phosphorus adds extra negative charges (elec-
trons) to the system. However, if we dope silicon with an element containing
fewer valence electrons (such as aluminum, Group IIIA), there will not be
enough valence electrons to fill the valence band. The result: Electrons in the va-
lence band can be easily excited to move to an unoccupied molecular orbital. This
semiconductor is electrically conductive if we apply a small electric field. We call
it a
p-type semiconductor because the addition of gallium can be thought of as
introducing positive holes in the valence band. The properties of the n- and
p-type semiconductors make them quite useful for the production of small elec-
tronic devices.
554 Chapter 13 Modern Materials
Conduction
band
(unfilled)
Valence
band
(filled)
Energy
Energy
Conduction
band
(partially filled)
p-type n-type
Valence
band
(partially filled)
Al
Si
P
Si
FIGURE 13.17
Doping the semiconductor. An n-type semi-
conductor contains extra electrons added
to the conduction band. A p-type semicon-
ductor is missing some electrons from the
valence band.
Video Lesson: Doped
Semiconductors

EXERCISE 13.4 The Color of Paint
The mineral greenockite (CdS) is best known as cadmium yellow and is used as a
yellow pigment in paints. Cadmium sulfide has a medium-sized band gap (4.2 ×
10
–19
J/photon). Judging on the basis of the information in Table 13.3, is greenock-
ite an insulator, a semiconductor, or a conductor? What color light does cadmium
sulfide absorb?
Solution
The band gaps in Table 13.3 are listed in units of kilojoules per mole, whereas this
exercise gives the size of the band gap for greenockite in joules per photon. We can
use Avogadro’s number to make the units the same.
4.2 ×10
−19
J
photon greenockite
×
6.022 ×10
23
photons
mol greenockite
×
1kJ
1000 J
=
250 kJ
mol greenockite
This value places cadmium sulfide in the semiconductor region. The promotion of
an electron from the valence band to the conduction band actually requires 4.165 ×
10
–19
J. Using the equations E = hv and c = λv, we find a frequency of 6.286 ×
10
14
Hz and a wavelength of 476.9 nm. This corresponds to the absorption of blue
light. A solution of cadmium sulfide appears yellow to the eye.
PRACTICE 13.4
What is the largest wavelength of light, in nanometers, that can be absorbed by a
particular semiconductor if the band gap is determined to be 0.95 eV? (1 eV/atom =
96.485 kJ/mol).
See Problems 27, 28, 35, and 36.
Perhaps the greatest benefit that a semiconductor offers is its ability to act as
a
photovoltaic device, in which light energy can be used to generate an electric
current. Photovoltaic cells capable of over 30% efficiency have been designed.
How is the sunlight converted into electricity? When silicon is doped with two
elements such as phosphorus (Group VA) and gallium (Group IIIA), the semi-
conductor contains both electron-rich and electron-deficient areas. Light energy
throughout the visible and IR range (the particular range of wavelengths depends
on the chemical composition of the semiconductor) provides enough energy for
electrons to “jump” the band gap and enter the conduction
band. This enables both negative charges (electrons) and posi-
tive holes to move so that the flow of electrons can be harvested
as energy.
Photovoltaic cells have been used in many applications,
from the solar-powered calculator to solar-powered water
heaters, space stations, and satellites. The use of photovoltaic
cells to make a solar-powered car, such as that shown in Figure
13.18, is the next big hurdle. Because our world demands fossil
fuels as asourceof energyfortransportation,and because of our
limited supply of this natural resource, alternative-energy auto-
mobiles will one day be commonplace. Currently, auto makers
and researchers have been able to develop solar-powered cars
that can travel an average of 50 miles an hour relying solely on
the photovoltaic cell. However, despite the estimated 60% de-
cline in costs to implement the use of photovoltaic cells over the
last 20 years, sales of solar-powered vehicles are still dwarfed by
sales of those powered by fossil fuels; solar-powered autos made
up less than 1% of the total auto sales in 2003.
13.2 Metals 555
FIGURE 13.18
The solar-powered car is currently being tested as a replacement
for the gasoline auto that we use today. This solar-powered
prototype is not too far from what we may be driving in the
near future. Note the photovoltaic cells on the roof of the car.
