Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

classify as “insoluble” in water, our universal solvent, can dissolve at least a little.
One such chemical is oxygen (O
2
), and that is fortunate for fish and other sea life
that depend on it. Another such chemical is carbon tetrachloride, a nonpolar in-
dustrial solvent; that is not so fortunate, because even in low concentrations in
water, it is considered an environmental hazard.
11.7 Measures of Solution Concentration
We have said that seawater is largely an aqueous solution of dissolved sodium
chloride. However, there are many other components of this solution, including
a good deal of Mg
2+
,SO
4
2–
, and Ca
2+
; smaller quantities of iron, phosphorus,
and copper; and really small amounts of dissolved oxygen, cadmium, and even
gold. What do we mean by “a good deal,” “smaller quantities,” and “really small
amounts”? It depends on whom you ask. And “it depends” is too vague in a sci-
entific community that requires clarity when communicating the results of mea-
surements. We need descriptions of concentration that have consistent meaning
to everyone reading the data.
Measures Based on Moles
Molarity (M)
We first examined molarity in Chapter 4 as a measure of moles of solute per liter
of solution.
M =
mol solute
L solution
Molarity is a standard concentration unit in the chemical laboratory. We can
speak of the initial molarity of a solute added to a solution, as in “What is the ini-
tial molarity of the sodium chloride?” We can also discuss the actual molarity of
each ion after the solute has dissolved, as in “What is the molarity of the sodium
ion in the solution?”
Molality (m)
Molality is a measure of moles of solute per kilogram of solvent.
m =
mol solute
kg solvent
The molality of a solute in solution is independent of temperature because it is
based on measuring the mass of the solvent, rather than the volume of the solution.
It is useful in exploring properties at a variety of temperatures, as we shall discuss
in Section 11.8. And because we can measure mass accurately and precisely,
molality can be determined to many significant figures, if necessary.
Mole Fraction (χ
i
)
The mole fraction of a substance is the ratio of the number of moles of a substance
present per total moles of all substances in the solution. If there are three solutes,
i, j, and k, in aqueous solution, then the mole fraction of solute i is
χ
i
=
mol i
mol i +mol j + mol k +mol water
It is important, when we calculate the mole fraction, to include the contribu-
tion of all components in the system. Note that the denominator in the equation
indicates that we add the number of moles of i, j, k, and water to obtain the total
number of moles in the system.
468 Chapter 11 The Chemistry of Water and the Nature of Liquids
Video Lesson: Molality
Video Lesson: Molarity and the
Mole Function
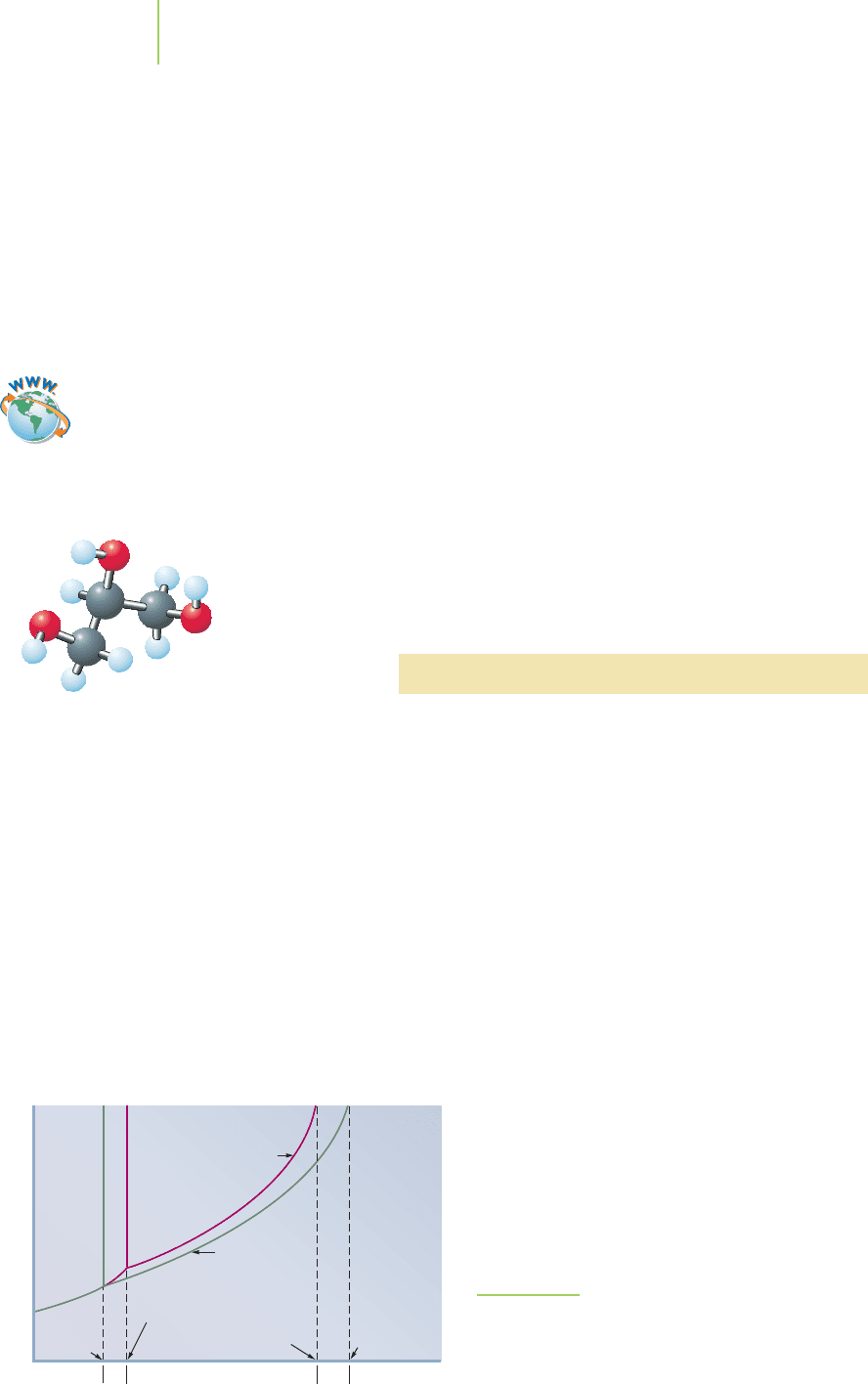
EXERCISE 11.8 Practice with Mole-Based Units of Concentration
Fructose is one of the three important “simple sugars,” the other two being galactose
and glucose. What are the values for molarity, molality, and mole fraction of 36.0 g
of fructose (C
6
H
12
O
6
) in 250.0 mL of a fruit-flavored drink? The density of the
water-based drink is
1.05 g/mL, and you may neglect the presence of flavorings.
