Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

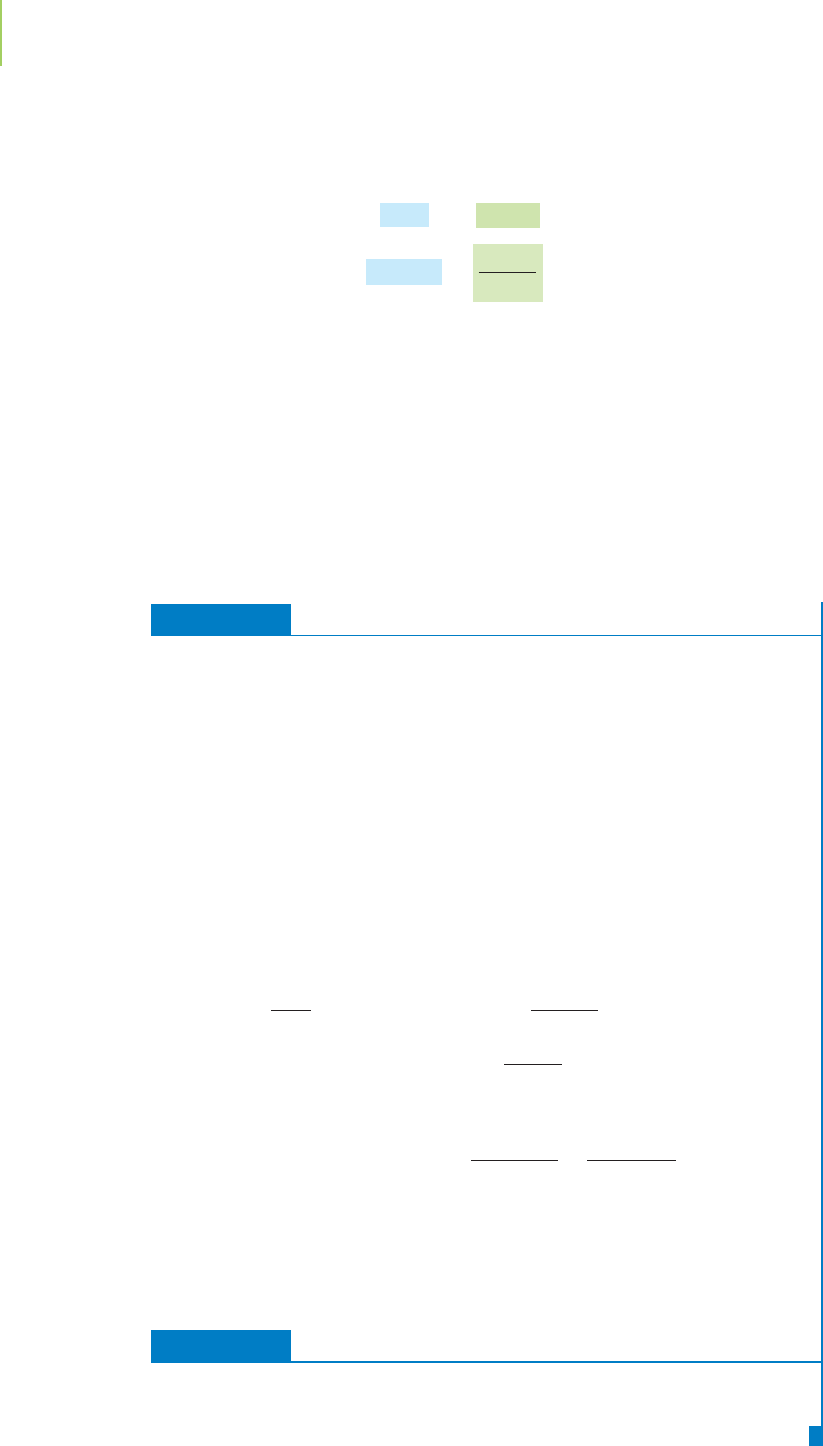
the intermolecular forces when boiling water than when melting ice. Boiling
causes all of the remaining hydrogen bonds keeping the water as a liquid to be
broken. The heat needed to boil 10.0 kg of water is 24,400 kJ.
q = mass ×
vap
H
10,000 g ×
2.44 kJ
g water
= 24,400 kJ
This amount of heat can be supplied by burning about 440 g of methane, over 3
times the total of the previous changes! The heat required from start to finish is
equal to the sum of the amounts of heat required for all the change along the way.
Total heat = q
ice warming
+ q
ice melting
+ q
water warming
+ q
water boiling
= 205 kJ + 3340 kJ + 4180 kJ + 24,400 kJ
= 32,100 kJ
It is possible to heat the water vapor to quite a high temperature, and the amount
of heat needed is related to the specific heat of water vapor, 1.84 J/g
·°C. This is
shown in Figure 11.16.
EXERCISE 11.5 Energy Changes Upon Heating the Water
Determine the heat required to raise the temperature of a 2.50 × 10
2
g block of ice
(equivalent to about a cup of water) in a 425 g aluminum pot from 0.0°C to room
temperature, 20.0°C. Roughly how many grams of methane must be burned in air
to supply this much heat? The heat of combustion of methane is –890 kJ/mol. Solid
aluminum has a specific heat of 0.880 J/g·°C. Assume that all heat goes to the ice and
the aluminum pot.
