Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

488 Chapter 11 The Chemistry of Water and the Nature of Liquids
65. Water and ethanol are said to be miscible.
a. Distinguish between a solute that is miscible with water
and a solute that is soluble in water.
b. What do miscible substances have in common?
66. What would you predict would occur if a saturated solution
of sodium chloride were warmed? What would happen if a
saturated solution of lithium sulfate were warmed?
Section 11.7 Measures of Solution Concentration
Skill Review
67. Calculate the molarity (M) of these solutions:
a. 42.0 g of NaOH dissolved in enough water to form 0.500 L
of solution
b. 10.0 g of C
6
H
12
O
6
(dextrose) dissolved in enough water to
form 0.250 L of solution
c. 25.0 g of urea (NH
2
CONH
2
) dissolved in enough water to
form 100.0 mL of solution
68. Using the information in Problem 67, calculate the molality
of each solution (assume the density of the solution is
1.0 g/mL).
69. Calculate the molality (m) of these solutions:
a. 12.5 g of ethylene glycol antifreeze (CH
2
OHCH
2
OH) dis-
solved in 0.100 kg of water
b. 53.0 g of sucrose (C
12
H
22
O
11
) dissolved in 500.0 g of water
c. 4.55 g of sodium bicarbonate (NaHCO
3
) dissolved in
250.0 g of water
70. Using the information in Problem 69, calculate the molarity
of each solution (assume the density of each solution is
1.0 g/mL).
71. Determine the mole fraction of solute in the following:
a. 22.7 g of benzene (C
6
H
6
) dissolved in 67.5 g of cyclo-
hexane (C
6
H
12
)
b. 15.0 g of formic acid (HCOOH, found in ants) dissolved
in 100.0 g of water
c. 0.195 g of acetaldehyde (C
2
H
4
O, found as the product of
ethanol metabolism) dissolved in 25.0 g of water
72. Using the information in Problem 71, calculate the molality
of each solutions (assume the density of water is 1.0 g/mL).
73. Fill in the missing information.
Chemical Applications and Practices
75. A typical cup of coffee may contain 75 mg of caffeine per
200.0 mL of water. If the density of the solution were approx-
imately 1.09 g/mL, what would you calculate as the mass per-
cent caffeine, the molarity, and the molality? (Hint: Yo u ’ l l
need the formula of caffeine to answer this problem.)
Caffeine
76. Perspiration has a slight acidity because of the presence of
lactic acid (C
3
H
6
O
3
). Suppose we analyze the perspiration
of a chemistry student running late to class. If the density of
the perspiration were 1.15 g/mL and the mass percent lactic
acid were found to be 4.88%, what would you calculate as the
