Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

508 Chapter 12 Carbon
Products of the Fractional Distillation of Crude Oil and Their Uses
Fraction Formulas Uses Boiling Point (°C)
Natural gas CH
4
to C
4
H
10
Fuel, cooking gas <0
Petroleum ether C
5
H
12
to C
6
H
14
Solvent for organic compounds 30–70
Gasoline C
6
H
14
to C
12
H
26
Fuel, solvent 70–200
Kerosene C
12
H
26
to C
16
H
34
Rocket and jet engine fuel, 200–300
domestic heating
Heating oil C
15
H
32
to C
18
H
38
Industrial heating, fuel for 300–370
producing electricity
Lubricating oil C
16
H
34
to C
24
H
50
Lubricants for automobiles >350
and machines
Residue C
20
H
42
and up Asphalt, paraffin Solid
TABLE 12.5
into further processing steps to convert them into useful products. Table 12.5 also
lists some of the major uses made of each fraction. We will explore some of these
uses in more detail, and in doing so we will investigate the chemical properties
and reactivities of the hydrocarbons in each fraction.
12.5 Processing Hydrocarbons
The hydrocarbons within the fractions obtained by distillation are normally sub-
jected to further processing before being used as fuels or as feedstocks for the
chemical industry. The two major types of processing are known as cracking and
reforming.
Cracking
Many of the most useful hydrocarbons are the smaller ones, with fewer than
10 carbon atoms. At the refinery, long-chain hydrocarbons are isolated from the
crude oil by fractional distillation. Then they are broken into a mixture of
shorter-chain hydrocarbons, including some branched and unsaturated hydro-
carbons, by means of a process called
cracking. This process uses heat to break
some of the carbon–carbon bonds in the long-chain hydrocarbons. The products
of cracking often contain short saturated and unsaturated hydrocarbons. There
are various types of cracking:
■
Thermal cracking uses heat alone.
■
Catalytic cracking uses heat plus a catalyst.
■
Hydrocracking uses heat plus a catalyst in the presence of hydrogen gas, en-
couraging formation of saturated rather than unsaturated products.
■
Steam cracking uses heat plus steam and a catalyst.
Reforming
Another crucial process at the refinery is called reforming. In this process hydro-
carbons distilled from petroleum are passed through a catalyst bed and converted
into more useful forms.When a mixture is reformed, the predominantly straight-
chain hydrocarbons are made into a mixture containing more aromatic and
branched-chain hydrocarbons.
Reforming is a key step in making the gasoline we use as automobile
fuel. Gasoline must contain a suitable proportion of branched and aromatic
12.5 Processing Hydrocarbons 509
hydrocarbons to prevent the problem known as knocking. This is the rapid explo-
sion of the gas–air mixture as it is compressed in the automobile cylinder before
the spark plug creates the spark that is intended to cause the mixture to explode.
In some cases knocking damages a car’s engine, but most often it results only in
poor engine performance and reduced gas mileage. Addition of reformed distil-
lates to gasoline reduces knocking.
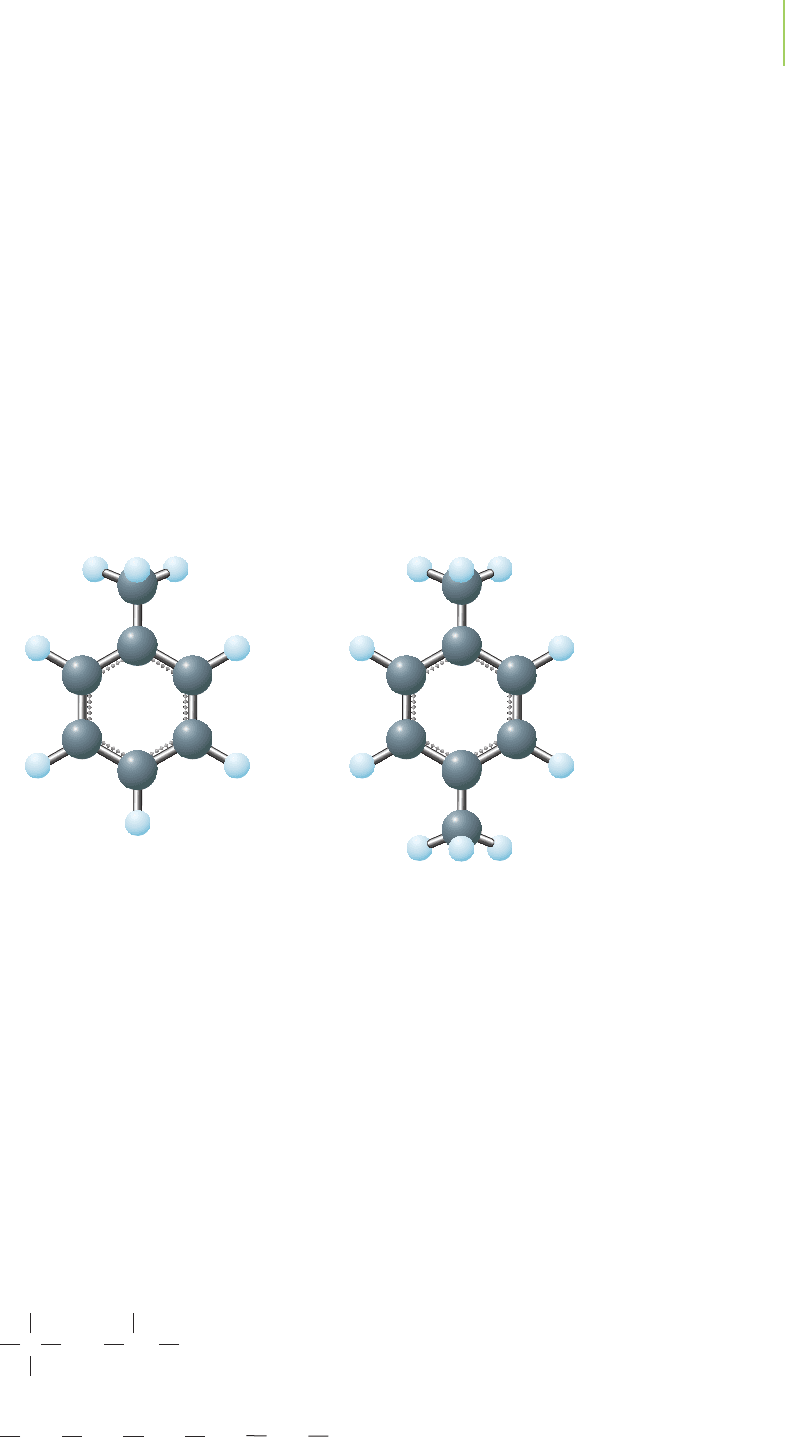
Gasoline
The gasoline we use to power automobiles is a complex mixture of well over a
hundred different hydrocarbons, which can include molecules such as pentane,
benzene, ethylbenzene, toluene (methylbenzene), and xylene (dimethylbenzene).
