Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

518 Chapter 12 Carbon
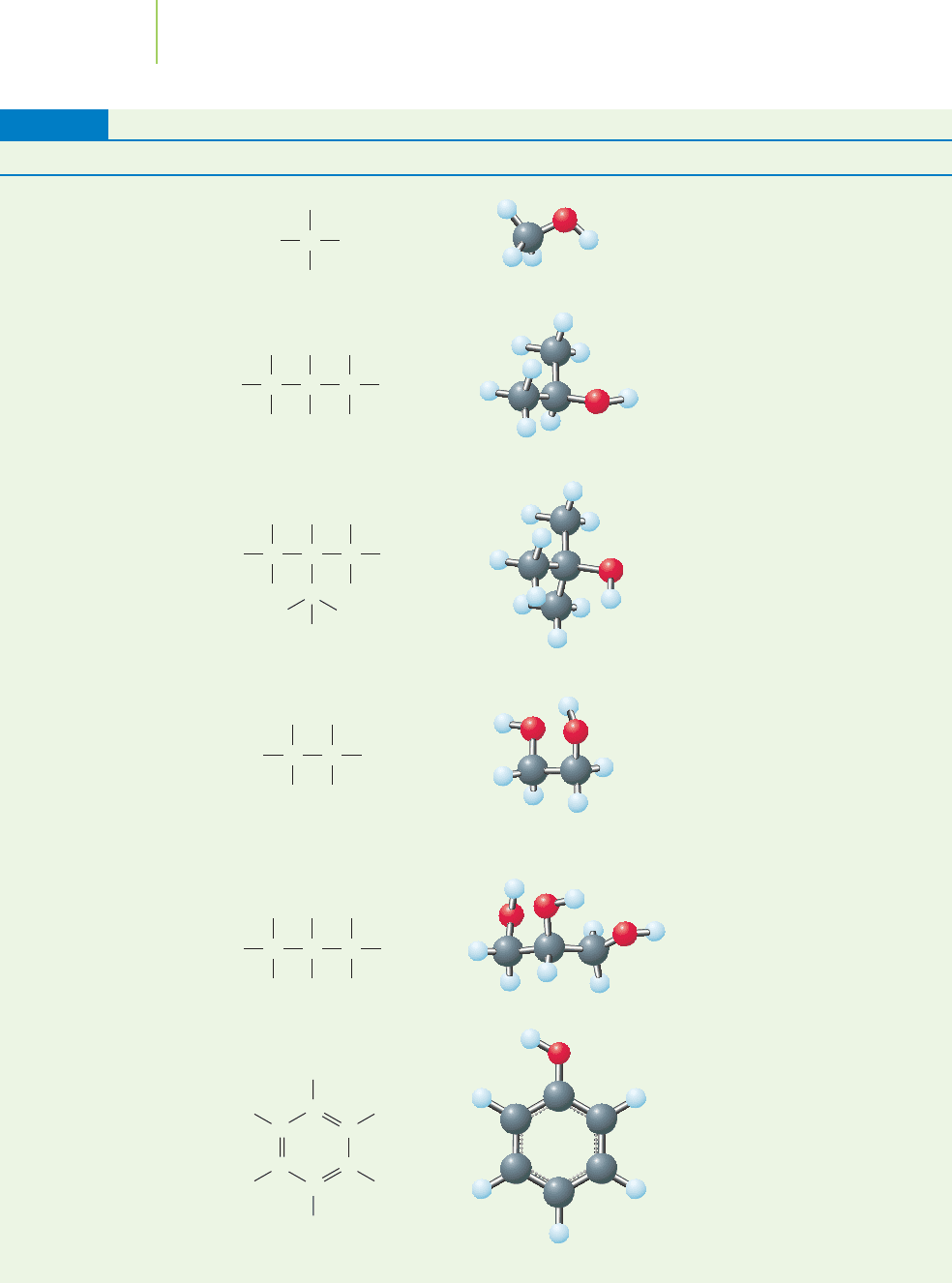
Structures and Uses of Selected Alcohols
Common Name Lewis Structure 3-D Ball-and-Stick Structure Selected Uses
Methanol Fuel, fuel additive
Rubbing alcohol
Phenol
Found in cosmetics and
foods
1,2,3-Propanetriol
(glycerol)
Antifreeze, de-icing
agent
1,2-Ethanediol
(ethylene glycol)
Solvent in chemical
synthesis
2-Methyl-2-propanol
(tertiary butyl alcohol)
2-Propanol
(isopropyl alcohol)
TABLE 12.8
HC
H
H
OH
HC
H
H
C
OH
C
H
H H
H
HC
H
C
OH
H
C
C
H
H
H
HH
H
HC
OH
C
OH
H
H H
HC
OH
C
OH
H H
C
OH
H
H
C
C
C
C
H
OH
C
H
H
H
H
C
Making resins (viscous
substances often used as
adhesives) for use in
plywood manufacture
and automotive
applications
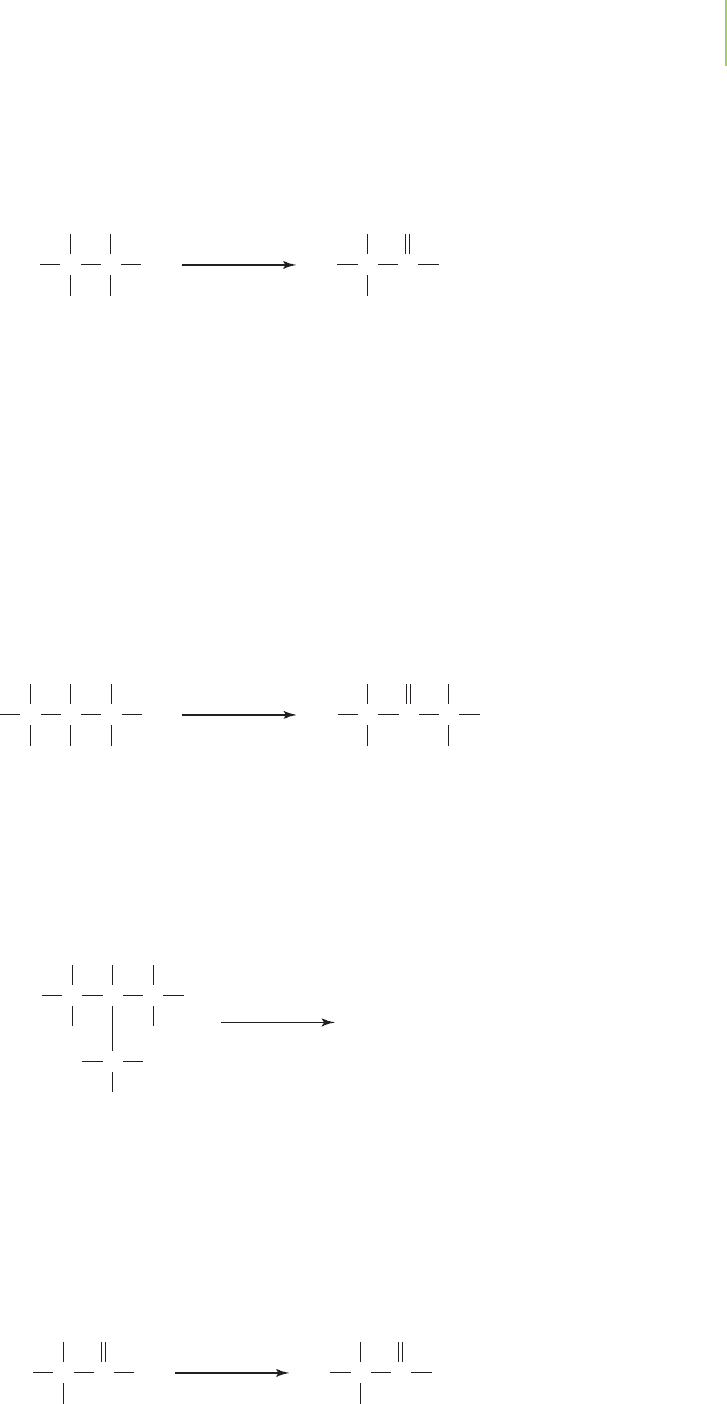
12.10 From Alcohols to Aldehydes, Ketones, and Carboxylic Acids 519
Note that this reaction is similar to the dehydrogenation reactions of the alkanes.
As another example, ethanol can be oxidized to ethanal. Ethanal, also known as
acetaldehyde, is used primarily in the production of polymers (Section 12.12)
and medicines.
An aldehyde is named by taking the name of the alcohol from which it is de-
rived and replacing the ending -ol with -al. No number is needed as part of the
name to indicate where the aldehyde group occurs in the compound, because it
always occurs at the end of a carbon chain.
The hydroxyl group of secondary alcohols can be oxidized to a carbonyl
group. In this case, compounds called
ketones are formed. Ketones differ from
aldehydes in that they have two carbon atoms bonded to the carbonyl carbon
atom. For example, 2-propanol can be oxidized to propanone (acetone).
Propanone is an excellent solvent because of its ability to dissolve both polar
and nonpolar molecules. It has a low boiling point and a low toxicity, finding use
as a solvent for paints and as a fingernail polish remover. For these reasons, over
3 billion kilograms of propanone are used each year in industry.