First Thoughts
What processes occur? The input of heat will melt the ice at constant temperature
(change of state) and then warm the ice from 0.0°C to 20.0°C. The aluminum pot
will be warmed from 0.0°C to 20.0°C.
Solution
Total heat = q
melting ice
+ q
warming liquid
+ q
warming aluminum
=
334 J
gice
× (2.50 ×10
2
gice)
+
4.184 J
gice·
◦
C
× (2.50 ×10
2
gice)
×(20.0°C −0°C)
+
0.880 J
gAl·
◦
C
× 425 g Al ×(20.0°C −0°C)
= 83,500 J + 20,920 J + 7480 J = 111,900 J = 112 kJ
Grams of CH
4
required =112 kJ
×
1 mol CH
4
890 kJ
×
16.0gCH
4
1 mol CH
4
= 2.0gCH
4
Further Insights
Even though the specific heat of the aluminum pan is a lot less than that of water, it
is a heavy pan, and a lot of heat is required to raise its temperature. A more realistic
picture would need to take into account the heat loss, and lots of it, to the air.
PRACTICE 11.5
How much energy does it take to convert 130.0 g of ice at –40.0°C to steam at
160.0°C?
See Problems 31, 32, 37, and 38.
458 Chapter 11 The Chemistry of Water and the Nature of Liquids

FIGURE 11.17
The moons of Jupiter as taken by NASA’s Galileo space probe.
11.4 Phase Diagrams
We now understand the changes that occur in water as we raise its temperature
from below freezing to the boiling point at normal pressure. However, much of
the known universe is nowhere close to our own “normal” pressure or tempera-
ture, and that affects the form water takes beyond our world. Figure 11.17 shows
recent photographs of the moons of Jupiter taken by NASA’s Galileo space probe.
Why do the moons look so different from one another, from their host planet,
and from anything else we’ve seen in the solar system? One reason apparently has
to do with ice—not the type of ice we find in the refrigerator, but ice that exists
under pressures of tens of thousands of atmospheres. Under these immense pres-
sures, the molecules of ice that form large parts of the interiors of the “Jovian”
moons are highly distorted. Hydrogen bonds are squeezed and shifted. Atoms are
brought closer together and bond lengths shortened so that the ice becomes un-
usually dense. Temperature changes deep inside these moons also lead to changes
in the nature of the ice. The consequences of all these changes are that the moons
contract and expand, causing huge cracks and grooves. We add to our under-
standing of the solar system by looking at the changes in ice as temperature and
pressure change.
A graph showing the way the phase of a substance, or a mixture of substances,
is related to temperature and pressure is called a
phase diagram. The heating
curves we discussed previously describe how water changes with temperature at
a constant pressure. However, as with the changes in the moons of Jupiter, a great
many processes do not happen at constant pressure, and a phase diagram gives us
a more comprehensive picture of these changes. In the chemical industry, phase
diagrams have many uses, including determining the conditions required for
manufacturing ceramics from clays (see Chapter 13). The composition of homo-
geneous mixtures of metals, called alloys, can also be changed on the basis of the
information in phase diagrams.
The phase diagram that includes the various types of ice in the core of plan-
ets is fairly complex, so let’s work first with a small portion of it, that for water at
more Earth-like temperatures and pressures, shown in Figure 11.18. Note in the
11.4 Phase Diagrams 459
