Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

10.7 Kinetic-Molecular Theory
Skill Review
73. Using the tenets of the kinetic-molecular theory, explain why
the absolute temperature is directly proportional to the pres-
sure of a trapped volume of gas.
74. Does the kinetic-molecular theory assist in the explanation
of Avogadro’s law? If so, how?
75. Two gases known for their bleaching power are ClO
2
and Cl
2
.
Assume you have separate 1-L containers containing 5 g of
each at 25°C.
a. Compare the average kinetic energy of the molecules of
each gas.
b. Compare the average speed of the molecules of each gas.
c. Which, if either, would be exerting more pressure?
d. If the samples were placed in the same container, which, if
either, would have the larger partial pressure?
76. Equal masses of two gases, B
2
H
6
and C
2
H
2
, are placed in sep-
arate containers at the same temperature.
a. Compare the average kinetic energy of the molecules of
each gas.
b. Compare the average speed of the molecules of each gas.
c. Which, if either, would exert a greater pressure?
d. If the samples were placed in the same container, which, if
either, would have the larger partial pressure?
77. Assuming that a single molecule of each of these gases is
moving at a speed of 1100 m/s, what would you calculate as
its kinetic energy?
a. CO
2
b. NH
3
c. Ne
78. Assuming that a single molecule of each of these gases is
moving at a speed of 1100 m/s, what would you calculate as
its kinetic energy?
a. H
2
b. C
2
H
6
c. Ar
79. Arrange these gases in order from lowest to highest average
molecular speed (assume that there are 1.00 mol of each at
the same temperature and pressure).
a. H
2
b. C
2
H
6
c. Ar
80. Arrange these gases in order from lowest to highest average
molecular speed (assume that there are 1.00 mol of each at
the same temperature and pressure).
a. CO
2
b. NH
3
c. Ne
81. At 25.0°C, what would be the root-mean-square speed of
propane (C
3
H
8
) used in bottle gas fuel systems?
B
2
H
6
ClO
2
Cl
2
438 Chapter 10 The Behavior and Applications of Gases
82. At extremely low temperatures, molecular motion also be-
comes relatively slow. What would the temperature be when
the root-mean-square speed of He was 100 m/s?
83. Calculate the rms speed of CO
2
and of H
2
O at 250.0 K.
84. What is the rms speed of a gas with a molar mass of
16.0 g/mol if the temperature is 45°C?
Chemical Applications and Practice
85. A device sometimes used in teaching the gas laws consists of
small marbles trapped inside a glass-walled container. The
container is open at one end, where it is fitted with a move-
able piston. Using each of the six points of the kinetic-
molecular theory, discuss how this device compares to a gas
sample in a container. In addition to the marbles obviously
being larger than gas molecules, where does the analogy fit
the kinetic-molecular theory and where does it not?
86. During exercise, our bodies heat up as a consequence of the
increase in metabolism. Using the kinetic-molecular theory,
explain how panting (breathing faster) can help reduce this
increase in temperature.
10.8 Effusion and Diffusion
Skill Review
87. Arrange these gases in order from lowest to highest rate of
effusion.
a. N
2
b. SO
3
c. CO
2
d. Xe
88. Arrange these gases in order from lowest to highest rate of
effusion.
a. O
2
b. Ar c. F
2
d. HF
89. A gas effuses at a rate of 0.300 m/s. A second gas has a molar
mass of 2.02 and effuses 6.00 times faster than the first gas.
What is the molar mass of the first gas?
90. Gas A diffuses 1.47 times as fast as gas B. If gas B has a molar
mass of 54.6 g/mol, what is the molar mass of gas A?
91. If a noble gas diffuses 0.317 times as fast as does helium at the
same pressure, which noble gas is it?
92. Whichwould takelonger to effuse, 1.00 mol Xegas or 2.00 mol
CO
2
gas? Assume both are initially at the same temperature
and pressure. How much faster would 1.00 mol Xe gas effuse
if there were 10.0 mol CO
2
gas at the same temperature and
pressure?
93. Would it be possible to separate a mixture of propane (C
3
H
8
)
and carbon dioxide (CO
2
) using Graham’s law of effusion?
Explain your answer.
94. Would it be possible to separate a mixture of diborane
(B
2
H
6
) and acetylene (C
2
H
2
) using Graham’s law of effusion?
Explain your answer.
Chemical Applications and Practice
95. An interesting visual demonstration to show diffusion rates
of gases is to introduce some NH
3
gas at one end of a hollow
tube, while simultaneously placing some HCl at the other
end. Within a few minutes, the two gases will diffuse
through the air inside the tube and react to form a white
NH
4
Cl precipitate when they meet. If the tube was 39 cm in
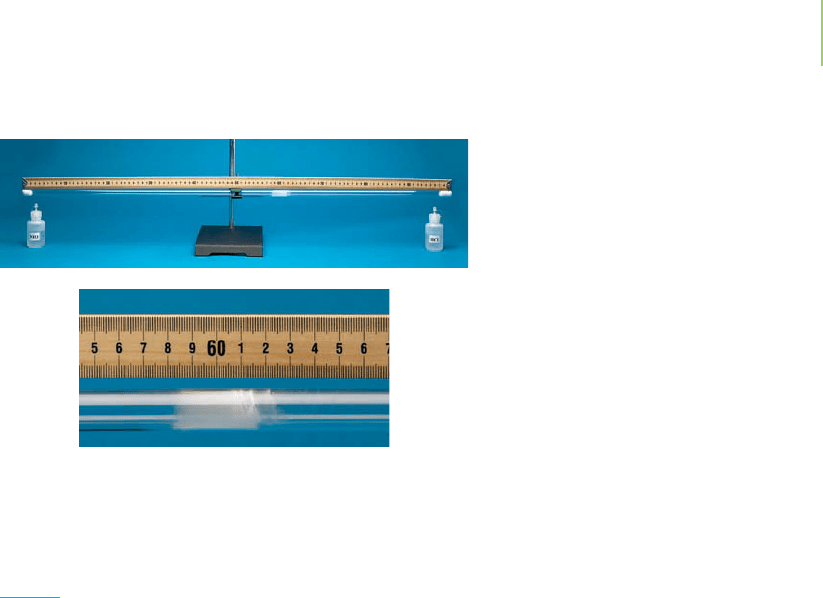
length, approximately where would the white precipitate
form inside the tube?
