Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

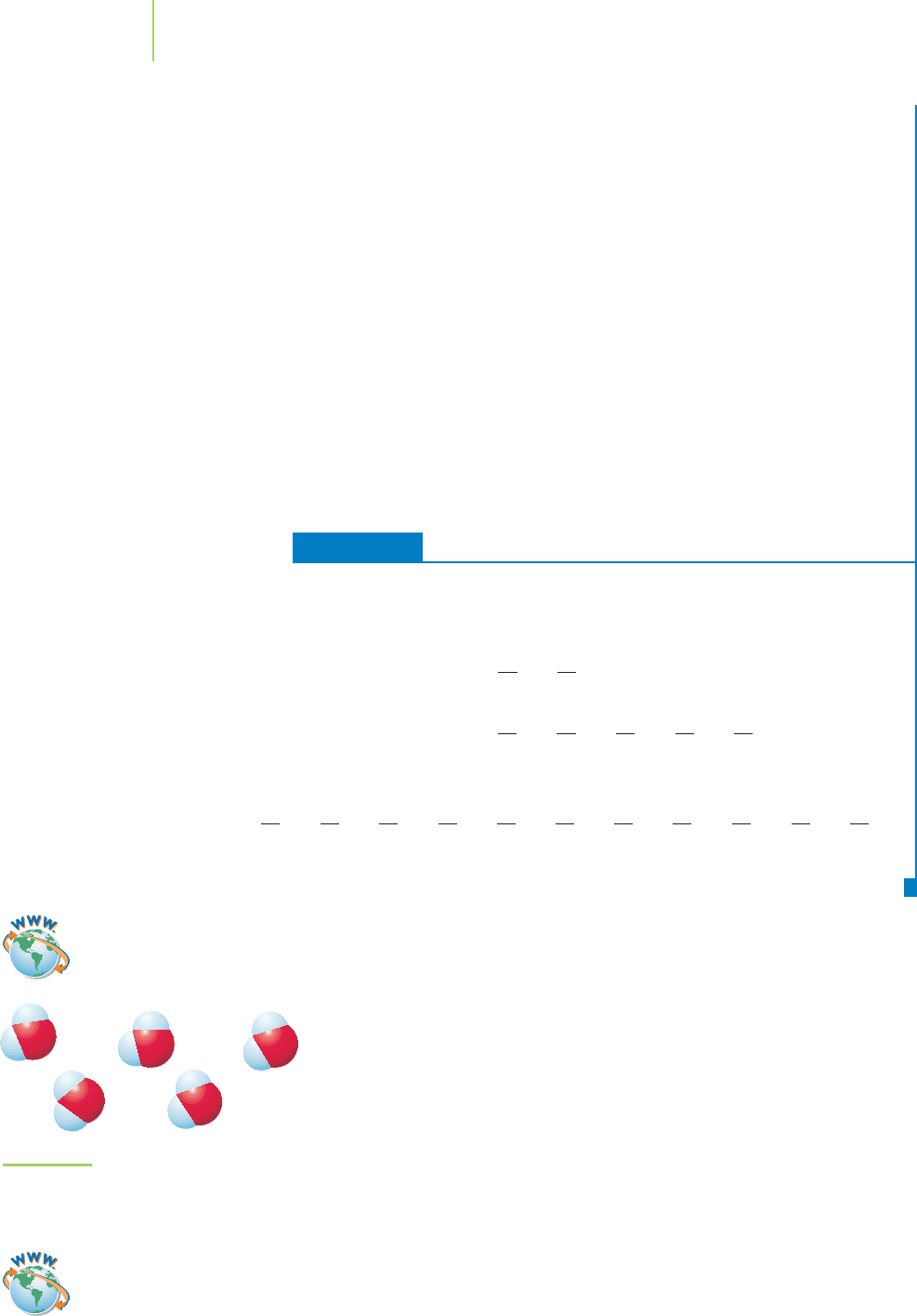
FIGURE 11.8
Interaction of the oppositely charged
poles of water molecules makes water
a liquid at room temperature.
Solution
Comparing the 12-carbon compounds, we note that dodecane has a straight-chain
structure, whereas 2,2,4,6,6–pentamethylheptane is highly “branched,” with many
of the atoms hidden from other molecules, so dodecane will have the higher boiling
point of the two. Pentadecane is a longer straight-chain molecule than dodecane, so
its boiling point will be the highest among the group. The actual boiling points of
the compounds are (from lowest to highest)
177.8°C 2,2,4,6,6-Pentamethylheptane
216.3°C Dodecane
270.6°C Pentadecane
Further Insights
Other factors affect the boiling (and melting) points of organic chemicals. One is
the way in which molecules fit together. Molecules that only have single bonds be-
tween carbon atoms fit together nicely, leading to higher melting and boiling points
than those that have double or triple bonds. The presence of other types of atoms
can also substantially affect boiling points.
PRACTICE 11.1
Propane (C
3
H
8
) is a gas at room temperature, hexane (C
6
H
14
) is a liquid, and
dodecane (C
12
H
26
) is a solid. Explain why these three molecules have their specific
properties.
Propane C
3
H
8
Hexane C
6
H
14
Dodecane C
12
H
26
See Problems 11, 25, 26, and 27a.
Permanent Dipole–Dipole Forces
The forces that give strength to the interactions among water molecules are a re-
sult of the permanent dipoles that exist in water. Water molecules organize to
maximize the energetic stability gained by attractions and to minimize the re-
pulsions, as shown in Figure 11.8. Dipole–dipole interactions are relatively
weak compared to covalent bonds, but they become stronger in molecules
with relatively large dipole moments. However, even permanent dipoles in a
molecule may not lead to significant intermolecular bonding at normal condi-
tions, as with the relatively small molecules HCl and H
2
S, which have boiling
points of only –85°C and –61°C.
Hydrogen Bonds
One very special type of interaction, the hydrogen bond, is of great importance.
Knowing about
hydrogen bonds, a term first used in 1912, furthers our under-
standing of why water is a liquid at room temperature. This knowledge also helps
us explain some very elegant and important ideas about protein and DNA struc-
ture (Chapter 22) that add to our collective insight into human biology.