Tertiary alcohols do not react with oxidizers to make carbonyl compounds.
For instance, there is no reaction when we attempt to oxidize 2-methyl-2-
propanol under the conditions typical for primary and secondary alcohols. This
lack of reactivity occurs because the carbon bearing the —OH group does not
have a hydrogen that can be removed to make the carbonyl (CPO).
Aldehydes have a characteristic biting and often unpleasant aroma. Ketones,
on the other hand, have an aroma that is not so unpleasant, but more medicinal.
Chemically, though, the important difference between aldehydes and ketones is
that the aldehydes can be oxidized further to form
carboxylic acids. The carboxylic
acids carry the
carboxyl functional group, the combination of a carbonyl and a
hydroxyl group. Ethanal, for example, can be easily oxidized to ethanoic acid.
Oxidation
HC
H
C
O
OH
H
C
O
HC
H
H
H
Ethanal Ethanoic acid
Oxidation
No reaction
2-Methyl-2-propanol
HC
H
C
OH
H
C
H
C
H
H
H
HH
Oxidation
HC
H
C
O
C
H
H
H H
C
H
H
H
C
OH
H
HC
H
H
2-Propanol Propanone
Oxidation
HC
H
C
O
H
H
C
OH
H
H
HC
H
H
Ethanol Ethanal
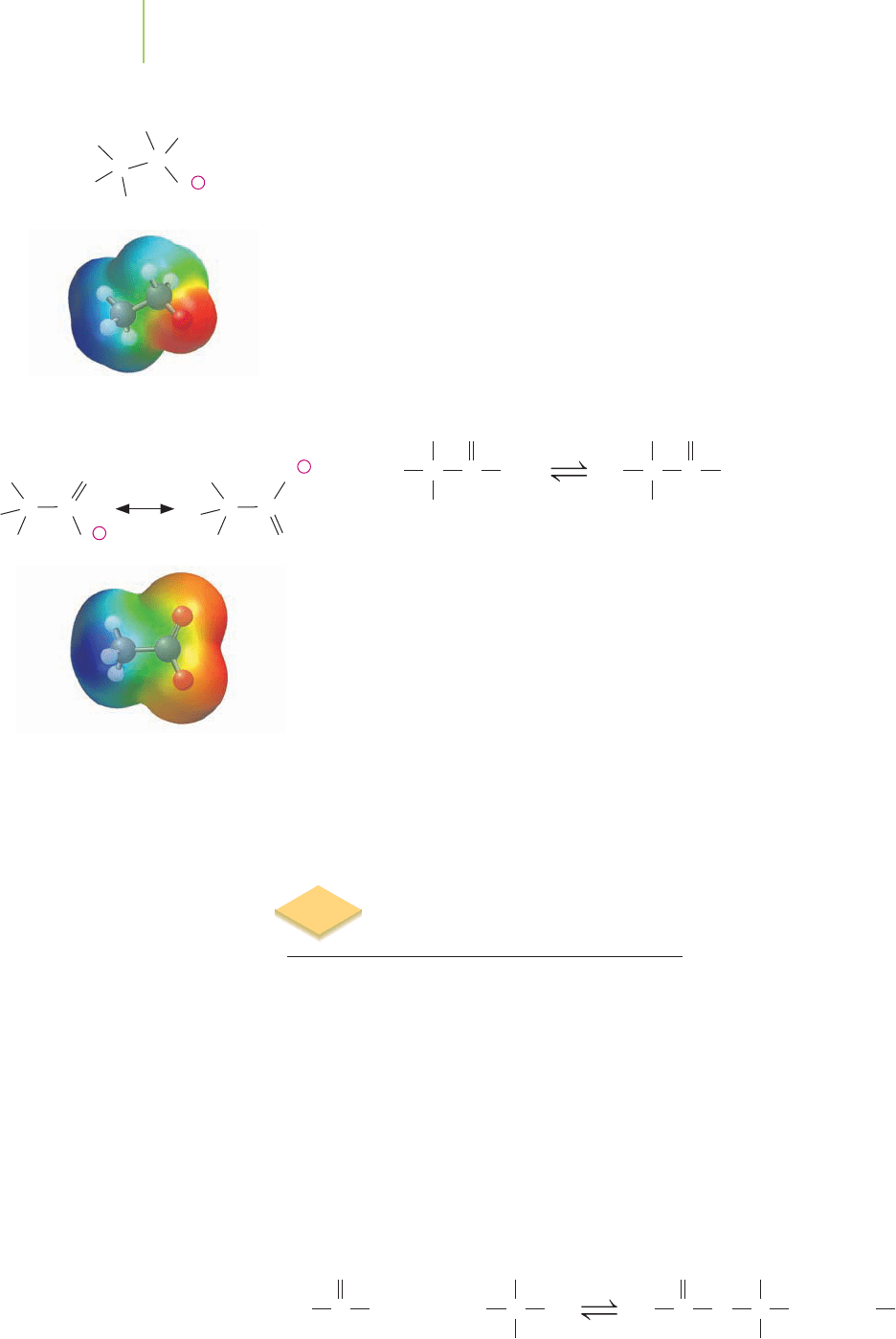
CC
O
–
H
HO
H
CC
O
–
H
HO
H
Ethanoate
520 Chapter 12 Carbon
Ethanoic acid (also known as acetic acid) is the organic acid that we use as
vinegar when it is in the form of a weak solution (typically about 4 to 5% by
mass) in water. Some bacteria can perform the conversion of ethanol to ethanoic
acid, which can cause wines to spoil when they “turn to vinegar” due to bacterial
contamination. However, this process can also be exploited purposefully to pro-
duce the wine vinegars that are widely used in cooking. Industrially, ethanoic acid
is used in the manufacture of plastics, pharmaceuticals, dyes, and insecticides.
The carboxyl functional group is weakly acidic due to the dissociation of a
hydrogen ion from the oxygen. The result is the formation of a
carboxylate anion
and a hydrogen ion (i.e., a proton). Although it would appear that any hydrogen
on an oxygen could dissociate in a similar manner, the carboxylic acids have