Solution
The addition of fructose changes the density of the solution compared to pure
water, so we would expect the molarity (which is based on the solution volume) and
molality (based on the solvent mass) to be different. When we calculate the mole
fraction, we must take into account both the moles of fructose and the moles of the
solvent, water. All three measures of concentration require us to know the number
of moles of fructose (C
6
H
12
O
6
).
mol fructose = 36.0 g fructose
×
1 mol fructose
180.0gfructose
= 0.200 mol fructose
Molarity of fructose =
mol fructose
L solution
=
0.200 mol fructose
0.2500 L solution
= 0.800 M
The molality calculation requires that we know the mass of water. We can find this
via the mass of the solution and its density.
Mass of solution = 250.0 mL solution
×
1.05 g solution
mL solution
= 262.5 g solution
(Note that we keep the extra figure for this calculation and round only the number
that will be our final answer.)
Mass of water = mass of solution − mass of fructose = 262.5 g − 36.0 g
= 226.5 g water
Molality
=
mol fructose
kg solvent
=
0.200 mol fructose
0.2265 kg water
= 0.883 M
We can covert the mass of water to moles for our mole fraction calculation.
mol water = 226.5 g water
×
1 mol water
18.0 g water
= 12.58 mol water
Mole fraction of fructose =
χ
fructose
=
mol fructose
mol fructose + mol water
=
0.200
0.200 +12.58
= 0.0156
Fructose
OC
CH
2
OH
C
HC
C
OH
H
CH
2
OH
OH
H
OH
11.7 Measures of Solution Concentration 469
PRACTICE 11.8
What mass of potassium hydroxide (KOH) is required to prepare 600.0 mL of a
1.40 M KOH solution? What is the mole fraction of water in this solution? (Assume
that the density of the solution is 1.07 g/mL.)
See Problems 67, 70, 71, and 73.
Measures Based on Mass
We discussed the two useful sets of mass-based concentration measures, weight
percent and
parts per million, parts per billion and parts per trillion, in Section 4.2.
We present them again here for purposes of review and for application in the
context of our discussion of seawater.
Weight Percent (wt %)
Weight percent is a measure of the mass fraction of a substance in a solution,
expressed as a percentage.
wt % =
g substance
g solution
× 100%
If you were to prepare a reference solution that has the same weight percent
of sodium chloride as seawater, it would contain 29.5 g of sodium chloride per
1.00 × 10
3
g of solution, a weight percent of 2.95% NaCl.
29.5gNaCl
1.00 ×10
3
g solution
×
100% = 2.95% NaCl
Other related units are weight-to-volume and volume-to-volume.
Parts per Million, Billion, and Trillion (ppm, ppb, ppt)
In discussing the concentration of possible health hazards in water, the Environ-
mental Protection Agency (EPA) cites its
maximum contaminant level (MCL) the
highest acceptable level in a solution, as 10 parts per million for nitrate and
5 parts per billion for cadmium. Very low solute concentrations
(sometimes called trace concentrations) are often expressed this
way. The density of very dilute aqueous solutions is close enough to
that of pure water, 1.0 g/mL, that we may use these volume-based
conversions that we derived in Section 4.2.
ppm =
1 g solute
10
6
g solution
≈
1 mg solute
L solution
ppb =
1 g solute
10
9
g solution
≈
1 g solute
L solution
ppt =
1 g solute
10
12
g solution
≈
1 ng solute
L solution
The average concentration, in parts per million, of the major ions
that are present in seawater are listed in Table 11.4.
EXERCISE 11.9 Conversion Between Mole and Mass Concentration Units
The maximum concentration of O
2
in seawater is 2.2 ×10
−4
M at 25
◦
C. What is this
concentration in parts per million of oxygen?