448 Chapter 11 The Chemistry of Water and the Nature of Liquids
CH
2
CH
3
CH
3
CH
2
CH
3
CH
2
CH
2
CH
3
CH
2
CH
2
CH
3
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
3
CH
2
–
–
–
–
–
+
+
+
+
+
Visualization: Intermolecular
Forces: Dipole–Dipole Forces
Visualization: Intermolecular
Forces: Hydrogen Bonding
Forces
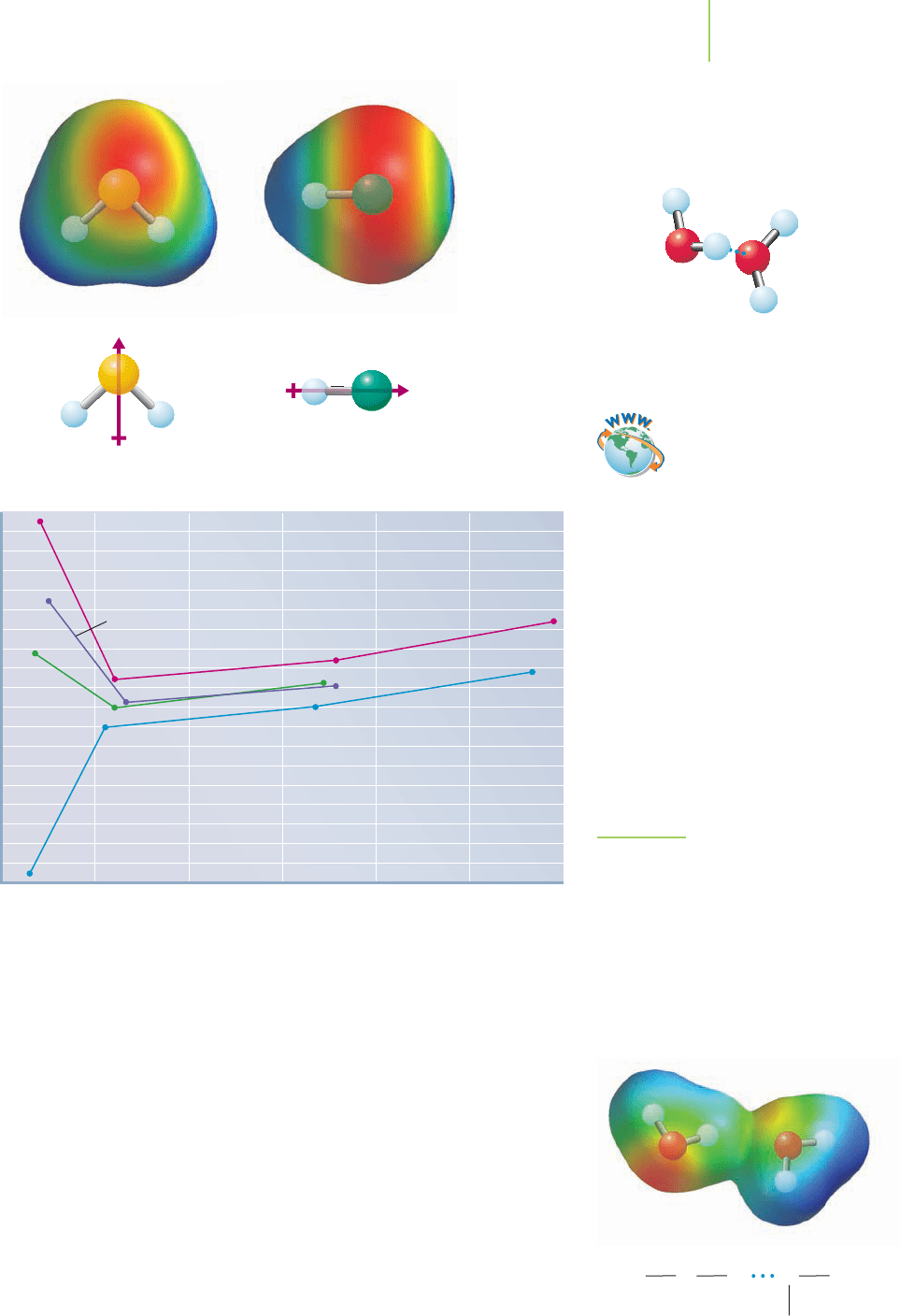
11.2 A Closer Look at Intermolecular Forces 449
Hydrogen sulfide Hydrogen chloride
Dipole moments
–270
–250
–230
–210
–190
–170
–150
–130
–110
–90
–70
–50
–30
–10
10
30
50
70
90
110
Boiling point (˚C)
Molecular mass (amu)
10 30 50 70 90 110 130
Group VIIA
hydrides
Group VA
hydrides
H
2
O
Group VIA
hydrides
H
2
Te
H
2
S
H
2
Se
NH
3
AsH
3
PH
3
HF
HCl
HBr
Group IVA
hydrides
CH
4
SiH
4
GeH
4
SnH
4
FIGURE 11.9
Hydrogen bonds keep water liquid at
room temperature. In fact, hydrogen
bonds are breaking and re-forming at
an incredible rate.
Judging on the basis of its low molar mass alone, we would never have as-
sumed that water is a liquid at room temperature. However, water isn’t unique in
this regard. Other molecules, such as some of those shown in Figure 11.9, exhibit
boiling points higher than their molar masses would lead us to expect. Why are
their boiling points higher than expected? Each possesses hydrogen bonds.
A hydrogen bond is largely a dipole–dipole interaction of unusual strength
compared to other intermolecular forces. It is formed when a hydrogen nucleus
(a proton) is shared between two highly electronegative atoms of oxygen, nitro-
gen, or fluorine. These electronegative atoms interact with the proton through
their available lone pair (or pairs) of electrons. In essence, the electronegative
atoms play tug-of-war with the proton, creating a very strong intermolecular
force of attraction. We can illustrate the hydrogen bond by drawing a series of
dots from one of the electronegative atoms to the hydrogen.
Recent data have shown that hydrogen bonds are at least partly—as much as
10%—covalent in nature. Why is a hydrogen bond so strong? The partial positive
charge on the tiny hydrogen atom gives it a relatively large charge-to-size ratio.
That helps it pack a large attractive punch. This permits a strong interaction with
HOHO
H
H
Water molecules play tug-of-war
with a proton.