Application
C
HEMICAL
ENCOUNTERS:
Photovoltaic
Devices
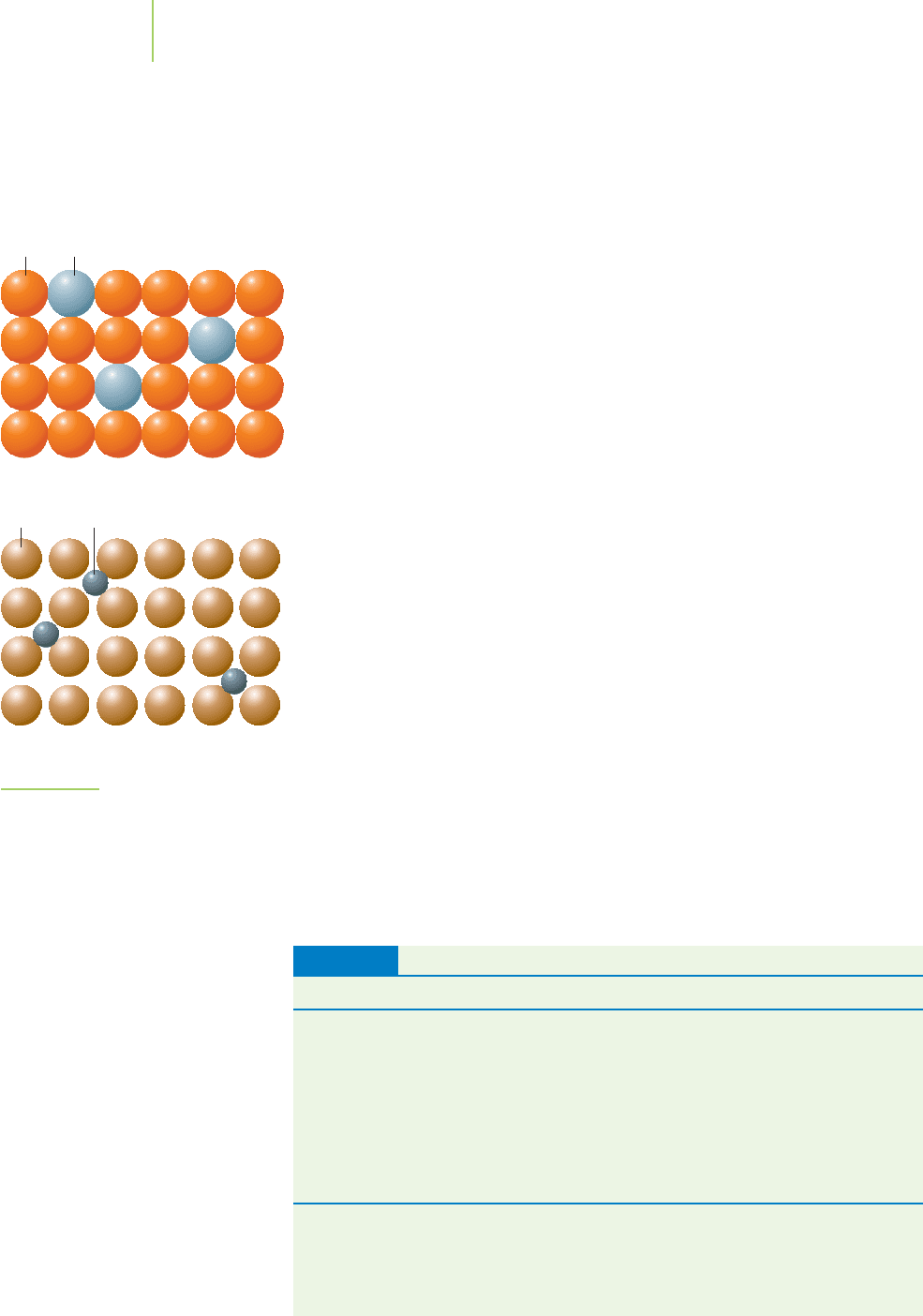
Alloys
We return now to our opening theme of materials used in the hospital by noting
that the limited properties of the metals don’t lend themselves well to any more
than routine medical tasks. For example, surgeons have a relatively limited num-
ber of choices in materials when replacing a hip joint. They need something as
strong and durable as metal, but they don’t use solid copper, silver, or gold.
Why don’t they use a coinage metal for a hip joint? Many of the pure metals
are too malleable and ductile to withstand the forces in a human body. A
gold hip joint would easily bend when a patient walked with it. Other factors,
such as the cost and toxicity of some of the pure metals, are also important.
In fact, it’s rare that we see pure metals used in the “real world.”Because there
are only a limited number of pure metals, and because the properties of
those pure metals are often not what is required, we look to mixtures of met-
als to give us the properties we need.
A homogeneous mixture of two or more metals is known as an
alloy.The
elements are typically mixed together in the molten state and then allowed
to cool to form the alloy. Alloys are useful because their properties can be
adjusted by changing the ratio in which the elements are combined. (The
Bronze Age is named after the discovery of one of the first of these metal
blends.) Typically, alloys are less ductile and less malleable than pure metals.