96. The demonstration described in Problem 95 was repeated,
this time using HF instead of HCl. Where would the precip-
itate form inside the tube?
97. During the Manhattan project, efforts were made to sepa-
rate
238
U from the fissionable isotope
235
U. The uranium
sample was converted to UF
6
, which is a gas at low pressure.
How much faster does
235
UF
6
effuse than
238
UF
6
?
98. Atmospheric scientists have recently detected very low con-
centrations of a long-lived greenhouse gas containing sulfur
and fluorine, SF
5
CF
3
. What is the ratio of the rates of effu-
sion of this gas and of its suspected precursor SF
6
, a mater-
ial used in high-voltage insulators?
Comprehensive Problems
99. Examine Table 10.2 and answer these questions.
a. Assuming SATP conditions, how many liters of ammonia
gas were produced in 2004?
b. How many liters of oxygen gas at SATP were produced?
c. How many metric tons of hydrogen gas were produced at
SATP?
d. How many moles of N
2
gas were produced in the United
States in 2004 at SATP?
100. When you exhale, you are releasing carbon dioxide, all of
the nitrogen you inhaled, and unused oxygen gas. Under the
same conditions of temperature and pressure, rank these
three gases in order from
a. least dense to most dense.
b. fastest rate of diffusion to slowest rate of diffusion.
101. During exercise, we breathe faster and obtain more oxygen
for metabolism. If a handball player absorbs oxygen at a rate
of approximately 75 mL per kilogram of body mass per
minute, how many molecules of oxygen would a player with
a mass of 86.8 kg absorb during a 30.0-min match at SATP?
102. Although the ideal gas equation is sufficient for most gas
law calculations, there are times when it needs to be modi-
fied. Explain the specific physical purpose behind the van
der Waals constants a and b in the modified equation.
103. Hydrogen gas continues to gain attention as a possible fuel
for modified cars of the near future. Compare the pressure
Focus Your Learning
439
of H
2
in a tank with a volume of 25 L that contains 45 g of
H
2
at 25°C, using calculations from the ideal gas equation
and the van der Waals modification to the gas equation.
104. In a quality test, one tennis ball is filled with N
2
gas. Another
tennis ball of the same volume is filled to the same pressure
as the first with air. If the two tennis balls were at the same
temperature, what else would also be the same?
105. If you collected some oxygen in an experiment at 298 K,
how much of a change in the temperature (in kelvins), at
constant pressure, would be needed to quadruple the vol-
ume? If that same sample of oxygen had its temperature
lowered by 25°C, what volume change, at constant pressure,
would be expected?
106. If the CO
2
trapped above your favorite carbonated drink is
exerting a pressure of 7.2 atm, yet the total pressure above
the aqueous drink is 7.9 atm, what are the partial pressure
and percent of water in the trapped space? (Assume that no
other gas is present.)
107. The helium in a pressure tank at a carnival may be pressur-
ized to 21.0 atm. If the temperature of a 27.0-L tank is
29.5°C, how many grams of helium are in the tank? If it was
your job to inflate 1.50-L balloons with the helium, how
many could you inflate so that each of them would have
1.11 atm of pressure at 27.5°C?
108. In 2004, 17.7 billion m
3
of hydrogen gas was produced in
the United States, as shown in Table 10.2. Most of the gas is
delivered via trucks, either as a compressed gas or as a liquid
stored at very low temperature. If, for example, 50% of
the volume of gas produced were liquefied and stored at
20 K, and each delivery truck held 6000 gal of liquid
hydrogen, how many truckloads of hydrogen would be
needed in one year? (Assume the density of liquid hydrogen
is 0.070 g/cm
3
.)
Thinking Beyond the Calculation
109. In Problem 108 we noted that hydrogen gas can be trans-
ported via tanker trucks either as a compressed gas (at about
400 atm) or as a liquid at 20 K. If you are charged with
deciding whether to ship the hydrogen as a compressed gas
or as a liquid, what chemical, physical, demographic, eco-
nomic, and other considerations would factor into your
decision? Use the Internet and examine how some large gas
production companies make these decisions.
110. Under STP conditions, 100.0 mL of an unknown hydrocar-
bon gas was combusted in excess oxygen. The only products
of the combustion were 300.0 mL of carbon dioxide and
400.0 mL of water vapor.
a. What are the intermolecular forces common to hydro-
carbon gases?
b. Does the behavior of these gases tend to resemble ideal
gas behavior? How might these gases be expected to
deviate from this behavior?
c. How many moles of carbon dioxide are produced in the
combustion?
d. What is the formula of the hydrocarbon?
e. If the combustion were performed at 2.50 atm and
500°C, how many liters of water vapor would you expect
to produce?
NH
3
and HCI are released at opposite ends of a glass tube.

The Chemistry
of Water and
the Nature
of Liquids
Water is a vital part of our lives. In the
developed world, most of us tend to take
clean water for granted. But many people,
like these girls in Nicaragua, are constantly
preoccupied with how they’ll obtain each
day’s supply of water.