Tutorial: Hydrogen Bonding
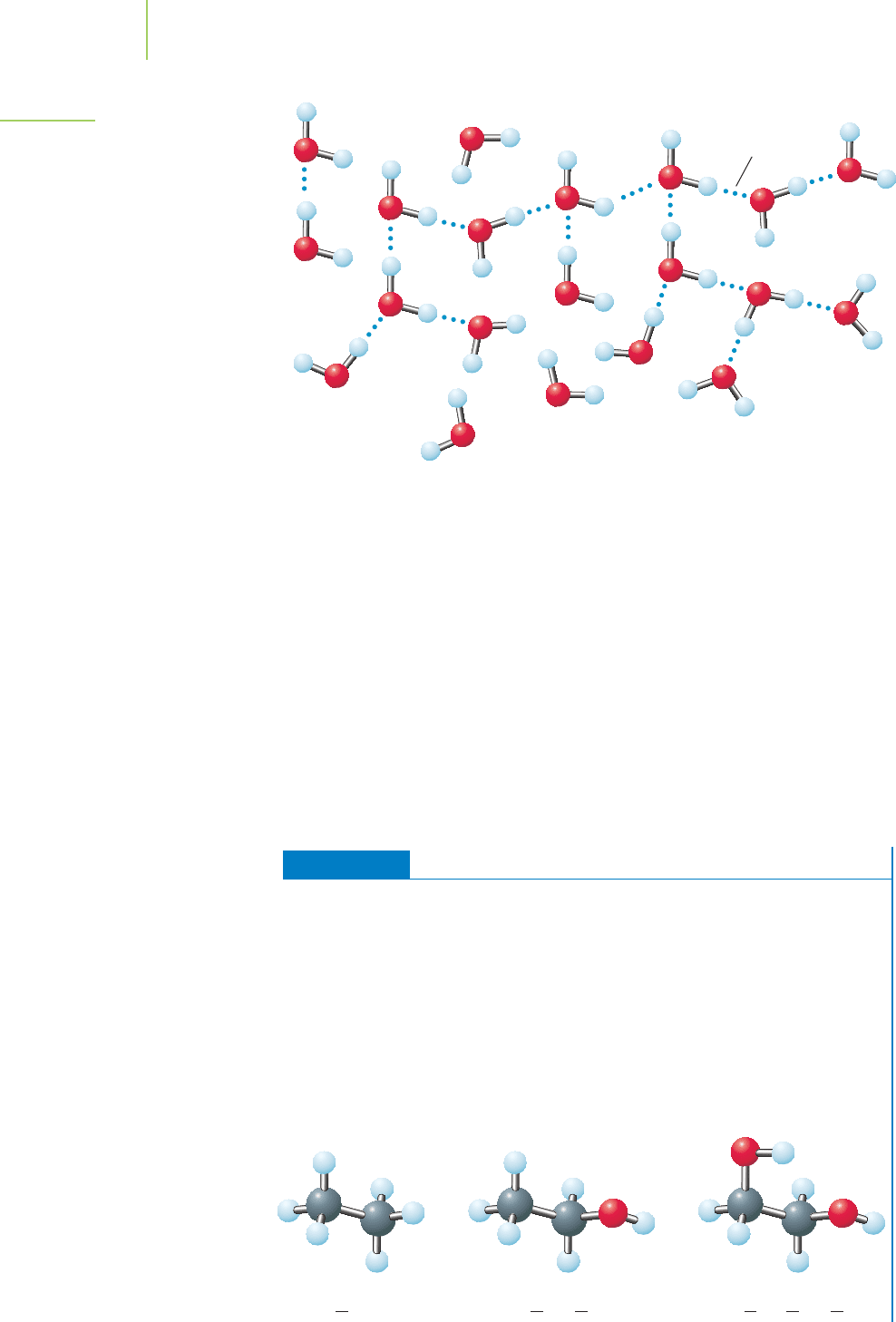
CH
3
CH
3
Ethane Ethanol Ethylene glycol
CH
3
CH
2
OH CH
2
CH
2
OHHO
450 Chapter 11 The Chemistry of Water and the Nature of Liquids
Hydrogen
bond
FIGURE 11.10
Compounds such as water, ammonia,
and hydrogen fluoride have much
higher boiling points than compounds
of similar size, as a consequence of
hydrogen bonding.
the highly electronegative fluorine, oxygen, or nitrogen atoms. How strong are
hydrogen bonds compared to covalent bonds? The O···H hydrogen bond in liq-
uid water has a measured bond energy of about 23 kJ/mol, compared to the aver-
age O—H covalent bond energy of 470 kJ/mol, which means that this hydrogen
bond is only about 5% as strong as the intramolecular O—H bond, but stronger
than the 9 kJ required to vaporize a mole of methane.
What does our understanding of hydrogen bonding tell us about the properties
of water?
Hydrogen bonds between neighboring water molecules, as shown in
Figure 11.10, keep water in the liquid state at STP. Hydrogen bonds break and
re-form billions of times per second, but on average, enough hydrogen bonds
exist at any one time to keep water liquid. This process of bonds breaking and
re-forming means that a particular set of three atoms, HOH, will not stay to-
gether very long. Covalent bonds become hydrogen bonds, and vice versa. A
flurry of activity is going on at the atomic level, even in a glass of water resting on
a tabletop.
EXERCISE 11.2 Comparing the Boiling Points
Ethane (C
2
H
6
) is an important starting material for the industrial production of
polyethylene plastics, used in items such as soft drink bottles. Ethanol (C
2
H
6
O)
second only to water as an industrial solvent, is used in the synthesis of other
compounds and in some blends of gasoline. Ethylene glycol (C
2
H
6
O
2
) is the main
component in conventional automobile antifreeze. Judging on the basis of their
structures, arrange these compounds from lowest to highest boiling point.
First Thoughts
Hydrogen bonds have a substantial impact on boiling point, especially when the
molecule itself is small, with many hydrogen-bonding sites. Molecules that have
—OH groups are likely candidates for hydrogen-bonding interactions.
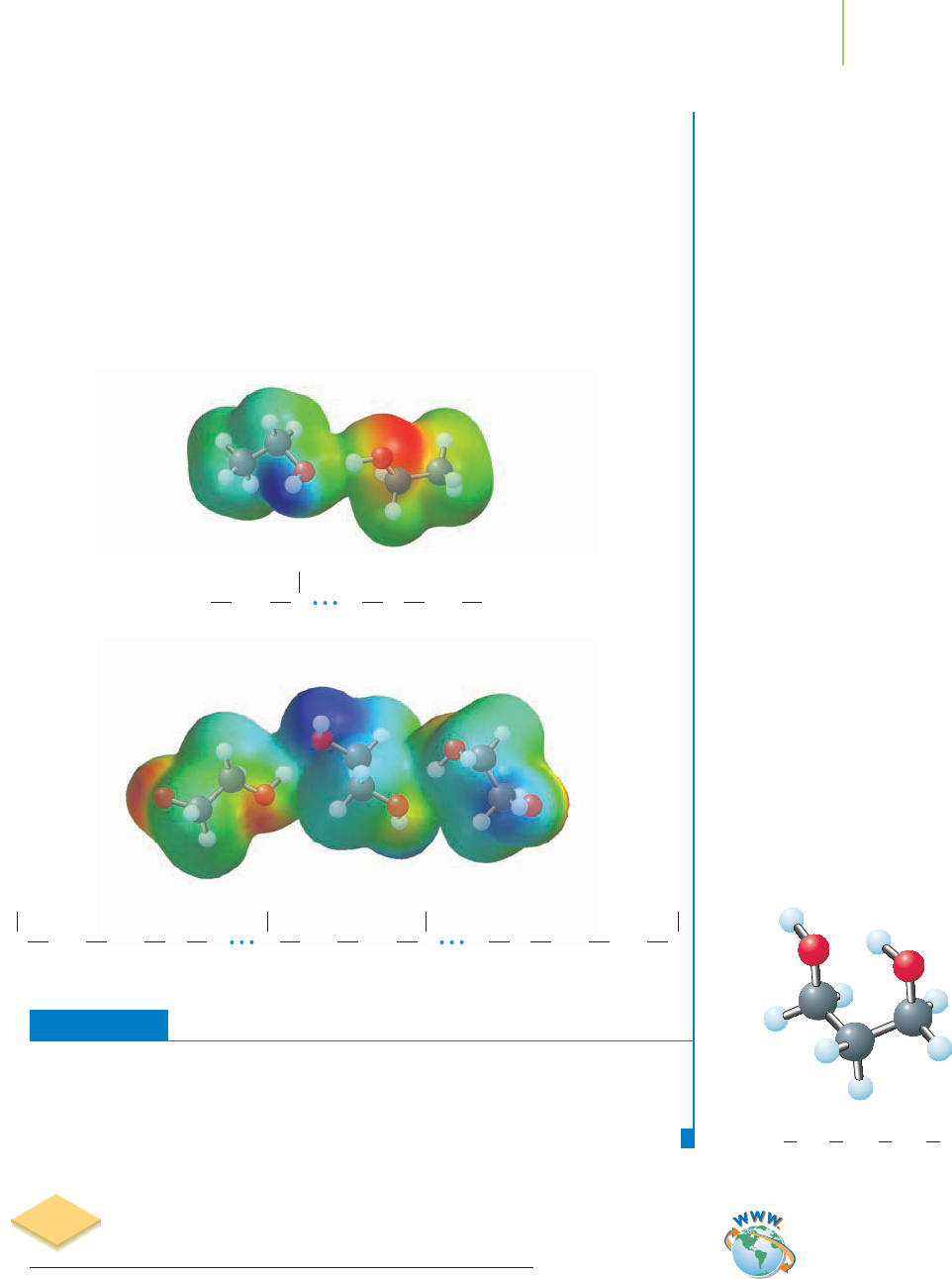
OCH
2
OO
Ethylene glycol hydrogen bonding
HHCH
2
OCH
2
OCH
2
CH
2
OCH
2
H H H H
Ethanol hydrogen bonding
H
OCH
2
CH
3
CH
3
OCH
2
H
Solution
Ethylene glycol has two polar sites at which hydrogen bonds can form, compared to
one on ethanol and none on ethane. Ethane therefore has the lowest boiling point,
–88.6°C, ethanol is next at 78.5°C, and ethylene glycol boils at 198°C.