The precise composition of the mixture varies with the brand of gasoline, the lo-
cation, and the time of year, but the hydrocarbon molecules in automobile gas
generally contain between 5 and 17 carbon atoms, many being in the C
5
-to-C
9
range. One factor influencing the optimum mixture of hydrocarbons is the need
to produce a fuel with a vapor pressure that allows it to evaporate to an appro-
priate degree under the conditions in which it will be used.
Toluene Xylene
The extent to which a particular type of gasoline will burn smoothly in an
engine (without knocking) is indicated by using the
octane rating. This is based
on the early twentieth-century discovery that pure isooctane produced minimal
knocking in an engine, whereas pure heptane produced a very high level of
knocking. On the octane scale, 2,2,4-trimethylpentane (also known as isooctane)
is given the octane rating of 100, and heptane is given the octane rating of 0. By
comparing mixtures of isooctane and heptane to different formulations of
gasoline, an octane rating for the gasoline can be assigned. For example, a high-
quality gasoline, which produces a level of knocking equivalent to a mixture of
90% isooctane and 10% heptane, is assigned an octane rating of 90. Low-quality
gasoline can be improved by adding other compounds with octane ratings that
are higher than 100, such as ethanol (octane rating 112) or toluene (octane rating
118). Brands of automobile gasoline are typically available with octane ratings in
the range of 83 to 98.
CH
3
C CH
2
CH CH
3
CH
3
CH
3
CH
3
2,2,4-Trimethylpentane (isooctane)
Heptane
CH
3
CH
2
CH
2
CH
2
CH
2
CH
2
CH
3
12.6 Typical Reactions of the Alkanes
Globally, the most significant reaction of the alkanes is their combustion in air. As
we have already seen, the complete combustion of alkanes generates carbon diox-
ide and water vapor and releases considerable amounts of energy. The energy can
be used to provide heat, both for warmth and for cooking, to generate electricity,
or to power vehicles.
The chemical industry also makes substantial use of alkanes in
substitution
reactions
, in which one or more hydrogen atoms are replaced by other types of
atoms, most commonly halogens such as bromine and chlorine. These substitu-
tion reactions of the alkanes are promoted by ultraviolet light, which causes the
halogen molecules to split into two atoms:
The halogen atoms generated in this process are known as free radicals, each hav-
ing an unpaired electron. The presence of the unpaired electron makes them
highly reactive. In the presence of an alkane, the free radicals can readily attack
the C—H bonds. The result is a halogenated alkane and a molecule of the hydro-
gen halide.
For example, the simplest hydrocarbon, methane, can be converted into four
different organic products and HCl by successive substitution reactions with
chlorine:
These four products can be put to a variety of uses. Chloromethane is used in the
manufacture of silicone-based polymers and as a solvent in the production of
certain types of rubber. Dichloromethane is widely used as a solvent in the chem-
ical industry and within products such as paint strippers.
Trichloromethane, also known as chloroform, was one of the first anesthetics,
pioneered as such in 1847 by the Scottish physician Sir James Simpson. Because
of the sweetness of this molecule, it was also used as the solvent in early cough
medicines. However, this compound was found to be both toxic and carcino-
genic, and ethanol has taken its place in medicines. Chloroform is still used as an
industrial solvent. Tetrachloromethane, also known as carbon tetrachloride, once
was the solvent most commonly used for the dry cleaning of clothes and other
fabrics. It was also used as a fire extinguisher agent because it is not flammable.
Unfortunately, it too is toxic and carcinogenic. It has now largely been abandoned
for dry cleaning and fire extinguishing in favor of less toxic compounds such as
liquid carbon dioxide (see Chapter 10). However, it is still used as a solvent in
various industrial processes.