a special feature that alcohols do not have (hence the latter are not acidic). Let’s
explore this property further.
Why does the carboxyl functional group dissociate?
When we draw Lewis dot structures of compounds, we must always consider
the possibility that the electrons could be placed in different locations (see Chap-
ter 8). This would generate a resonance structure of the compound. The car-
boxylate anion possesses this ability. In fact, we can draw two good resonance
structures for this ion. The resonance spreads the large negative charge across the
functional group and helps to stabilize the carboxylate by lowering the energy of
the anion.
Why doesn’t the alcohol functional group also dissociate? If a hydrogen ion
were to leave the molecule, a negative charge would reside on the oxygen. Unfor-
tunately, there aren’t any other resonance structures that we can draw for this
alkoxy anion. Therefore, the electrons cannot be spread to other atoms by reso-
nance, and the resulting alkoxyl anion is not energetically stabilized.
12.11 From Alcohols and
Carboxylic Acids to Esters
An extremely important reaction, widespread throughout both natural and in-
dustrial chemistry, takes place between the alcohol and carboxylic acid functional
groups. These two groups can react, often with an acid catalyst such as sulfuric
acid, to eliminate a molecule of water in a reaction known as a
condensation reac-
tion
. In the reaction, the carbonyl carbon from the carboxyl group becomes
bonded to the oxygen atom from the alcohol group in what is known as an ester
linkage or ester bond. Compounds containing this arrangement of atoms
(carbonyl–oxygen–carbon) are known as
esters. The simplest possible ester is
methyl methanoate (also known as methyl formate), formed when methanol
reacts with methanoic acid. Note that the first part of the name of an ester is
derived from the alcohol that can be used in its formation, and the second part of
the name is derived from the carboxylic acid component.