440
Contents and Selected Applications
Chemical Encounters: Worldwide Water Use
11.1 The Structure of Water: An Introduction to Intermolecular
Forces
11.2 A Closer Look at Intermolecular Forces
11.3 Impact of Intermolecular Forces on the Physical Properties
of Water, I
11.4 Phase Diagrams
Chemical Encounters: CO
2
as a Dry Cleaning Solvent
11.5 Impact of Intermolecular Forces on the Physical Properties
of Water, II
11.6 Water: The Universal Solvent
11.7 Measures of Solution Concentration
Chemical Encounters: Composition of Seawater
11.8 The Effect of Temperature and Pressure on Solubility
Chemical Encounters: Impact of the Solubility of Oxygen in Fresh Water
11.9 Colligative Properties
Chemical Encounters: Meeting Municipal Water Needs
Go to college.hmco.com/pic/kelterMEE for online learning resources.
441
The Tampa Bay area has a severe water short-
age brought about by a fivefold increase in popula-
tion since 1950. With over 2.5 million residents and
population growth estimated at over 50,000 per year, even
the abundant rainfall of Florida’s coastal areas cannot keep up
with the skyrocketing demand for water. Tampa Bay is not at all
unique in the world, or even in the United States, in its thirst for this pre-
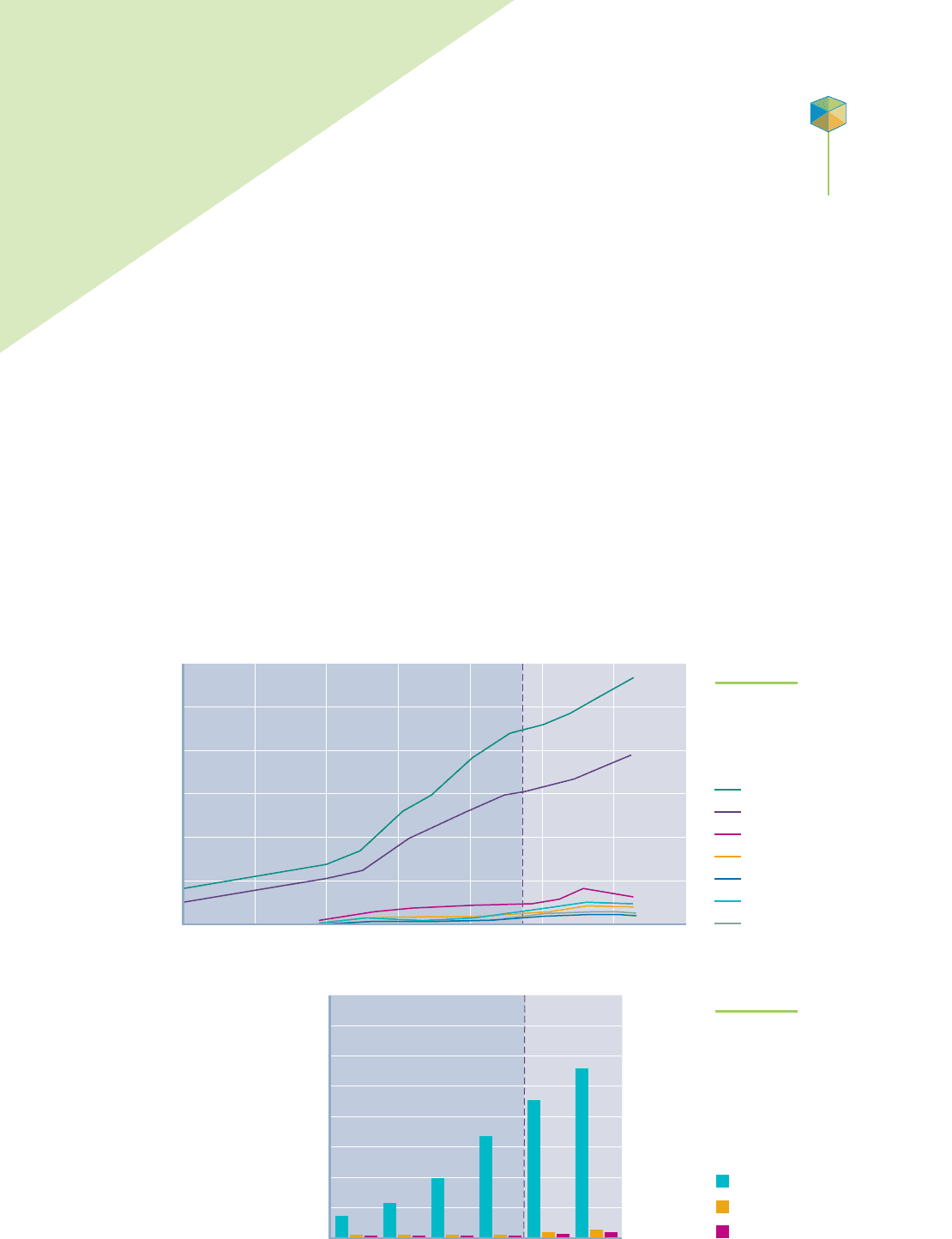
cious natural resource. Figure 11.1 shows the expected trends in water use by
continent through the year 2025. Most of the world’s increase in water consump-
tion will be driven by population growth and rapid industrialization in Asian coun-
tries. Localized dramatic increases are also predicted in other areas with pockets of
rapid population growth, such as southern Florida and the southwestern states of
Nevada and Arizona. This will further stress the already limited water supplies.