Tutorial: Phase Diagrams
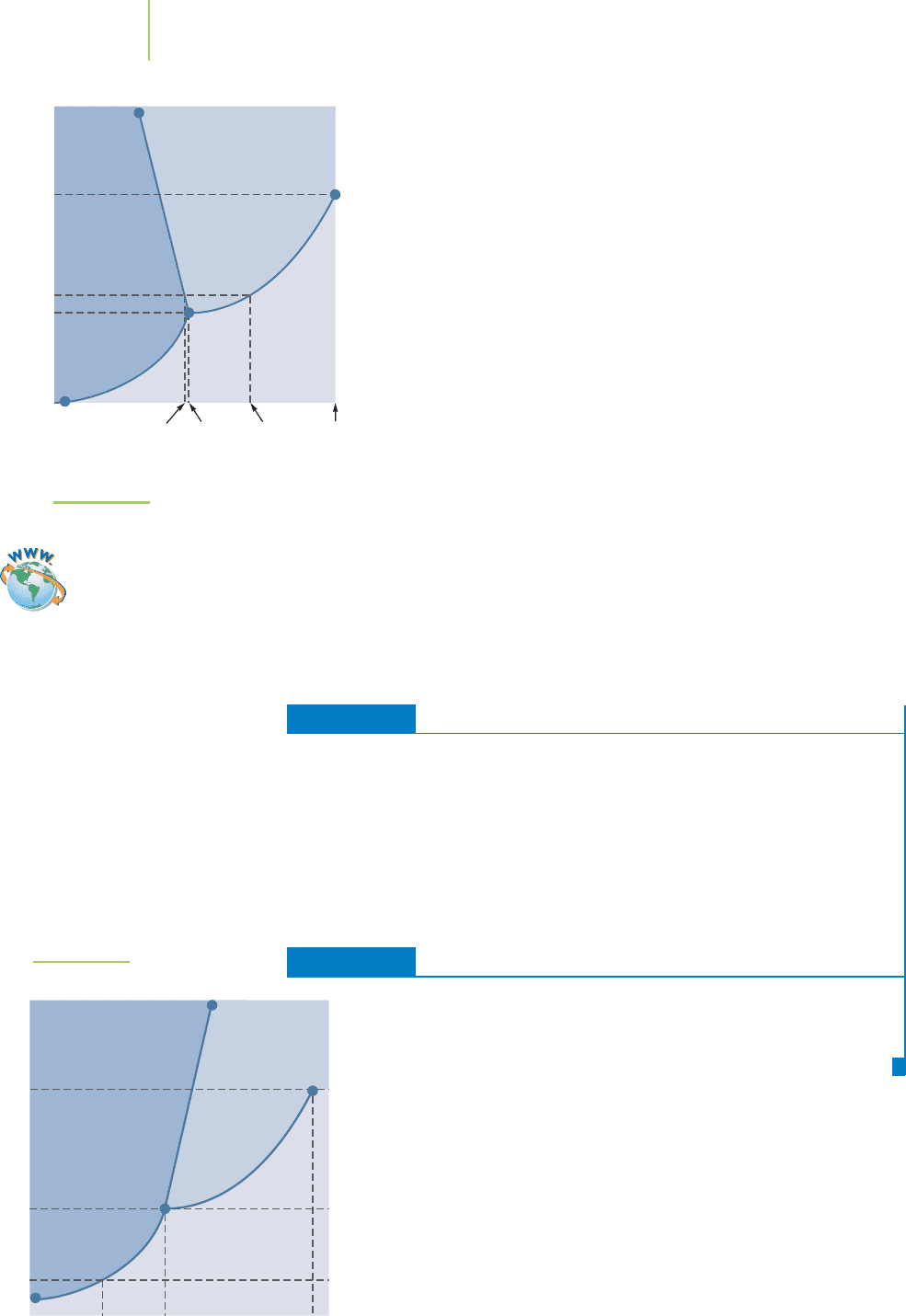
figure the three normal phases: solid, liquid, and gas (water vapor).
There are also bold lines, called
phase boundaries, at which two phases
exist in equilibrium. How does water change state as we heat it from
well below 0°C and at 1 atm (the dotted line on Figure 11.18)? Keep in
mind that these are the common changes that we experience every day
as we interact with water by boiling it to make tea or coffee or cause it
to melt when we add it as ice to a soda.
Our water sample begins as ice. We raise the temperature to 0°C,
where it arrives at a phase boundary at which ice and liquid water exist
in equilibrium. This is the normal melting (and freezing) point of
water. As we continue to raise the temperature, the water remains liq-
uid until 100°C, the boiling point, which is at the liquid/gas phase
boundary. Above 100°C, the water will always be a gas if the pressure is
kept constant at 1 atm. This is the same information we gleaned from
the heating curve of water, but a phase diagram gives us so much
more. We can tell, for example, that there is a
triple point of water (Fig-
ure 11.18) where all three phases exist in equilibrium. For water, this
occurs at 0.01°C and 4.6 torr. The triple points of elements and com-
pounds are useful because they serve as reference points with which temperature
scales for thermometers are defined, and against which the highest-quality ther-
mometers are calibrated. If we follow the liquid/gas phase boundary (the curved
line segment AB, the boiling point) toward increased temperature and pressure,
we get to a point above which the substance can no longer be liquefied and the
gas and liquid have the same density—that is, we have a
supercritical fluid. This
point (B) is called the
critical point, and it is associated with a critical temperature.
The pressure at the critical temperature is the
critical pressure.
EXERCISE 11.6 Reading Phase Diagrams
What does the line segment AD on the phase diagram of water represent? What
does line AC indicate?
Solution
Line segment AD represents the point of equilibrium between solid and liquid—the
melting (freezing) point at which the solid and liquid exist in equilibrium. Line seg-
ment AC is point of equilibrium between solid and gas for water; sublimation and
deposition occur at equilibrium anywhere along this line.
PRACTICE 11.6
What is the approximate boiling point of water when the external pres-
sure is 0.70 atm? Does this agree with our previous discussion about boil-
ing points at high altitudes?
See Problems 39–48.
The phase diagram of CO
2
, shown in Figure 11.19, looks similar to that
of water, with a triple point, critical temperature and pressure, and sev-
eral phase boundaries. At 1 atm, CO
2
changes directly between the solid
and gas phases (sublimation/deposition). You might have seen dry ice,
which does not melt but, rather, sublimes directly from a solid into a
gas. At pressures greater than about 5 atm, CO
2
will liquefy from the
solid phase. This liquid is used for dry-cleaning clothing to replace
460 Chapter 11 The Chemistry of Water and the Nature of Liquids
C
A
D
B
218
1.00
0.0060
0 0.0098 100
374
Solid
Liquid
Gas
Temperature (
°C)
Pressure (atm)
FIGURE 11.18
Phase diagram of water.
72.8
1.00
5.1
Solid
Liquid
Gas
Temperature (°C)
Pressure (atm)
–78 –56.6 31
FIGURE 11.19
Phase diagram of carbon dioxide.