Another important reaction of the alkanes is their
dehydrogenation to pro-
duce the corresponding alkenes. In a dehydrogenation reaction, a molecule of
CH
4
+ Cl
2
CH
3
Cl
CH
3
Cl + Cl
2
CH
2
Cl
2
CH
2
Cl
2
+ Cl
2
CHCl
3
CHCl
3
+ Cl
2
CCl
4
+ HCl
+ HCl
+ HCl
+ HCl
Chloromethane
Dichloromethane
Trichloromethane
(chloroform)
Tetrachloromethane
(carbon tetrachloride)
hv
hv
hv
hv
Cl
2
hv
Cl Cl
510 Chapter 12 Carbon
12.7 The Functional Group Concept 511
hydrogen is removed from the starting material. The result is an unsaturated
hydrocarbon, an alkene. For example, dehydrogenation of ethane produces
ethene. The dehydrogenation of propane produces propene, and so on:
12.7 The Functional Group Concept
The processes of adding chlorine atoms or CPC double bonds to the basic struc-
ture of alkanes are examples of adding
functional groups to a hydrocarbon frame-
work. A functional group is an atom or group of atoms with a characteristic set
of chemical properties. Chemists often make sense of the infinite variety of
chemical reactions in organic chemistry by treating each molecule as a hydrocar-
bon framework with a particular set of functional groups incorporated within
that framework.
A chlorine atom bonded to a hydrocarbon is an example of an
alkyl halide
functional group, a term that covers any of the halogen atoms bonded to a hydro-
carbon framework. These and many other examples of functional groups are
listed in Table 12.6. For example, the CPC double bond (the alkene functional
group) is a functional group whose properties depend on the type of bonding
between the atoms involved. The CqC triple bond (the alkyne functional group)