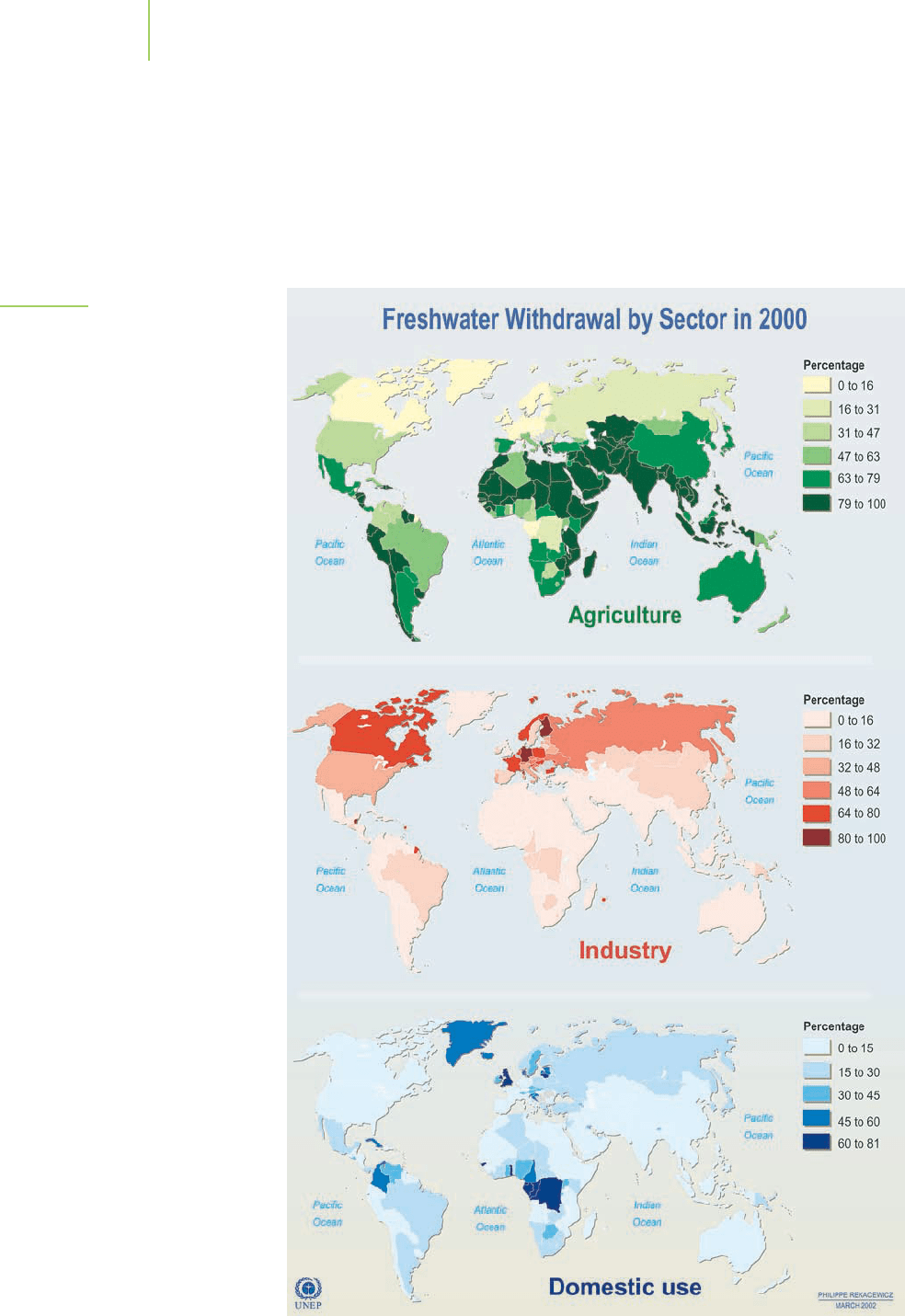
What uses are there for water, and why is it so important in our lives? Domestic
use for washing, cooking, and drinking generates part of the demand, but agricul-
ture accounts for most of our consumption of water (Figure 11.2). Large amounts
of water are also used for industrial production, including the generation of electric-
ity. The patterns of water demand also reveal a great deal about the development of
a country’s economy. Examining these data across the countries of the world, as is
done in Figure 11.3, illustrates that water is used differently in developed countries
than in underdeveloped countries. For example, Figure 11.4 shows that the United
States earmarks most of its water supply for power generation and agriculture.
Application
C
HEMICAL
ENCOUNTERS:
Worldwide
Water Use
0
500
1000
1500
2000
2500
3000
1900 1920 1940 1960 1980 2000 2020 2040
Water consumption (km
3
/year)
Assessment Forecast
FIGURE 11.1
UNESCO data showing the trends
in water consumption.
Assessment Forecast
1900
0
400
800
1200
1600
2000
2400
2800
3200
1925 1950 1975 2000 2025
Global water consumption (km
3
)
FIGURE 11.2
Agriculture is a major consumer
of water around the world.
S. America
Africa
Australia
Europe
N. America
Asia
Wor ld
Agricultural
Domestic
Industrial
442 Chapter 11 The Chemistry of Water and the Nature of Liquids
FIGURE 11.3
The use of water can be broken down
into agricultural, industrial, and domestic
(personal) uses.
Water is so fundamental to life that the search for it has extended to the outer
reaches of the solar system. Detecting water elsewhere in the universe could provide
important information about the chemistry of the universe and, perhaps, the origin
of life.
What is it about the structure and properties of water that makes it so pervasive and so
vital to our world?
How can these properties help us to understand water’s many and

varied uses—and assist us in our efforts to provide clean water for places like
Tampa Bay? This chapter focuses on the nature of water. We will compare it to
other liquids in order to highlight its own special character. As you might sense,
water is truly unique, vital in so many ways, and it’s worth knowing why.
11.1 The Structure of Water: An Introduction to Intermolecular Forces 443
FIGURE 11.4
According to the U.S. Geological
Survey (USGS), more than half of
the water used in the United States
in the year 2000 was used for ther-
moelectric power generation and
agriculture.