HOHHC
O
O
H
HC
H
HC
H
H
HO
Methanol
C
O
OHH
Methanoic acid Methyl methanoate
H
HC
H
C
O
O
H
C
O
OHC
H
H
H
Ethanoic acid Ethanoate
Ethoxide
C
C
O
–
H
H
H
H
H
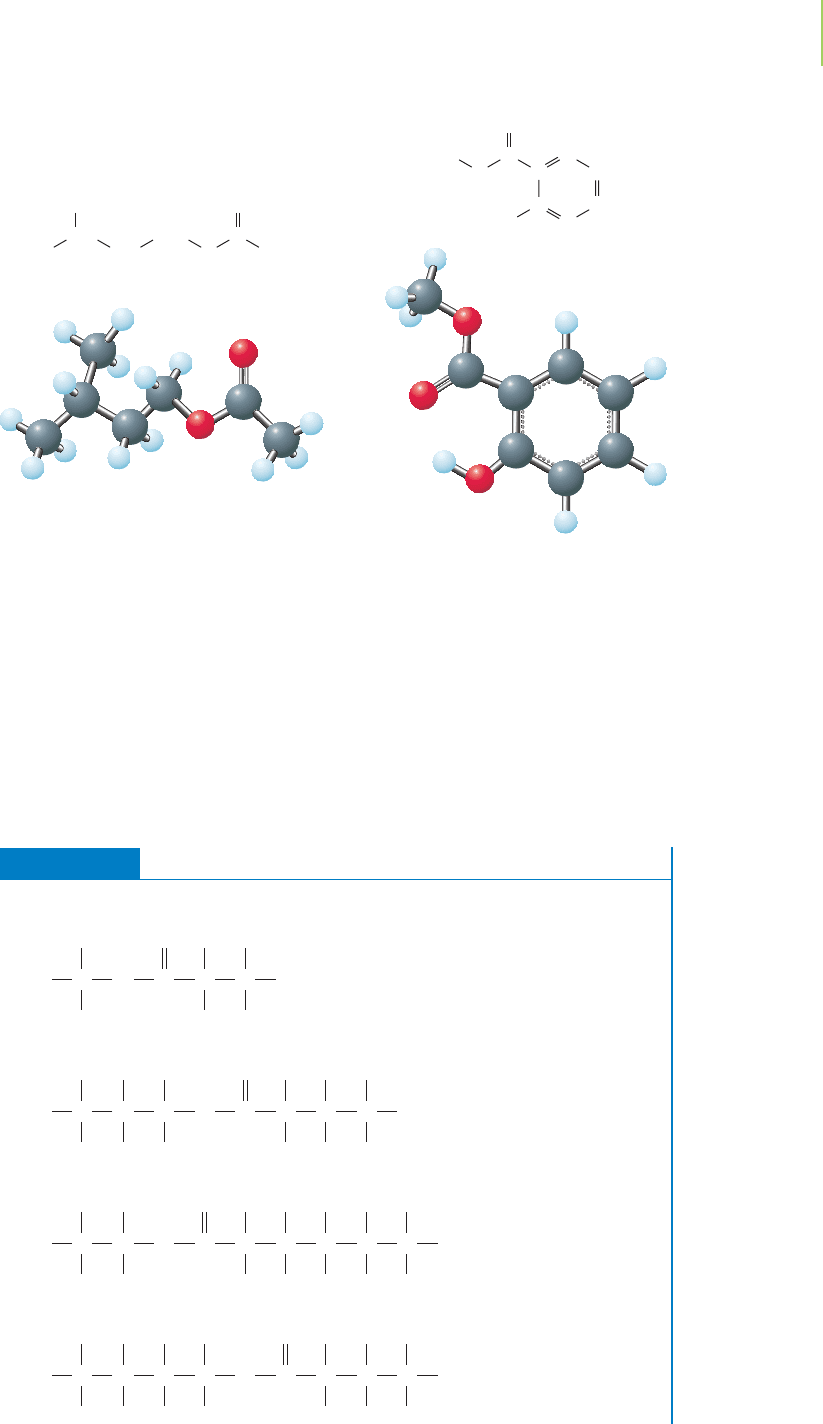
Compounds that contain the ester functional group often have a very fruity
aroma and in nature are found in plant oils. For example, isopentyl ethanoate has
the odor of ripe bananas, and methyl salicylate smells like wintergreen. For this
reason, esters are often used in industry to lend flavors and odors to products that
we buy. Esters are also used in industry as solvents, due to their ability to dissolve
both polar and nonpolar solutes. For example, ethyl ethanoate (commonly
known as ethyl acetate) is found in cleaners and glues.
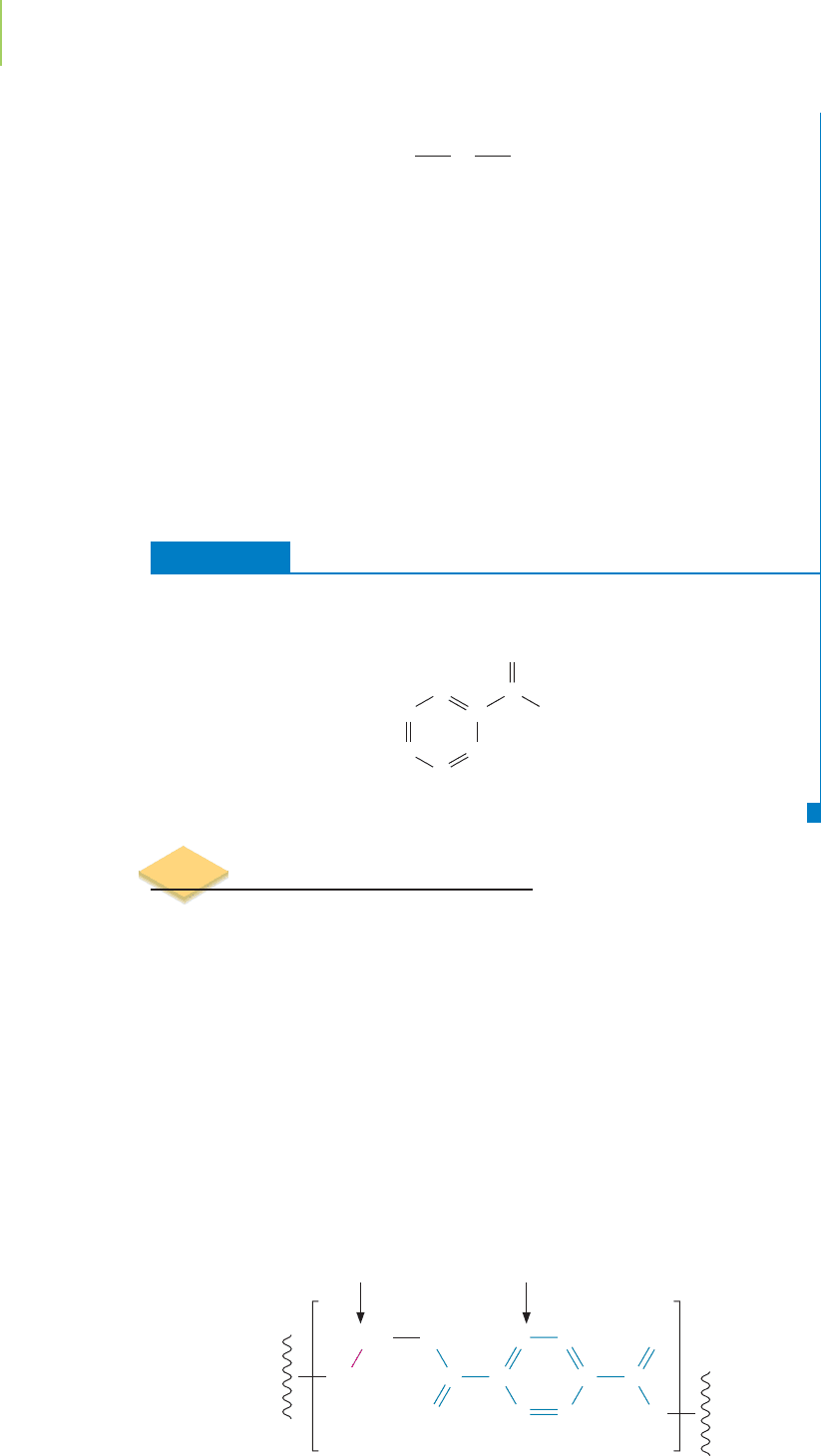
EXERCISE 12.6 Nomenclature of Esters
Provide names for each of these esters.
