Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

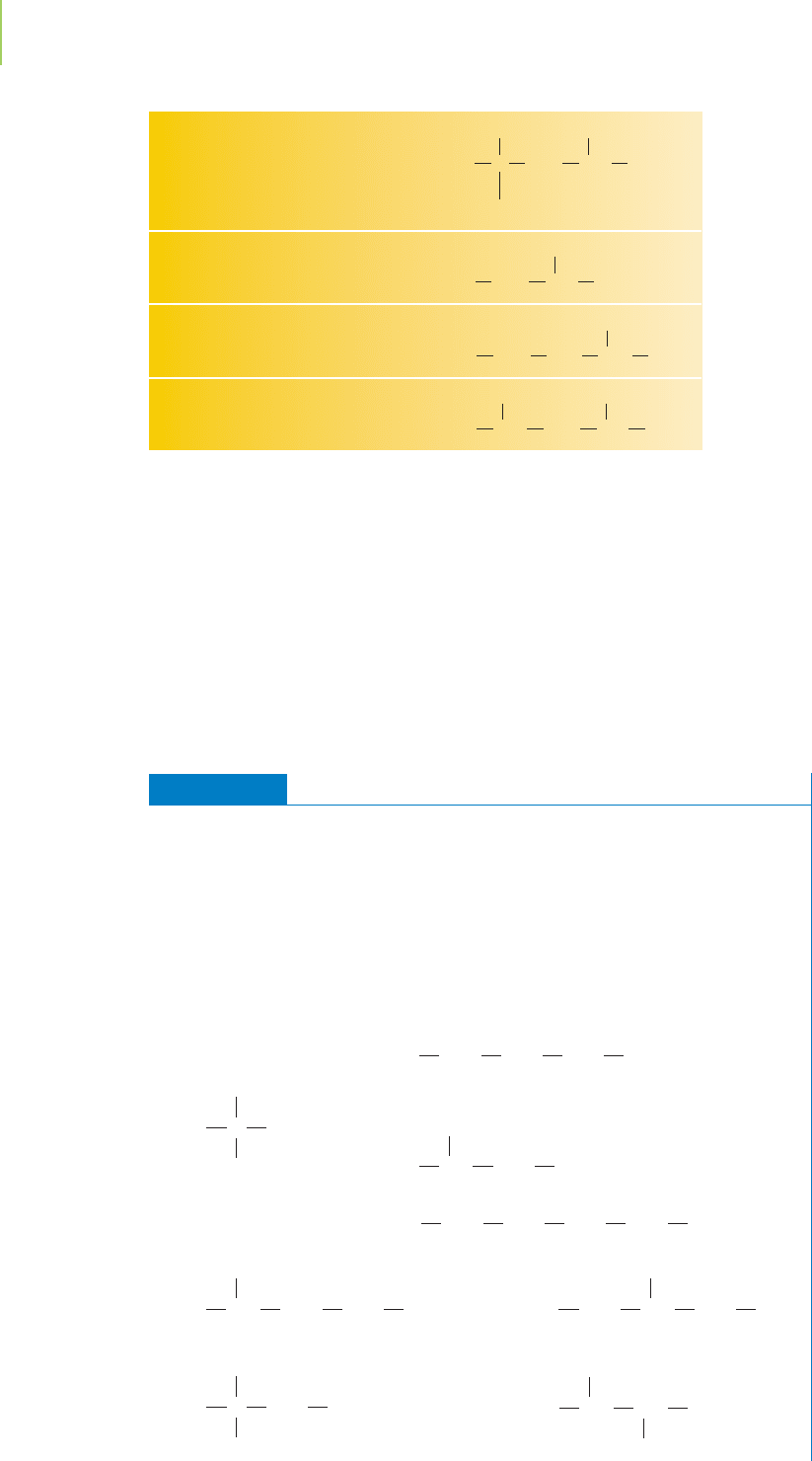
Branched-Chain Alkanes: Isomers of the Normal Alkanes
Crude oil also contains a significant proportion of alkanes with “branched”
chains. Each branched-chain alkane has the same formula as a corresponding
straight-chain (normal) alkane, so each one can be regarded as a
structural isomer
of a normal alkane. Structural isomers always have the same formula but differ in
the way the atoms are attached. We say that they share the same molecular for-
mula but have different molecular structures. Structural isomers do not have the
same properties. For instance, the structural isomers pentane and 2,2-dimethyl-
propane have the same formula (C
5
H
12
). Their boiling points, however, are much
different: 36°C for pentane and 10°C for 2,2-dimethylpropane.
EXERCISE 12.1 Finding the Isomers
How many structural isomers of pentane (C
5
H
12
) exist? How many structural iso-
mers are there for hexane (C
6
H
14
)?
First Thoughts
Isomers must have the same atoms arranged in different ways. Isomers of alkanes
differ in their skeleton of bonded carbon atoms. We can find all the isomers by
drawing the possible different carbon skeletons then adding the hydrogen atoms
needed to ensure that each carbon atom has four bonds.