11.1 The Structure of Water:
An Introduction to Intermolecular Forces
When we get thirsty, we can pour ourselves a glass of water. Huge numbers of
water molecules stream out of the faucet in the
liquid state. They fall into our glass
and, like the molecules of all liquids, conform to the shape of their container. We
can freeze water to make it a
solid, which has its own shape, or boil it, forming a
gas, which completely fills a container no matter how much is present. To make
our water hot, we pipe the water into a water heater, which may be operated using
another substance vital to our way of life,
natural gas (mostly methane, CH
4
).
+
–
+
–
444 Chapter 11 The Chemistry of Water and the Nature of Liquids
Both water and natural gas arrive at our homes via a system of underground
pipes. Even though they possess similar molar masses (18 g/mol for H
2
O and
16 g/mol for CH
4
), water is a liquid and methane is a gas. Why is this so? The
properties, chemical behavior, and day-to-day uses of these compounds are based
on their structures. As we’ve noted in previous discussions, the structure of a
molecule determines its properties.
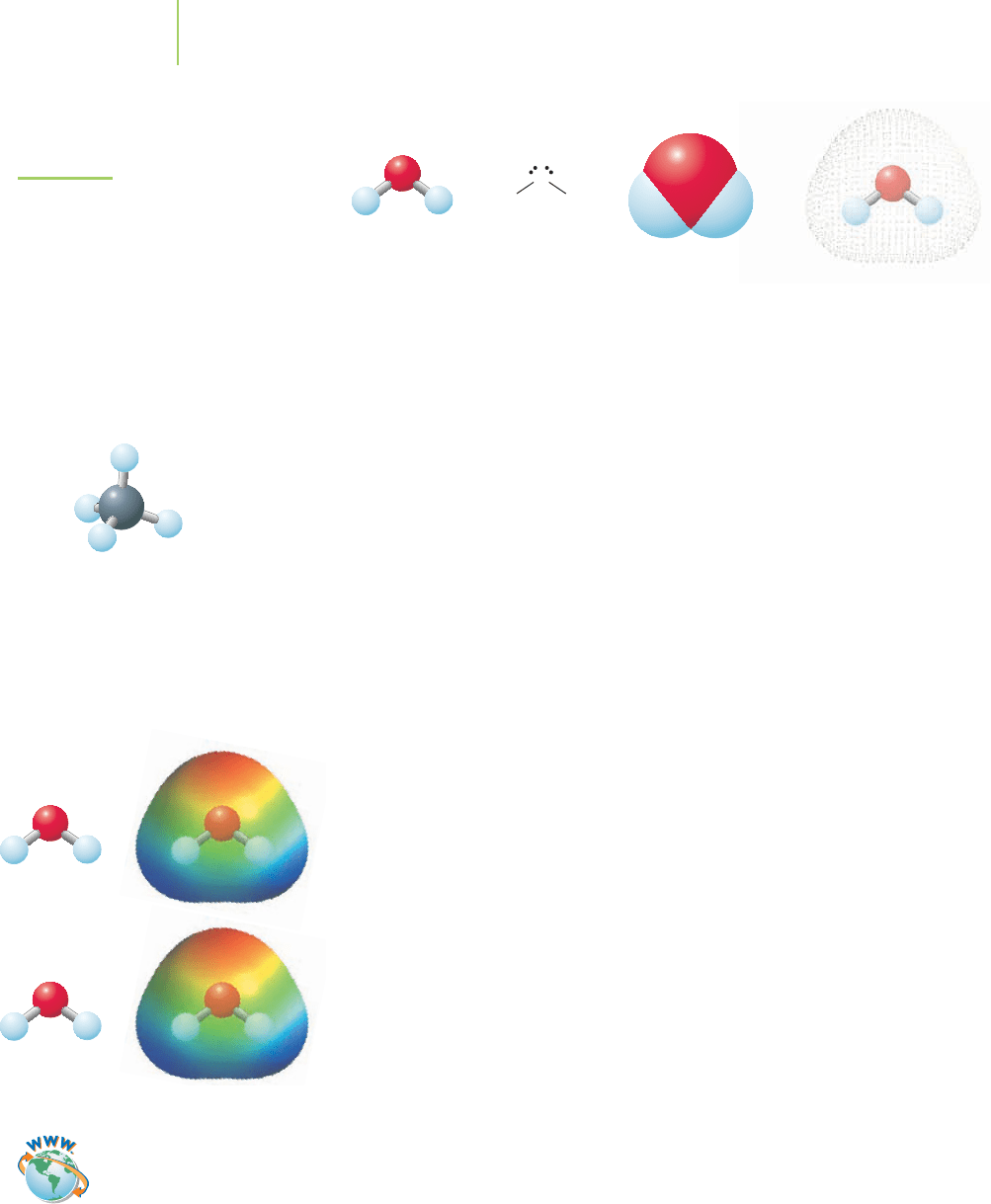
Figure 11.5 provides several representations of a water molecule. Recall from
Chapter 7 that oxygen is substantially more electronegative than hydrogen,
leading to poles of charge on the individual O—H bonds in water. Using the
VSEPR model from Chapter 8, we saw that water is a bent molecule, with an
H—O—H bond angle of 104.5°. This led to our discovery of a net dipole mo-
ment for the polar molecule. Unlike water, methane is made up of atoms with
similar electronegativity values. Additionally, the VSEPR structure of CH
4
is
tetrahedral, and the molecule lacks a net dipole moment.