Further Insights
An important idea in chemistry is that small changes in structure can lead to large
changes in properties. Having an alcohol group (—OH, see Chapter 12) substitute
for a hydrogen atom increases the boiling points of the compounds by over 100°C
for each such substitution. The effect is less in larger compounds. Why is this so?
Think about the types of intermolecular interactions that become possible.
11.3 Impact of Intermolecular Forces on the Physical Properties of Water, I 451
PRACTICE 11.2
Recent blends of “Green” (environmentally benign) antifreeze solutions use propy-
lene glycol (C
3
H
8
O
2
) which is far less toxic than ethylene glycol. Is its boiling point
higher or lower than that of ethylene glycol?
See Problems 12, 19–23, 27b, and 103.
11.3 Impact of Intermolecular Forces on
the Physical Properties of Water, I
A First Look at Phase Changes
A glass of pure water resting on a tabletop seems to be just that: at rest. But on a
molecular level, the system is deliriously active. The water molecules are moving
randomly and at great speed. Intermolecular hydrogen bonds are being broken
and re-formed at an incredible rate. Water molecules on the surface of the liquid
have enough energy to break free of their hydrogen bonds and go into the vapor
Propylene glycol
CH
2
CH
2
OHHO CH
2
Video Lesson: Properties
of Liquids
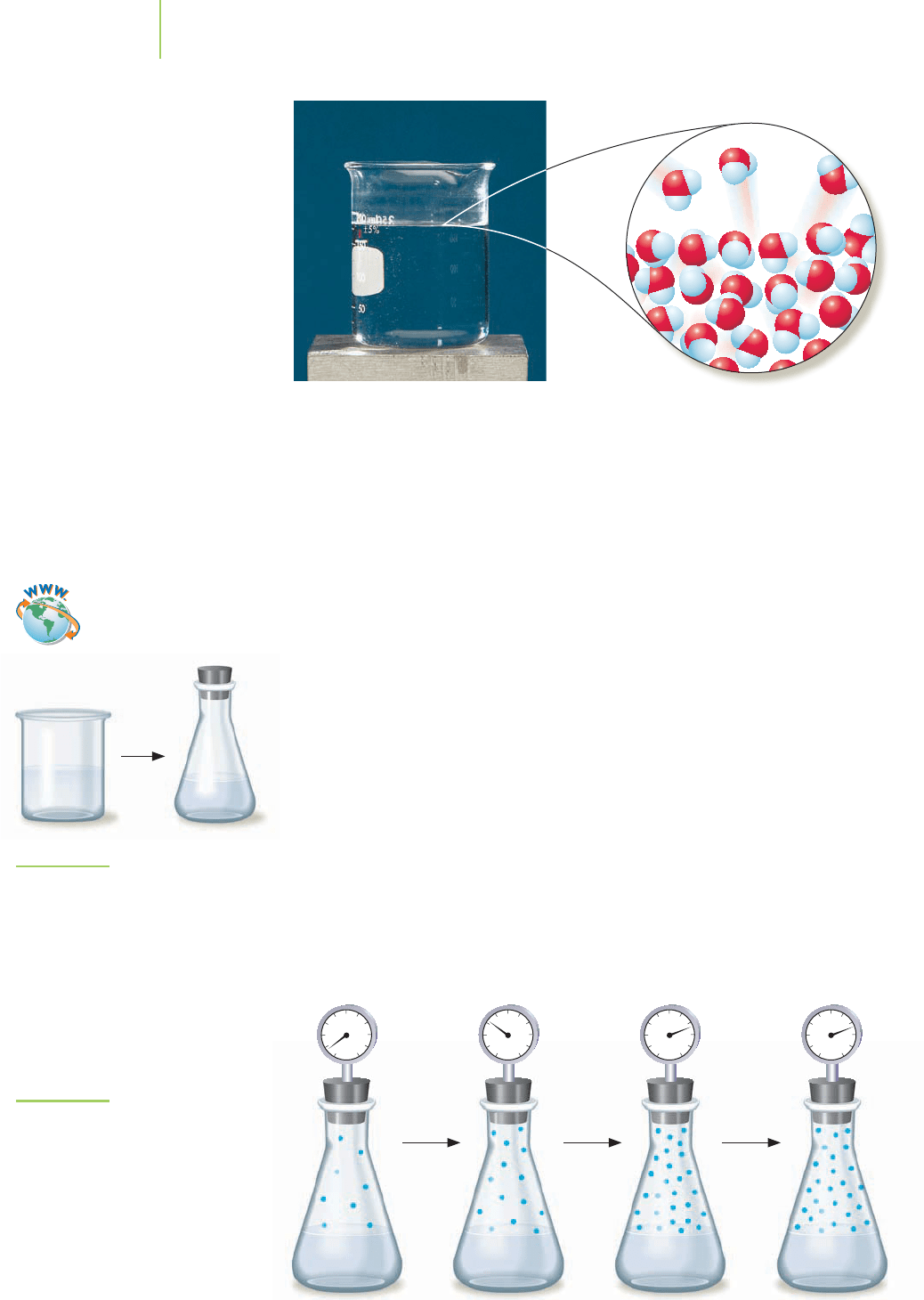
452 Chapter 11 The Chemistry of Water and the Nature of Liquids
Evaporation of water from the surface of the liquid.
FIGURE 11.11
How will the behavior of the liquid and
vapor change if we use a stoppered
(sealed) flask?