Solution
Isomers of pentane:
Isomers of hexane:
CH
3
CH
2
CH
2
CH
2
CH
3
CH
3
CH
2
CH
2
CH
2
CH
2
CH
3
CH
3
CH
CH
3
CH
2
CH
3
C
CH
3
CH
3
CH
3
CH
3
498 Chapter 12 Carbon
CH
2
CH
3
CH
3
CH
3
CH
2-Methylbutane (isopentane)
CH
2
CH
3
CH
3
CH CH
3
CH
2
2-Methylpentane (isohexane)
CH
CH
3
CH
3
CH CH
3
CH
2
CH
3
2,4-Dimethylpentane
C
CH
3
CH
2
CH
3
CH
3
CH
3
CH
3
CH
2,2,4-Trimethylpentane (isooctane)
CH
3
CH CH
2
CH
2
CH
3
CH
3
C
CH
3
CH
3
CH
3
CH
2
CH
3
CH
2
CH
3
CH CH
2
CH
3
CH
3
CH
3
CH CH CH
3
CH
3
CH
3
CH
3
CH
2
CH
2
CH
3
CH
3
CH CH
3
CH
2
CH
2
CH
3
CH
3
CH
CH
2
CH
3
CH
2
CH
2
CH
2
CH
3
CH
3
CH
CH
3
CH
3
CH
3
CH
3
CH CH CH
3
CH
3
CH
3
CH
3
CH CH
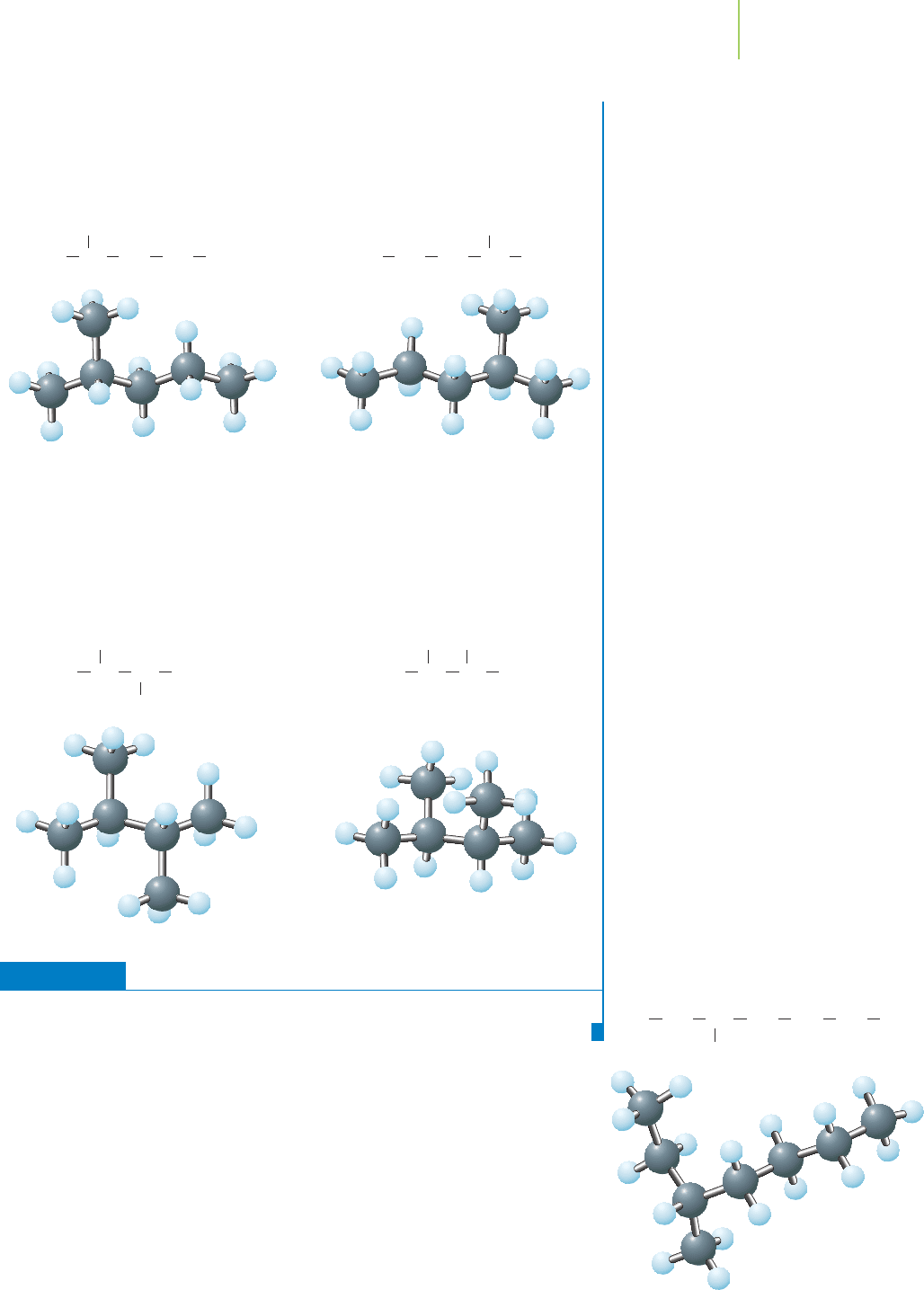
Further Insights
Why are the two structures below not counted as different isomers of hexane?
Although at first glance they look different, they are actually the same molecule just
flipped over.
12.3 Hydrocarbons 499
Here are two more examples of structures that initially appear different but are
in fact identical. You can tell that they’re identical by noting the attachments of the
carbon atoms. In each case, there are four carbons in a row, with the branched
carbons emanating from the middle two carbon atoms. You need to be careful to
eliminate such identical structures when searching for isomers. Working with three-
dimensional models, rather than two-dimensional drawings, can make this easier to
follow.
PRACTICE 12.1
Draw all of the isomers of heptane (C
7
H
16
).
See Problems 13, 14, 21, and 22.
Naming the Alkanes
With over 16 million known organic chemicals, it is useful to have an interna-
tionally agreed-upon set of nomenclature rules. We hinted at such a system with
the first 10 normal alkanes in Table 12.4. These names form the basis of the
more complex names given to branched-chain alkanes. This molecule is called
3-methylheptane. Why?
1. We identify and name the longest straight chain in the molecule, which in this
case is derived from heptane.
3-Methylheptane
2. We name the branch, using the name of the alkane with the same number of
carbon atoms as are in the branch (methane, in this case). However, we replace
the -ane ending with -yl to indicate that the group is a branch. The word
methyl then is added to the front of our molecule’s name, which becomes
methylheptane.
3. We identify the location of the branch by associating it with the numbered
carbon atom of the main chain to which it is attached. We number the main
chain from the end that gives the branch point the lowest number. The final
name is 3-methylheptane.
Various other rules guide us in naming more complex alkanes. These rules,
collectively, are known as IUPAC nomenclature rules after the International
Union of Pure and Applied Chemistry, whose members developed the naming
system. All compounds in the world can be named using IUPAC nomenclature,
but many compounds have less structured names. For instance, as we learned in
Chapter 2, dihydrogen monoxide is commonly known as water. The common
names, unfortunately, have few rules—and many exceptions. It is best to learn the
common names as you are exposed to the compound, though with all but the
most common compounds, we will use the IUPAC nomenclature.