is another example.
Much of the chemistry done with hydrocarbons from oil involves the addi-
tion of specific functional groups. Chemists can modify the structure of the basic
hydrocarbon chain, adjusting its length and degree of branching, and then
add whatever selection of functional groups will create the molecules they are
seeking.
Ethane
CH
500˚C
Pt
H
H
CH
H
H
C H + H
2
H
CH
H
Propane
Ethene
Propene
CH
500˚C
Pt
HH
H
C
H
C
H
H
CH
H
H
C H + H
2
H
CH
H
Chloroform
CHCl
3
Carbon tetrachloride
CCl
4
512 Chapter 12 Carbon
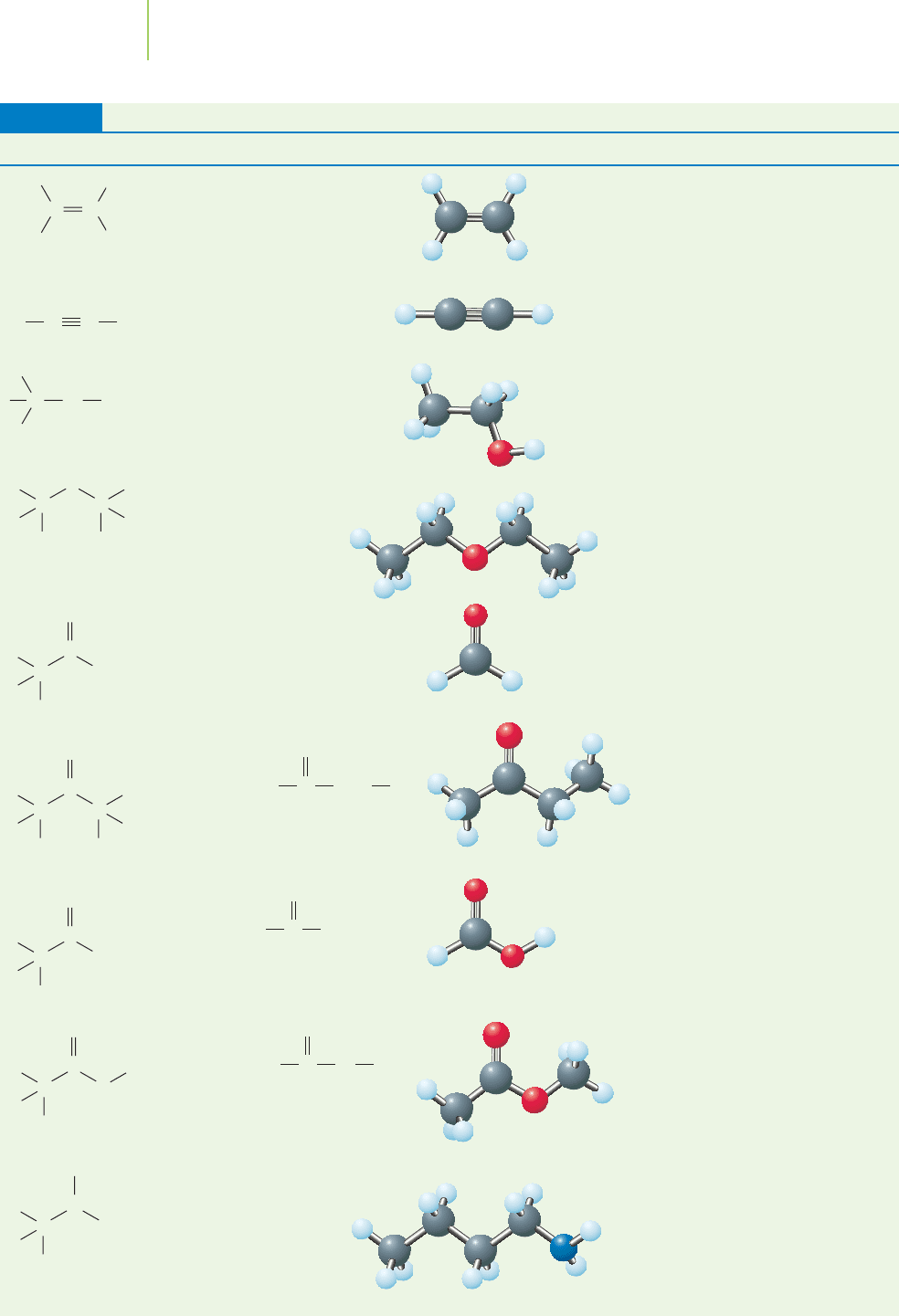
Selected Functional Groups
Structure Group Example 3-D Structure Name Selected Uses
Alkene CH
2
PCH
2
Alkyne HCqCH Acetylene
Alcohol CH
3
CH
2
OH Ethanol
Ether CH
3
CH
2
OCH
2
CH
3
Diethyl ether
Aldehyde CH
2
PO Formaldehyde
Ketone
Carboxylic Formic acid
acid
Ester Methyl acetate
Amine CH
3
(CH
2
)
3
NH
2
Butylamine
Manufacture
of rubber,
insecticides
Paint remover,
pharmaceutical
manufacture
Manufacture of
textiles, pesticides;
electroplating
Solvent in
synthetic rubber
industry
Methyl ethyl
ketone
Bactericide,
fungicide, chem-
icals production
Industrial
solvent
Liquors,
industrial solvent
Welding,
cutting, brazing
C C
Refrigerant,
production of
polyethylene
Ethene
(ethylene)
TABLE 12.6
(Continued)
C C
C OH
CC
O
CH
C
O
CC
C
O
COH
C
O
CO
C
O
C
N
CH
3
CH
3
C
O
CH
2
CH
3
CH
3
C
O
O
HC
O
OH
12.7 The Functional Group Concept 513
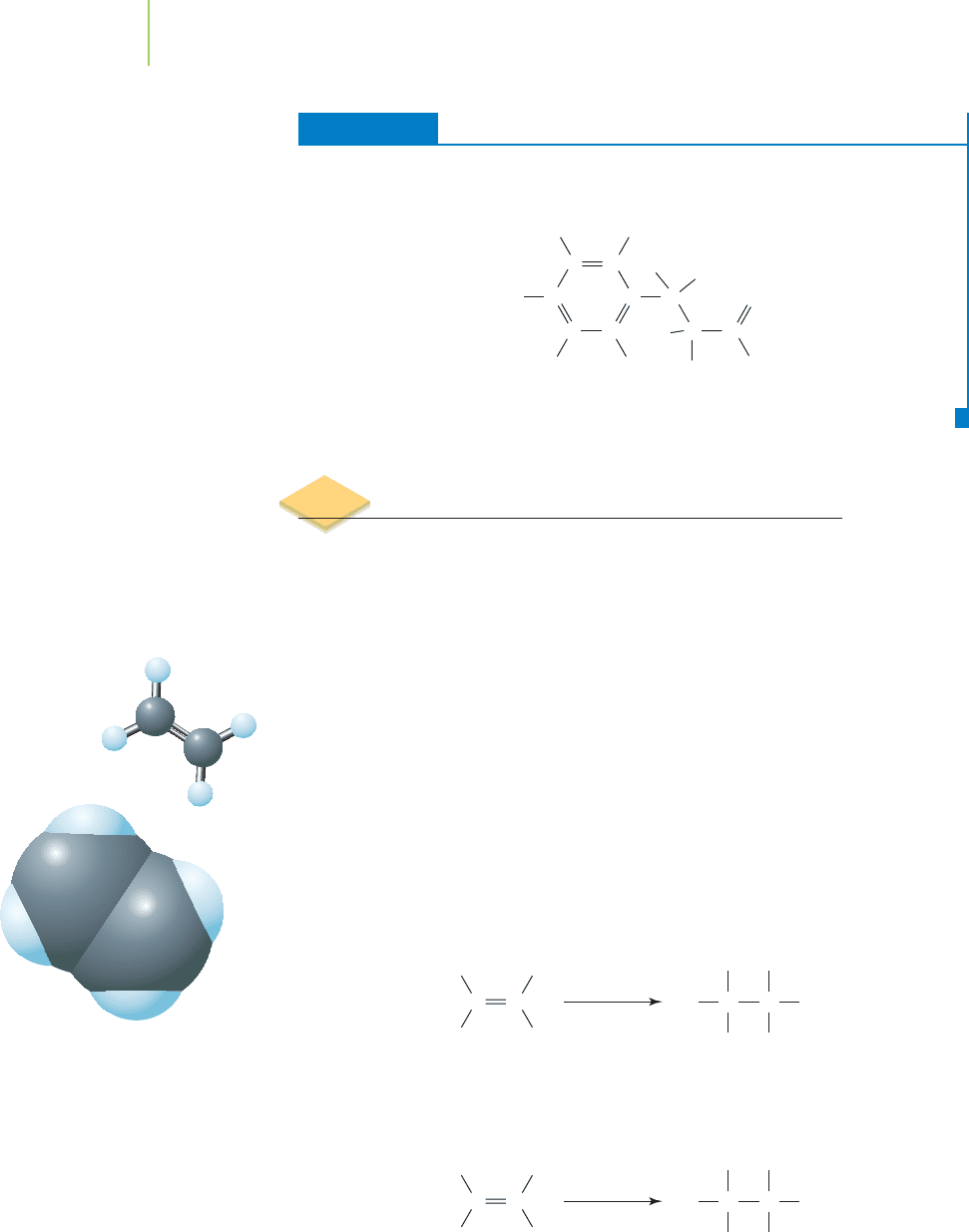
EXERCISE 12.5 Functional Groups and You!
Circle and name each of the functional groups in cyclexanone, a pharmaceutical
agent used to prevent coughing (an antitussive agent).