470 Chapter 11 The Chemistry of Water and the Nature of Liquids
Application
C
HEMICAL ENCOUNTERS:
Composition of Seawater
Application
Average Composition
of Major Ions in Seawater
Element Parts per Million
(main form in seawater) (mg/L)
Chlorine (Cl
−
) 19,000
Sodium (Na
+
) 10,500
Magnesium (Mg
2+
) 1,250
Sulfur (SO
4
2−
) 900
Calcium (Ca
2+
) 400
Potassium (K
+
) 380
Bromine (Br
−
)65
Bicarbonate (HCO
3
−
)30
Strontium (Sr
2+
)12
Source: HPS Certified Reference Materials.
TABLE 11.4

11.8 The Effect of Temperature and Pressure on Solubility 471
Application
C
HEMICAL ENCOUNTERS:
Impact of the
Solubility of Oxygen
in Fresh Water
Solubility of O
2
in Fresh Water at
Several Temperatures (in air at 1 atm)
Temperature (°C) Parts per Million (mg/L)
0 14.6
5 13.1
10 11.3
15 10.1
20 9.1
25 8.3
30 7.6
35 6.9
Solution
We can solve this via dimensional analysis, as follows.
2.2 ×10
−4
mol O
2
L seawater
×
32 g O
2
mol O
2
×
1000 mg O
2
1gO
2
= 7.0 ppm O
2
Does the answer make sense? We expect the solubility of (nonpolar) oxygen to be
quite low in water, and our answer confirms that.
PRACTICE 11.9
The MCL for arsenic in drinking water is 10 ppb, according to Environmental
Protection Agency guidelines. Convert this value to molarity.
See Problems 74–76, 79, 80, and 111.
11.8 The Effect of Temperature
and Pressure on Solubility
Temperature Effects
We saw in Exercise 11.9 that oxygen is nearly insoluble in seawater, yet 7.0 ppm
at 25
◦
C is still enough to allow the seas to teem with life. The concentration of
oxygen in the seas and in freshwater lakes, ponds, and rivers varies as natural
processes such as photosynthesis and respiration cycle oxygen into and out of the
water. The other important factor that determines oxygen’s solubility in water
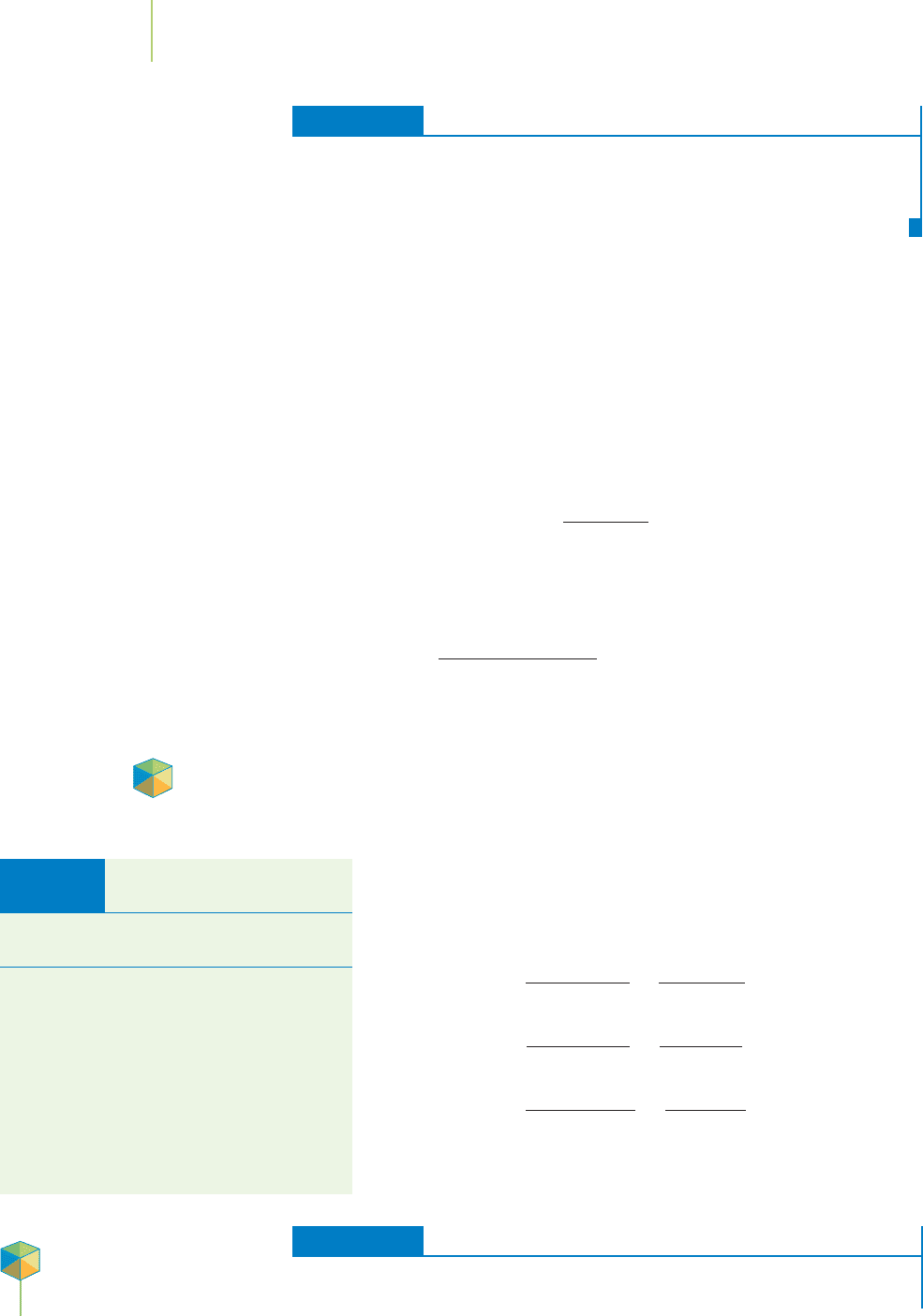
(see Table 11.5) is temperature. Note the trend, followed by all gases, that solubil-
ity of a gas decreases with temperature. Figure 11.28 shows this behavior for several
common gases.