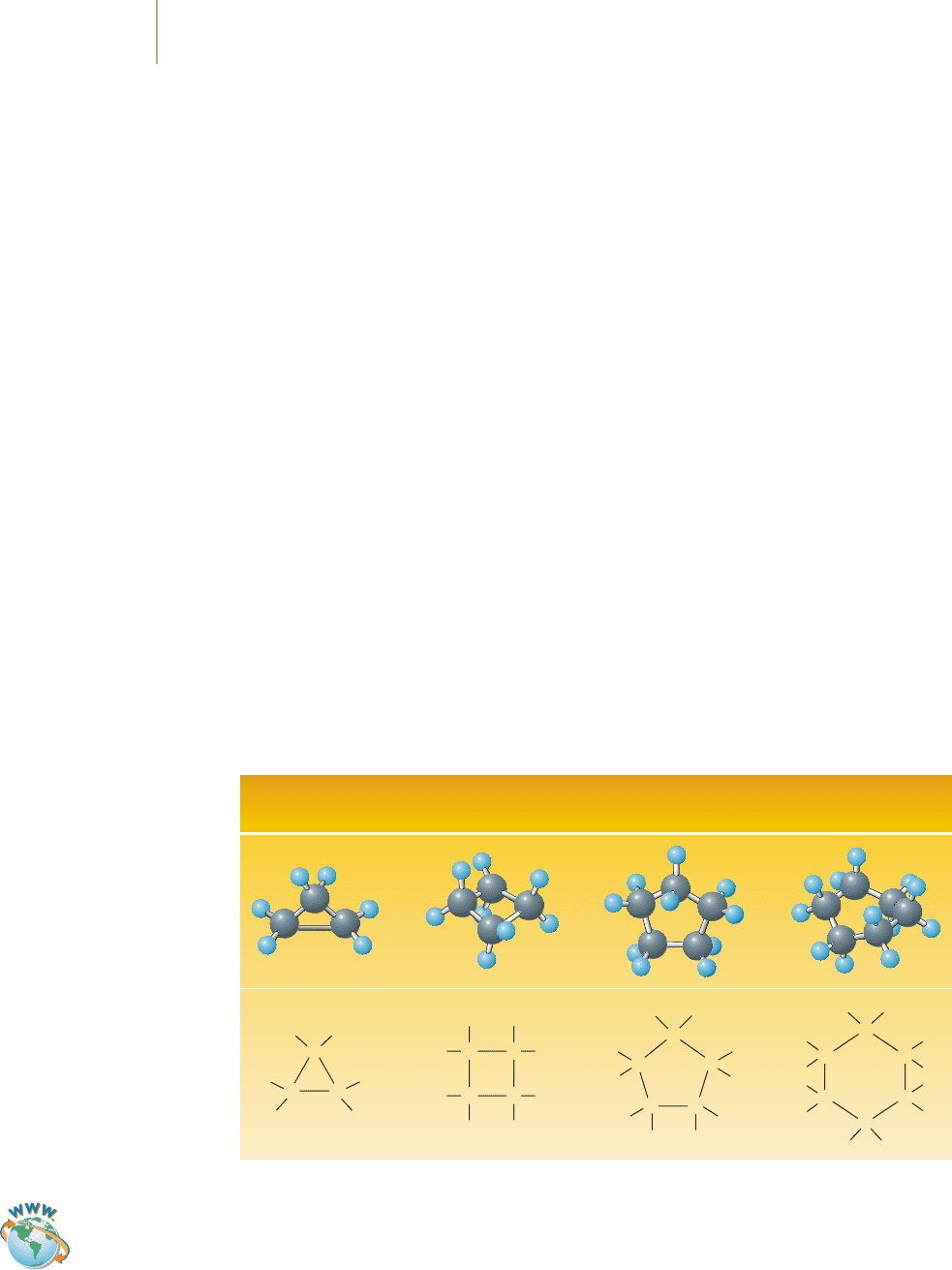
Cyclic Alkanes
Carbon atoms can be bonded into rings, to form cyclic alkanes. The four simplest
cyclic alkanes are shown below. Cyclohexane is used in the preparation of nylon
fiber and is a nonpolar solvent. Cyclopentane is increasingly employed as a
foaming agent—a substance that is needed to create foam—in the preparation of
insulation for use in refrigerators. It replaces some of the chlorofluorocarbons
implicated in global warming.
Alkenes
Alkenes are hydrocarbons that contain at least one carbon-to-carbon double
bond (CPC). The members of this class of compounds are important to the
chemical industry as starting materials in the manufacture of a host of organic
compounds. Only tiny amounts of alkenes are found in crude oil, but certain
alkenes are made from crude oil in huge quantities, in processes that we will
consider shortly. The simplest three alkenes are shown at the top of the next page.
500 Chapter 12 Carbon
CC
C
C
CC
C
C
C
C
C
C
C
CC
HH
HH
C
H
H
H
HH
HH
HH
HH
H
HH
H
HH
H
CC
HH
HH
HH
H
H
H
H
H
H
HH
Cyclopropane
C
3
H
6
Cyclobutane
C
4
H
8
Cyclopentane
C
5
H
10
Cyclohexane
C
6
H
12
Video Lesson: Alkenes and
Alkynes
FIGURE 12.9
Photograph of oxy-acetylene welding.
High temperatures are obtained during
the combustion of acetylene. This melts
the metals, allowing a strong weld to form.
12.3 Hydrocarbons
501
Ethene
C
HH
HH
C
Propene
C
HH
H
H
H
HC
C
1-Butene
C
HH
H
H
H
H
H
HC
C
C
Ethene Ethane
+
+
HH
Pt
Pt
CH
H
H
H
H
CH
C
HH
HH
C
Alkynes
Hydrocarbons with a carbon-to-carbon triple bond are known as alkynes. Those
with only one triple bond form a homologous series with the general formula
C
n
H
(2n–2)
. The first three members of this series are shown below. Ethyne (acety-
lene) is burned with oxygen to release the energy that generates very high
temperatures in oxy-acetylene welding, shown in Figure 12.9.
Ethyne
H C HC
Propyne
H C CC
H
H
H
1-Butyne
H C CC
H
H
C
H
H
H
The names of the alkenes are derived from the names of the corresponding alka-
nes, but with -ene at the end instead of -ane. The simple alkenes, those with only
one double bond, form a homologous series with the general formula C
n
H
2n
.
This general formula is also the same as that of the cyclic alkanes.
Alkenes are known as
unsaturated hydrocarbons because, unlike the alkanes,
they are not “saturated” with hydrogen. Each CPC bond in an alkene can react
with one hydrogen molecule, under suitable conditions, to generate the corre-
sponding alkane. For example, the
hydrogenation (addition of a molecule of hy-
drogen) of ethene gives ethane. This is a general reaction of the alkenes. Platinum
metal is a catalyst in this reaction and therefore is indicated over the arrow in the
chemical equation that represents the reaction.