mole fraction, molarity, and molality of the lactic acid?
77. The saline solution used in some medical procedures is a
5.00% NaCl solution. What would be the molarity of sodium
ions (Na
+
) in such a solution if the density were assumed to
be approximately 1.02 g/mL?
78. Sodium alkylbenzene sulfonate (C
18
H
29
SO
3
Na) is often used
as a synthetic detergent. This ingredient helps prevent scale
buildup in “hard” water applications. If a detergent solution
were 0.100 M in sodium alkylbenzene sulfonate, how many
grams would be dissolved per liter of solution?
79. Hard water is the name given to water containing relatively
high concentrations of metal ions, such as calcium and mag-
nesium. For instance,if a particular water sample were said to
contain 130.0 ppm Ca
2+
, it would be classified as hard water.
a. What would be the mass percent of calcium ion
containing 130.0 ppm Ca
2+
?
b. What would be the same concentration expressed as parts
per billion?
80. The concentration of potassium ion in human blood cells
varies over a healthy range. If the concentration of K
+
in a
red blood cell were listed at 0.95 mol/mL, what would you
report as the molarity and parts per million?
Section 11.8 The Effect of Temperature and Pressure
on Solubility
Skill Review
81. This represents a system at equilibrium: O
2
(aq) → O
2
(g)
a. In which direction will the equilibrium be shifted by an
increase in pressure?
b. In which direction will the equilibrium be shifted by an
increase in temperature?
82. This a system at equilibrium: CO
2
(g) → CO
2
(aq)
a. In which direction will the equilibrium be shifted by an
increase in pressure?
b. In which direction will the equilibrium be shifted by an
increase in temperature?
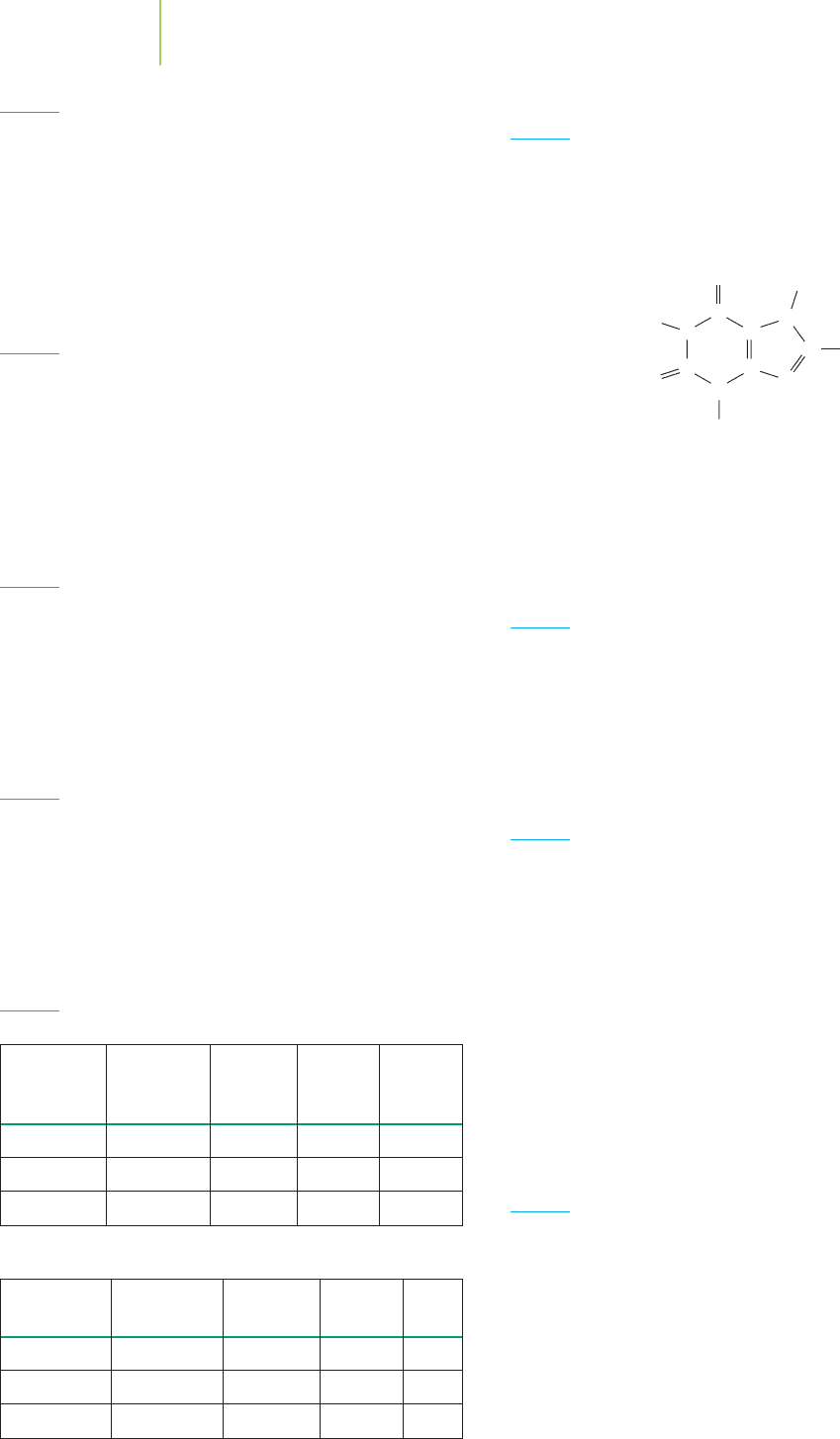
CH
3
CH
3
C
O
C
N
N
C
C
CH
3
O
N
CH
N
Mole
Grams of Grams of Fraction
Compound Compound Water of Solute Molality
NH
4
Cl 12.5 g 95.0 g
KNO
3
125 g 0.155
C
6
H
12
O
6
250.0 g 0.115
Grams of Grams of
Compound Compound Water Mass % ppm
NH
4
Cl 1.0 g 99.0 g
KNO
3
125 g 125
C
6
H
12
O
6
300.0 g 15.8
74. Fill in the missing information.
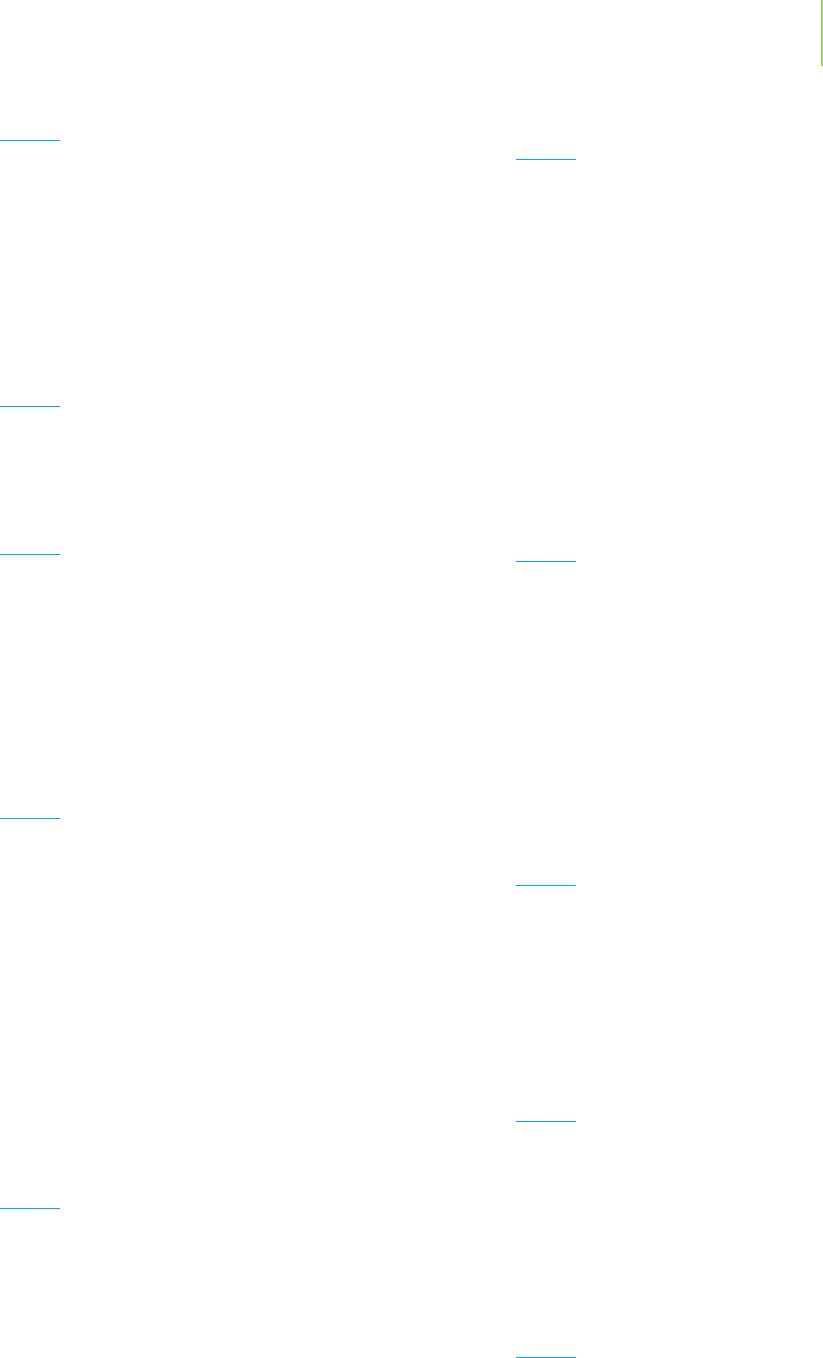
Focus Your Learning 489
Chemical Applications and Practices
83. Underwater activities require assisted breathing techniques.
When using pressurized air, divers must be aware of Henry’s
law. Assuming that air contains 78% nitrogen, what is the
concentration of N
2
in blood at 1 atm? (The Henry’s law con-
stant for N
2
(g) at 25
◦
C is 1540 atm/M.)