Solution
C
O
C
H
H
HH
H
H
H
H
H
H
H
H
H
C
C
C
C
C
C
C
C
H
CH
2
CH
2
H
H
H
C
N
C
H
H
H
H
Ether
Amine
A
lkene
Ketone
C
C
O
C
O
C
H
H
HH
H
H
H
H
H
H
H
H
H
C
C
C
C
C
C
C
C
H
CH
2
CH
2
H
H
H
C
N
C
H
H
H
H
C
C
O
(Continued)
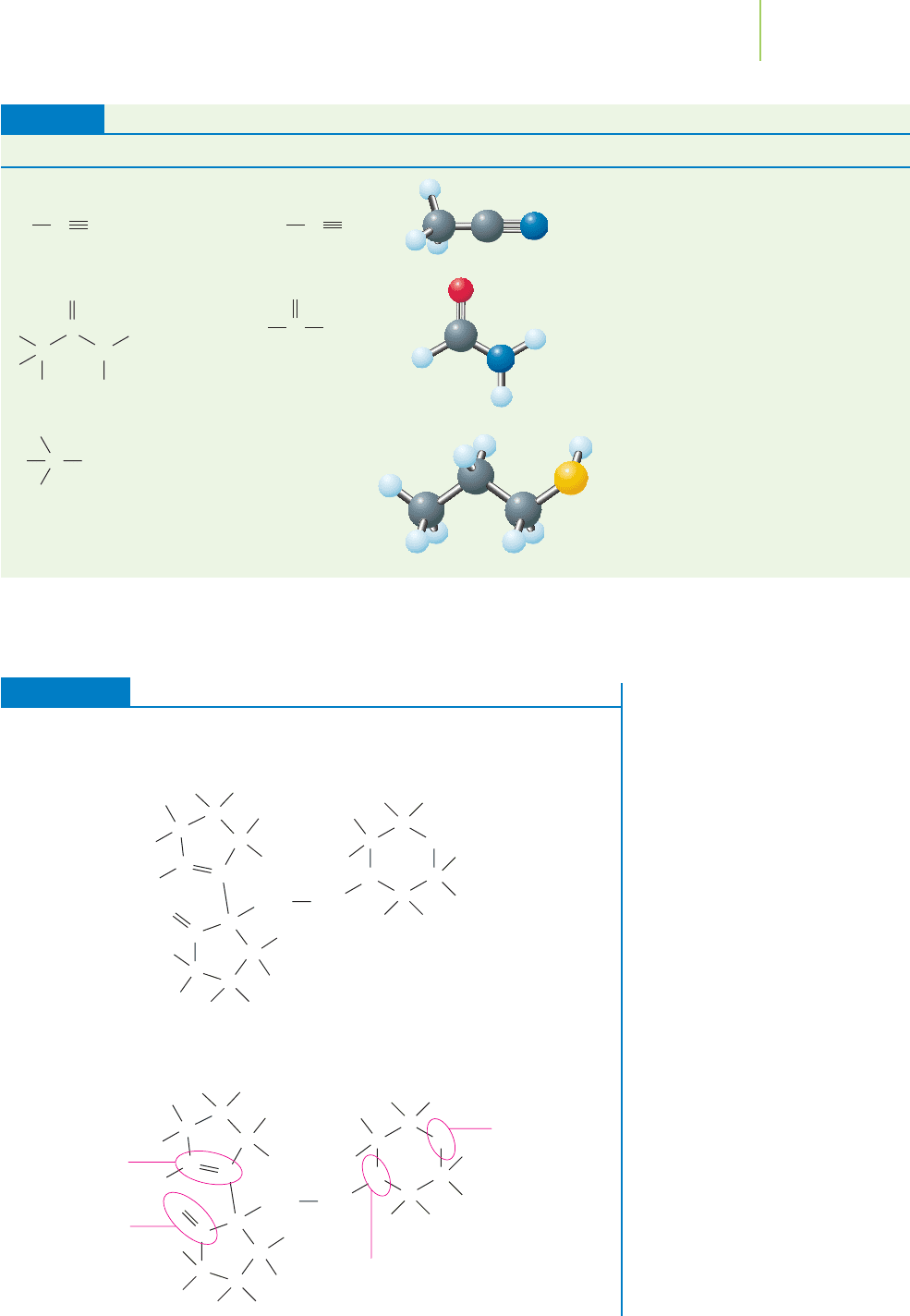
Structure Group Example 3-D Structure Name Selected Uses
TABLE 12.6
Nitrile Acetonitrile
Amide Formamide
Thiol CH
3
CH
2
CH
2
SH Propanethiol Herbicide, flavor-
ing agent, additive
to odorless poiso-
nous gases
Manufacture
of paper, glue;
industrial solvent
Industrial solvent,
pharmaceutical
manufacture
Source: Kelter, Scott, and Carr. Chemistry, A World of Choices, 2nd Ed; McGraw-Hill, 2003: New York; and New Jersey
Department of Health & Senior Services Right-to-Know Program Fact Sheets.
C N
CH
3
C N
CN
C
O
HNH
2
C
O
C SH
PRACTICE 12.5
Circle and name each of the functional groups in tyrosine,one of the building blocks
used to make proteins.
See Problems 51–54.
12.8 Ethene, the CPC Bond, and Polymers
One of the most versatile of the hydrocarbons produced by catalytic cracking is
ethene (ethylene). It serves as the central feedstock molecule that is converted
into a vast range of organic chemicals utilized in modern life. The chemistry of
ethene is dominated by the reactivity of its CPC double bond. Especially impor-
tant is the
addition reaction, in which two parts of another chemical species be-
come added to the atoms at either end of the double bond. The CPC of ethene
is converted into a single bond in the process. The pi electrons of the double bond
end up participating in the new bonds holding the groups that have become
added “across” the double bond. We’ve already examined one of the addition re-
actions with ethene, hydrogenation.