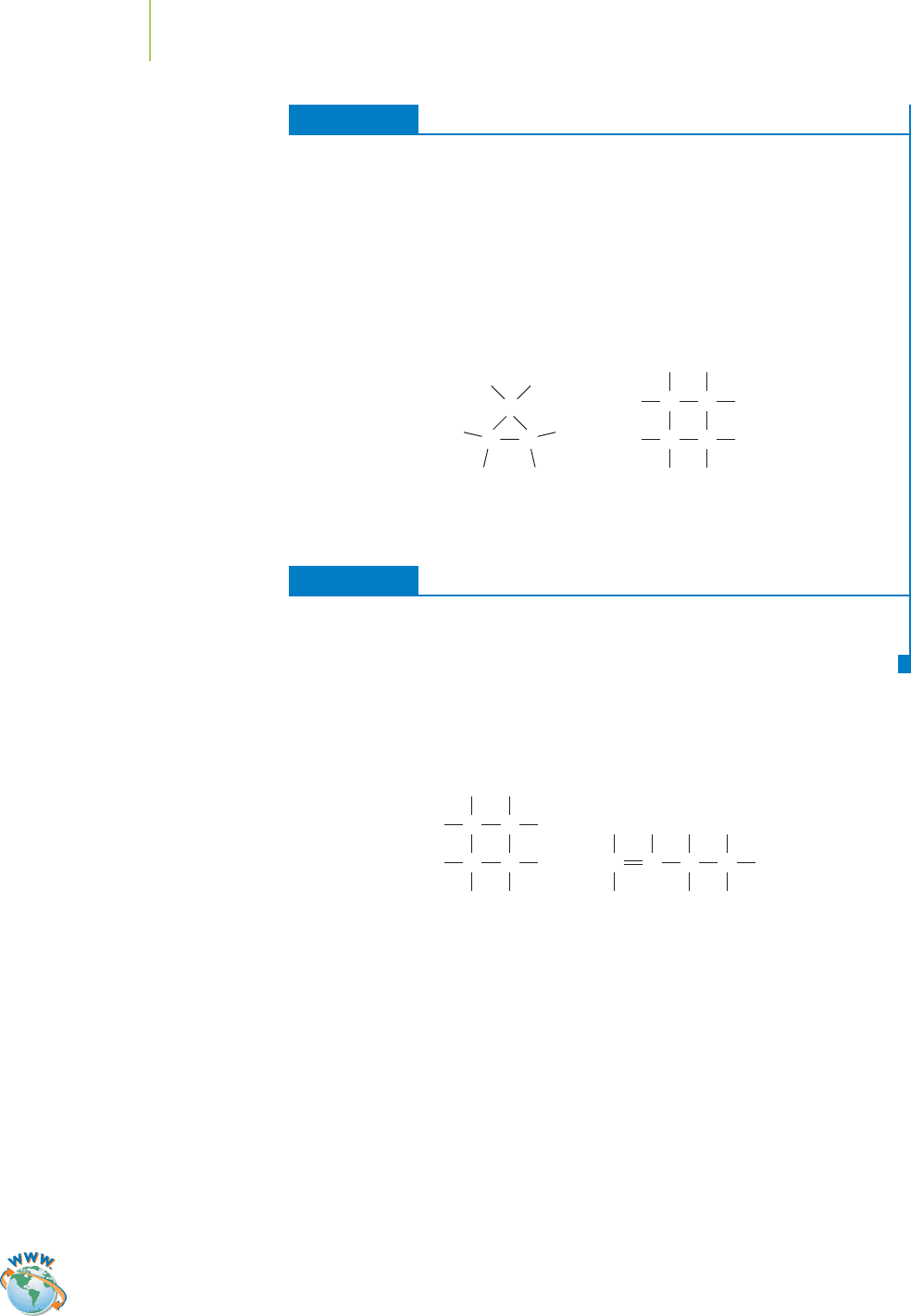
EXERCISE 12.2 Care with General Formulas
We have seen that the general formula for the alkenes is C
n
H
2n
. Does this mean that
every hydrocarbon with that general formula must be an alkene?
Solution
Can you draw any hydrocarbon structure that has twice as many hydrogen atoms as
carbon atoms, but no double bonds? It cannot be done using straight chains or
branched chains of carbon atoms, but it can be done if the carbon chain bonds back
on itself, to form a cyclic hydrocarbon. As you can verify from the examples below,
cyclic alkanes share the general formula C
n
H
2n
with alkenes.
PRACTICE 12.2
Provide a structural drawing for a cyclic hydrocarbon that would be an isomer of
hexene.
See Problems 23 and 24.
The general formula C
n
H
2n
is not unique to alkenes. We saw in Exercise 12.2 that
each alkene with three or more carbon atoms will have a cyclic alkane as one of
its isomers. For example, cyclobutane is an isomer of butene:
Isomers do not always have to belong to the same homologous series; they just
have to have the same atoms bonded in different ways.
The naming of alkenes and alkynes is complicated by the need to distinguish
exactly where the double or triple bond lies within the molecule. This is handled
by attaching a number in front of the name of the molecule. The number corre-
sponds to the location of the first carbon in the double or triple bond. Just as in
naming branched alkanes, the number we use must be the lowest number that
can be generated by numbering the longest carbon chain. For example,
CH
3
CHPCHCH
2
CH
3
is 2-pentene, not 3-pentene. In this way, we see that there
are only two alkenes with the formula C
4
H
8
: 1-butene (CH
2
PCHCH
2
CH
3
) and
2-butene (CH
3
CHPCHCH
3
).
Geometric Isomers
Geometric isomers are common in the alkenes. What is a geometric isomer? Look-
ing at the boiling points of two of the isomers of C
4
H
8
shown at the top of the
next page will help us arrive at an answer to this question.
1-Butene
C
4
H
8
CC
H
H
C
H
H H
C
H
H
H
Cyclobutane
C
4
H
8
HC
H
C
H
H
HC
H
C H
H
Cyclobutane
C
4
H
8
HC
H
C
H
H
HC
H
C
H
H
Cyclopropane
C
3
H
6
H
C
H
C
H
C
HH
H
502 Chapter 12 Carbon
Video Lesson: Isomers
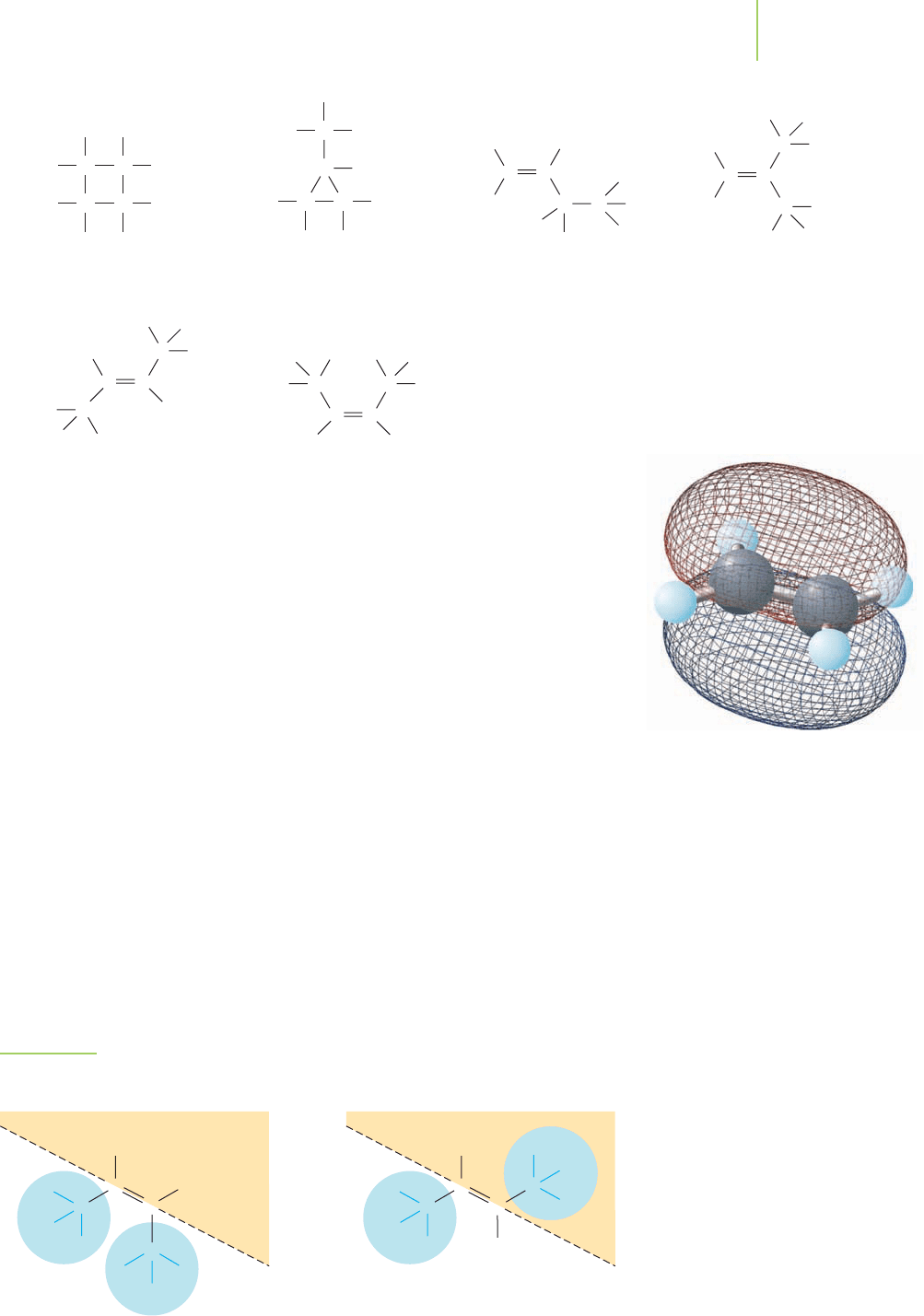
We see that two cyclic molecules, cyclobutane (A) and methylcyclopropane (B),
are the only alkanes we can draw. The remainder of the molecules with the for-
mula C
4
H
8
are alkenes, including 1-butene (C), 2-methylpropene (D), and two