84. What would be the concentration of N
2
in blood if the N
2
pressure were increased to 3.0 atm? (The Henry’s law con-
stant for N
2
(g) at 25
◦
C is 1540 atm/M.)
Section 11.9 Colligative Properties
Skill Review
85. In theory, what concentration of ions would you expect to
find dissolved in 500.0 mL of a 0.00100 m solution of CaCl
2
?
86. What value is expected for the van’t Hoff factor in very dilute
solutions of each of these?
a. AlCl
3
c. Mg(OH)
2
b. (NH
4
)
3
PO
4
d. C
6
H
12
O
6
87. Although several factors are necessary to explain the magni-
tude of the vapor pressure of a sample, some general trends
can be noted. Arrange these substances in order of decreasing
vapor pressure.
Water (H
2
O)
Glycerol (HOCH
2
CHOHCH
2
OH)
Pentane (CH
3
CH
2
CH
2
CH
2
CH
3
)
88. a. Complete this sentence by inserting the appropriate term
in the blank: As external air pressure ________ (increases,
decreases) the boiling point of a liquid decreases.
b. Explain why the term you selected is correct.
89. For each of these solutions, determine the boiling point of
the solution. You may need to use Table 11.6 to help with this
problem. (Assume these solutions behave ideally.)
a. 0.75 m NaCl in water
b. 0.040 m glucose (C
6
H
12
O
6
) in water
c. 0.250 M CaCl
2
in water (Assume the density of the
solution is 1.00 g/mL.)
d. 0.25 mol fraction naphthalene in benzene (Assume the
density of the solution is 0.88 g/mL.)
90. For each of these solutions, determine the boiling point of
the solution. You may need to use Table 11.6 to help with this
problem. (Assume these solutions behave ideally.)
a. 1.00 m sucrose (C
12
H
22
O
11
) in ethanol
b. 0.14 m Na
2
SO
4
in water
c. 0.250 M glucose (C
6
H
12
O
6
) in water (Assume the density
of water is 1.00 g/mL.)
d. 500.0 ppm FeCl
3
in methanol
91. For each of these solutions, determine the melting point of
the solution. You may need to use Table 11.8 to help with this
problem. (Assume these solutions behave ideally.)
a. 0.500 m glucose (C
6
H
12
O
6
) in water
b. 0.055 m LiOH in water
c. 0.125 m methanol in phenol
d. 1.20 m benzoic acid in water
92. For each of these solutions, determine the melting point
of the solution. You may need to use Table 11.8 to help with
this problem. (Assume these solutions behave ideally.)
a. 0.200 mol fraction glucose (C
6
H
12
O
6
) in water
b. 0.055 m CoCl
2
in water
c. 0.125 m benzene in phenol
d. 1.20 m HCl in water
93. For each of these solutions, determine the vapor pressure of
the solution at STP. (Assume these solutions behave ideally.)
a. 0.200 mol fraction glucose (C
6
H
12
O
6
) in water
b. 0.055 mol fraction CoCl
2
in water
c. 0.125 mol fraction benzoic acid in water
d. 1.20 m HCl in water
94. For each of these solutions, determine the osmotic pressure
of the solution at STP. (Assume these solutions behave
ideally.)
a. 0.200 M FeCl
2
in water
b. 0.055 m CoCl
2
in water (Assume the density of the
solution is 1.0 g/mL.)
c. 0.125 mol fraction glucose (C
6
H
12
O
6
) in water (Assume
the density of the solution is 1.02 g/mL.)
d. 0.0945 mol fraction NaCl in water (Assume the density of
the solution is 1.10 g/mL.)
Chemical Applications and Practices
95. An amount of water in a closed container will have a certain
vapor pressure when the temperature is held constant.
Varying the shape of the container can change the surface
area of the volume of water. However, this does not change
the vapor pressure. Explain this observation, and reconcile
it with the observation that the surface area of a sample of
water does change the rate of evaporation in an open
container.
96. The vapor pressure of water at 25
o
C is 23.76 mm Hg. What
would you calculate as the new vapor pressure of a solution
made by adding 50.0 g of ethylene glycol (HOCH
2
CH
2
OH,
antifreeze) to 50.0 g of water? You may assume that the
vapor pressure of ethylene glycol at this temperature is
negligible.
97. Explain why 0.10 m solutions of nonelectrolytes have ap-
proximately the same boiling point regardless of the iden-
tity of the solute. Why doesn’t this same statement apply to
electrolytes?
98. Two solutions are placed in a −1.0
◦
C storage cooler. One
solution is labeled 5.0% C
6
H
12
O
6
and the other 15.0%
C
6
H
12
O
6
. When they were to be retrieved the next day, the
labels of both had loosened and fallen off the containers.
Would you be able to identify the solutions on the basis of
their freezing points? Prove your answer.
99. How many grams of antifreeze (HOCH
2
CH
2
OH) would
have to be added to 5.0 kg of water in an automobile cool-
ing system to keep it from freezing during a cold Nebraska
winter with temperatures of −32
◦
C?
100. Freezing-point depression can be used to determine the
molecular mass of a compound. Suppose that 1.00 g of an
unknown molecule were added to 20.0 g of water and the
freezing point of the solution determined. If the new freez-
ing point of water were found to be −1.50
◦
C, what would
you predict to be the molecular mass of the compound?
101. a. The outer membrane of many fruits acts as a semiper-
meable membrane through which osmosis can take
place. Diagram the cell of a cucumber before and after it
has been set in a highly concentrated salt solution.

490 Chapter 11 The Chemistry of Water and the Nature of Liquids
b. Why is it important to know the osmotic pressure of
human fluids before administering any fluids to a
patient?
102. One of the first stages in healing a small cut takes place
when a blood clot begins to form. A key enzyme in this
process is thrombin. This large enzyme has a molar mass of
nearly 34,000 g/mol. What would be the osmotic pressure
of a solution that contained 0.20 g of thrombin per milli-
liter, at 37.0
◦
C?
Comprehensive Problems
103. The compounds guanine and cytosine are two bases that
make up part of the structure of your DNA. Use the struc-
tures shown to indicate how three hydrogen bonds can
form between these two molecules.
104. Explain why you would expect the heat of vaporization for
water to be so much larger than the heat of fusion.