Video Lesson: Phase Diagrams
Tetrachloroethylene
C
2
Cl
4
nonpolar, but environmentally hazardous, solvents such as tetrachloroethylene
(C
2
Cl
4
). These solvents rid clothing of nonpolar grease and dirt.
Liquid CO
2
is an excellent and relatively benign reusable alternative. Clothes
are cleaned in CO
2
at 15°C and 50 atm pressure, which is along the liquid/gas
boundary in the CO
2
phase diagram. The clothes are kept close to room temper-
ature, and about 98% of the CO
2
is recycled. The critical temperature and pres-
sure of CO
2
are 304.2 K and 70.8 atm. Supercritical carbon dioxide, typically in
the range of 310 to 400 K and 75 to 100 atm, can be used to clean industrial
machinery.
11.4 Phase Diagrams 461
The moons of the outer planets make
most elegant chemical laboratories,
because conditions on these worlds are so very dif-
ferent from those here at home. When we look at
photos of Jupiter’s moons, Europa and Ganymede
(Figure 11.17), we see some vexing geological fea-
tures. How do we know what caused these features?
We can’t visit the moons, at least not directly. How-
ever, our space probes can gather various types of
electromagnetic radiation—including light, ultra-
violet radiation, and X-rays—that give us data
from which to draw conclusions.
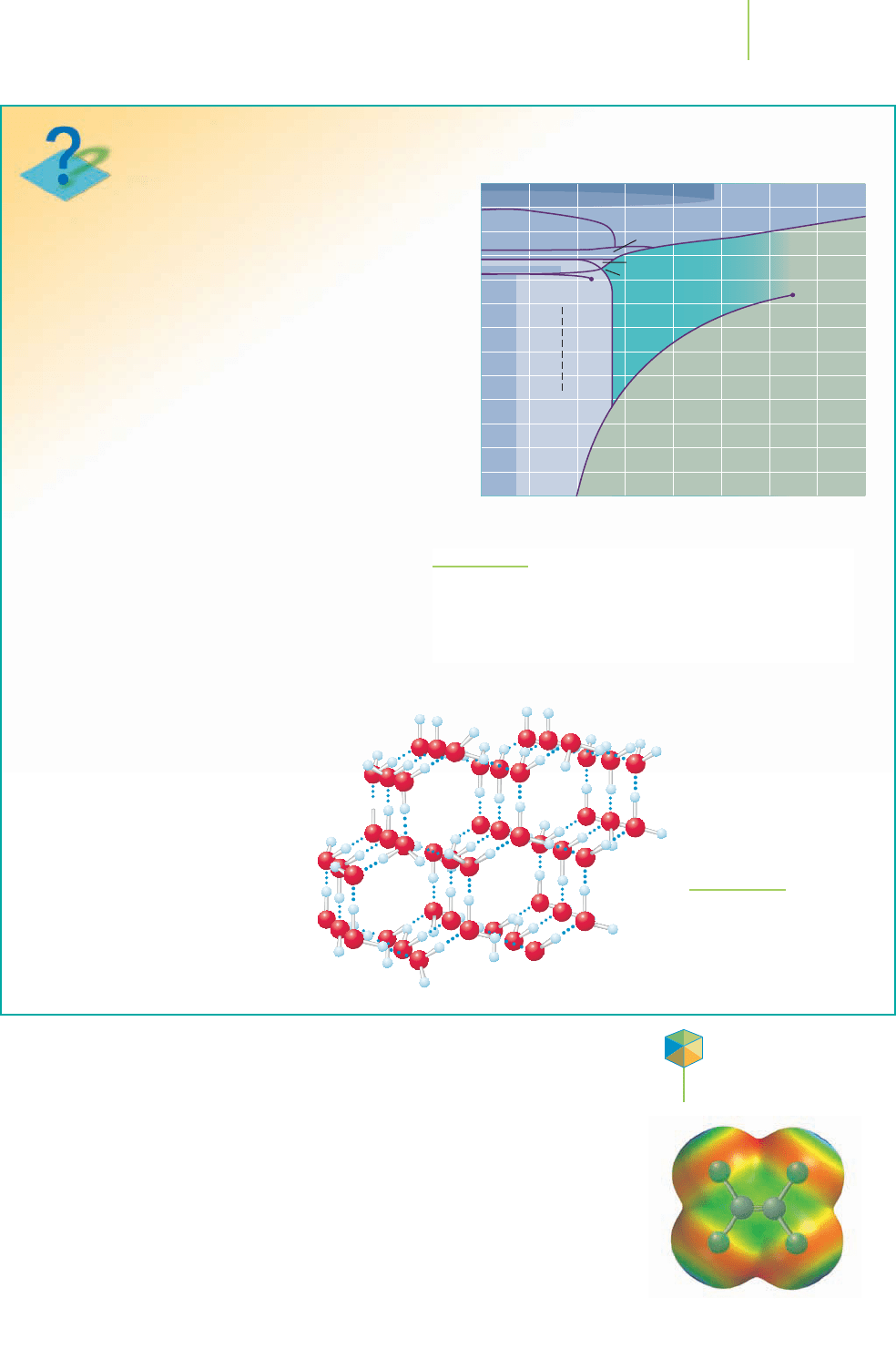
The probes’ data seem to show that deep within
the surface of these moons, there are layers of ice,
each in a different phase, mixed with rocks. The phase
diagram of water that we showed as Figure 11.18
is inadequate to account for the low temperatures
and massive pressures found within these moons.