different 2-butenes (E and F). Are the two 2-butenes really different? We could
imagine that if we rotated the CPC bond around, we would have the same struc-
tures. However, the CPC bond doesn’t allow rotation to occur, so these two mole-
cules cannot be redrawn to show the same structure. In fact, their physical prop-
erties indicate to us that they are completely different. This is where the boiling
points prove important. Molecule (F) has a boiling point of 3.7°C, whereas mol-
ecule (E) has a boiling point of 1°C, giving evidence for two different molecules.
To distinguish between these two different molecules, we use the designations
cis and trans to describe their structures, as illustrated in Figure 12.10. These pre-
fixes are always given in italics. In the
cis form, the groups on either side of the
CPC are on the same side of the molecule (Figure 12.10). (As a remembering de-
vice, think of cis as “sisters.”) The molecule with the 3.7°C boiling point is cis-2-
butene. In the
trans form, the groups on either side of the CPC are on opposite
sides of the molecule (Figure 12.10). (Think about the groups having to transfer
from one side to the other.) This molecule is trans-2-butene.
In general, geometric isomers are isomers that differ in the location of the
atoms only because of the geometry of the carbon to which they are attached. Cis
and trans isomers are geometric isomers because the carbons of the alkene group
have a fixed geometry.
12.3 Hydrocarbons 503
A
HC
H
C
H
H
HC
H
C H
H
B
HC
H
H
C
H
HC
H
C H
H
C
CC
H
H
H
C
H
H
H
C
H
H
E
H
H
H
CC
C
HC
H
H
H
H
F
H
H
HH
CC
H
CC
H
H
H
D
H
H
H
CC
H
HC
C H
H
H
H
H
H
H
H
cis trans
C
C
C
C
H
HH
H
H
H
H
H
H
H
C
C
C
C
H
FIGURE 12.10
Cis and trans isomers of the alkenes. The cis isomer contains groups on the same side
of the alkene. The trans isomer contains groups on opposite sides of the alkene.
The side-to-side overlap of adjacent
p orbitals within the C
PC bond gives
rise to the pi bond between the carbon
atoms. As an atom on one end of the
bond begins to rotate the p orbital over-
lap decreases. Thus, the pi bond restricts
rotation of the atoms at either end.
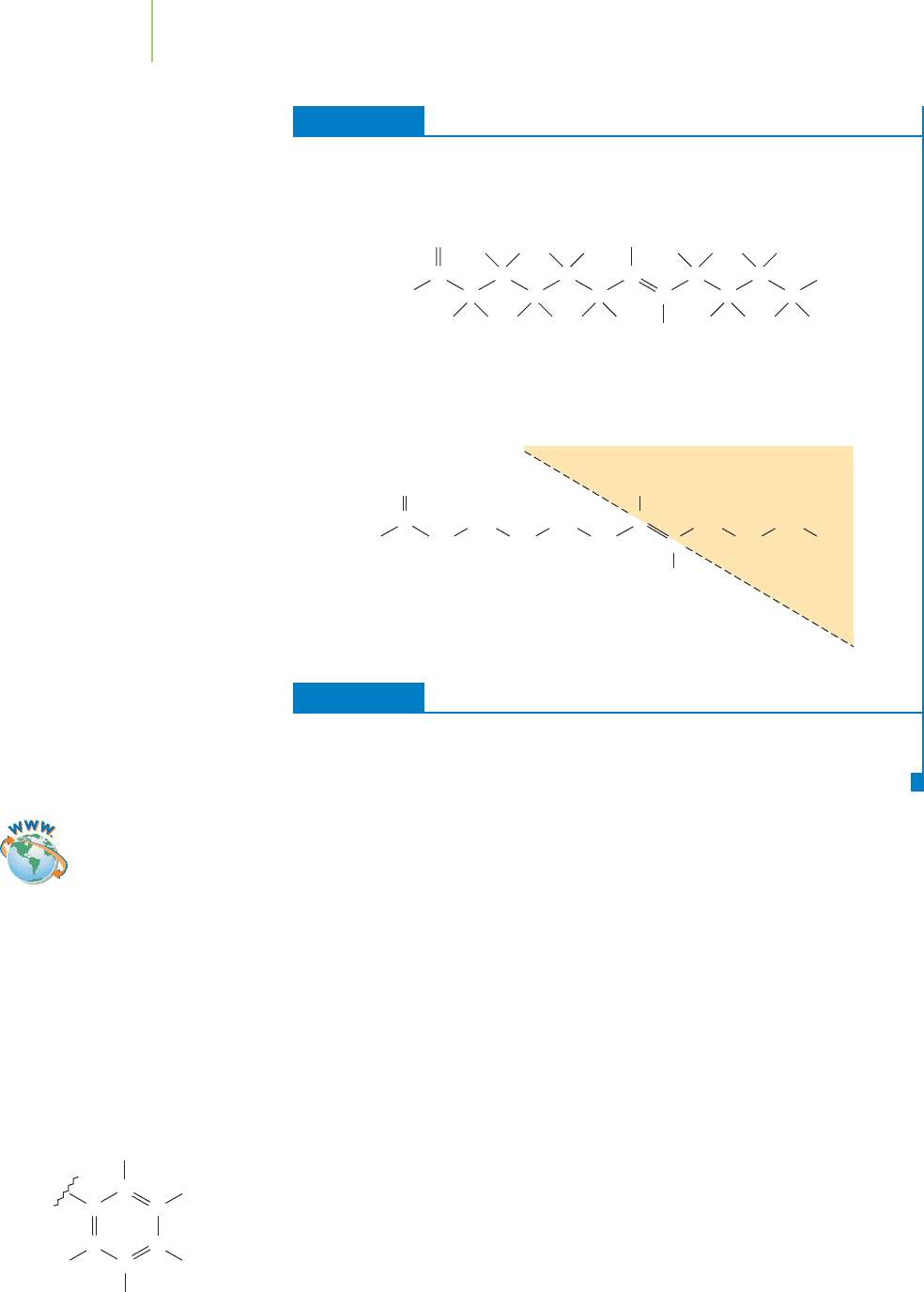
EXERCISE 12.3 Cis and Trans
Consuming trans fatty acids is considered bad for your health. They arise when
vegetable oil is hydrogenated. The resulting partially hydrogenated vegetable oils con-
tain a small amount of trans fats. Is the compound below a cis or a trans fatty acid?