105. If the combustion of methane were used as the source of
heat for Problem 37, how many grams of CH
4
would be
needed?
c
H for CH
4
= 55.5 kJ/g.
106. When preparing some solutions for analysis, chemists must
be aware that some solution processes are very exothermic.
Sodium hydroxide solutions are often used when analyzing
acid solutions.
a. The heat of solution for NaOH is −44.5 kJ/mole. Using
three factors, describe the dissolving of solid NaOH
pellets in water.
b. What temperature change would you calculate for a
solution, starting at 25.0°C, made when 100.0 g of water
is mixed with 10.0 g of NaOH? Assume the heat capacity
of the solution is the same as that for water.
107. Carbon tetrachloride (CCl
4
) and bromoform (CHBr
3
) both
have tetrahedral structures. One has more than twice the
surface tension of the other. Which has the higher surface
tension, and what factor gives rise to this difference?
108. The viscosity of glycerol is over a thousand times larger than
that of chloroform (CHCl
3
). Explain what factor accounts
for this large difference.
109. Suppose you are on a camping trip in the mountains of
Colorado. While boiling water to cook some dried food,
your friends notice, with their handy thermometer, that the
water is boiling at only 90
◦
C. How would you explain to
your perplexed friends that turning up the gas on the stove
will not increase the temperature of the water?
110. The final production of the writing paper you may be using
to solve this problem involves several chemical treatments.
One compound used in the process is aluminum sulfate.
What would be the molarity of an industrial solution if
Cytosine
NH
2
HC
HC
N
H
N
O
Guanine
O
C
C
N
C
NH
NH
2
N
HC
N
H
3250 g of Al
2
(SO
4
)
3
were dissolved in enough water to make
20.0 L of solution?
111. Sulfuric acid (H
2
SO
4
) solutions are used as the primary
electrolyte in the lead storage batteries found in automo-
biles. One of the most common ways to check the charge
level in such a battery is to determine the density of the so-
lution. A sulfuric acid solution had a density of 1.58 g/mL
and was known to contain 35.6% by mass H
2
SO
4
. What is
the molarity of the solution?
112. According to Figure 11.1, the world’s water consumption in
2020 will be 2700 km
3
/year. Assuming a density of 1.00 g/mL,
how many metric tons of water will be consumed each year?
113. Hexane is a common liquid used in the chemistry labora-
tory as a solvent.
a. How many carbon atoms are there in hexane?
b. Is there a relationship between the number of carbon
atoms in a straight-chain (normal) alkane and the boil-
ing point of the alkane? If so, what is it?
c. What intermolecular forces are present in hexane?
114. In the electron density maps shown throughout the text, the
red color indicates regions of the molecule that possess par-
tial negative change (greater electron density). The blue
color indicates regions of the molecule with partial positive
charges (reduced electron density). Compare the electron
density maps of water (page 127) and of hydrogen bonded
water (page 449). Do you notice any striking differences
between the two images? If so,what are those differences and
what does that imply about the effects of hydrogen bonding?
115. As a beaker of water boils, bubbles develop at the bottom of
the beaker and rise to the surface (see the figure on
page 455).
a. What is the origin of the bubbles?
b. How many liters of water vapor could be produced from
a beaker containing 250 mL water (d 1.00 g/mL) if the
beaker is boiled to dryness? (Assume the temperature of
the water vapor is 100°C at 0.95 atm.)
c. How much heat would be required to complete the
conversion of 250 mL water at 25°C into water vapor at
100°C?
Thinking Beyond the Calculation
116. The fuel most commonly used in portable lighters is butane
(C
4
H
10
). The normal boiling point of butane is −0.50
◦
C.
a. Draw the Lewis dot structure for butane where all of the
carbons are in a row.
b. What types of intermolecular forces of attraction would
you predict for this molecule?
c. Does the low boiling point of butane make sense?
d. Is this compound soluble in water?
e. If 3.50 g of butane were burned in oxygen, how many
moles of water would be formed? (The other product is
carbon dioxide.)
f. Considering that −0.50
◦
C is so much lower than typical
room temperatures,why doesn’t the butane in the lighter
boil?
g. Relative to butane, estimate the normal boiling point of
pentane (C
5
H
12
) and propane (C
3
H
8
).

491
Contents and Selected Applications
12.1 Elemental Carbon
12.2 Crude Oil—the Basic Resource
Chemical Encounters: Crude Oil—the Basic Resource
12.3 Hydrocarbons
12.4 Separating the Hydrocarbons by Fractional Distillation
12.5 Processing Hydrocarbons
12.6 Typical Reactions of the Alkanes
12.7 The Functional Group Concept
12.8 Ethene, the C
PC Bond, and Polymers
12.9 Alcohols
12.10 From Alcohols to Aldehydes, Ketones, and Carboxylic Acids
12.11 From Alcohols and Carboxylic Acids to Esters
12.12 Condensation Polymers
12.13 Polyethers
12.14 Handedness in Molecules
12.15 Organic Chemistry and Modern Drug Discovery
Carbon
Oil, like that stored aboard this
tanker, is responsible for heating
our homes, running our cars, and
serving as the feedstock for pro-
duction of an extraordinary number
of organic chemical compounds.
The processing of oil provides plas-
tics that we use every day. From
grocery bags to the shirts on our
backs, oil is vital to our current way
of life.
Go to college.hmco.com/pic/kelterMEE for online learning resources.

Deep beneath the surface of the Earth, a
spinning drill bit from an oil exploration plat-
form breaks through the hard rock and hits pay dirt—
oil. The dark liquid escapes from its high-pressure vault to
emerge at the surface as a gushing fountain of chemical possi-
bilities. The famous “gushers” of the past (see Figure 12.1) may be
rare now, but oil remains the chemical foundation of a huge industry
sustaining our modern way of life. The gas and diesel fuels that power our
vehicles, along with plastics, paints, modern textiles, and a vast range of medi-
cines, are derived from the petrochemical industry. The raw material that flows out of
the Earth and into that industry is crude oil, petroleum. The element at
the heart of the chemistry of oil is carbon. This carbon is not pure but is
bonded together with hydrogen and other elements to form a rich mix-
ture of molecules that can be separated, modified, and exploited to make
so many of the products that sustain our modern way of life.