As we’ve seen in previous discussions, opposite charges attract. This is appar-
ent in the structure of table salt (NaCl), in which the positive charge on the
sodium ion is attracted to the negative charge on the chloride ion. But this at-
traction isn’t limited to complete charges. Even slight distortions of electron dis-
tribution give rise to this attraction. If we recall the concept of energy (Chapter 5)
as that which is needed to oppose a natural force, we can deduce that energy is re-
leased when opposite poles attract. This concept helps us to understand why water
is liquid under normal conditions: The hydrogen atoms at positive poles of the
dipole moment on water molecules can interact with the negative poles of oxygen
atoms on other molecules. The resulting release of energy is the key to the stabil-
ity of liquid water at room temperature. This interaction is an example of an
in-
termolecular force
. Unlike water, methane, does not have sufficiently strong inter-
molecular forces to exist as a liquid or solid at normal conditions, so it travels to
our homes as a gas.
The result of the attraction between opposite poles in a sample of water is the
interaction of 3 to 6 water molecules with each other at any one time, with an av-
erage of about 4.5. These molecules change partners constantly as they swirl
about in the sample exchanging intermolecular forces of attraction. Although the
number of interactions among the molecules of a substance does say something
about its properties, the relative strength of each individual intermolecular inter-
action is particularly important.
How do intermolecular (between molecules) forces compare with the
intramolecular (within molecules) forces that we call bonds? Consider what hap-
pens when we boil water. We produce water vapor (H
2
O(g)), but we do not pro-
duce H
2
and O
2
gas. In boiling water, the individual intramolecular forces
(bonds) within each water molecule have not been broken. Instead, the intermol-
ecular interactions between molecules have been disrupted.
Why is this so? About
44 kJ of energy is required to convert 1 mol of liquid water to the vapor, via
the breaking of intermolecular attractions. It takes about 940 kJ to break the
O—H bonds within a mole of water. This idea can be reinforced by examining
similar forces in a sample of methane. About 9 kJ of energy is needed to vaporize
O
H H
(a) (b) (c) (d)
FIGURE 11.5
There are several ways to represent
a water molecule: (a) ball-and-stick
model, (b) Lewis dot structure,
(c) space-filling model, and
(d) electron dot surface model.
VSEPR structure of methane.
Dipoles attract each other.
Video Lesson: An Introduction
to Intermolecular Forces and
States of Matter

11.2 A Closer Look at Intermolecular Forces 445
Water boils and creates steam.
a mole of liquid methane, compared to 1650 kJ to break the four C—H bonds in
a mole of methane! We can extend this information to make the more general
statement that intermolecular interactions are much weaker than intramolecular
interactions. Less energy is required to boil a liquid than to break it into its
component elements.
11.2 A Closer Look at Intermolecular Forces
Can we use our understanding of intermolecular forces to explain the nature
of liquids other than water? In short, yes; the forces by which molecules come
together as liquids are based on the intermolecular interaction of oppositely
charged poles. They are collectively known as
van der Waals forces, after the Dutch