Solution
This compound is a trans fatty acid. The groups on either end of the CPC are on
opposite sides of the molecule
PRACTICE 12.3
Draw all of the alkenes with the formula C
5
H
10
and indicate whether each is cis or
trans.
See Problems 25 and 26.
Aromatic Hydrocarbons
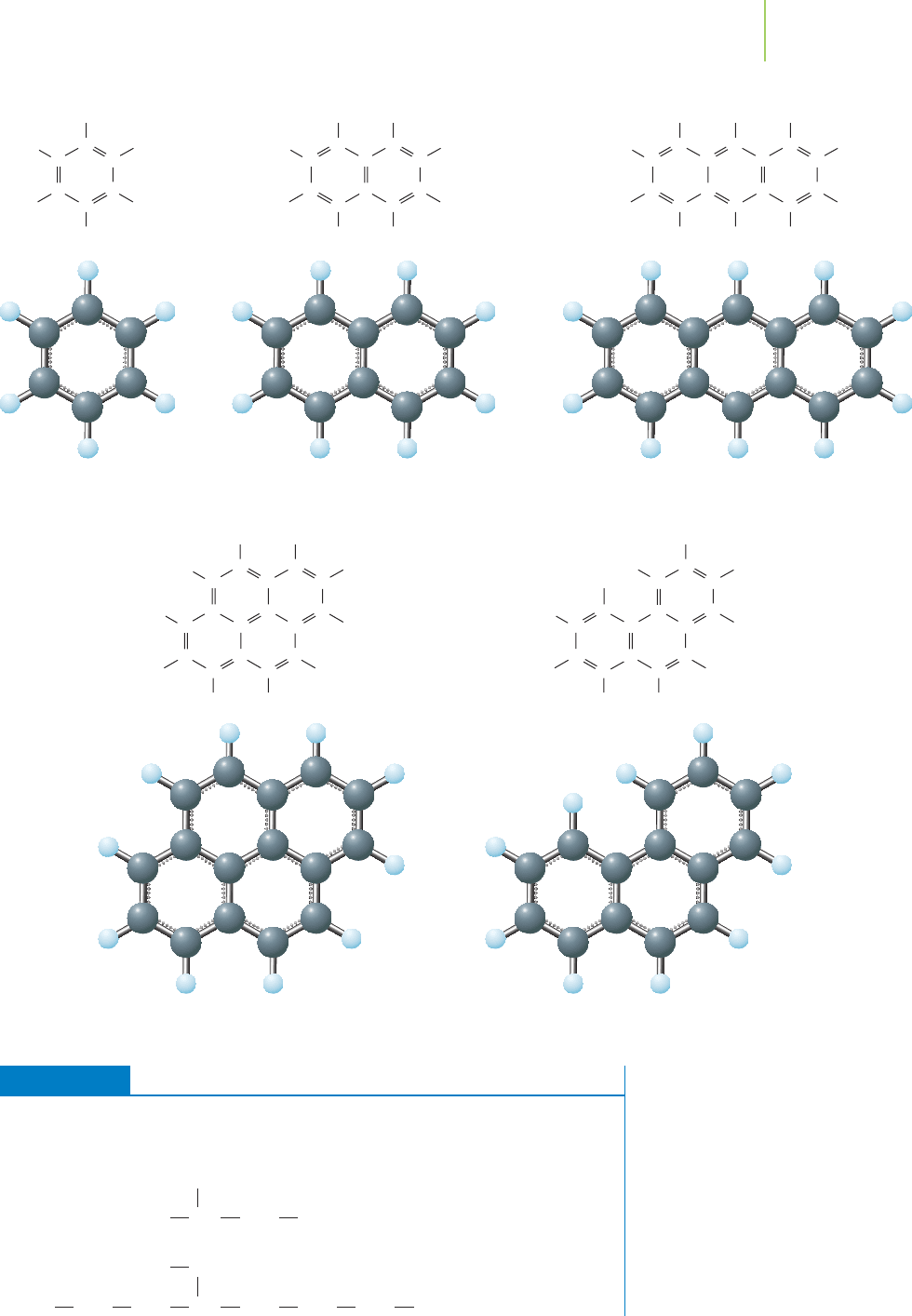
Aromatic hydrocarbons contain one or more aromatic rings, which were discussed
in Chapter 9. A common aromatic compound is benzene. This compound and its
derivatives are used in the production of a vast array of consumer products such
as polystyrene and other plastics, nylon, detergents, and pharmaceuticals. The
molecule consists of three alternating carbon-carbon double bonds in a six-
carbon ring. Benzene and several more complex aromatic compounds are shown
on page 505.
Alkyl Groups
Many hydrocarbons can be regarded as being composed of a main chain of car-
bon atoms with various hydrocarbon branches attached. These branches are
known as
alkyl groups, and many have a name derived from the name of the
alkane on which they are based. The names of other structures are derived from
common names. The names of alkyl groups are placed in front of the parent
name in alphabetical order.
—CH
3
is a methyl group.
—C
2
H
5
is an ethyl group.
—C
3
H
7
is a propyl group.
—C
4
H
9
is a butyl group.
—C
5
H
11
is a pentyl group.
H
H
C
C
O
CH
2
HO
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
3
C
C
H
O
C
CHO
H
H
HH H
H
H
C
H
H
C
H
H
H
C
H
H
C
H
C
H
H
C
C
C
H
H
H
C
504 Chapter 12 Carbon
H
H
H
Phenyl group
H
C
C
C
H
C
C
C
—C
6
H
13
is a hexyl group.