Coal, another carbon-based treasure, is mined from natural out-
croppings both above and below ground, such as the one shown in
Figure 12.2. The use of this resource helped fuel the industrial revolution
of eighteenth and nineteenth centuries. Since then, coal has been em-
ployed to make a wide range of useful carbon-based substances, including
gasoline, diesel and jet fuels, and some chemicals. Coal also remains one
of the most important fuels for generating electricity (see Table 12.1).
Our planet is made most interesting by the chemistry of carbon. In ad-
dition to its vital role as the primary component of fossil fuels, the chem-
istry of carbon is also important to perhaps the most precious treasure of
all, life. All of the key molecules of life are built around a framework of
bonded carbon atoms. These carbon-based molecules, were initially
known as
organic compounds because this class of compounds was found in
living organisms. It was later determined that organic compounds do not
appear exclusively in living organisms, but the name stuck. In fact, the oil
and coal we use as a source of carbon-based compounds were themselves
formed from the carbon-based chemicals in ancient ani-
mals and plants. For this reason, the study of carbon com-
pounds, known as
organic chemistry, focuses on the reactions
and properties of a vast number of organic compounds.
FIGURE 12.1
Black gold. An oil derrick becomes
engulfed in a spray of crude oil.
FIGURE 12.2
Coal is obtained from huge open-pit mines
and from deep underground in large pockets.
This natural resource is useful in heating
homes, in fueling electric plants, and in
making steel.
2004 Fuel Sources of Energy in the United States
Source Percent of Total BTU*
Coal 32.2%
Natural gas 27.5
Petroleum 16.4
Hydroelectric 3.9
Nuclear 11.7
All others 8.3
Total Production = 70.369 quadrillion BTU (quadrillion = 10
15
)
*1 BTU = 1055 joules
Source: U.S. Department of Energy, Energy Information Agency.
492
TABLE 12.1
12.1 Elemental Carbon
Carbon is a very versatile element that can be used to prepare a nearly endless
array of carbon-based compounds. It also exists in three different and distinct
forms as the element. Different forms of the same element, known as
allotropes,
are not exclusive to carbon. Other elements, such as phosphorus, oxygen (as O
2
and O
3
,) and sulfur, also can be found in a variety of allotropes. The allotropes of
carbon are diamond, graphite, and fullerenes.
Diamond
Figure 12.3 illustrates the first of the three allotropes of
carbon, diamond. The carbon within a diamond is bonded
to four neighboring carbons in a giant covalent network,
as shown in Figure 12.4. The bonds in a diamond are the
result of the overlap of identical sp
3
hybridized orbitals
that are arranged tetrahedrally around each atom. This
makes diamond one of the hardest substances known,
because the network of many strong covalent bonds must
be disrupted to break or distort a piece of diamond. The
strong bonding in diamond explains why it forms such an
enduring gemstone and why it can be used to cut other
materials.
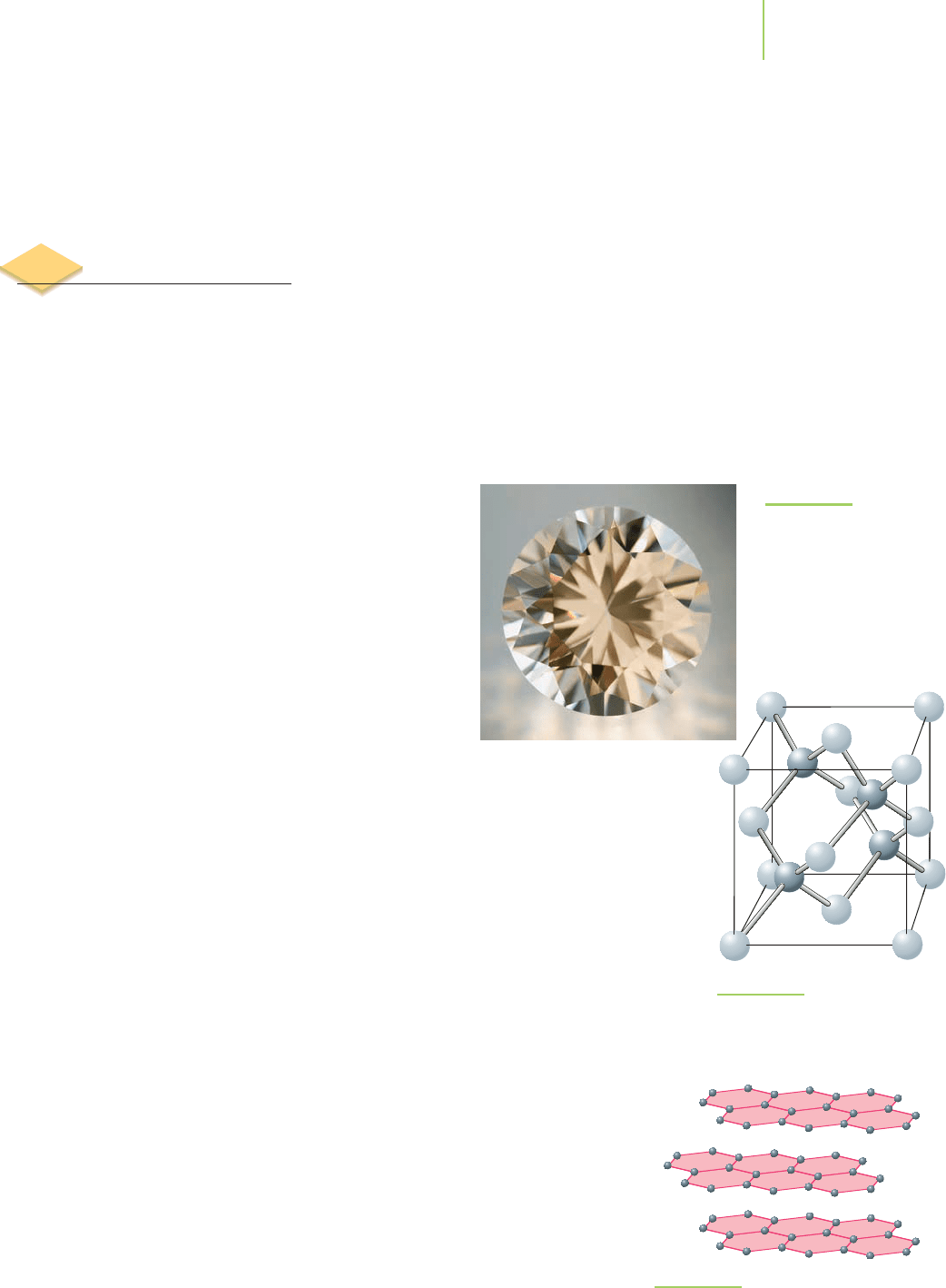
Graphite
Compare the structure of diamond to that of graphite, another of the allotropes
of carbon shown in Figure 12.5. The sp
2
hybridized carbon atoms in graphite
share covalent bonds to three neighboring atoms, creating a repeating hexagonal
network of carbon atoms in extended layers. Each atom in this structure con-
tributes one unbonded electron to a system of delocalized π electrons above and
below each hexagonal layer. Unlike the sp
3
hybridized carbons in diamond, the
delocalized and mobile electrons in graphite allow it to conduct electricity. This
means that graphite can be used as the electrodes in industrial processes such as
the Hall–Heroult process for producing aluminum (discussed in Chapter 19).