Small changes in temperature do not dramatically affect the
solubility of oxygen, but a large increase in temperature can sig-
nificantly lower the oxygen concentration in a waterway, and
the harm done can be felt throughout the aquatic food chain.
The artificial raising of the ambient water temperature is called
thermal pollution and is of concern in the design of nuclear
power plants, in which river water is used to cool the nuclear
core of the reactor (see Chapter 21). The heated river water is
passed through a
cooling tower (Figure 11.29, on page 472)
before it flows back to the river.
Temperature (°C)
Solubility (mg gas/100 g H
2
O)
4.00
3.50
3.00
2.50
2.00
1.50
1.00
0.50
010203040506070
Total air
N
2
O
2
80 90 10
0
FIGURE 11.28
The solubility of gases decreases with temperature.
This is especially important with O
2
, where “ther-
mal pollution” can have important consequences
for the aquatic food chain.
TABLE 11.5
Video Lesson: Temperature
Change and Solubility
FIGURE 11.30
The solubility of some salts in water as a function of
temperature. Note that the solubility of the majority of
these salts increases as the temperature increases. Li
2
SO
4
decreases solubility as the temperature increases.
In contrast to gases, the solubility of ionic solids in water generally increases
with temperature, as shown in Figure 11.30. For example, the solubility of sodium
chloride in water at 0
◦
C is 35.7 g/100 mL, and at 100
◦
C it is 39.1 g/100 mL. Some
solids show a much more marked increase in solubility. Sodium carbonate
decahydrate (Na
2
CO
3
· 10H
2
O) has an aqueous solubility of 22 g/mL at 0
◦
Cto
420 g/mL at 100
◦
C, a 22-fold increase. A few salts, such as manganese(II) sulfate
hexahydrate (MnSO
4
· 6H
2
O) and sodium sulfate (Na
2
SO
4
), have lower solubility
with increasing temperature.
Pressure Effects
The solubilities of solid solutes in water are not especially responsive to modest
pressure changes. The solubility of gases in water, on the other hand, is quite sen-
sitive to the external pressure. This is important in the preparation of soft drinks,
in which CO
2
is combined with water, sweetener, and other flavorings at pres-
sures between about 6 and 15 atm. The “fizz” in the soda is due to the dissolved
CO
2
. When the can is opened, the CO
2
above the soda escapes from the container,
and the pressure of the can suddenly drops to atmospheric pressure. The lower
pressure decreases the solubility of the CO
2
in the soda, and bubbles of CO
2
form.
William Henry (1775–1836), an English chemist, noted this behavior.
Henry’s
law
says that at constant temperature, the solubility of a gas is directly propor-
tional to the pressure that the gas exerts above the solution.
P
gas
= k
gas
C
gas
where P
gas
= the pressure of the gas above the solution
C
gas
= the concentration of the gas in the solution
k
gas
= a constant relating the gas pressure above the solution and its
concentration
472 Chapter 11 The Chemistry of Water and the Nature of Liquids
FIGURE 11.29
A cooling tower lowers the temperature of water
that has been heated as part of the operation of
power plants. This water is close to the tempera-
ture of the waterway from which it was taken
and to which it will be returned, so the impact
of thermal pollution is minimized.
Temperature (°C)
Solubility (g solute/100 g H
2
O)
140
160
180
200
220
240
260
120
100
80
60
40
20
201003040506070
80 90
100
KNO
3
NaNO
3
NaBr
KBr
KCl
NaCl
Na
2
SO
4
Li
2
SO
4
Ce
2
(SO
4
)
3
Application
Carbon dioxide is released from a
glass of freshly opened soda.
Video Lesson: Pressure Change
and Solubility
In pure water at 1 atm and 0
◦
C, the solubility of CO
2
is 3.48 g/L. According
to Henry’s law, if we triple the CO
2
pressure, the solubility will roughly triple.
Overall, this law holds best for gases such as N
2
and O
2
that do not significantly
interact with the solvent. It does not hold well for HCl, which ionizes in water to
form the hydrated ions H
+
(aq) and Cl
−
(aq). However, as we have noted, even
molecules such as O
2
interact with water to some degree.
EXERCISE 11.10 Henry’s Law and CO
2
in Soda
In air in which the pressure of CO
2
is 3.4 × 10
−
4
atm at 0
◦
C, the solubility of CO
2
in water is
1.18 × 10
−3
g/L water. If the pressure of the CO
2
above the water
is increased to 6.00 atm, what will be the solubility of the CO
2
in the water? Do
these data lead to a conclusion consistent with our prior assertion that sodas go
“whoosh” when they are opened?