C
H
C
H
H
H
C
O
C Od. H
H
C
C
H
H
H
H
H
C
H
H
C
H
H
H
C
H
C
H
H
H
O
O
C Cc. H
H
C
C
H
H
H
H
H
C
H
H
C
H
H
H
C
H
C
H
H
H
H
C
O
O
H
C
b. H
H
C
C
H
H
H
C
H
H
H
C
H
O
H
C
O
C
H
H
C
H
Ha. H
H
12.11 From Alcohols and Carboxylic Acids to Esters 521
Isopentyl ethanoate Methyl salicylate
H
H
CCCH
3
CHO
CHCO
CH
3
CH
3
CH
3
CH
2
O
CH CH
2
C
O
CHC
O
First Thoughts
All ester names are in the form “ yl anoate.” The first part of the name is
derived from the part of the molecule that was originally an alcohol, and the second
part of the name is derived from the part of the molecule that was originally a car-
boxylic acid. Therefore you need to find the alcohol component, which is the alkyl
group linked to the O atom, count its carbon atoms to get the first part of the name,
and then count the carbon atoms in the rest of the molecule to get the second part
of the name.
Solution
The names are (a) methyl propanoate, (b) propyl butanoate, (c) ethyl hexanoate,
and (d) butyl butanoate.
Further Insights
Working in reverse, you can figure out the name of an ester that will form between
any alcohol and any carboxylic acid, just by linking their names in the correct order
and manner. Trace through that process for the esters considered above.
PRACTICE 12.6
Shown below is the structure of benzoic acid. Can you draw the structure of ethyl
benzoate? ...ofbenzyl ethanoate?
See Problems 81–84.
12.12 Condensation Polymers
Industrial preparation of polyethylene, polyvinylchloride, and polystyrene occurs
by addition polymerization. But that is not the only way to make a polymer. A
major class of polymers that are very useful in our lives are constructed by a
condensation reaction. Condensation reactions entail the removal of a small
molecule as two larger molecules are connected. If a polymer is the product of the
reaction, we call the process
condensation polymerization.
Esters can be made by condensation reactions. The process,
esterification, can
be used to link a variety of monomers into long polymer chains. The products of
the reaction are
polyesters, which may be familiar to you as parts of clothes,
bedding, fabrics, upholstery, ropes, belts, and so on. They are called polyesters
because of the many ester linkages that hold the monomer units within the
structure of the polymer. One of the most common polyesters is polyethylene
terephthalate (PETE), which as used for such things as soft drink and mouthwash
bottles.
HC
HC
O
C
C
H
H
C
CH
C
OH
522 Chapter 12 Carbon
HC CH O
HC CH O
n
CC
O
O
CCH
2
C
Ethylene Terephthalate
CH
2
Another functional group that is a part in many important condensation
polymerization reactions is the
amino functional group, —NH
2
. Many amines,
unfortunately, have a particularly foul odor. For instance, the common names of
some of the amines indicate the types of environments from which they were first
isolated; putrescine (1,4-butanediamine) and cadaverine (1,5-pentanediamine)
are examples. When an amine group participates in a condensation reaction with
a carboxylic acid group, an
amide bond is formed.
The amide bond holds together the monomer units of polymers known as the
polyamides. The fabrics we know as nylons, for example, are polyamides. Nylons
are named with numbers to help indicate the structure of the polymer. For ex-
ample, Nylon [6,6] is made of monomers that each contain six carbon atoms.
Nylon [6,6] can be made when an acid chloride functional group (COCl) on one
molecule reacts with an amine functional group on another molecule. This reac-