Table 13.4 lists some of the common alloys, along with a typical composi-
tion for each and several uses to which each is put. Manufacturers vary the
actual composition of each type of alloy to change its properties slightly.
Two different classes of alloys exist, the
substitutional alloys and the inter-
stitial alloys
, illustrated in Figure 13.19. Substitutional alloys include metals
such as brass, sterling silver, pewter, and solder. In a substitutional alloy, spe-
cific atoms within the lattice structure of a metal are replaced with other
metal atoms. In the example of sterling silver, an average of 7 silver atoms
out of every 100 are replaced with copper atoms. Interstitial alloys are made
of metals with “impurities” trapped in the spaces of the lattice structure. By
far the most important of these is steel. Steel, which we discussed in Section 7.2,
contains carbon atoms located in the spaces between the atoms in an iron atom
lattice.
556 Chapter 13 Modern Materials
Common Alloys and Their Uses
Name Common Composition (mass %) Uses
Substitutional
Brass 90 Cu : 10 Zn Decorative fittings
Pewter 85 Sn : 7 Cu : 6 Bi: 2 Sb Plates, jewelry, figurines
Medal bronze 92–97 Cu : 1–8 Sn : 0–2 Zn Medals, artwork
14-carat gold 58 Au : 30 Ag : 12 Cu Jewelry
Sterling silver 92.5 Ag : 7.5 Cu Jewelry, kitchenware
Coinage silver 90 Ag : 10 Cu Silver coins
Plumber’s solder 67 Pb : 33 Sn Pipe solder
Interstitial
Steel 98–99.9 Fe: 0–2 C Building materials
Stainless steel >10.5 Cr: <89.5 Fe Eating utensils,
structural materials
TABLE 13.4
An interstitial alloy
Fe
C
Cu
A substitutional alloy
Ag
FIGURE 13.19
Substitutional versus interstitial alloys.
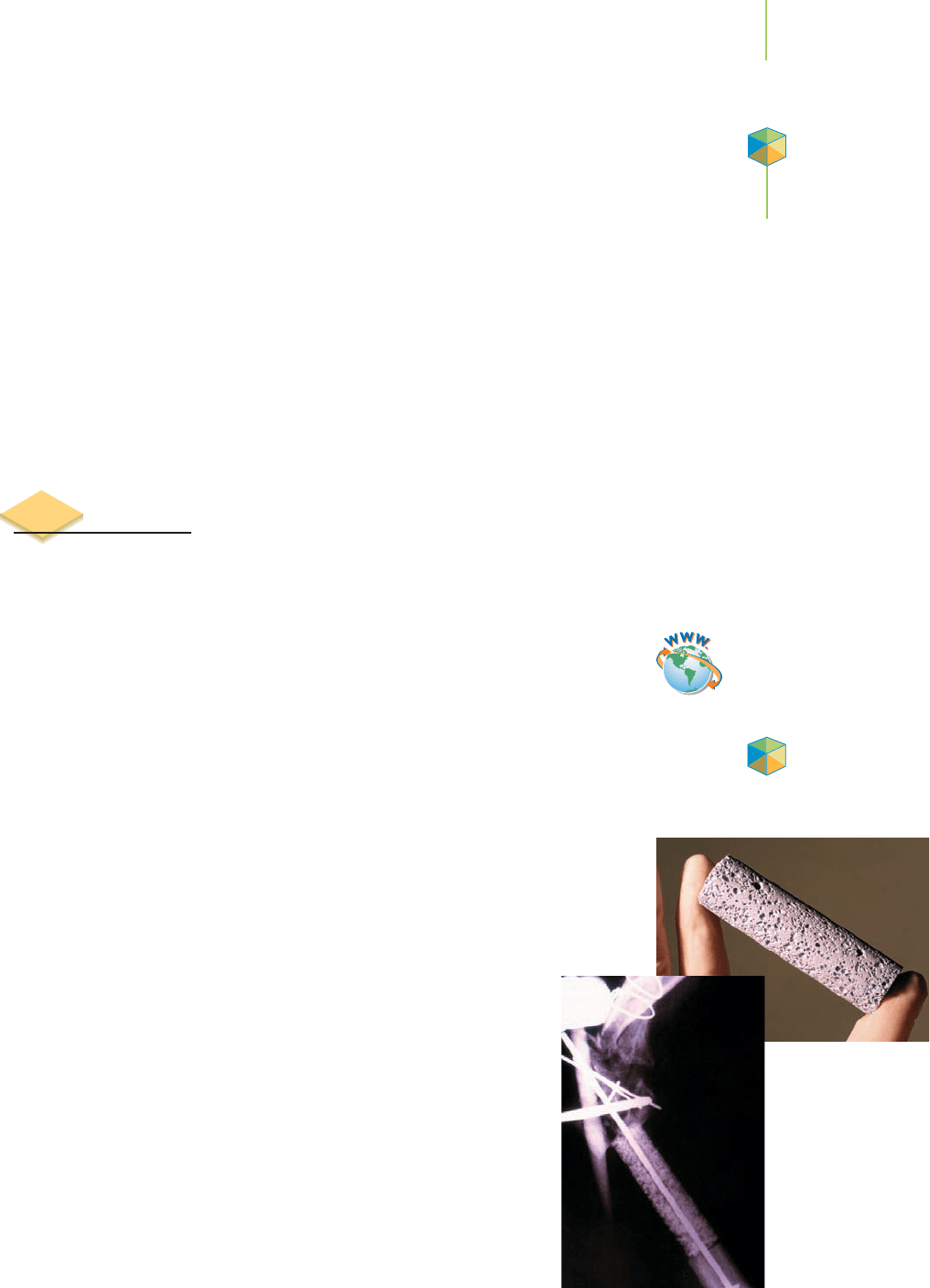
Amalgams
An amalgam is an alloy containing the metal mercury. Dentists use an amalgam
for filling teeth by mixing a powder containing silver and tin and then adding
mercury. The amalgam immediately begins to form at room temperature, mak-
ing a complex and very hard mixture of Ag
2
Hg
3
and Sn
7
Hg.
14Ag
3
Sn + 65Hg n 21Ag
2
Hg
3
+ 2Sn
7
Hg
Other amalgams do exist. For instance, the amalgamation of gold with mer-
cury is one way in which gold dust was extracted from powdered gravel in the
California gold rush era of the mid- to late nineteenth century. Because of
the hazards of mercury poisoning, the use of mercury has been supplanted by
other means of extracting gold (see Chapter 4). Unfortunately, it continues to be
used by individuals and small companies in the poorer countries of the develop-
ing world. Its use has led to mercury poisoning of the workers and the local water
supplies. Other amalgams include zinc and cadmium, used in batteries to in-