Solution
The CO
2
pressure is being increased by a huge factor, so we would expect the solu-
bility to increase about proportionately. Assuming proportionality, we can elimi-
nate the need to calculate the Henry’s law constant and just solve the problem with
ratios.
1.18 ×10
−3
g/L
3.4 ×10
−4
atm
=
x g/L
6.00 atm
x = 21 g/L
The solubility of the gas increased sharply at high external CO
2
pressure, in accor-
dance with Henry’s law. As is consistent with much of our discussion on solutions,
the formulas we cite work best for very dilute solutions. When the can of soda is
opened, the pressure of CO
2
above the liquid sharply decreases, lowering the solu-
bility of the gas and therefore contributing to the escape of gas that we hear when
we open the soda.
PRACTICE 11.10
A researcher adds 22.7 g of NaCl to 55.0 mL of water. Examine Figure 11.30. Will all
of the salt dissolve in the given amount of water at 25
◦
C? If not, how much water
will need to be added to just dissolve the salt completely?
See Problems 83 and 84.
11.9 Colligative Properties
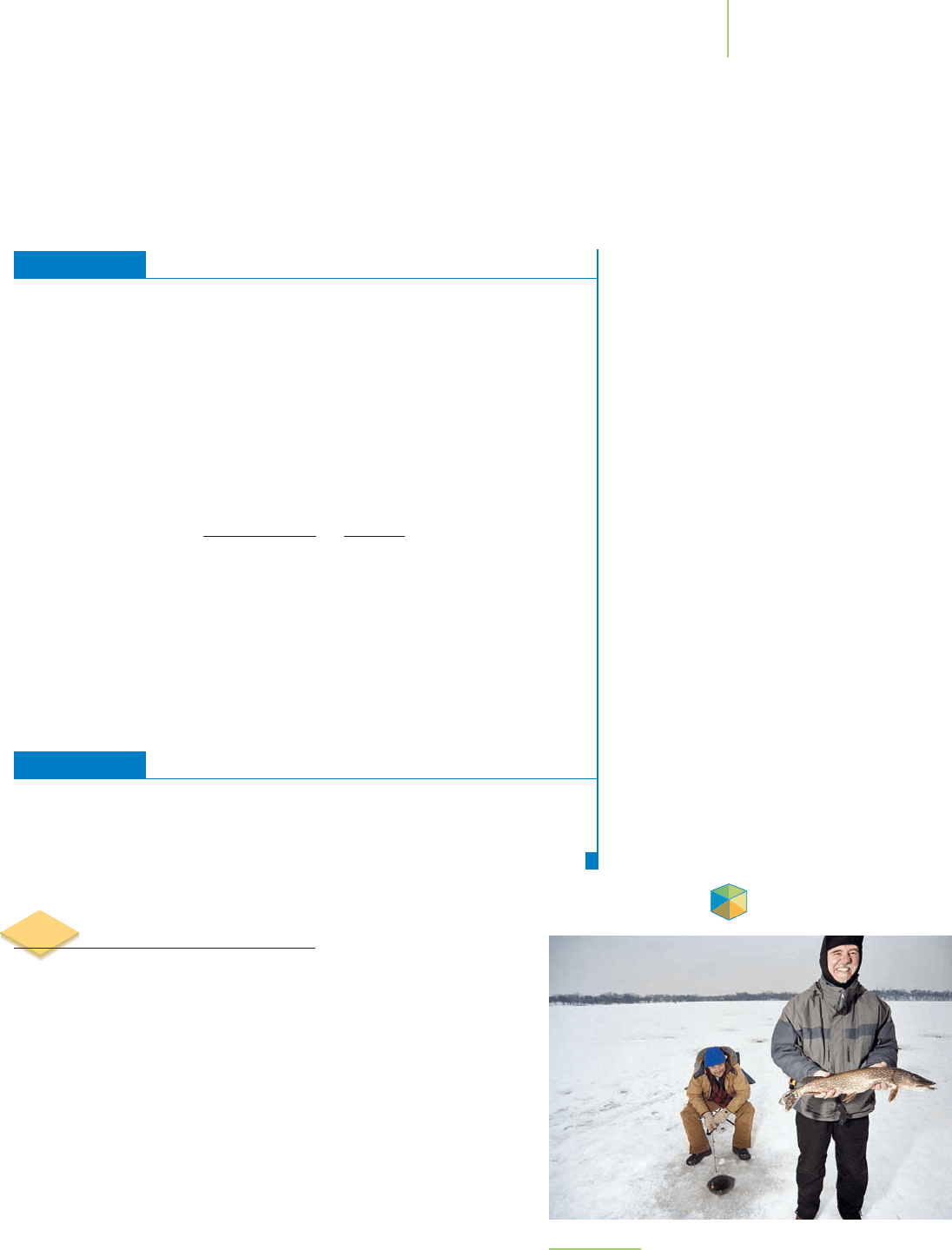
In the dead of winter at Lake Riley in Minnesota, it is common to see
people brave the harsh winter weather (Figure 11.31) to sit around
meter-deep fishing holes in the ice. The ice fishing season here typically
runs from late January through mid-March. We take it for granted, but
ice fishing is possible only because the water in the freshwater lake has
a relatively low concentration of dissolved solutes and has a freezing
point just below 0
◦
C. If Lake Riley were as salty as the Red Sea or the
Persian Gulf, with sodium and chloride ion concentrations equal to 40
parts per thousand, its freezing point would be about −2.5
◦
C, and the
ice fishing season would be shorter (if not nonexistent), as anglers
waited for sufficient ice to form to make the lake safe for the fishing
shacks and vehicles that transport them. Why does the presence of the
salt lower the freezing point of water? Does the amount of salt affect
the freezing point, and does the nature of the salt matter? Are there any
other solution properties that are affected in this way?