physicist Johannes Diderik van der Waals, who noted their existence in 1879 and
whose correction for the behavior of real gases we discussed in Chapter 10. van
der Waals forces can be quite weak, as reflected in the low boiling points of
methane, –164°C (109 K), and N
2
, –196°C (77 K), at 1 atm. van der Waals forces
connecting molecules, such as water, are different in nature and much stronger.
The stronger forces result in a much higher boiling point for water (boiling
point = 100°C at 1 atm). What are these forces and how do they vary among
types of molecules?
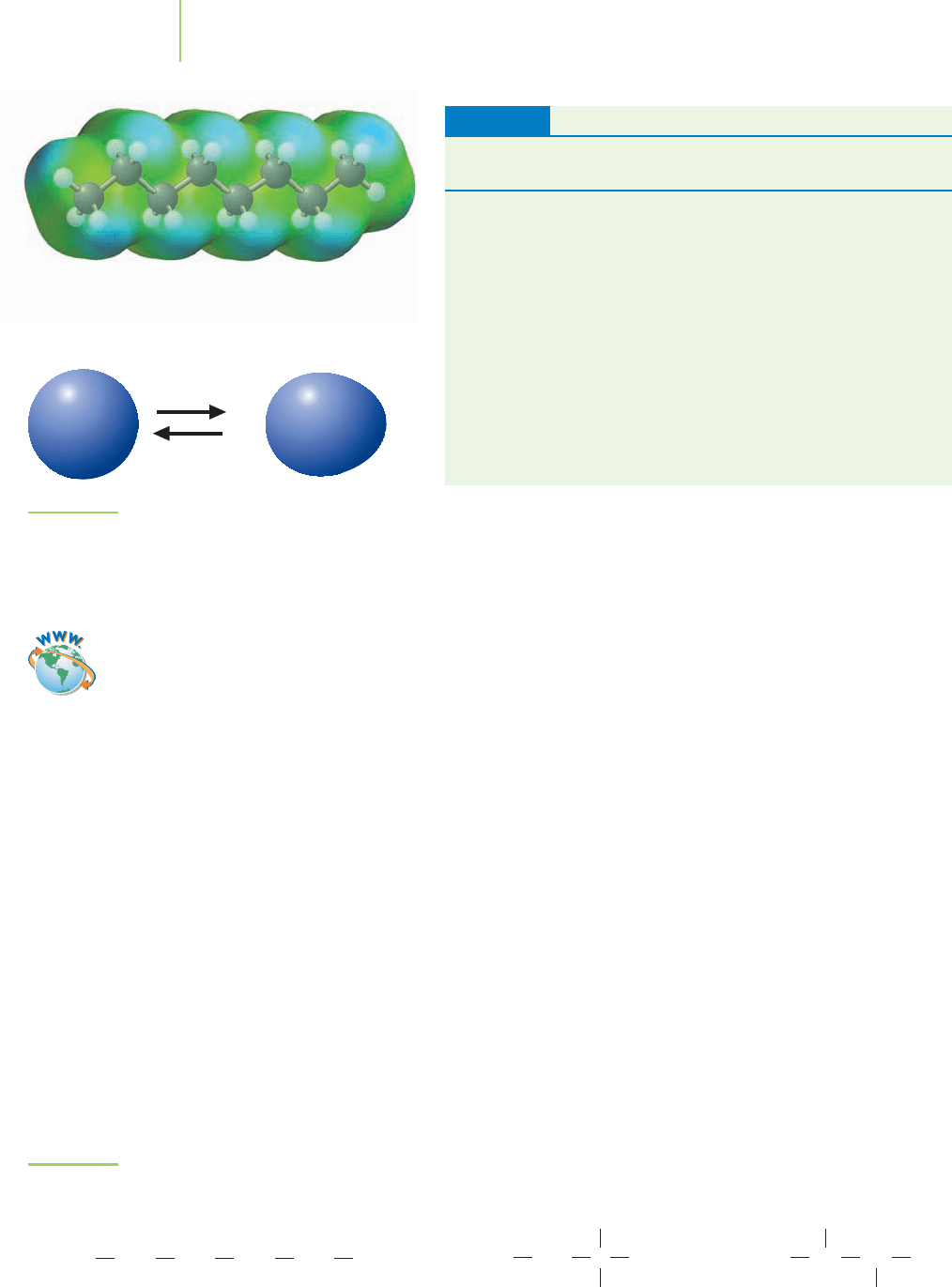
London Dispersion Forces—Induced Dipoles
In our comparison of water and methane, we noted that the polarity of water ex-
plains why it exists as a liquid at normal temperatures. Yet the ability of nonpolar
methane to liquefy at very low temperatures (boiling point =−164°C) suggests
that there is something even in nonpolar substances that can hold molecules
together. The molecule octane (C
8
H
18
) is nonpolar, yet it is a liquid at room
Five molecules of liquid water interact
with each other. Note the positions of
the hydrogen atoms. They are being
shared with neighboring oxygen atoms.
Video Lesson: Intermolecular
Forces
Octane
C
8
H
18
δ+δ –
FIGURE 11.6
In this representation, instantaneous induced dipoles
are shown on atoms.
temperature. It has a boiling point of 125.7°C, which is higher than that of our
small polar water molecule. How can we reconcile these two observations?
The physicist Fritz London, who defined a concept we now call
London forces,
gave the explanation to this question in 1929. The key to his concept is a property
called
polarizability, the extent to which electrons can be shifted in their location
by an electric field. Polarized electrons produce an
induced dipole, an uneven dis-
tribution of charge caused (or “induced”) by the electric field. In the same way,
atoms or molecules can have temporary dipoles induced by instantaneous distor-
tions in neighboring electron positions, as shown in Figure 11.6. The larger the
number of electrons in the system, the larger the polarizability. The larger polar-
izability results in a larger induced dipole moment. In short, stronger attractive
forces between molecules are observed when the induced dipole moment is
larger.
Do London forces explain the differences among boiling points of nonpolar
substances? Table 11.1 shows that the boiling points of substances are quite reli-
ably related to their size, such that, for example, fluorine is a gas at room temper-
ature, whereas bromine is a liquid and iodine is a solid. Similarly, ethane (C
2
H
6
)
is a gas, whereas hexane (C
6
H
14
) is a liquid and tetracosane (C
24
H
50
) is a solid.
However, the structure of a molecule does have some effect on its polarizabil-
ity. From Table 11.1 we see that the boiling point of hexane is 69°C. However,
2,2-dimethylbutane, an isomer of hexane (that is, it has the same chemical
formula but a different structure) shown in Figure 11.7, boils at 50°C, and
2,3-dimethylbutane boils at 58°C.
Why is this so? Polarizability is highly related to
distance. Within a molecule of hexane, electrons are relatively close to those on
446 Chapter 11 The Chemistry of Water and the Nature of Liquids
Hexane
bp 68C
CH
2
CH
3
CH
2
CH
2
CH
3
CH
2
2,2-Dimethylbutane
bp 50C
CH
2
C
CH
3
CH
3
CH
3
CH
3
2,3-Dimethylbutane
bp 58C
CH CH
CH
3
CH
3
CH
3
CH
3
FIGURE 11.7
The “linear” molecule hexane has a higher boiling point than the two
isomers in which some polarizable electrons are “hidden.”