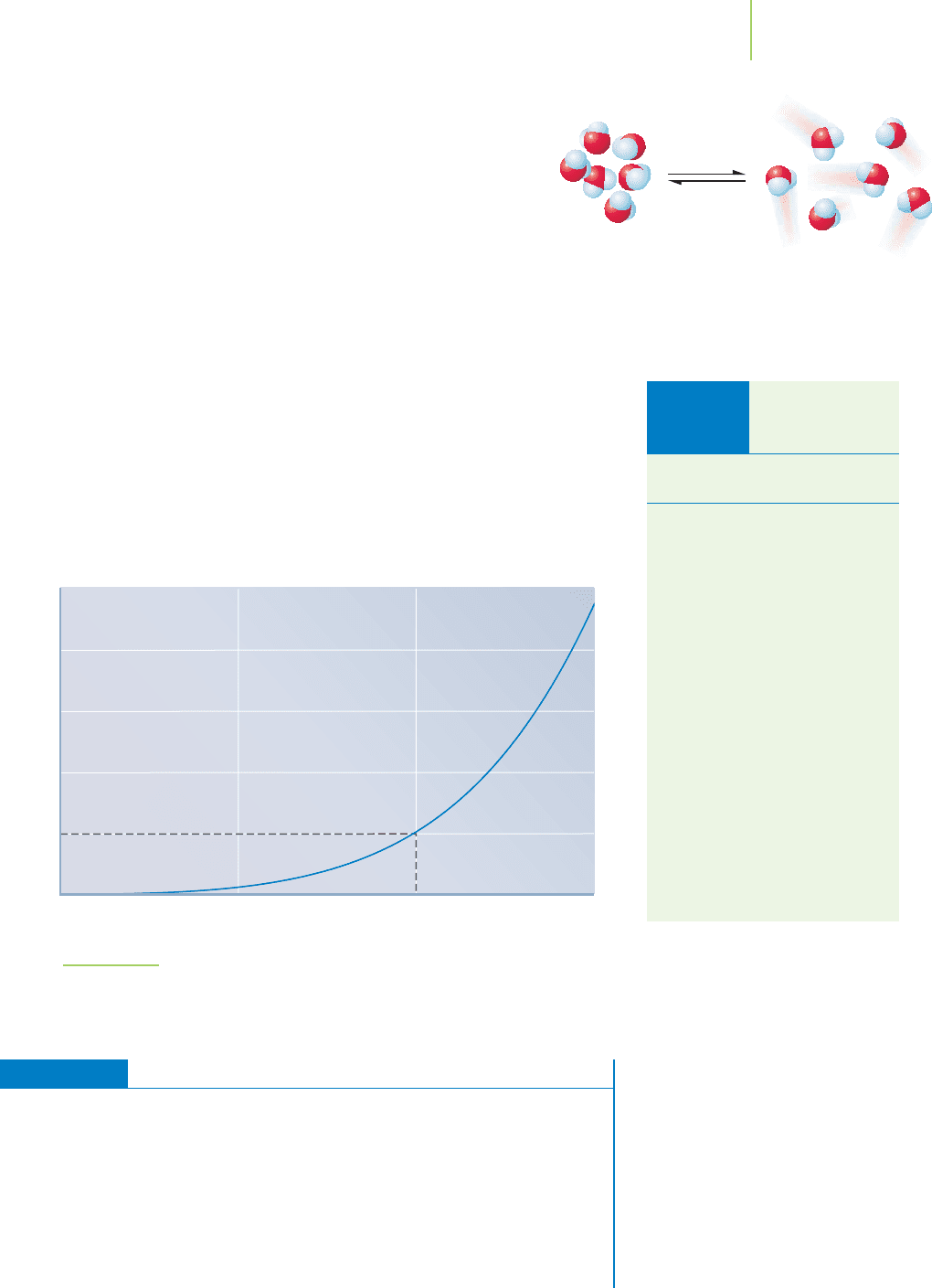
Time Time Time
PPPP
FIGURE 11.12
In a sealed flask, the system will come
to equilibrium, with the rate of evapora-
tion being equal to the rate of
condensation.
state. With higher temperature comes increased average kinetic energy of the sys-
tem. That means greater molecular motion, more breaking of hydrogen bonds,
and more molecules leaving the surface in the process we call
evaporation.Some
of the surrounding molecules return from the vapor to the liquid, attaching to
the surface via hydrogen bonding, in the process of
condensation. The corre-
sponding escape of molecules from a solid (such as ice) to the vapor is called
sublimation, and their return from the vapor to the solid is known as deposition.
Vapor Pressure
If we were to let our glass of pure water sit long enough, more molecules would
evaporate than would condense, and we would be left with an empty glass.
Let’s change the set-up by enclosing the water in a sealed flask, as shown in
Figure 11.11. How will the behavior of the system change?
Initially, those water molecules with sufficient energy will break free of their
intermolecular hydrogen bonds and escape from the surface. The resulting
vapor will collide with air molecules in the flask and with the walls of the con-
tainer many times each second, exerting a force per unit area that we measure as
pressure. Recall from Chapter 10 that a pressure of 760 torr equals 1 atm.
In our sealed flask, as illustrated in Figure 11.12, the more vapor that exists,
the more collisions per second with the surroundings and the greater the pres-
sure. As the concentration of vapor builds up in the flask, some of it will con-
dense. Eventually, the air in the flask will hold as much vapor as it possibly can,
and, as might occur on a sultry summer afternoon, the air will become
saturated
Video Lesson: CIA
Demonstration: Boiling Water
at Reduced Pressure
11.3 Impact of Intermolecular Forces on the Physical Properties of Water, I 453
with water vapor. At this point, the rate of evaporation from the
surface of the water will equal the rate of condensation back to
the surface. When the rates of the forward (evaporation) and reverse
(condensation) processes are equal, they are said to be in
equilibrium.
We indicate that both evaporation and condensation can occur by
using double arrows.
As with every process we study, bond breaking and bond forming
are always vigorously occurring, even though to our eyes all seems
quiet. We often indicate that the molecular-level equilibrium is dy-
namic, rather than static, by calling it
dynamic equilibrium. The pressure exerted
by a vapor in equilibrium with its liquid is called the
equilibrium vapor pressure,
or, commonly, the
vapor pressure, of the liquid at a given temperature. The vapor
pressure of hot water at 80°C is 355.1 torr; that of cool water at 20°C is 17.5 torr;
this means that more water molecules are in the gas phase at relatively higher
temperatures than at lower temperatures. The vapor pressure of a liquid increases
with temperature. Figure 11.13 and Table 11.2 show this dependence for water.
Does this make sense at the molecular level? A higher system temperature
means higher average kinetic energy. Water molecules are moving faster than at
lower temperature, so more molecules have enough energy to break their hydro-
gen bonds and evaporate.
Vapor Pressure of
Water at Selected
Temperatures
Temperature Vapor
(°C) Pressure (torr)
0 4.58
10 9.21
15 12.8
20 17.5
21 18.7
22 19.8
23 21.1
24 22.4
25 23.7
26 25.2
30 31.8
35 41.2
40 55.3
50 92.5
60 149.4
70 233.7
80 355.1
90 525.8
Source: Chemical Rubber Company.