11.9 Colligative Properties 473
Application
FIGURE 11.31
Ice fishing on Lake Riley in Minnesota can be rewarding.
Let’s answer the last question first. Properties of a solution that approximately
depend only on the number of nonvolatile solute particles, irrespective of their
nature, are called
colligative properties (from the Latin colligatus, which means
“collected together”). There are four useful colligative properties: vapor pressure
lowering, freezing-point depression, boiling-point elevation, and osmotic pres-
sure. Understanding vapor pressure lowering will help us answer our questions
about dissolving salts in water, as well as give us insight into the other three
colligative properties.
Vapor Pressure Lowering
1,2,3-Propanetriol (C
3
H
8
O
3
) is the systematic name for the nonvolatile substance
we commonly call glycerol or glycerin. The colorless, viscous liquid is used as a
lubricant and moistener, especially in cosmetics, and to reduce swelling in med-
ical procedures, such as eye examinations. The presence of three OH groups on
the molecule leads to significant hydrogen bonding, making glycerol completely
soluble in water. We noted in Section 11.3 that water has a vapor pressure equal
to 23.8 torr at 25
◦
C. (Judging on the basis of our discussion in that section about
intermolecular forces, structure, and vapor pressure,
does it make sense that
water should be volatile, whereas glycerol is nonvolatile?
) Glycerol has essentially
no vapor pressure at room temperature. When glycerol and water are mixed, the
total vapor pressure of the resulting solution is dependent only on the vapor pres-
sure of pure water,
P
o
H
2
O
,
multiplied by its mole fraction,
χ
H
2
O
, in the solution.
Vapor pressure of the solution =
P
solution
= χ
H
2
O
P
o
H
2
O
For example, if we add enough glycerol to water so that the mole fraction of the
water is reduced to 0.900, the resulting vapor pressure of the solution will be re-
duced. At 25
◦
C, the vapor pressure of the solution would be
P
solution
= χ
H
2
O
P
o
H
2
O
P
solution
= 0.900 ×23.8torr= 21.4torr
The relationship of the vapor pressure of the solution, P
solution
, to the mole
fraction, χ
solvent
, and vapor pressure, P°
solvent
, of the volatile solvent holds true for
any ideal solution containing a nonvolatile solute. It is known as
Raoult’s law,
named after the French chemist Francois-Marie Raoult (1830–1901).
P
solution
= χ
solvent
P
o
solvent
An ideal solution exists when the properties of the solute and solvent are not
changed by dilution. This means that other than being diluted, combining solute
and solvent in an ideal solution does not release or absorb heat, and the total vol-
ume in the solution is the sum of the volumes of the solute
and solvent. Only very dilute solutions approach ideal
behavior, so although Raoult’s law is a good first approxi-
mation, actual measurements are required to properly
describe vapor pressure changes in mixtures of solutions.
Figure 11.32 shows the general trend: The vapor pressure is
depressed with the addition of a nonvolatile solute.
474 Chapter 11 The Chemistry of Water and the Nature of Liquids
FIGURE 11.32
The vapor pressure of water (red line) is lowered by the
addition of a nonvolatile solute. This is described for an
ideal solute by Raoult’s law.
1 atm
Pressure (atm)
∆T
f
∆T
b
Freezing
point of
solution
Freezing point
of solvent
Boiling point
of solvent
Boiling point
of solution
Temperature (°C)