crease their lifetime (see Chapter 19).
13.3 Ceramics
According to the American Academy of Orthopedic Surgeons, most of us will
have two bone fractures in our lifetime, and many of these accidents will require
immediate medical attention. Some of the casts used to hold fractured bones in
place as they heal are made of fiberglass or plastic, but the less expensive plaster
cast is still widely used. The plaster, also known as plaster of Paris, is a ceramic
material made from calcium sulfate hemihydrate (CaSO
4
·
1
⁄2 H
2
O) and water.
Why does plaster harden? This is the subject of the accompanying NanoWorld/
MacroWorld feature.
Ceramics are a class of nonmetallic materials that are made of inorganic com-
pounds. They can be either amorphous or crystalline in structure. Examples of
ceramics include glass, clay pottery, cement, bricks, and china dinner plates. Be-
cause ionic bonds exist within a ceramic material, the band gap is typically quite
large. These compounds therefore act as insulators with low electrical conductiv-
ity, high melting point, and a lack of malleability and ductility, but they do have
good resistance to corrosion. With the exception of glass, melting does not
reshape ceramics. Typically, a mixture of the finely ground ceramic and some
binders is used to mold the material into the desired shape. After heating of the
new shape (called firing), the ceramic becomes hard. Compare the properties of
the ceramics with those of other materials (see Table 13.5 on page 559).
Research into the uses of ceramics as bone replacements or sup-
plements is currently under way. One goal of the research is the
ability to replace badly damaged bones with synthetic material that
later becomes bone, rather than using bone from elsewhere in the
patient’s body. Ceramics made from hydroxyapatite (see Chapter 8)
show some promise in this area because they contain the same
sponge-like structure as real bone. This enables new bone to grow
and integrate into or replace the ceramic material.
The large band gap and the relatively low coefficient of thermal
expansion in a ceramic material allow design engineers to use
ceramics in places where insulation against extremely high tempera-
tures is needed. Of particular interest is the use of ceramics as
insulation on the space shuttle. During a typical mission, the space
shuttle’s hull is subjected to temperatures of –156°C during orbit
and up to 1650°C as the shuttle reenters the Earth’s atmosphere.
13.3 Ceramics 557
Application
C
HEMICAL
ENCOUNTERS:
Dental Amalgams
Hydroxyapatite
(above) can be
formed into
replacements for
bones, as seen in
the X-ray (left).
Application
Video Lesson: Ceramics and
Glass