tion is accompanied by the release of HCl. Carbon chains of different lengths can
be found within other nylon polymers.
Amide bond
Methanoic acid
(formic acid)
Methanamine
H
N
H H
OH
C OHH++H
O
H
C
H
N-Methylmethanamide
(N-methylformamide)
H
N
H
CH
O
H
C
H
12.12 Condensation Polymers 523
CH
2
N
H
C
H
N
O
CH
2
CH
2
CH
2
C CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
O
n
N
H
Amide bond
Nylon 6-6
CH
2
Cl
C
Cl
O
CH
2
CH
2
CH
2
C+H
N
H
CH
2
CH
2
CH
2
CH
2
CH
2
NH
2
CH
2
O
CH
2
Cl
C
HCl
O
CH
2
CH
2
CH
2
C
+
H
N
CH
2
CH
2
CH
2
CH
2
CH
2
NH
2
CH
2
O
Visualization: Synthesis of Nylon
Video Lesson: CIA
Demonstration: The Synthesis of
Nylon
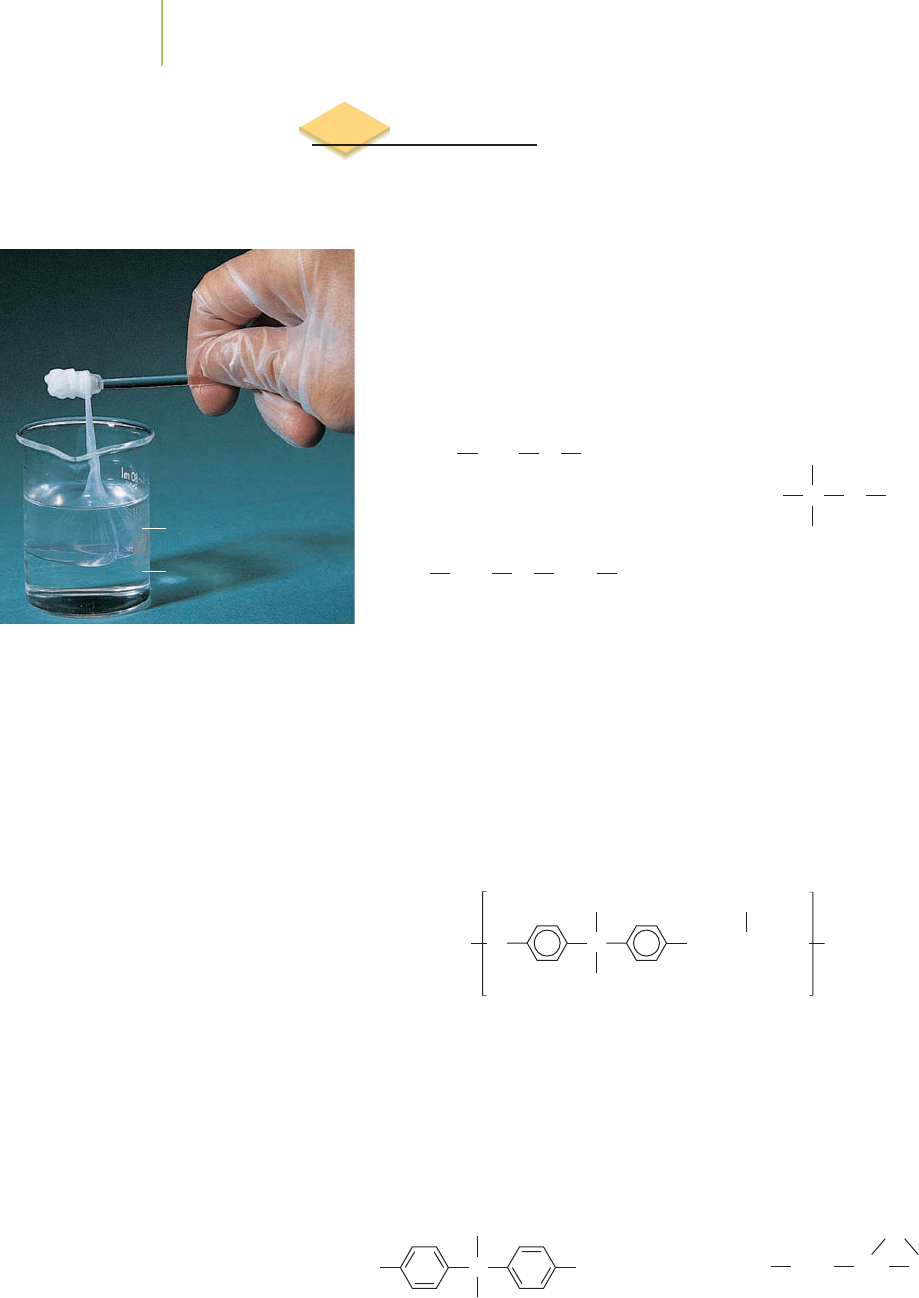
12.13 Polyethers
Another important category of polymers contains the ether functional group at
regular intervals along the polymer chain. The ether group is —C—O—C—, an
oxygen atom linking two carbon atoms. Three simple examples of ethers are ethyl
methyl ether, diethyl ether, and methyl t-butyl ether (MTBE). Diethyl
ether is the “ether” that, like the chloroform mentioned earlier, was used
as an anesthetic in the early days of medicine. It is often still used in biol-
ogy classes to anesthetize and determine the characteristics of Drosophila
flies. MTBE is the ether used as a fuel additive. Its octane rating is 116,
and for this reason it can help boost the rating on otherwise poor-quality
gasoline.
The properties of ethers make them ideal as solvents. Because they lack the
ability to form hydrogen bonds with themselves, they have boiling points similar
to those of the alkanes. However, the presence of an oxygen atom within the
structure indicates that they can participate in hydrogen bonding as acceptors
with other molecules that can donate a hydrogen atom. This means that they
make good solvents for chemical reactions.
Polyethers, which incorporate repeating ether linkages within very long
chain-like molecules, are widely used in adhesive resins. An example of a poly-
ether containing the ether functional group is found in the
epoxy resins. These
rather viscous polymers, used mostly as construction and household adhesives,
contain an ether linkage formed between one carbon atom that is part of a phenyl
ring, and another that is part of a methylene (CH
2
) group. The monomers that
make up an epoxy resin contain the phenol functional group and a triangular
ether structure known as an
epoxide.
C OCH
2
CHCH
2
CH
3
CH
3
OH
n
O
CH
3
O
Diethyl ether
CH
2
CH
2
CH
3
CH
3
O
Ethyl methyl ether
CH
2
CH
3
524 Chapter 12 Carbon
A phenol
An epoxide
Diamine solution
Acid chloride solution
The nylon rope trick. Nylon can be made
at the interface of two solutions con-
taining an acid chloride and a diamine.
The film that forms can be pulled out
of the mixture slowly to make a long
strand of nylon polymer.
CHO OH
CH
3
CH
3
HO CH
2
CH
2
CH
O
Methyl t-butyl ether
(MTBE)
CH
3
OC
CH
3
CH
3
CH
3
12.14 Handedness in Molecules 525
HERE’S WHAT WE KNOW SO FAR
■
The major components of crude oil include hydrocarbons.
■
Crude oil has many uses, including serving as fuel and supplying feedstock
molecules for industrial processes.
■
Manipulation of the hydrocarbons can yield a variety of molecules containing
different functional groups.
■
Functional groups are common specific arrangements of atoms within a mol-
ecule that impart similar physical and chemical properties to the molecule.
■
Organic molecules are named by applying a set of rules established by the
International Union of Pure and Applied Chemistry (IUPAC).