Vapor pressure
of pure solvent
Vapor pressure
of solution
Glycerol
C
3
H
8
O
3
Visualization: Vapor Pressure
Lowering: Liquid/Vapor
Equilibrium
Visualization: Vapor Pressure
Lowering: Addition of a Solute
Visualization: Vapor Pressure
Lowering: Solution/Vapor
Equilibrium
Video Lesson: Vapor Pressure
Lowering
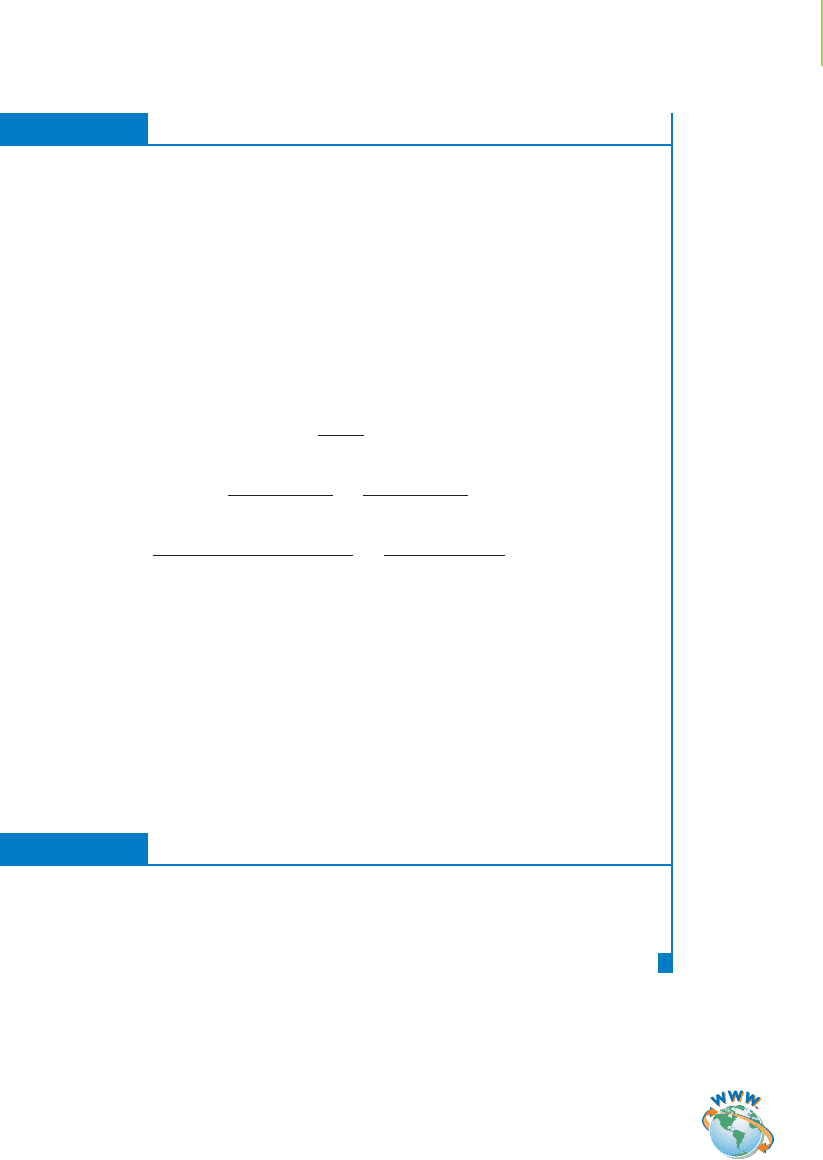
EXERCISE 11.11 Vapor Pressure Lowering
You stir 3 teaspoons (45.0 g) of sucrose, table sugar (C
12
H
22
O
11
, molar mass =
342 g/mol), into a cup of tea containing 250.0 mL of water at 90.0
◦
C (density =
0.965 g/mL). What is the new vapor pressure of the solution?
P
o
H
2
O
=
526 torr at
90.0
◦
C.
First Thoughts
The key problem-solving hurdle is calculating the mole fraction of water in the
solution. To do this, we must calculate the number of moles of each component. We
will retain an extra, nonsignificant figure until the end of the calculations.
Solution
mol sucrose = 45.0 g
×
1 mol
342 g
= 0.1316 mol sucrose
g water = 250.0 mL
×
0.965 g water
1 mL water
×
1 mol water
18.02 g water
= 13.39 mol water
χ
water
=
mol water
mol glucose + mol water
=
13.39
0.1316 +13.39
= 0.9903
P
solution
= χ
water
P
o
water
P
solution
= 0.9903 ×526 torr = 520.9torr≈ 5.2 × 10
2
torr
Further Insights
As expected, the vapor pressure of the solvent decreases as a consequence of the ad-
dition of the sucrose. Note the use of the symbol ≈, which means “is approximately
equal to.” Raoult’s law is strictly followed only for ideal solutions. This solution has
enough sucrose in it that it does not approach ideal behavior.
PRACTICE 11.11
The vapor pressure of ethanol (C
2
H
5
OH) at 40
◦
C is 135.3 torr. Calculate the vapor
pressure of a solution containing 26.8 g of glycerin (C
3
H
8
O
3
) a nonvolatile solute,
in 127.9 g of ethanol.
See Problems 87, 88, 93, 95, and 96.
Boiling-Point Elevation
At 1 atm, the temperature of any solution must be elevated until its vapor pres-
sure equals 760 torr in order to reach the boiling point. Concentrated solutions
possess lowered vapor pressures (which we discussed earlier), because the solu-
tion must have a vapor pressure equal to the atmospheric pressure in order to
boil. The more solute is dissolved in a solution, the more the vapor pressure of the
solution is lowered, and the higher the boiling point. The temperature of the so-
lution must be elevated for the vapor pressure to reach that of the surroundings.
For fairly dilute solutions of nonelectrolytes, the following formula approximately
describes the
boiling-point elevation due to the addition of a nonvolatile solute.
T
b
= K
b
m
where T
b
= the change in boiling point in
◦
C
K
b
= the boiling-point elevation constant, which depends on the solvent,
in units of °C/m
m = the molality of the solute in moles of solute per kilogram of solvent
11.9 Colligative Properties 475
Visualization: Boiling-Point
Elevation: Liquid/Vapor
Equilibrium
Visualization: Boiling-Point
Elevation: Addition of a Solute
Visualization: Boiling-Point
Elevation: Solution/Vapor
Equilibrium
Video Lesson: Boiling-Point
Elevation and Freezing-Point
Depression

The value of K
b
for water is 0.512
◦
C/m. This means that a
2.00 molal aqueous sugar solution would have a boiling-point
elevation of approximately 1.02
◦
C (2.00 m × 0.512
◦
C/m) and a
boiling point of about 101
◦
C. We say “about” because the
boiling-point elevation constant begins to deviate significantly
from the ideal solution value at higher concentrations.