A more comprehensive phase diagram for water,
emphasizing its solid phases, is given as Figure 11.20.
The ice that is made in our kitchen freezer or found
in an ice storm—what we might call “normal ice”—
is technically called Ice Ih. Its structure is shown in
Figure 11.21. There are 11 other forms
of ice that have been made in the laboratory or
simulated by computer.
The phase diagram shows that as the
temperature and pressure inside the moons
change, different types of ice are formed.
Each ice has its own density. As layers
form versions of ice of different
densities, they form the geological
features, such as cracking and
grooves, that we see on the surface
of these incredible satellites. Part
of the task of scientists is to
develop phase diagrams that
tell us about the conditions at
which each type of ice exists.
How do we know?
The moons of Jupiter
1
10
3
10
6
10
9
10
12
0 100 200 300 400 500 600 700 800
Solid
IhIc
III
V
VI
VII
VIII
IX
XI
II
X
XI
Liquid
Vapor
Temperature (K)
Pressure (Pa)
FIGURE 11.20
This is an expanded phase diagram for water, taking into account
the phase changes among the various solid (ice) forms. These forms
of ice are thought to occur in layers beneath the surface of some
of the moons of the outer planets.
FIGURE 11.21
The arrangement of water
molecules within a crystal
of “normal” ice (Ice Ih).
Application
C
HEMICAL ENCOUNTERS:
CO
2
as a Dry Cleaning Solvent

FIGURE 11.22
A water strider (Gerris remigis) on the surface
of a pond. Note that the water indents as it
supports the weight of the water strider.
462 Chapter 11 The Chemistry of Water and the Nature of Liquids
11.5 Impact of Intermolecular Forces on
the Physical Properties of Water, II
Viscosity
Making chocolate at home is difficult, because so much can go wrong, including
unintended air bubbles, cracking, and a greasy look and feel to the final product.
Having these things occur on an industrial manufacturing scale can be especially
damaging. One of the most important properties that cause these and other in-
consistencies in chocolate is called
viscosity, which is a measure of resistance to
flow. In the chocolate process industries, a viscometer is used to constantly moni-
tor the viscosity of fully melted chocolate so that it flows into the molds and
produces a final product that is consistent from batch to batch. Viscosity mea-
surements are also important to the fuel industry, where the ability of fuels to
flow is important to consider when storing, pumping and, within engines, inject-
ing the fuel. Viscosity is reported in units of millipascal seconds (mPa·s). A highly
viscous liquid, ethylene glycol (C
2
H
6
O
2
), is combined with water to form com-
mercial antifreeze and has a viscosity of 16.1 mPa·s at 25°C and 1 atm. The water
with which it is combined has a viscosity of 0.890 mPa·s at the same conditions.
Why does the viscosity differ among liquids?
As with so many things related to chemistry, we gain understanding by look-
ing at behavior at the molecular level. The molecules that constitute viscous liq-
uids do not move well relative to each other.
Why is this so? Part of the answer has
to do with intermolecular forces. More such interactions tend to increase vis-
cosity. Size is also important. It is hard to move large molecules.And, as with boil-
ing point, molecular shape is relevant because molecules must be able to move
well among each other in order to flow easily. Octane (C
8
H
18
) has a viscosity of
0.508 mPa·s at 25°C and 1 atm, whereas the viscosity of pentane (C
5
H
12
) mea-
sures 0.224 mPa·s at these conditions. Temperature is also a key factor because, as
we have seen, molecules with high average energy move relatively quickly and can
overcome intermolecular forces, leading to low viscosity. For example, the viscos-
ity of water changes from 0.890 mPa·s at 25°C to 0.378 mPa·s at 75°C. That’s why
chocolate, as well as oil in your car’s engine, flows more smoothly at higher than
at lower temperatures.
Surface Tension
The water strider glides from place to place along the water’s surface, as shown in
Figure 11.22. Why can the insect travel on the water’s surface? The key is a prop-
erty of liquids called
surface tension. This property is also responsible for the
Motor oil viscosity is rated for use on
the basis of the type of engine and the
outside temperature.
beads of water that run down your window in the rain or across the surface of
your car after it has been waxed. Surface tension is a measure of the energy per
unit area on the surface of a liquid. To better understand what this measure
means, we note that hydrogen bonding in water makes it more energetically
stable. Water molecules on the interior of the solution can hydrogen-bond in all
directions, whereas surface molecules can form these bonds only toward the
solution itself. In order to maximize the number of hydrogen bonds (and, there-
fore, the energetic stability of the liquid), water forms a shape with the minimum
surface area, a sphere. This is why small droplets of water form beads and water
striders can glide on water.
The chemical industries pay close attention to surface tension in the design of
many consumer products. For example, soaps and detergents are designed to
have very low surface tension in order to interact well with clothing and dishes.
Surface tension is also considered in the design of pharmaceuticals and plays a
large role in how tablets and gelcaps dissolve after they are swallowed.