The layered structure of graphite also makes it soft and slippery. Each layer is
only weakly attracted to the layers above and below. This enables us to use
graphite as pencil “lead”; portions of the layers will slide off the tip of the pencil
and onto the paper. Graphite’s slipperiness also makes it useful as a solid
lubricant in specialized machinery. Diamond, by contrast, in which the entire
structure is a network of covalent bonds, is a terrible lubricant but an excellent
grinding agent.
Fullerenes
A third allotrope of carbon, shown in Figure 12.6, was discovered in 1985. This
allotrope consists of sp
2
hybridized carbon atoms folded into structures that
resemble balls and tubes. The core member of this class of molecules is called
Buckminsterfullerene (C
60
) because of its similarity to the geodesic domes de-
signed by the architect Buckminster Fuller. Buckminsterfullerene (also known as
12.1 Elemental Carbon 493Carbon 493
FIGURE 12.3
Diamond, an allotrope
of carbon that humans
find particularly valu-
able when cut and
polished.
FIGURE 12.4
The covalent network structure
of diamond.
What is the nature of these organic chemicals? How do we make them? How do we
chemically manipulate them to produce the stunning range of goods based on the
crude oil that generates over a quarter of a trillion dollars in sales for oil and gasoline
companies per year?
FIGURE 12.5
The structure of graphite.

494 Chapter 12 Carbon
Nanotube BuckminsterfullereneBuckminsterfullerene
FIGURE 12.6
Fullerenes, one of the allotropes of
carbon, includes ball-shaped structures
and hollow tube structures. Each of the
carbon atoms in these structures is sp
2
hybridized.
Geodesic domes have been used to
construct spacious homes.
Application
C
HEMICAL
ENCOUNTERS:
Crude Oil—the
Basic Resource
(a) Anticlinal trap (b) Fault displacement trap
FIGURE 12.7
Geology of a crude oil reservoir.
Sandstone
Impervious rock such as shale
Oil
“buckyball”) is prepared when an electric discharge arcs between two graphite
rods surrounded by helium gas at high pressure. Although C
60
and graphite are
similar in the presence of sp
2
hybridized carbon atoms, the carbon atoms in buck-
minsterfullerene are held in both six-membered and five-membered rings. The
five-membered rings cause a curvature in the overall structure of the compound.
Buckminsterfullerene is a member of an increasingly varied group of related
structures known as
fullerenes. These include cage-like molecules both smaller
and larger than the C
60
cage, as well as tubes of bonded atoms that can occur
either alone or in concentric nested patterns, as shown in Figure 12.6. Many
research teams are busy trying to find medical and technological applications
for these structures.
12.2 Crude Oil—the Basic Resource
Crude oil, also known as petroleum, is not an allotrope of carbon. Instead, it is a
very complex mixture of hundreds of compounds containing carbon and other
atoms. In general terms, crude oil contains many different kinds of
hydrocarbon
molecules (molecules composed of only carbon and hydrogen atoms), together
with smaller amounts of molecules derived from hydrocarbons, often including
atoms of sulfur, nitrogen, oxygen, and various metals. Table 12.2 lists the typical
classes of compounds that can be found in crude oil.
Crude oil is generally found deep beneath the surface of the Earth, trapped
between layers of rock in pockets like that shown in Figure 12.7. It is found nearly
everywhere in the world, under both land and sea. Unfortunately, removing the
oil from these pockets is an involved and expensive process, but it is done because
of the incredible usefulness of the compounds found in crude oil. Table 12.3 on
page 496 lists the countries that export the most crude oil and indicates how
much oil they ship each day. Note how many of these countries are global “hot
spots” of regional conflict.
Why do we so passionately seek out oil? Why are nations willing to go to war
over it? Why are national economies dependent on the prevailing price of oil? The

12.2 Crude Oil—the Basic Resource 495
Composition of Crude Oil
Class of Compound Percent of Oil Typical Example
Hydrocarbons
Aliphatics 25%
(hydrocarbons containing
no double or triple bonds)
Aromatics 17%
(containing aromatic groups,
first defined in Chapter 9)
Naphthenes 47%
(cyclic hydrocarbons with
all single-bonded carbons)
Nonhydrocarbons
Sulfurous <8%
Nitrogenous <1%
Oxygenated <3%
Metals <<< 1% Fe, Mn, Zn, V
TABLE 12.2
HCC
H
H
C
H
H
C
H
H
H
H
C
H
H
H
H
C
H
C
C
C
C
C
H
C
H
H
H
H
H
C
H
H
H
HH
CC
H
H
CC
C
H
H
H
H
H
H
CC
H
CC
S
H
H
H
C
H H
C
C
C
C
C
H
C
N
H
C
H
H
H
C
H O
C
C
C
C
C
H
C
H
OH
H
Crude oil.
answers include our heavy use of oil to produce the gasoline and other fuels for
cars, trucks, trains, boats, and jet airplanes. But the uses of oil go well beyond
these things. We are surrounded by products made from oil, including plastics,
clothing, furniture, carpeting, eyeglasses, and compact disks; the list is almost
endless.