Handbook of Chemistry and Physics, 66th
ed.; CRC Press: Boca Raton, FL, 1986.
Water vaporWater
0
0
760
1520
2280
3040
3800
50 100 150
Temperature (˚C)
Vapor pressure (torr)
FIGURE 11.13
The vapor pressure of liquids is related to temperature, as shown for water.
The line on the graph indicates the boiling point of water at 1 atm.
EXERCISE 11.3 Comparing Vapor Pressures
Which substance, water or methanol (CH
3
OH), would have a lower vapor pressure
at 50°C? Explain your choice.
Solution
The molecule that has more extensive hydrogen bonding will have the lower vapor
pressure at a given temperature. The entire structure of water encourages hydrogen
bonding. Methanol can also hydrogen-bond, but it also has nearly nonpolar C—H
bonds. The vapor pressure of water is 93 torr at 50°C, compared to about 400 torr
for methanol.
TABLE 11.2
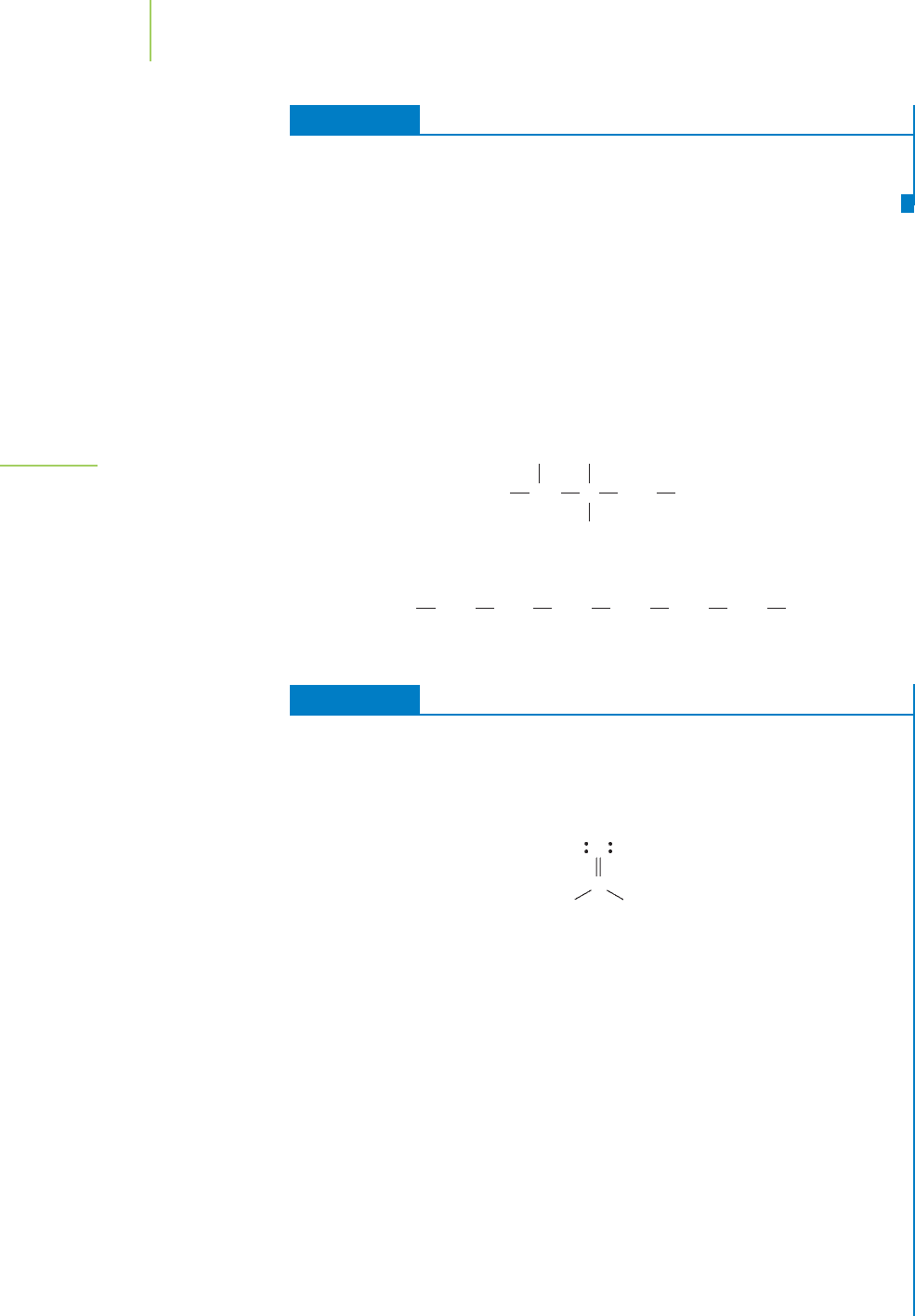
EXERCISE 11.4 An Implication of Vapor Pressure
Acetone (propanone, C
3
H
6
O), is shown below. It is a polar compound with a vari-
ety of industrial uses as a solvent and in the manufacture of plastics and pharma-
ceuticals. It occurs naturally in plants and animals. If acetone and water were both
accidentally spilled on a lab bench, which would be likely to completely evaporate
first at 20°C?
First Thoughts
To “completely evaporate” means to fully change from the liquid to the gaseous
state. Even though evaporation is not an equilibrium process (that is, molecules are
leaving the liquid’s surface more rapidly than they are condensing back), we can use
the vapor pressure as a guide. What contributes to a high vapor pressure? A low-
molar-mass molecule, even a polar one, that cannot form hydrogen bonds to itself
will probably have a higher vapor pressure than that of water.
Solution
Acetone at 20°C has a vapor pressure of about 185 torr, much greater than water’s
17.5 torr. Acetone (boiling point = 57°C) will completely evaporate first.
Further Insights
Looking a bit more deeply, we note that there are hydrogen bond donors, such as
water, that can use a hydrogen atom to engage in hydrogen bonding with, for exam-
ple, another water molecule. Water is also a hydrogen bond acceptor, because its
oxygen can accept a hydrogen bond from another water or, for example, from
ethanol. On the other hand, acetone is only a hydrogen bond acceptor (as in the
C
CH
3
CH
3
O
454 Chapter 11 The Chemistry of Water and the Nature of Liquids
PRACTICE 11.3
Name a substance that would have a lower vapor pressure at 50°C than either water
or methanol. Explain your reasoning.
See Problems 33–35 and 36a.
We now know that the ability to form hydrogen bonds is one important fac-
tor leading to lower vapor pressure at a given temperature, but it is not the only
factor. Molar mass and structure are also important because, as we have already
learned, London forces can be significant in relatively large molecules. In order to
develop a vapor pressure of 400 torr, octane (C
8
H
18
) must be heated to 104°C.
The branched molecule 2,3,3–trimethylpentane, shown in Figure 11.14, has this
vapor pressure at 92.7°C, and water reaches it at 83.0°C.