12.14 Handedness in Molecules
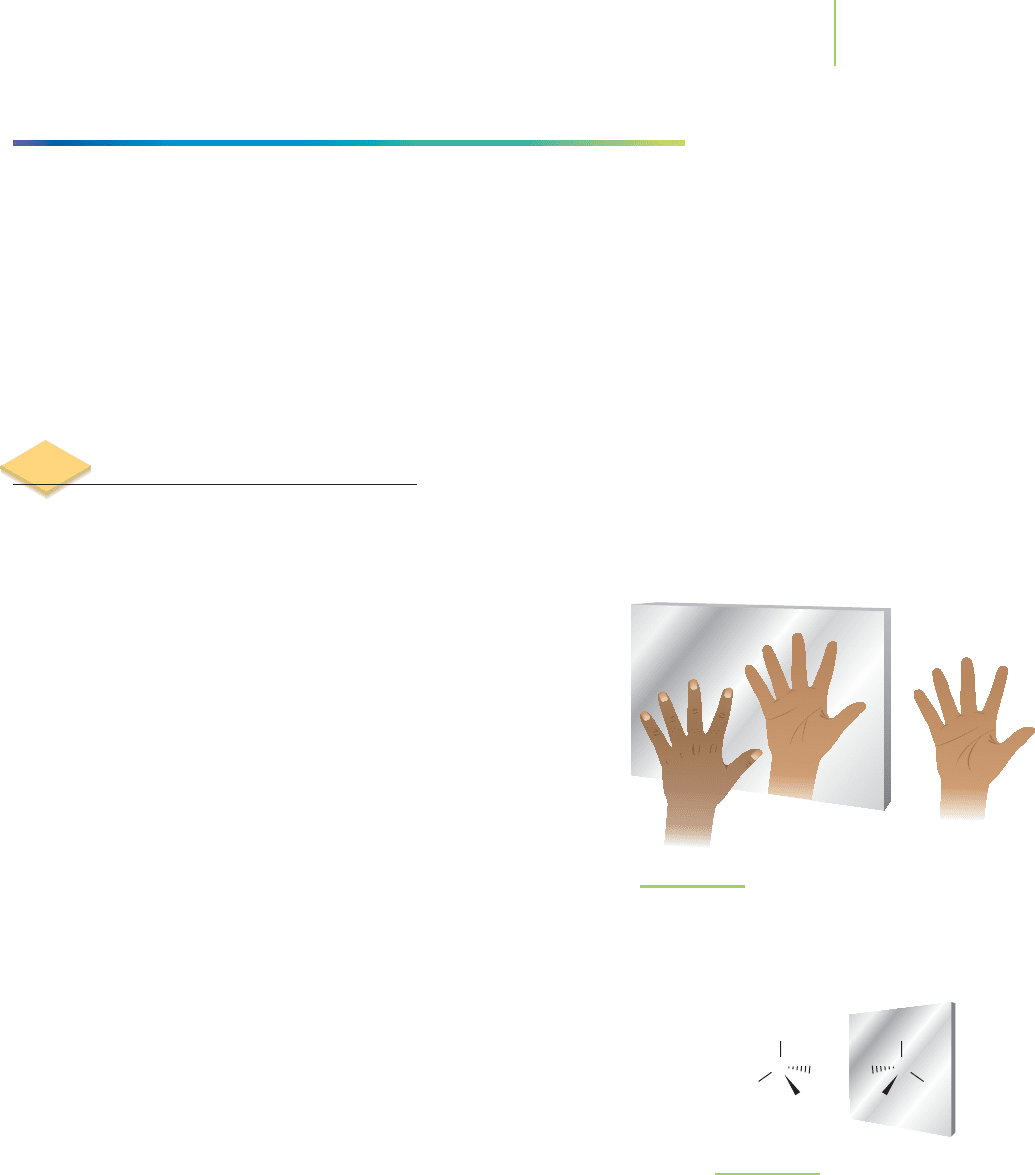
Symmetry abounds in nature. Take, for instance, the symmetry of a person. The
left-hand side of your friend appears to be a mirror image of the right-hand side.
Proof of this can be found in your own hands. Hold your hand to a mirror such
as that shown in Figure 12.15. What do you see? Your other hand is the
mirror image. What’s more interesting is that your left and right hands
(despite being mirror images) are not superimposable. That is to say,
your left hand and your right hand cannot be lined up perfectly. Try
placing a left-handed glove on your right hand. It just doesn’t fit.
Molecules can also exhibit this handedness. In chemistry, we say
that “handed” molecules are
chiral, a term derived from the Greek cheir
(“hand”). A chiral object has a nonsuperimposable mirror image.
Those molecules that lack
chirality are said to be achiral, and they have
a superimposable mirror image. Every chiral molecule possesses a twin
that has the same chemical formula, the same structure, but a different
arrangement of atoms in three-dimensional space.
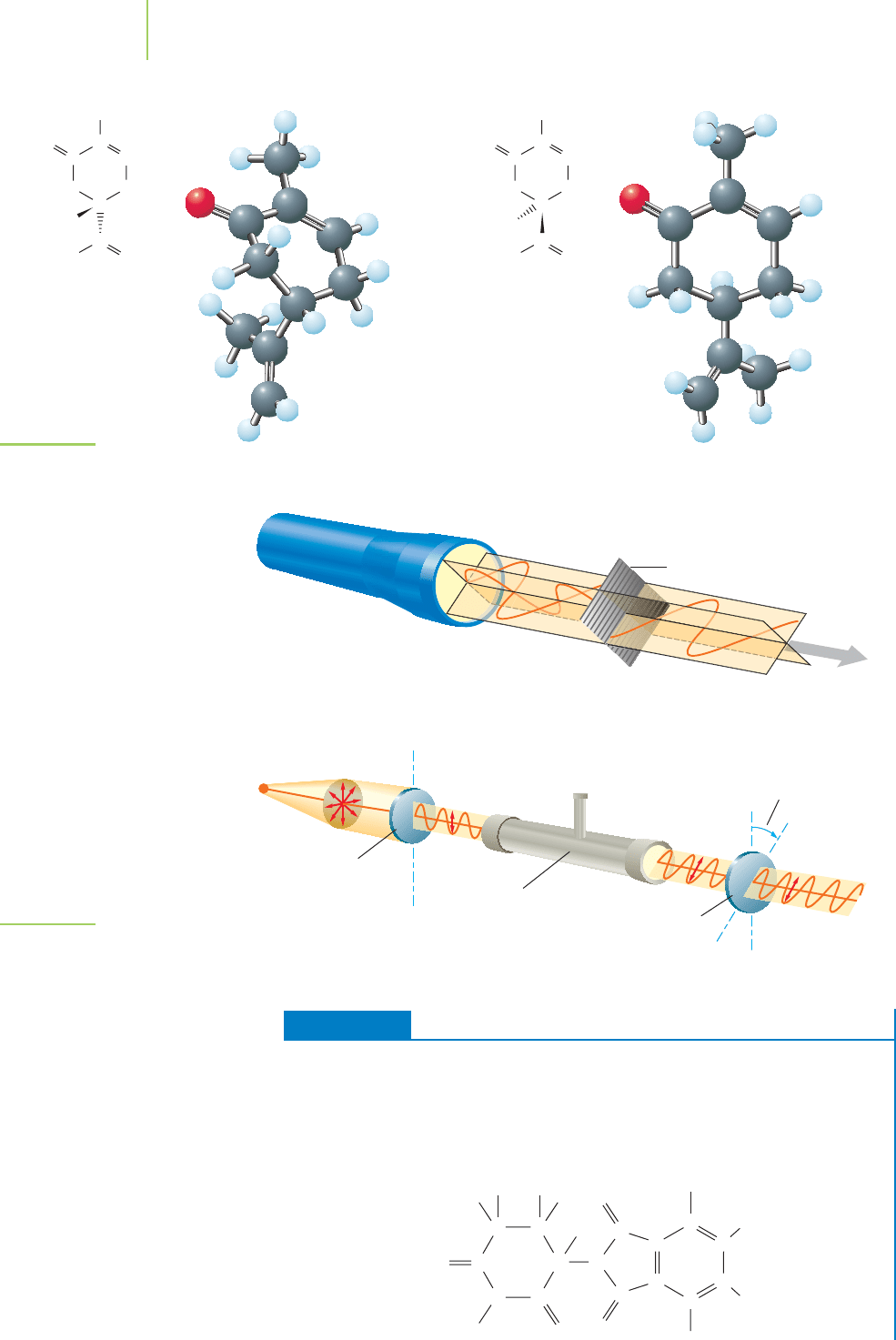
What makes a molecule chiral? Carbon-based molecules are chiral
when they contain a carbon atom that is attached to four different groups.