—C
7
H
15
is a heptyl group.
—C
8
H
17
is an octyl group.
—Ph (C
6
H
5
) is a phenyl group.
Video Lesson: Aromatic
Hydrocarbons
H
H
C
CHH
HH
Benzene
C
C
C
C
H
H
C
CHH
HH
Naphthalene
Pyrene Phenanthrene
C
C
H
H
C
C
C
C
C
C
H
H
CC
CHH
H
H
C
C
H
H
C
C
C
C
C
C
HH
C
C
CC
C
H
H
CC
CHH
H
H
C
C
H
C
C
C
C
C
C
H
C
H
HC
C
H
H
C
CHH
HH
Anthracene
C
C
H
H
C
C
C
C
H
H
C
C
C
C
C
C
12.3 Hydrocarbons
505
EXERCISE 12.4 Naming with Alkyl Groups
Fill in the blanks, corresponding to the type of alkyl group, to complete the names
of the hydrocarbons shown.
CH
2
CH
2
CH
3
CH
3
CH CH
2
CH
2
CH
2
CH
3
4-[ ] octaneb.
CH
2
CH
3
CH CH
2
CH
3
CH
3
2-[ ] butanea.
CH
2
CH
3
CH
3
CH C CH
3
CH
2
CH
3
CH
3
]-2,2-[3-[ ] pentaned.
CH
3
CH CH CH
3
CH
3
CH
3
2,3-[ ] butanec.
506 Chapter 12 Carbon
Solution
a. 2-methylbutane c. 2,3-dimethylbutane
b. 4-ethyloctane d. 3-ethyl-2,2-dimethylpentane
PRACTICE 12.4
Draw the structure of 2,2-dimethyl-4-propyloctane.
See Problems 17 and 18.
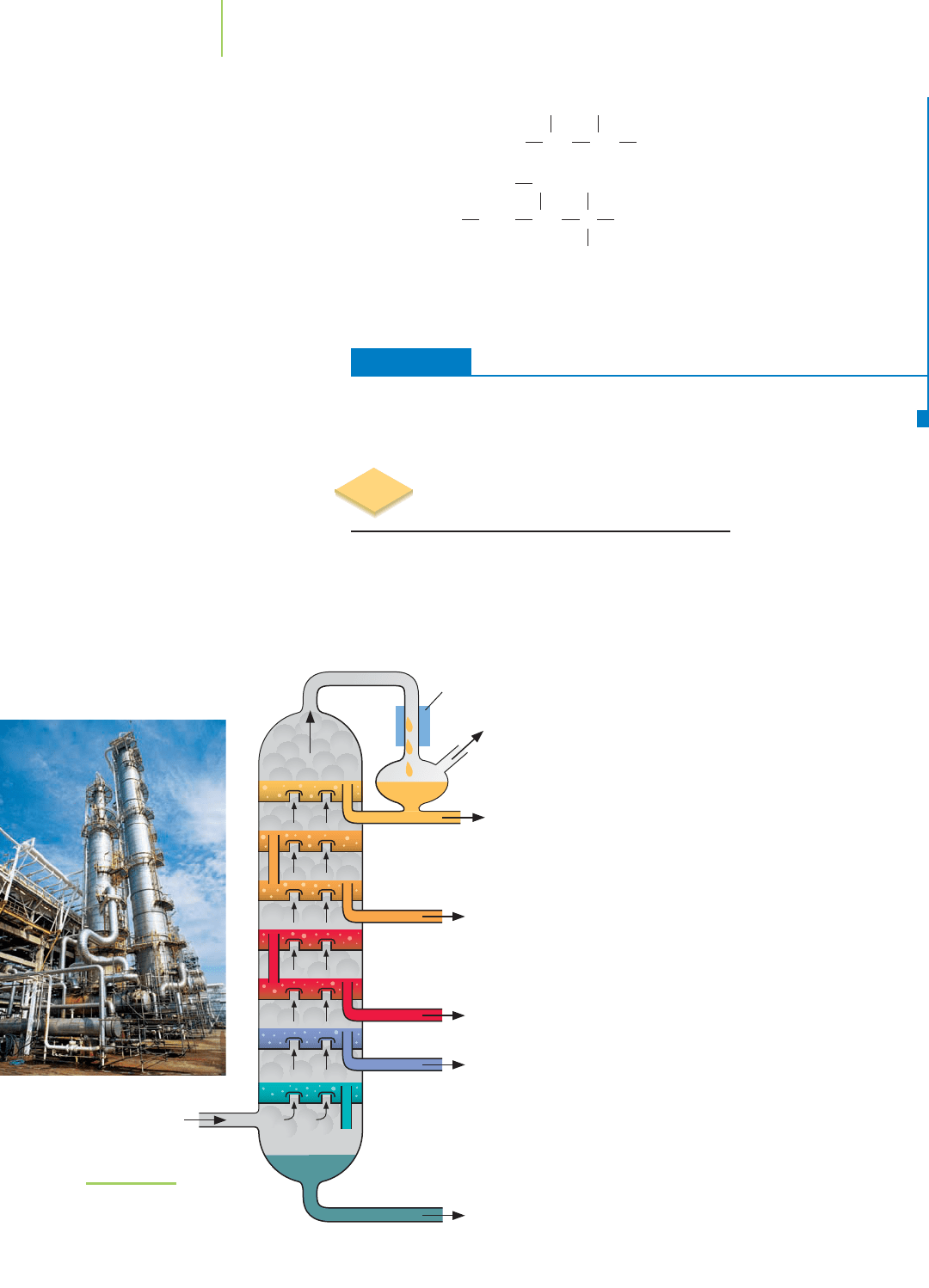
12.4 Separating the Hydrocarbons
by Fractional Distillation
Crude oil contains a wide variety of carbon-based compounds. Separating those
compounds so that they can be used to make fabrics, medicines, and other com-
pounds we need everyday is an important procedure. The first step in this process
is to separate the oil into several “fractions” using fractional distillation, as shown
in Figure 12.11. Each of the fractions contains a selection of hydrocarbons with
generally similar physical properties. Because
a hydrocarbon’s boiling point is broadly re-
lated to its molecular size, each fraction con-
tains molecules that are similar in molecular
size. The oil is heated to around 400°C, caus-
ing much of it to vaporize. The tarry residue
that remains is a mixture of very large hydro-
carbon molecules. This residue is collected as
one of the basic fractions produced by the
fractional distillation process.
The vaporized portion of the oil rises up
the fractionating column (Figure 12.11),
which can be around 60 m in height. The
vapor cools as it rises, causing the various
fractions of the oil to condense back into
liquid form at different heights up the col-
umn, depending on their boiling points. At
selected levels, the hydrocarbons that are in
liquid form gather on collecting trays and are
piped away. The smallest (and lowest-boiling)
hydrocarbons do not condense into liquid at
all but emerge at the top of the column as
“refinery gas.”