Boiling Points of Compounds at 1 atm
Molar Mass Boiling Point
Compound Name (g/mol) (°C)
F
2
Fluorine 38 –188.1
Cl
2
Chlorine 71 –34.6
Br
2
Bromine 160 58.8
I
2
Iodine 254 184.4
CH
4
Methane 16 –164
C
2
H
6
Ethane 30 –88.6
C
4
H
10
Butane 58 –0.5
C
6
H
14
Hexane 86 69
C
6
H
14
2,2-Dimethylbutane 86 50
C
6
H
14
2,3-Dimethylbutane 86 58
C
10
H
22
Decane 142 174.1
C
24
H
50
Tetracosane 339 391
TABLE 11.1
Visualization: Intermolecular
Forces: London Dispersion
Forces
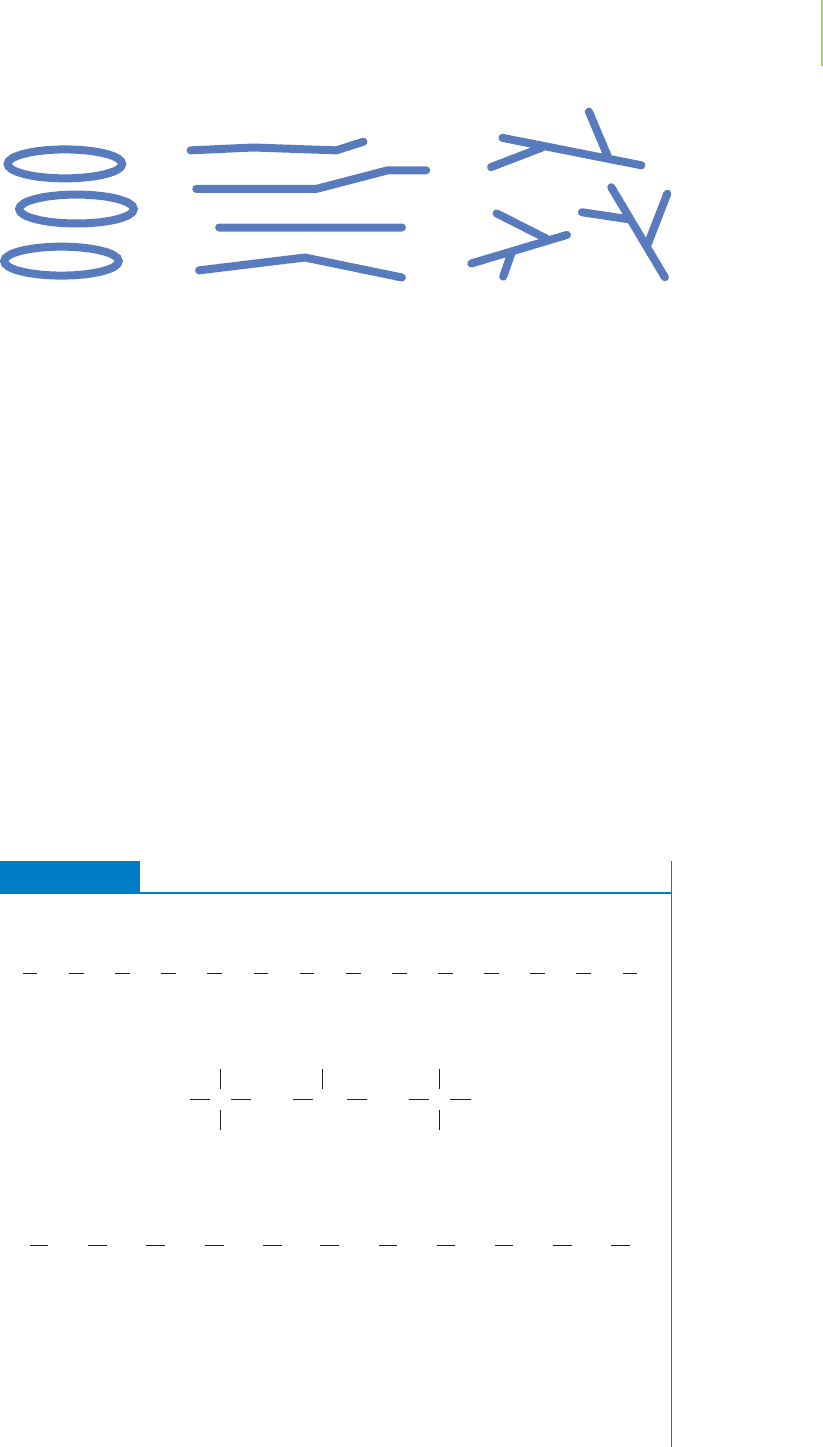
other hexane molecules. On the other hand, some of the carbon atoms in the iso-
mers of hexane are “hidden,” farther away from other neighboring molecules, so
the polarizability of compact molecules is not as great. Moreover, within a series
of molecules of approximately the same molecular mass, those molecules that
can interact more with their neighbors have a higher boiling point. Branched
molecules lack some of this interaction; linear molecules have more such interac-
tion but, because of their flexibility, do not interact perfectly; cyclic molecules
have the most interaction with their neighbors and can stack together like dinner
plates. This greater intermolecular interaction leads to higher boiling points.
The data in Table 11.1 also show us that the molar mass (related to the num-
ber of electrons in a molecule) of nonpolar substances is a major factor in their
boiling points. The impact of molar mass on the boiling point is much greater
than the effect observed in the isomers of hexanes. By comparing the boiling
points of the straight-chain molecules in the table (such as ethane, –88.6°C;
butane, –0.5°C; hexane, 69°C; decane, 174.1°C), we can see that as the molar mass
gets larger, the boiling point increases. In fact, with a sufficiently large molar
mass, it is possible for a nonpolar molecule to have a boiling point higher than
that of water. Why is it that water itself has such a high boiling point? Water must
have additional intermolecular forces of attraction that the nonpolar molecules
do not possess.
EXERCISE 11.1 Molar Mass, Structure, London Forces, and Boiling Point
One of the following nonpolar substances is a gas at 200°C. All the others are liquids.
Which one has the lowest boiling point at 1 atm, and why?
First Thoughts
The two most important criteria in determining the relative boiling points of non-
polar substances are their molar masses and their structure. When we evaluate the
structures to see which substance has the lowest boiling point, what we are looking
for is an especially low molar mass, or perhaps a nonlinear structure.
Dodecane
C
12
H
26
CH
2
CH
3
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
3
CH
2
2,2,4,6,6-Pentamethylheptane
C
12
H
26
C
CH
3
CH
3
CH
3
CH
2
CH C
CH
3
CH
2
CH
3
CH
3
CH
3
Pentadecane
C
15
H
32
CH
2
CH
3
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
3
11.2 A Closer Look at Intermolecular Forces 447
Cyclic molecules
Branched molecules
Linear molecules
Cyclic, linear, and branched molecules
interact through London forces differ-
ently. Those molecules that can get
closer to each other have greater inter-
molecular forces of attraction and
higher boiling points.