2,3,3-Trimethylpentane
CH C
CH
3
CH
3
CH
3
CH
3
CH
2
CH
3
Octane
CH
2
CH
3
CH
2
CH
2
CH
2
CH
2
CH
3
CH
2
FIGURE 11.14
The London forces are greater in octane than in
the highly branched 2,3,3-trimethylpentane. Which
would require a higher temperature to reach a
vapor pressure of 400 torr?
present example), so it cannot internally hydrogen-bond. However, it can form such
bonds with water and is soluble in it. There are millions of organic (carbon-based)
compounds that have a vast range of properties. Chapter 12 will introduce the com-
pounds and chemistry that make organic chemistry well worth knowing.
PRACTICE 11.4
Compare acetone and hexane (C
6
H
12
) using the same lab bench scenario as in
Exercise 11.4.
See Problems 29 and 30.
HERE’S WHAT WE KNOW SO FAR
■
The vapor pressure of a liquid increases with temperature.
■
More hydrogen bonding leads to lower vapor pressure.
■
All other things being equal, heavier molecules have lower vapor pressures
than lighter molecules.
■
Straight-chain molecules have lower vapor pressures than their branched
isomers.
■
When several of these factors come into play, it is hard to predict which will
dominate. We then run experiments or look up information in data tables.
Boiling Point
Let’s return to our glass of pure water. As we heat it, the vapor pressure of the liq-
uid increases along with the temperature. If we are at sea level on a day when the
surrounding pressure is 1 atm, the liquid will start to bubble from within as the
temperature approaches 100°C. As it does so, the vapor pressure of the liquid will
edge ever closer to the atmospheric pressure. When the temperature reaches
100°C, bubbles burst forth throughout the water in a familiar phenomenon we
call
boiling. We discussed boiling earlier in the chapter, and you understand its
general meaning from all the years you have been boiling water to make tea or
cook vegetables. Now we are ready to look at it in more depth. Boiling is not just
a surface process like evaporation, because it involves the entire liquid. We define
the
boiling point as the temperature at which the pressure of the liquid’s vapor
(rather than the vapor pressure, which is defined for an equilibrium process) is
equal to the surrounding pressure. If that pressure is 1 atm, at or near which so
many of life’s normal activities take place, the temperature at which a liquid boils
is called its
normal boiling point.
A good portion of the world’s population does not live at sea level, and for
them,“normal”is anything but. In mile-high Denver, the atmosphere is less dense
than at sea level, so the atmospheric pressure is correspondingly lower, about
0.82 atm (620 torr). If our glass of water were heated in Denver, it would boil at
about 95°C. The difference in boiling point with pressure is even more dramatic
in Mexico City, at 2240 m (7340 ft), where the atmospheric pressure is only
0.76 atm (580 torr). The boiling point at that altitude is only about 90°C (194°F).
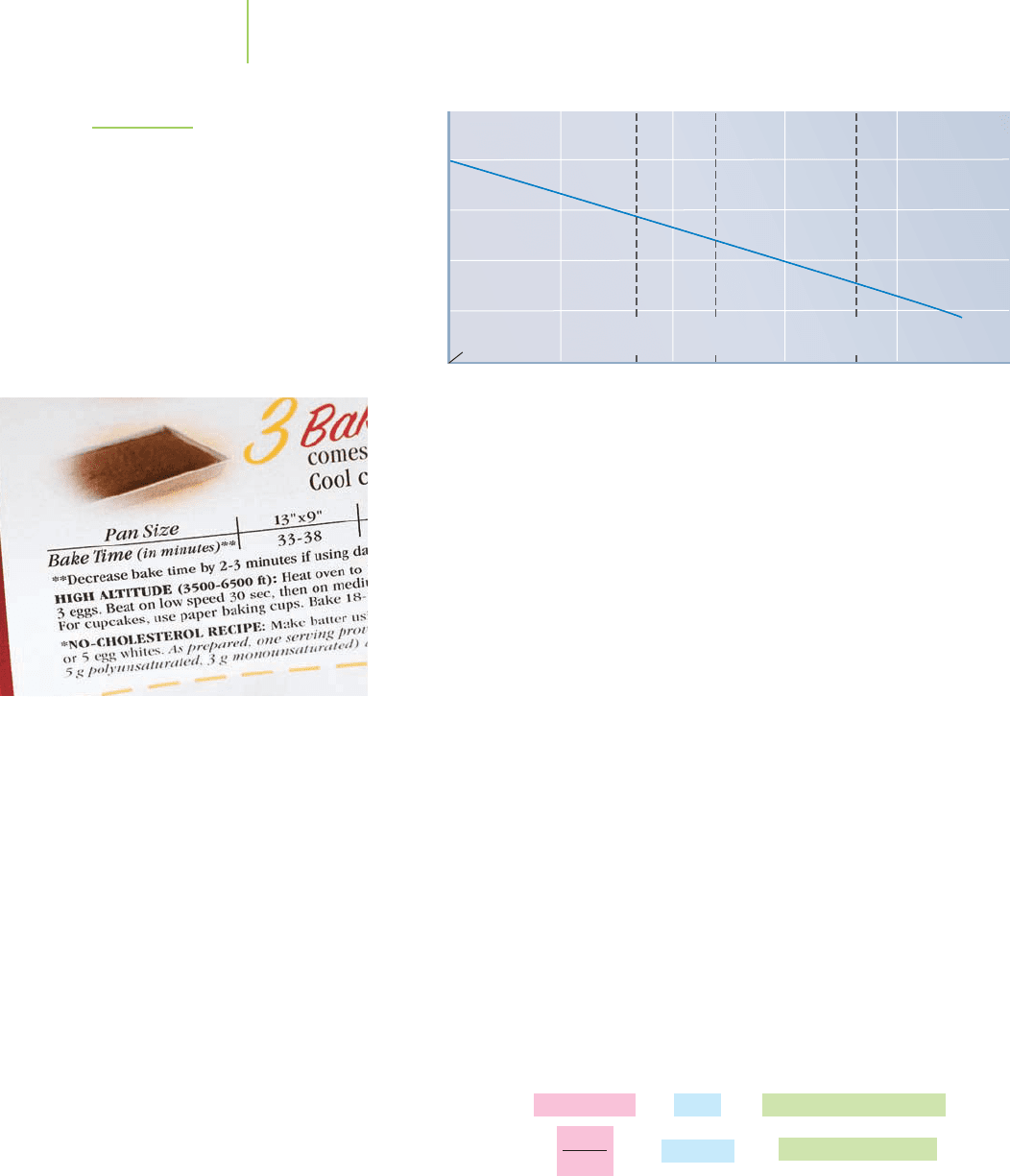
Figure 11.15 shows the decrease in the boiling point of water as the altitude
increases and atmospheric pressure consequently decreases. Food manufacturers
take advantage of the increase in boiling point with pressure when they process
foods by canning them at high pressure, allowing the food item to be heated to a
relatively high temperature, typically 107°C, for at least 3 minutes, killing any
bacteria within. A quick way to cook soup is to use a pressure cooker, which in-
creases the pressure within from 1 to 2 atm, raising the boiling point of the soup
11.3 Impact of Intermolecular Forces on the Physical Properties of Water, I 455
Application
Water boils at 100°C at 1.0 atm. At this
temperature, the vapor pressure equals
the atmospheric pressure.