These
chiral centers exist in many molecules in nature. Let’s examine
a derivative of methane to explain this feature. Bromochlorofluo-
romethane (CHBrClF) contains a carbon atom attached to four differ-
ent groups. A mirror image of this molecule (see Figure 12.16) shows
this molecule to have handedness. These two molecules are
stereoisomers. They
are a special kind of structural isomers that differ in their 3-D arrangements of
atoms, rather than in the order in which the atoms are bonded.
The right-handed molecule and the left-handed molecule have almost identi-
cal physical properties because they have the same chemical structure. They are
different, however. For instance, the receptors in your nose perceive different aro-
mas based on their interaction with molecules. Carvone, shown in Figure 12.17,
a molecule your nose might encounter in the kitchen, possesses a chiral carbon
and therefore exists as two stereoisomers. One of the isomers smells like caraway
seeds; the other smells like spearmint.
Chiral molecules also exhibit the ability to rotate the plane of polarized light.
Light, passed through a polarizing filter (such as the one in most sunglasses), is
composed of electromagnetic waves that are aligned in one direction (such as the
up-and-down direction). This polarized light interacts with the two stereoiso-
mers of a chiral molecule to the same degree, but one of the isomers rotates the
light to the left and the other to the right, as shown in Figure 12.18.
FIGURE 12.15
Chiral objects have a nonsuperimposable mirror
image. The mirror image of an achiral object is
superimposable.
C
H
C
H
Br Br
Cl Cl
FF
FIGURE 12.16
Some organic molecules are also chiral.
The image and the original are non-
superimposable.
526 Chapter 12 Carbon
CH
3
C
O
C
H
C
CHC
CH
2
CH
2
H
2
C
H
3
C
CH
3
C
O
C
H
C
CHC
CH
2
CH
2
H
2
C
H
3
C
Caraway seed oil Spearmint oil
FIGURE 12.17
The isomers of carvone.
Unpolarized
light
Polarized
light
Polarizer
Light
source
Polarizer
prism
Prism
axis
Unpolarized
light
Polarized
light
Rotated
polarized
light
Degree of
rotation
Analyzer
prism
Sample tube
(containing
an optically
active compound)
FIGURE 12.18
Plane polarized light is rotated as it interacts
with chiral molecules.
EXERCISE 12.7 Chiral Drugs
Thalidomide was used as a drug that relieved morning sickness in pregnant women
in the 1950s and 1960s. Although the FDA was hesitant (because of some neurolog-
ical side effects) to approve the drug for use in the United States, thalidomide found
widespread use in Europe and Canada. On the basis of the structure given here,
identify the chiral center in the molecule.
C
H
C
CO
H
H
C
N
N
C
C
C
C
C
C
C
C
C
H
H
H
H
H
H
HO
O
O
First Thoughts
We’ll examine the molecule, looking for carbons that are bonded to four different
groups. A helpful starting point is to ignore all carbons that contain double bonds
or more than one hydrogen atom.
Solution
Further Insight
The existence of a chiral center in thalidomide implies that there are two molecules
with the same structure and physical properties. It also implies that each of the two
stereoisomers might have different interactions with receptors in the body. In fact,
this is the case. Whereas one of the stereoisomers does exert a powerful sedative ef-
fect and reduce nausea, the other isomer is a powerful teratogen. Teratogens cause
terrible birth defects.Women who used the drug during pregnancy gave birth to ba-
bies with a wide variety of defects, such as deformed or missing limbs. Thalidomide
isn’t used to treat morning sickness anymore, though it is used to treat some people
with leprosy.
PRACTICE 12.7
Identify the chiral centers in each of the molecules below.
See Problems 95 and 96.
12.15 Organic Chemistry and
Modern Drug Discovery
Perhaps one of the most profound impacts of organic chemistry on modern
society has been its key role in pharmaceutical science. Historically, chemists and
medical researchers have analyzed natural product extracts for effectiveness
in curing diseases. Many times these plant extracts are the same ones identified in
folk medicine as curatives for common maladies. Ultimately, there must be some
component or collection of compounds present in the extract mixture that is
responsible for their effectiveness. Organic chemists can purify the active con-
stituents of complex mixtures originating from such natural sources, and very
often, this active constituent turns out to be a single compound. These com-
pounds are nearly always based on a carbon framework. Organic chemists can
determine the structure of these compounds and can even synthesize them using
simple starting materials. They can also make clever improvements on these nat-
ural products by rationally changing structural features (and, therefore, specific
properties) of the molecule.
H
HH
CC
F
F
OC
C
F
H
H
HCC
H
H
C
H
Br
C
H
H
H
H
H
C
H
C
CO
H
H
C
N
N
C
C
C
C
C
C
C
C
C
H
H
H
H
H
H
HO
O
O
12.15 Organic Chemistry and Modern Drug Discovery 527