12.3 Hydrocarbons
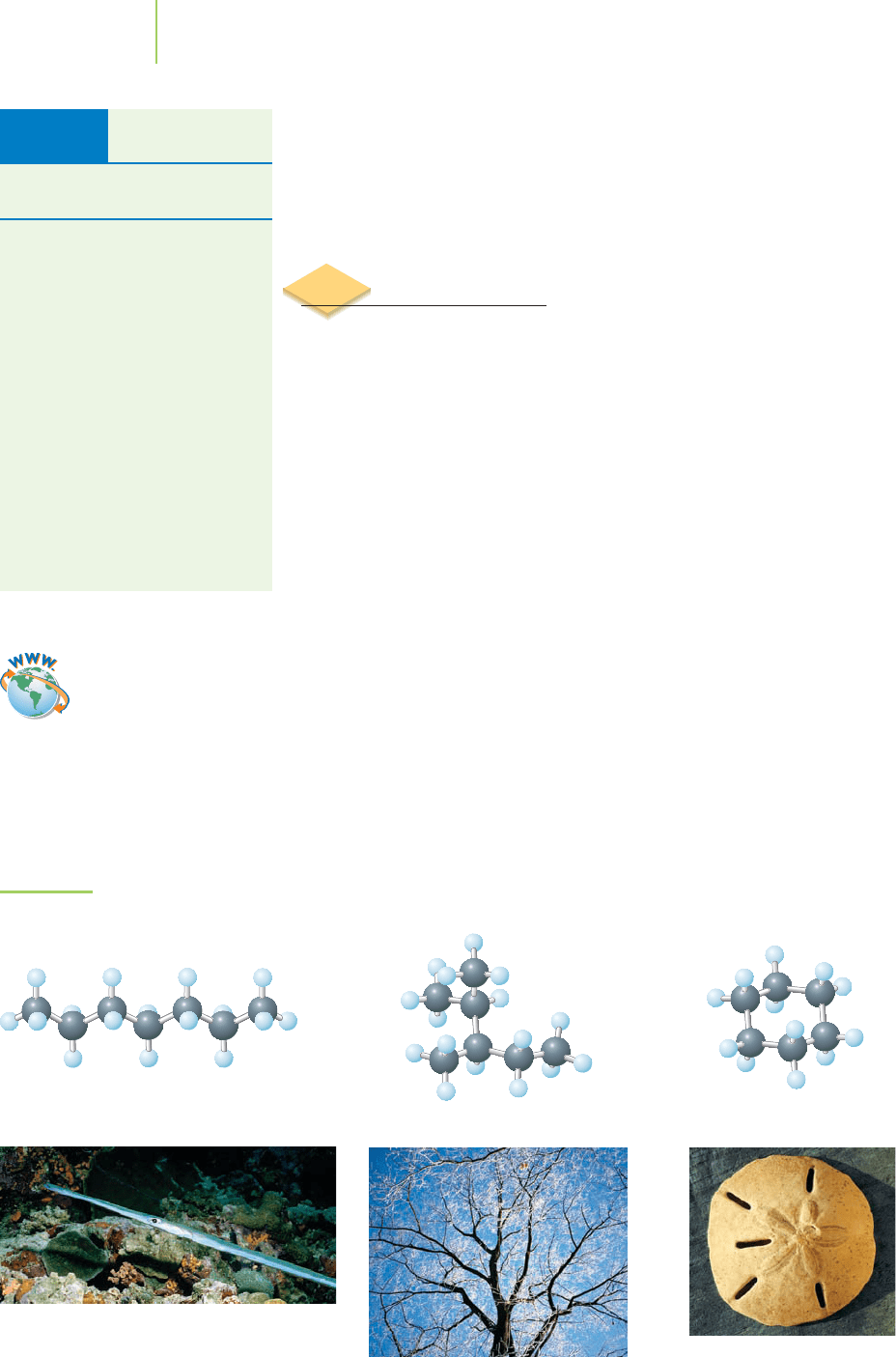
The carbon atoms of hydrocarbons are bonded into straight chains, branched
chains, rings, or more complex combinations of these three basic structures
(Figure 12.8). The hydrogen atoms are bonded to the carbon atoms so that each
carbon has a total of four covalent bonds. Some of the carbon atoms in hy-
drocarbons can be bonded together by carbon–carbon double bonds or carbon–
carbon triple bonds, reducing the number of hydrogen atoms that can bind to
the carbon atoms.
Hydrocarbons that contain no double or triple bonds are known as
saturated
hydrocarbons
,or aliphatic compounds. They are “saturated” with hydrogen, which
means that the carbon atoms are bonded to the maximum possible number of
hydrogen atoms, because none of the electrons are involved in double or triple
bonds. This is the origin of the term saturated fats, which might be familiar to you
from food labels. All fats include hydrocarbon chains as part of their structure,
and the saturated fats have no CPC double bonds.
Alkanes
Saturated hydrocarbons are also known as alkanes, and they make up the largest
fraction of any class of crude oil compounds (see Table 12.2). The simplest pos-
sible alkanes are the
normal alkanes, which have no branched chains or rings of
carbon atoms. The simplest normal alkane—and also the simplest hydrocarbon—
contains a single carbon atom bonded to four hydrogen atoms. This molecule is
known as methane.
496 Chapter 12 Carbon
Straight-chain Branched Cyclic
FIGURE 12.8
The three main classes of hydrocarbons: straight-chain, branched, and cyclic.
Top Daily Producers
of Crude Oil in 2004
Million Barrels
Country per Day
Saudi Arabia 8.73
Russia 6.67
Norway 2.91
Iran 2.55
Venezuela 2.36
United Arab 2.33
Emirates
Kuwait 2.20
Nigeria 2.19
Mexico 1.80
Algeria 1.68
Iraq 1.48
Libya 1.34
Kazakhstan 1.06
Qatar 1.02
Source: Energy Information Administration,
Non-OPEC Fact Sheet.
TABLE 12.3
The three classes of hydrocarbons have structures
that we see elsewhere in nature. A needlefish, tree
branches, and a sand dollar are representative of
straight-chain, branched-chain, and cyclic
hydrocarbons.