Capillary Action
A buret, shown in Figure 11.23, is an essential piece of glassware for measuring
volume when performing laboratory analyses with liquids. Water has been added
to the buret in Figure 11.24. Why does the water seem to rise up the sides of the
glass? And given this curvature, what do we say is the volume of water in the
buret? The answer to the first question again lies in one of our chemical themes,
the impact of hydrogen bonding on the structure of water. The forces that keep
the large group of water molecules together are called
cohesive forces. Then there
is the glass in which the water sits. The glass is largely silicon dioxide (SiO
2
) but
with a surface containing many polar oxygen and hydrogen atoms. As is shown in
Figure 11.25, the hydrogen atoms on water interact with the oxygen atoms on the
surface of the glass. The net result is that some molecules of water adhere to
the glass, a process called,
adhesion. The effect in which water seems to crawl up
the sides of a thin tube such as a buret is called
capillary action. In this case, the ad-
hesive forces between water and the glass are stronger than the cohesive forces
within water itself. The weight of the water prevents it from rising up even higher
11.5 Impact of Intermolecular Forces on the Physical Properties of Water, II 463
FIGURE 11.23
A buret is essential labware
in the analytical laboratory.
FIGURE 11.24
The meniscus of water within
a buret is readily observable.
Water beads on a freshly waxed car.
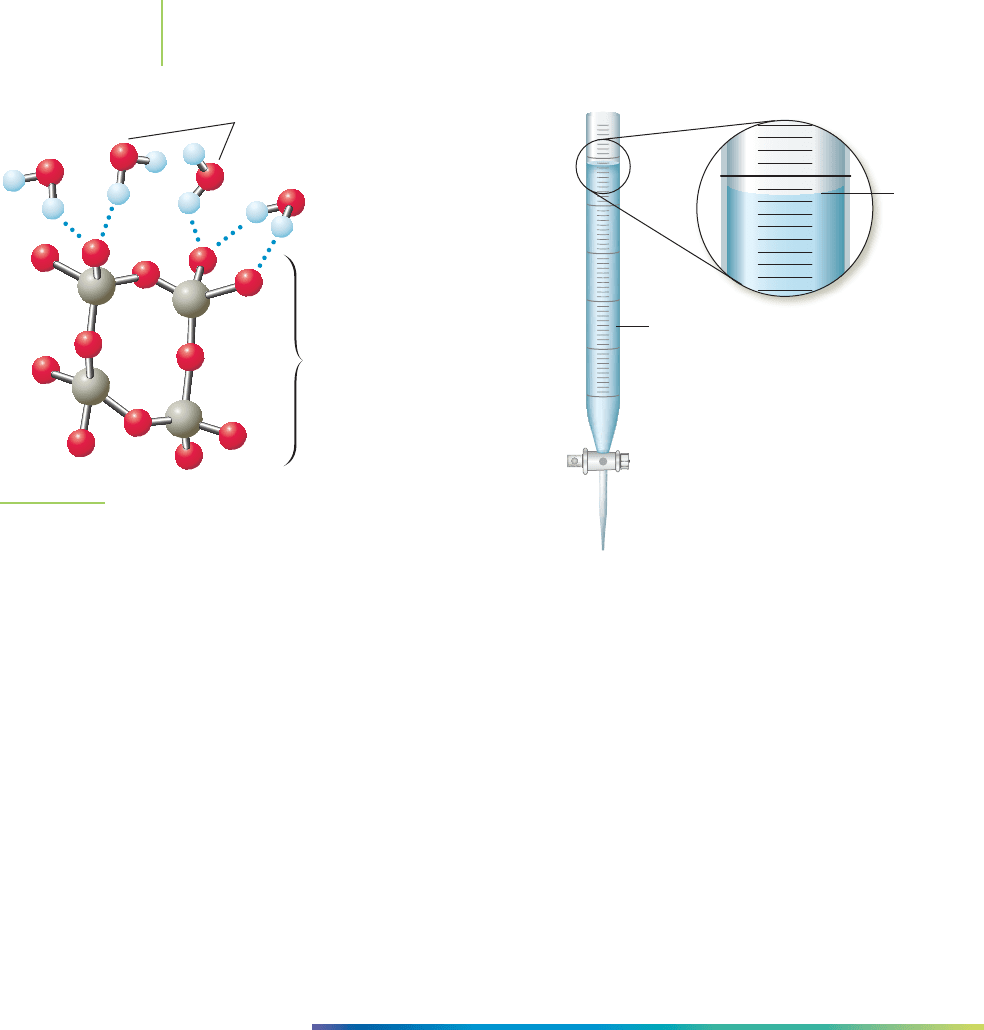
FIGURE 11.25
The interaction of water with the surface of glass
results in adhesion. Hydrogen bonds between the
water molecules and the glass surface are illustrated.
464 Chapter 11 The Chemistry of Water and the Nature of Liquids
Hexane in a buret.
within the buret. Mercury in a glass tube (such as in a thermometer) shows the
opposite behavior, bulging upward within the glass, in response to the domi-
nance of cohesive over adhesive forces.
Adhesive forces lead to the formation of a
meniscus, the concave shape at the
surface of the water in the buret. By convention, the volume of liquid delivered
from the buret is read at the bottom of the meniscus, as shown in Figure 11.24, so
our reading of the buret is 40.03 mL. Because burets are graduated from the top
(0 mL) down, there are 9.97 mL of water remaining in our 50.00-mL buret.
The balance between forces is not always easy to assess. For example, hexane
(C
6
H
14
) shows a concave meniscus similar to that of water, though not quite so
pronounced.