As another example, ethene can be converted into ethanol (CH
3
CH
2
OH) by
reaction with water in the presence of phosphoric acid (H
3
PO
4
) catalyst at 330°C.
Industrially, ethanol can be made in large quantities using this process. However,
because of the value of ethene as a feedstock for other organic molecules, much
of the ethanol that is made in the United States today comes from the fermenta-
tion of sugars found in corn. Ethanol is an example of an
alcohol, a compound
carrying the
hydroxyl group (—OH group).
Another addition reaction converts ethene into dichloroethane (ethylene
dichloride), an intermediate in the production of the seemingly ubiquitous ma-
terial polyvinyl chloride (PVC).
Ethene is the starting material for a great many of the plastics that are such a
common feature of modern life (see Chapter 13). The simplest of these plastics is
polyethene, which is made when huge numbers of ethene molecules participate in
addition reactions with themselves to generate long chain-like polyethene mole-
cules. In the reaction, one molecule of ethene adds to an adjacent molecule of
ethene, which adds to another molecule of ethene, and so on. The result is a very
long chain of carbon atoms.
C
H
H
H
H
C
Cl
2
HC
Cl
C
Cl
H
H H
C
H
H
H
H
C
H
2
O
H
3
PO
4
330C
HC
H
C
OH
H
H H
H
NH
2
H
CC
HH
C
C
H
H
C
CHO
OH
C
O
CC
H
514 Chapter 12 Carbon

12.8 Ethene, the CPC Bond, and Polymers 515
Polyethene, which is more commonly known as polyethylene, is an example of a
polymer (literally,“many units”), a compound composed of large molecules made
by the repeated bonding together of smaller
monomer (literally, “one unit”) mol-
ecules, ethene in this case. Because the reaction that bonds the ethene monomers
together is an addition reaction, polyethylene is known as an
addition polymer,
formed by the process of
addition polymerization.
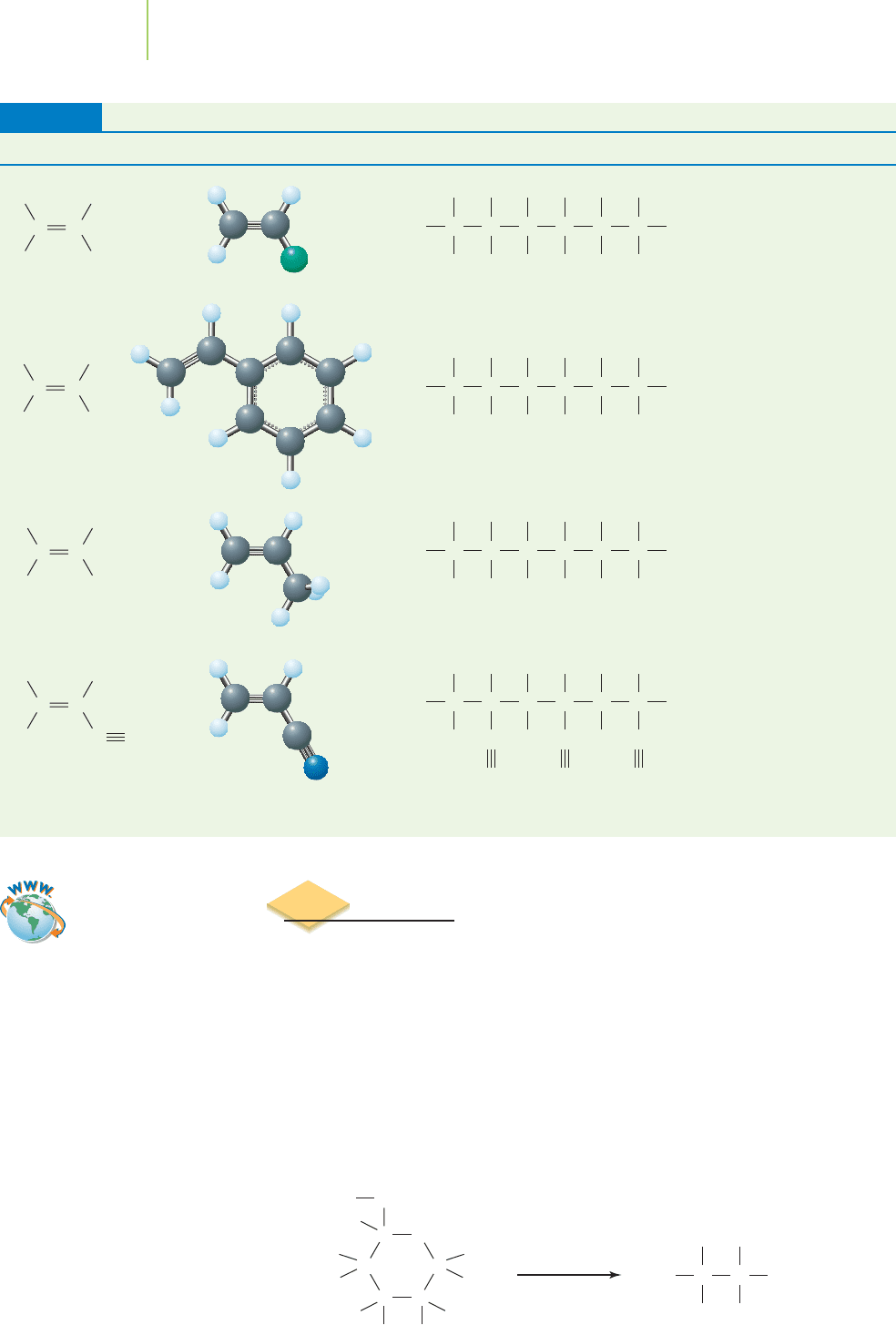
Different types of polyethylene can be made by varying the temperatures and
pressures of the reaction, as well as changing the type of catalysts and initiating
substances used. These different forms are classified into two main types:
high-
density polyethylene (HDPE)
, in which the molecules are long and linear with no
branch points, and
low-density polyethylene (LDPE), with shorter, branched-chain
molecules, as shown in Figure 12.14. The length of the polyethylene chain im-
parts different properties to the resulting molecule. For instance, because we
know that shorter alkanes have lower melting points, you might expect a shorter
polyethylene chain to have a lower melting point than a longer polymer.