This primary fractional distillation pro-
cess is typically used to separate the crude
oil into six major fractions, summarized in
Table 12.5. These fractions can be subjected to
secondary fractional distillation or can be fed
Residue, asphalt
C
25
and up
Hot petroleum
(crude oil)
Gasoline
40–200°C
C
4
to C
12
Petroleum gas
<40°C
C
1
to C
3
Kerosene, jet fuel
200–250°C
C
12
to C
16
Heating oil
250–300°C
C
15
to C
18
Lubricating oil
300–370°C
C
19
and up
Condenser
FIGURE 12.11
Fractional distillation—a
diagram of the process and the
main fractions.
12.4 Separating the Hydrocarbons by Fractional Distillation 507
On March 24, 1989, the oil tanker Exxon Valdez ran
aground in Prince William Sound, Alaska, spilling over 40 million liters
of crude oil into the sea. Despite the massive clean-up effort, small
amounts of crude oil are still found
along the shores near the accident.
Toward the end of 1990, samples of
these oil residues were collected as
part of research to monitor the fate
of the oil as the environment slowly
recovered. It turned out, however,
that some of the samples could not have come from
the Exxon Valdez, because they contained the wrong
mixture of hydrocarbons. The debris from older oil
spills was complicating the picture. How was this
determined? It was done using a technique called
gas chromatography (GC), one of the three most
common methods for analyzing the hydrocarbon
content of crude oil.
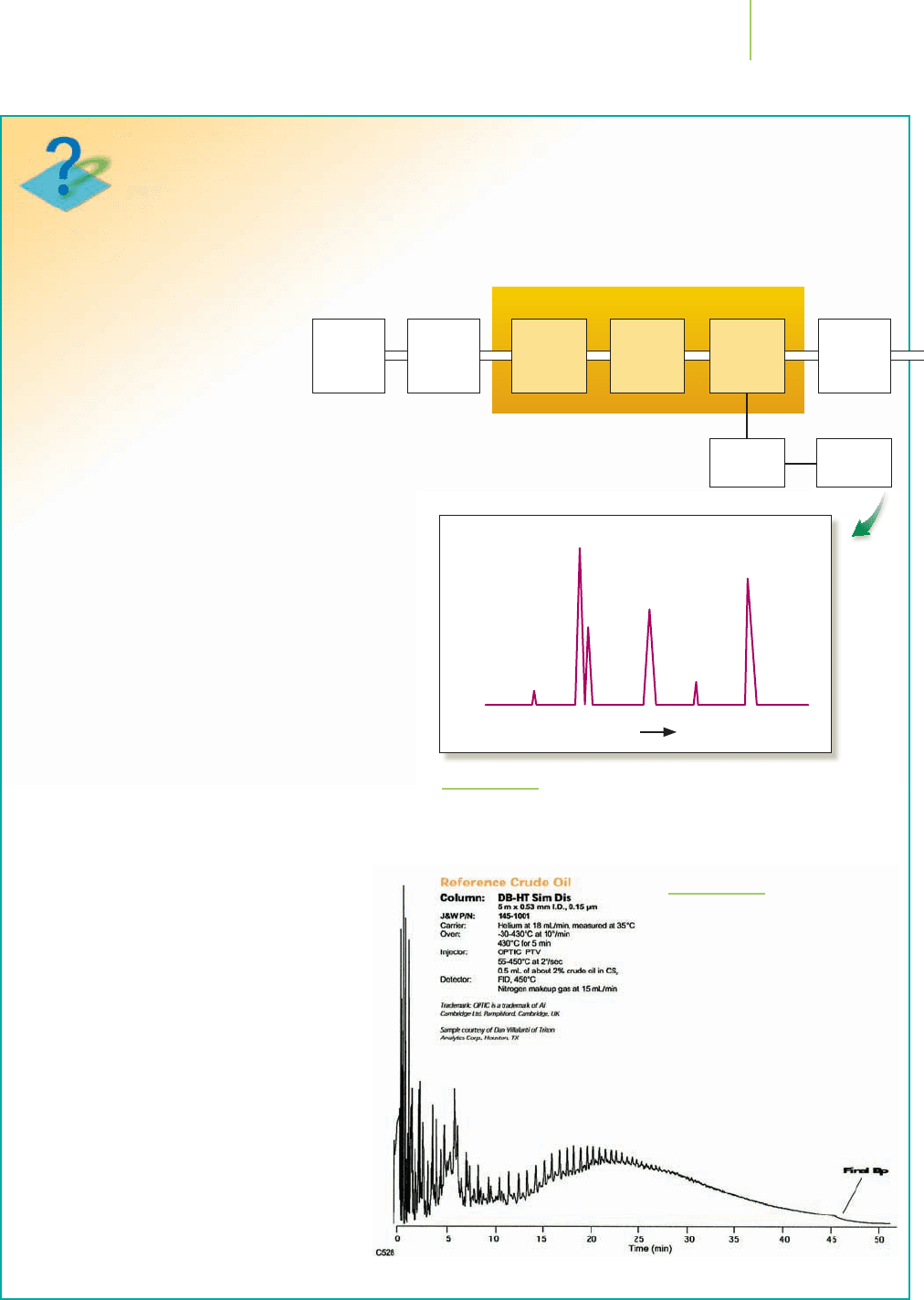
In gas chromatography, a sample such as crude
oil that is to be analyzed is converted into vapor by
heating and then carried by a flow of inert gas (such
as He) through a heated column packed with solid
powder such as silica, as shown in Figure 12.12. In
some cases, the particles of solid in the column may
be coated with a nonvolatile liquid, a technique
known as gas–liquid chromatography (GLC). As the
sample flows through the column, the components
of the mixture travel at different speeds because of
the differing extents to which they are adsorbed
onto the solid phase or are soluble in the liquid
phase (in GLC). The components emerge from
the column separately and are detected,
often by their effect on a detecting flame.
The apparatus will have previously been
calibrated using a range of known hydro-
carbons, so specific hydrocarbons in the
sample can be identified by comparison
with the results of the calibration.
An example of the results of an analysis
of one type of crude oil is shown in Fig-
ure 12.13. The technique is very powerful,
particularly in providing a GC “fingerprint”
of different samples from different origins,
but it has significant limitations. In particu-
lar, it cannot readily distinguish between
some of the different structural isomers of
hydrocarbons. For this reason, more thor-
ough analysis of the hydrocarbons in crude
oil also makes use of the techniques of mass
spectrometry and nuclear magnetic reso-
nance spectroscopy.
How do we know?
Which hydrocarbons are in crude oil?
FIGURE 12.13
A gas chromatogram showing the
components found in crude oil.
Pressure
regulator
Carrier
gas tank
Detector
Sample
injection
chamber
Flow
meter
Column
Oven
Amplifier Recorder
Time
Response of detector
FIGURE 12.12
The operating principles of gas chromatography and a sample
readout (a gas chromatogram).