Boiling-point elevation constants for several liquids are listed in
Table 11.6.
Colligative properties depend on the number, not the nature,
of the particles in the solution. We would expect solutions con-
taining compounds at the same concentration to have the same
elevated boiling point. However,we must consider the total num-
ber of particles that result from making the solution. For example, what would
happen to the boiling point of our aqueous sucrose solution if our solute were 2.00
molal cobalt(II) chloride (CoCl
2
) instead of sucrose? Because cobalt(II) chloride
is a strong electrolyte that dissociates to form cobalt ion and chloride ion,
H
2
O
CoCl
2
(s)
−−−−−→
Co
2+
(aq) + 2Cl
−
(aq)
we would expect three moles of particles (ions, in this case) for every mole of
CoCl
2
added to the solution. That is, the concentration of ions in the solution
should be 6.00 molal, and the boiling point of the solution should be raised by
(6.00 m × 0.512
◦
C/m) = 3.07
◦
C. This suggests that when dealing with strong
electrolytes, we can modify our boiling-point elevation formula to take into
account the dissociation of strong electrolytes into i particles.
T
b
= iK
b
m
For CoCl
2
, i =3 if the solution behaves ideally. The actual boiling-point elevation
for this solution is 4.6
◦
C, not 3.1
◦
C, which tells us that at this relatively high con-
centration (2.00 m), the solution does not behave even close to ideally. The value
i is known as the
van’t Hoff factor, after J. R. van’t Hoff (1852–1911), a chemist
from the Netherlands who suggested its use in the 1880s. Table 11.7 shows the
van’t Hoff factors for several electrolytes. Note that there is significant deviation
from the expected values as the solute concentration increases, so results are only
approximate even at relatively low concentrations.
EXERCISE 11.12 Boiling-Point Elevation
Recipes for cooking spaghetti often call for putting a little table salt in the water
before boiling it and adding the spaghetti. Does this help the spaghetti cook faster?
Assume that we add 10.0 g of NaCl to 6.00 L of water and that the density of the
solution is 1.00 g/mL. Also assume that this very dilute solution behaves ideally, so
i = 2. The value of K
b
for water is 0.512
◦
C/m.
Solution
mol NaCl = 10.0 g NaCl
×
1 mol NaCl
58.5gNaCl
= 0.171 mol NaCl
kg water = 6.00 L water
×
1.00 kg water
L water
= 6.00 kg water
Molality of NaCl =
mol NaCl
kg water
=
0.171 mol NaCl
6.00 kg water
= 0.0285 m NaCl
T
b
= iK
b
m
= 2.0 ×
0.512
◦
C
m
× 0.0285 m
T
b
= 0.03
◦
C
476 Chapter 11 The Chemistry of Water and the Nature of Liquids
Boiling-Point Elevation Constants
for Several Liquids
Solvent K
b
(
◦
C/m) T
b
(
◦
C)
Acetone 1.7 56.5
Benzene 2.6 80.1
Carbon tetrachloride 5.0 76.7
Ethanol (ethyl alcohol) 1.2 78.5
Methanol (methyl alcohol) 0.80 64.7
Water 0.51 100.0
TABLE 11.6
Jacobus Henricus van’t Hoff
(1852–1911) was a Dutch chemist who
initially shook the basic ideas of chem-
istry with his description of the three-
dimensional nature of molecules. In
1901 he won the first Nobel Prize in
chemistry for his work on solutions.
Video Lesson: Boiling-Point
Elevation Problem

This shows that there is nearly no elevation in the boiling point of water with the
addition of a little table salt. The salt is added for taste.
PRACTICE 11.12
What is the predicted boiling point of each of these solutions? (Assume that each
follows ideal behavior.)
a. 1.00 m NaCl in water
b. 0.35 m FeCl
3
in water
c. 1.50 m KCl in methanol
See Problems 89, 90, and 97.
In contrast to the minimal impact of adding a dash of table salt to water when
cooking spaghetti, making an aqueous solution that is 40% by volume ethylene
glycol (C
2
H
6
O
2
) elevates the boiling point to 105°C (221
◦
F). This solution, called
“antifreeze,” helps protect your automobile engine in hot weather.
11.9 Colligative Properties 477
van’t Hoff Factors for Several Electrolytes
Expected
Compound Value of im = 0.005 m = 0.01 m = 0.05 m = 0.10 m = 0.20 m = 1.00 m = 2.00
HCl 2 1.95 1.94 1.90 1.89 1.90 2.12 2.38
NH
4
Cl 2 1.95 1.92 1.88 1.85 1.82 1.79 1.80
CuSO
4
2 1.54 1.45 1.22 1.12 1.03 0.93 —
CoCl
2
3 2.80 2.75 2.64 2.62 2.66 3.40 4.58
K
2
SO
4
3 2.77 2.70 2.45 2.32 2.17 — —
TABLE 11.7
Ethylene glycol is a major
component of many
antifreeze solutions.
40 50 60 70 80 90 100 110 120
0
200
400
600
800
1000
1200
Temperature (˚C)
760 torr
Pure water
Vapor pressure (torr)
Boiling point
of water
Boiling point
of 10 m
ethylene glycol
10 m ethylene glycol
The boiling point of a 40% by volume solution of ethylene glycol is 105°C.
Video Lesson: Colligative
Properties of Ionic Solutions