The high-density part of HDPE is a result of the close packing of the linear
molecules. This makes HDPE a strong plastic, used to make such things as blow-
molded bottles and toys. LDPE has a more flexible, less crystalline structure; it is
used to make such things as plastic trash and grocery bags, packaging films, insu-
lation sleeves around electrical cables, and squeeze bottles. The properties of
polyethylene can be modified by incorporating some other atoms, such as chlo-
rine or sulfur, into its structure as it is formed. This can make the resulting mod-
ified polyethylene more resistant to oxidation, for example, or more flexible.
Many of the polyethylene-based materials around us are actually composed of
such modified polyethylenes.
We can prepare polymers that have properties different from those of poly-
ethylene, and yet are closely related to it in chemical structure, by using
monomers in which one or more of the hydrogen atoms of ethene are replaced
with other atoms or larger chemical groups. Table 12.7 lists some of these.
C
H
H
H
H
C
Polymerize
Polyethene (polyethylene)
C
H
C
H
H H
C
H
C
H
H H
n
C
H
H
C
H
H
C
H
H
C
H
H
C
H
H
C
H
H
C
H
H
C
H
H
C
H
H
C
H
H
C
H
H
FIGURE 12.14
HDPE and LDPE. The molecular structures
of HDPE and LDPE are similar, but their
processing gives them unique properties
for use in different materials.
Video Lesson: Organic Polymers
516 Chapter 12 Carbon
12.9 Alcohols
As the alcohol found in alcoholic beverages, ethanol is the best known of the al-
cohols. Ethanol can be made by hydrating ethene. However, because of the value
of ethene as a feedstock for other organic molecules, and because of the toxicity
of some of the by-products of this process, much of the ethanol that is typically
used for human consumption is made by the biological activity of yeast cells. In
this reaction, the yeast consumes glucose in a process known as
fermentation (see
Chapter 14). The waste products of the process are ethanol and carbon dioxide.
These waste products are toxic to the yeast and are excreted from the cell. Fortu-
nately, the industrial chemist can easily harvest the ethanol by distilling it from
the solution. The chemist also can capture the CO
2
gas given off in the reaction in
order to make other products.
C
CC
C
CO
CH
2
H
OH
H
OH
H
HO
HO
H
H
HO
Yeast
H2C
H
H
C
OH
H 2CO
2
H
Polymerization of Some Common Monomers
Monomer 3-D Structure Polymer Uses
Vinyl chloride Polyvinylchloride (PVC)
Styrene Polystyrene
Propene Polypropylene
Acrylonitrile
Polyacrylonitrile
C
H
C
H
H C
C
H
C
H
H C
C
H
C……
H
H C
NNN
C
H
H
H
CN
C
C
H
C
H
H CH
3
C
H
C
H
H CH
3
C
H
C……
H
H CH
3
C
H
H
CH
3
H
C
C
H
C
H
H C
6
H
5
C
H
C
H
H C
6
H
5
C
H
C……
H
H C
6
H
5
C
H
H
C
6
H
5
H
C
C
H
C
H
H Cl
C
H
C
H
H Cl
C
H
C……
H
H Cl
C
C
HH
H
Cl
TABLE 12.7
Indoor plumbing, toys,
plastic wrap, vinyl
siding
Outdoor furniture,
insulation, packing
“peanuts”
Contained in
indoor–outdoor
carpeting