Would we have predicted this fact? The surface of the glass can, pre-
sumably, induce a dipole in hexane, so we can explain the behavior. However,
making predictions when several effects are important is sometimes quite
challenging.
HERE’S WHAT WE KNOW SO FAR
■
A liquid fills its container from the bottom up and flows easily.
■
Intermolecular forces of attraction determine the properties of a substance.
The number and strength of these forces contribute to the attractions between
adjacent molecules.
■
London forces are typically the weakest forces between molecules. Because
every molecule has the ability to become polarized, every molecule possesses
London forces. In very large molecules, these forces can be significant.
■
Dipole–dipole interactions are much stronger than London forces. They exist
in molecules that contain a nonzero dipole moment, and their strength is
related to the strength of the dipole moment.
■
Hydrogen bonds are the strongest of the van der Waals forces. They exist pri-
marily between the interaction of a hydrogen atom and two atoms of F, O,
and/or N.
■
The strength of the intermolecular forces of attraction determines the shape of
a meniscus, the surface tension of the liquid, its vapor pressure, and other
physical properties associated with the liquid.
Buret
Meniscu
s
Water
Glass
11.6 Water: The Universal Solvent
In the introduction to this chapter, we raised three questions related to the pro-
jected water shortage in Tampa Bay: What is it about water that makes it unique
among molecules? How can we use our understanding of the nature of water to
explain its properties? And how can we use these properties to help in the search
for clean water in places like Tampa Bay? We have answered the first question, fo-
cusing especially on the polar nature of water and its ability to form the special
interaction called a hydrogen bond. This enabled us to explain a variety of
properties of water, including its unusually high boiling point, its vapor pressure,
and its phase diagram, giving us the understanding to answer much of the second
question.
Implicit in the third question about the search for clean water is recognition
that the water found in nature is not pure. It is filled with all manner of
solutes,
substances that dissolve in it. Water is often called the
universal solvent because of
its ability to dissolve so many chemicals to form homogeneous mixtures called
solutions. Aqueous solutions (water-based solutions) can have solutes that are
gases, liquids, or solids. A special class of large-molar-mass molecules that finely
disperse in solution are called colloids.
Why Do So Many Substances Dissolve in Water?
We can answer the question that opens this section by looking at seawater, in
which dissolved sodium chloride is one of the primary solutes. Chemically, we
can understand what happens when salt dissolves in water by considering the
process of dissolving as a series of discrete steps, as shown in Figure 11.26. Al-
though the process does not, in fact, occur in steps, we may use this procedure to
enhance our understanding of the dissolution process. At each of the steps along
our imaginary solution-forming pathway, ask yourself, “Is this likely to be an
endothermic or an exothermic process?”
■
Solute separation: In the first step, the salt must separate into sodium ions
and chloride ions. The electrostatic forces that keep the ions together must
be overcome, so energy is required. This part of the process is endothermic
(H =+).
11.6 Water: The Universal Solvent 465
This water contains many dissolved
salts, compounds, and molecules. It
also contains particles too big to
dissolve; these particles remain sus-
pended and make the water cloudy.
H
∆H
1
+ ∆H
2
∆H
3
∆H
soln
Solute
Separated solute Separated solvent
Solvent Solution
+
+
FIGURE 11.26
The processes necessary, along with
their energy exchanges, for the disso-
lution of a salt, such as NaCl, in water.
The overall change in energy can be +
or −, depending on the ion–dipole
interaction.
■
Solvent separation: Energy is required to break hydrogen bonds holding water
molecules together in order to accommodate the incoming ions from the
solute. This part of the process is endothermic (H =+).
■
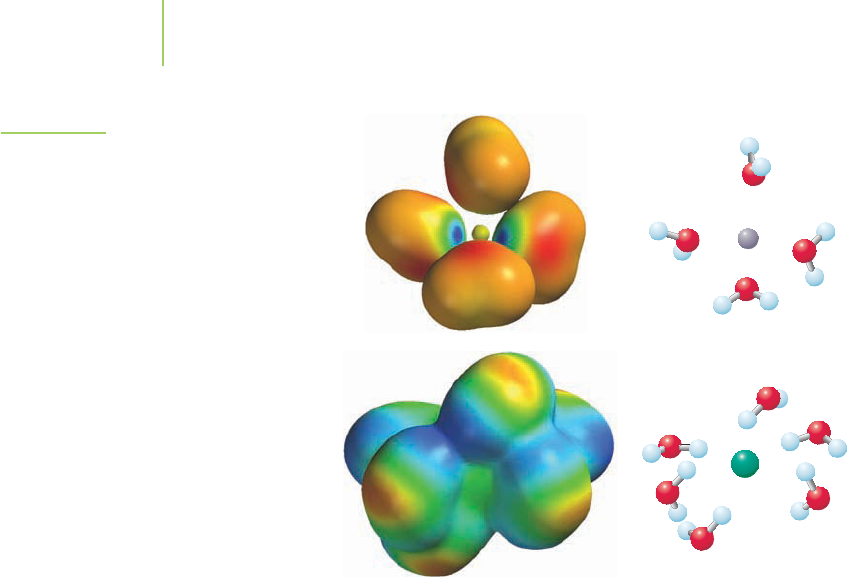
Electrostatic interaction between the solvent and solute ions: Electrostatic at-
traction is the great stabilizer. As shown in Figure 11.27, sodium cations are
attracted to the negatively charged dipole at oxygen on each water molecule.