Video Lesson: Alkanes
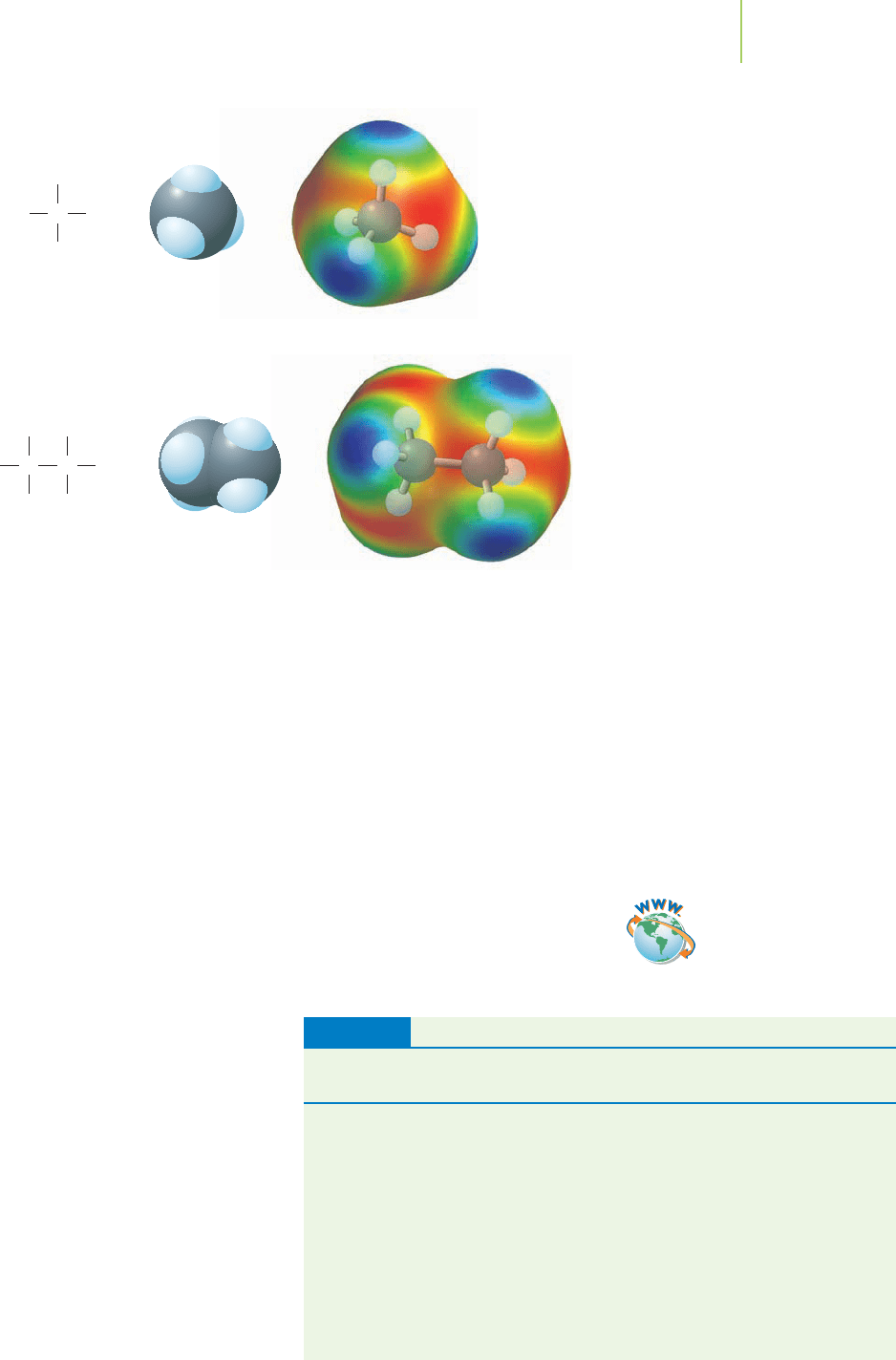
12.3 Hydrocarbons 497
CHH
H
H
Methane
CH
H
H
CH
H
H
Ethane
Methane is the principal component of “natural gas,” which is commonly
found trapped above petroleum deposits and is piped into homes, offices, and
factories as a fuel for heating and cooking.
The next-simplest hydrocarbon has two carbon atoms bonded together, with
each carbon atom bonded to three hydrogen atoms. This is ethane. As you can see
from Table 12.4, the normal alkanes form a regular series in which each member
has one more —CH
2
— group, a methylene group, than the preceding member of
the series. These alkanes are members of a
homologous series of compounds; this
means they all share the same general formula, C
n
H
(2n+2)
for the normal alkanes.
They have similar chemical and physical properties, which change in a gradual
manner as we move through the series.
Although we call the normal alkanes
straight-chain alkanes, in reality the car-
bon chain zig-zags, as you can see in Figure 12.8. This is because the four bonds
of each carbon atom are formed when four sp
3
hybridized orbitals of the atom
overlap with the orbitals of neighboring carbon atoms and hydrogen atoms.
The bonds around each carbon atom adopt a tetrahedral arrangement, as we dis-
cussed in Chapter 9.
The major direct use we make of
the alkanes in petroleum is as fuels.
Hydrocarbons burn in oxygen (or in air,
which is one-fifth oxygen) to generate
mostly carbon dioxide and water and re-
lease considerable amounts of heat. The
combustion of hydrocarbons helps us
heat our homes, cook our food, and
power our cars. Like most of the other
hydrocarbons in crude oil, the alkanes
also serve as valuable and versatile “feed-
stock” molecules—molecules that can be
modified by the chemical industry to
generate many useful products.
The First Ten Normal Alkanes
Number Molecular Structural Boiling
Name of Carbons Formula Formula Point (°C)
Methane 1 CH
4
CH
4
−161.5
Ethane 2 C
2
H
6
CH
3
CH
3
−88.6
Propane 3 C
3
H
8
CH
3
CH
2
CH
3
−42.1
Butane 4 C
4
H
10
CH
3
CH
2
CH
2
CH
3
−0.5
Pentane 5 C
5
H
12
CH
3
(CH
2
)
3
CH
3
36.0
Hexane 6 C
6
H
14
CH
3
(CH
2
)
4
CH
3
68.7
Heptane 7 C
7
H
16
CH
3
(CH
2
)
5
CH
3
98.5
Octane 8 C
8
H
18
CH
3
(CH
2
)
6
CH
3
125.6
Nonane 9 C
9
H
20
CH
3
(CH
2
)
7
CH
3
150.8
Decane 10 C
10
H
22
CH
3
(CH
2
)
8
CH
3
174.1
TABLE 12.4
Video Lesson: Organic
Nomenclature