Orlon, Acrilon clothing,
yarns, wigs
Video Lesson: Alcohols,
Ethers, and Amines
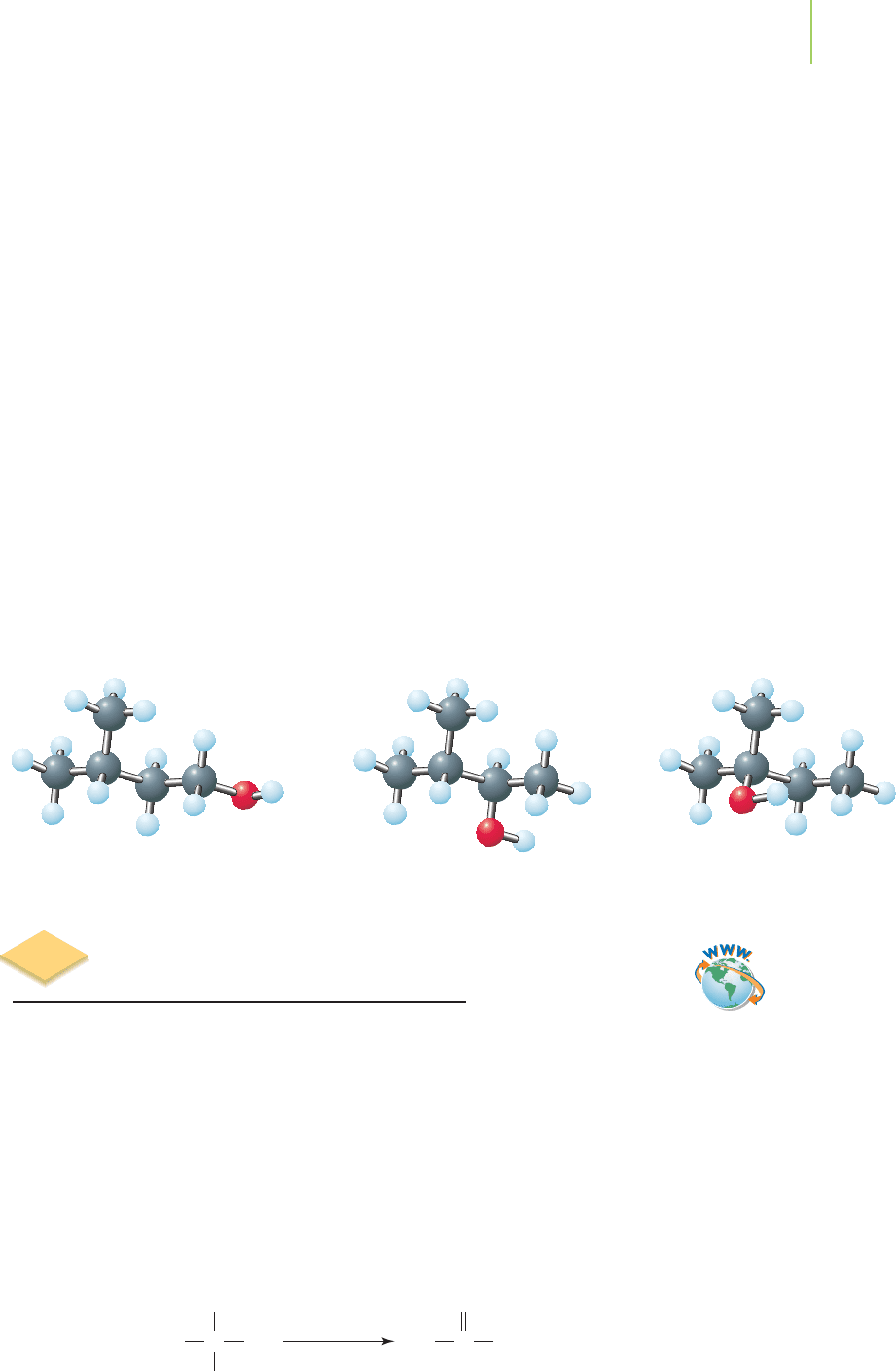
12.10 From Alcohols to Aldehydes, Ketones, and Carboxylic Acids 517
12.10 From Alcohols to Aldehydes,
Ketones, and Carboxylic Acids
In addition to determining the rate of reaction, the distinction between primary,
secondary, and tertiary alcohols is important in determining what types of mol-
ecules can be made from the alcohol. For example, primary alcohols can undergo
oxidation to form
aldehydes, which carry a carbonyl functional group (CPO) with
at least one H atom bonded to the carbonyl carbon atom. The oxidation is usu-
ally performed with a highly oxidized metal such as chromium (VI). In the lab,
however, special reactions must be performed to prevent the overoxidation of
the aldehyde. Methanol, for example, can be oxidized to the aldehyde methanal
(H
2
CPO), also known as formaldehyde. The solution formed when form-
aldehyde is dissolved in water is most commonly known as formalin, used to pre-
serve biological specimens.
Oxidation
HC
O
HHC
OH
H
H
Methanol Methanal
Primary alcohol Secondary alcohol Tertiary alcohol
The ethanol that is distilled from the fermentation mixture unavoidably con-
tains a small amount (roughly 5%, by volume) of water. For beverages, this water
is not a problem. However, if the ethanol is to be used as an additive to fuel used
in automobiles, the water must be removed. The process used to make 100%
ethanol by fermentation often leaves behind traces of toxic compounds, which
render the pure ethanol undrinkable.
Other alcohols of note include methanol, 2-propanol, the “dihydroxy alcohol”
1,2-ethanediol, and the “trihydroxy alcohol”1,2,3-propanetriol, whose structures
and uses can be found in Table 12.8. Note the way in which numbers are used
to indicate which carbon atom carries the —OH (hydroxy) group or groups in
alcohol structures. We can also use the structures themselves to explain the way
in which alcohols are classified into categories known as primary, secondary, or
tertiary alcohols, a type of classification that can be applied to other functional
groups as well.
Primary alcohols have only one carbon atom bonded to the carbon atom that
carries the hydroxyl group. This means that ethanol is a primary alcohol.
Sec-
ondary alcohols
have two carbon atoms bonded to the carbon atom that carries
the hydroxyl group, so 2-propanol is a secondary alcohol.
Tertiary alcohols have
three carbon atoms bonded to the carbon atom that carries the hydroxyl group,
so 2-methyl-2-propanol is a tertiary alcohol. This classification system is well cor-
related with the reactivity of the alcohols. For instance, primary alcohols undergo
many reactions more rapidly than do secondary or tertiary alcohols. For exam-
ple, primary alcohols react fastest, and tertiary alcohols slowest, in the formation
of esters.
Video Lesson: CarbonyI-
Containing Functional Groups