Conversely, chloride anions are attracted to the positively charged hydrogen
poles. Although there is constant ion movement in the solution, at any one
time it is typical to have up to six water molecules surrounding each ion. This
ion–dipole interaction contributes to the solution process in two ways. First,
for the process involving sodium chloride and water, the interaction is highly
exothermic (H =“–”). In order to have favorable formation of a solution,
the value of the exothermic interaction of the solute and solvent is typically
larger than the endothermic processes in the first two steps. Second, dissolv-
ing salt ions in water results in dispersal of the cations and anions through-
out the solution. This ion–solvent interaction is called
solvation, and when
the solvent is water, it is known as
hydration. The heat of solution (
sol
H) can
be viewed as the sum of the heats of solute separation, solvent separation,
and ion–dipole interaction.
sol
H =
solute
H +
solvent
H +
solvation
H
An overall exothermic heat of solution (–
sol
H) favors solution formation
but is not by itself an indicator of solubility. The additional dispersal of the ions
in the solvent (see Chapter 14) is also important. For example, NaCl has a value
of
sol
H =+3 kJ/mol, yet it dissolves in water. So does potassium hydroxide,
KOH (
sol
H =–57.6 kJ/mol). In fact, the heat of solution of KOH is so large that
adding 100 g to a liter of water will raise its temperature to nearly boiling, with
sol
H = –103 kJ for the 100 g.
As a rule of thumb, like dissolves like. Polar molecules typically dissolve many
salts via ion–dipole interactions. Similarly, polar liquids mix as a consequence of
the stability gained from dipole–dipole and, when possible, hydrogen-bonding
interactions. Nonpolar substances, such as the industrial solvents benzene (C
6
H
6
)
and toluene (C
7
H
8
) are often miscible (mix in all proportions) with other nonpo-
lar substances. Part of the reason lies with London forces and part with the sta-
bility gained by dispersion as the liquids mix.
466 Chapter 11 The Chemistry of Water and the Nature of Liquids
FIGURE 11.27
The interaction of the sodium ion with the
negative end of the dipole on water and
that of the chloride ion with the positive
end of the dipole on water contribute to the
energetic stability that permits NaCl to dis-
solve in water. Note in the electrostatic
potential maps how the water molecule’s
electron potential changes as it interacts
with the ions.
EXERCISE 11.7 Predicting Solubility
Which of the following substances is (are) soluble in water: ethanol (C
2
H
5
OH),
ethylene glycol (C
2
H
6
O
2
), and cyclohexane (C
6
H
12
)?
First Thoughts
The ability to form electrostatic attractions to water, including hydrogen bonds, dic-
tates the solubility of a substance in water. Which of these compounds can have
such attractions?
Solution
Ethanol and ethylene glycol are infinitely soluble in water. In fact, both can act as a
solvent or solute with water. Cyclohexane is not appreciably soluble in water.
Further Insights
11.6 Water: The Universal Solvent 467
Ethanol
C
H
H
H
H
H
COH
Ethylene glycol
C
H
OH
H
H
H
COH
Cyclohexane
C
CH
H
HH
C
HH
C
C
C
H
H
H
H
H
H
Both benzene (C
6
H
6
) and toluene (C
7
H
8
) are excellent solvents for cyclohexane.
Industrial manufacturing of polymer-based plastics often involves dissolving non-
polar solutes in nonpolar solvents.
PRACTICE 11.7
Which of the following substances is (are) soluble in hexane: water, carbon tetra-
chloride (CCl
4
), and iodine crystals (I
2
)?
See Problems 58–60 and 62.
The maximum amount of a solute that can dissolve in a solvent is its solu-
bility
, often given in grams of solute per 100 mL of solvent (
g solute
100 mL solvent
). If we
added any more solute, it would not dissolve. At that point, we say that the solu-
tion is
saturated (compare this with the use of the term saturated for the vapor of
the pure liquid). Sometimes the solubility of a substance in water depends on the
relative size of the polar and nonpolar parts of a molecule of the substance.
Table 11.3 shows that the series of alcohols gets progressively less soluble in water
as the nonpolar end of each becomes larger. However, even substances that we
Solubility of Some Alcohol Compounds in Water
Compound Name Solubility (g/L)
CH
3
OH Methyl alcohol (methanol) Infinite
C
2
H
5
OH Ethyl alcohol (ethanol) Infinite
C
3
H
7
OH Propyl alcohol (1-propanol) Infinite
C
4
H
9
OH Butyl alcohol (1-butanol) 79
C
5
H
11
OH Pentyl alcohol (1-pentanol) 27
C
6
H
13
OH Hexyl alcohol (1-hexanol) 5.9
C
7
H
15
OH Heptyl alcohol (1-heptanol) 0.9
C
10
H
21
OH Decyl alcohol (1-decanol) <0.04
Source: Kelter, Carr, and Scott. Chemistry: A World of Choices; McGraw-Hill: New York, 2003.
TABLE 11.3
Methanol
Ethanol
n-Propanol
Visualization: Saturated
Solution Equilibrium
Video Lesson: Types of Solutions