Visualization: Boiling Water
with lce Water
Video Lesson: Vapor Pressure
and Boiling Point
to about 120°C (250°F). Commercial cake mixes often have two sets of instruc-
tions: one for cooking the batter at sea level (normal boiling point) and one for
higher altitudes.
Heating Curves
We opened this chapter by looking at drinking water in the Tampa Bay area. Now
let’s look north, perhaps above the Arctic Circle, to a group of hikers who want to
get drinking and washing water by melting some ice and then purifying it by boil-
ing. What happens as they heat a 10.0-kg block of the ice at –10.0°C? To fully un-
derstand the process that occurs, we must consider four changes: (1) warming the
ice; (2) melting the ice; (3) heating the water, and (4) boiling the water. As we pro-
ceed, think about what happens to the energy we add, the molecular motion, and
the resulting system temperature. We will assume that all of the heat goes into the
ice (the system), not into the surroundings.
Change 1: Warming the Ice
The temperature of our ice is –10.0°C. As we add heat, the molecules that are
fairly rigidly held in place begin to move a bit more. We still have ice, but the
average energy has increased, so the temperature rises. Figure 11.16 displays a
heating curve, a plot of the temperature of a compound versus time as it is heated.
The plot indicates the specific temperature ranges for solid, liquid, and gas
phases.
Theheatneededtowarm1gofasubstance enough so that its temperature
rises 1.0°C is its
specific heat (see Section 5.4), which for ice is equal to 2.05 J/g·°C.
Raising the temperature of our 10.0-kg block of ice from –10.0°C to its
melting point, at which it changes from a solid to a liquid, requires that 205 kJ of
heat, q, be added to the system.
q = specific heat × mass × change in temperature
q =
2.05 J
g·
◦
C
× 10,000 g × (0.0° C −
−
10.0°C)
= 205,000 J
= 205 KJ
This much heat would be supplied by, for example, burning about 4 g of methane
(natural gas is typically over 90% methane) in air.
Change 2: Melting the Ice
Our ice is now at its melting point of 0.0°C. As we add more heat, water molecules
move more freely and randomly, beginning the transformation from the solid
456 Chapter 11 The Chemistry of Water and the Nature of Liquids
Directions for baking a cake at high
elevations.
0 1000
Miami,
Florida
Denver,
Colorado
Mexico
City
La Paz,
Bolivia
2000 3000 4000 5000
80
85
90
95
100
105
Altitude (m)
Boiling point of water (˚C)
FIGURE 11.15
The boiling point of water goes down
as the altitude rises. This is because air
pressure is lower at higher altitude
(remember the definition of boiling
point).
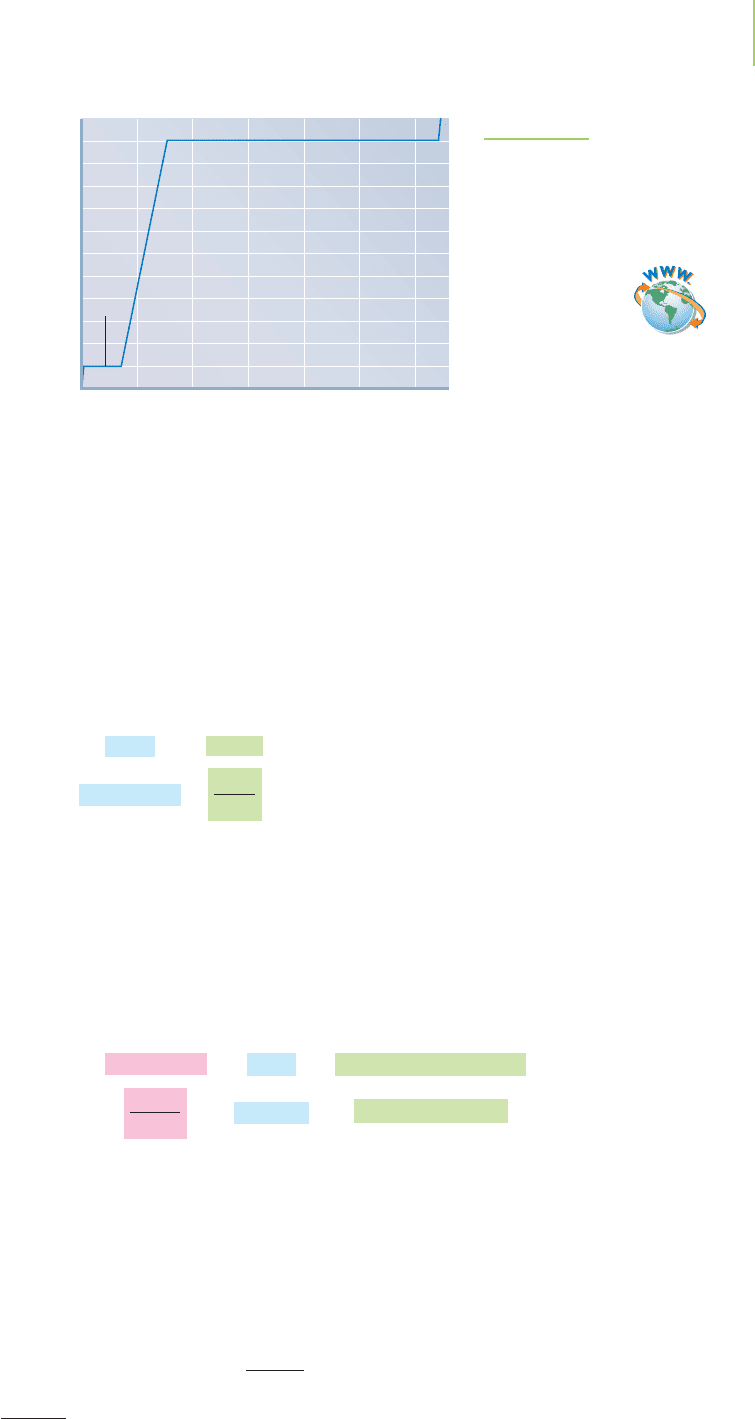
Temperature (°C)
0
10
20
30
40
50
60
70
80
90
100
110
–10
Ice
Water
Water and steam
Steam
Ice
and
water
0 5,000 10,000 15,000 20,000 25,000 30,000
Heat added (kJ)
crystal to the liquid state. The temperature at this change of state, the melting
point, is constant (Figure 11.16), because the added heat is going into breaking
hydrogen bonds, rather than increasing the average kinetic energy of the water
molecules. The amount of heat needed to convert the solid to liquid at the melt-
ing point at constant pressure is called the
heat of fusion (
fus
H), which for water
is 334 J/g, or 6.01 kJ/mol. Melting is an endothermic process, which means that
freezing (the opposite process) is an exothermic process. Heat is released when a
liquid freezes.
How much heat is needed to melt 10.0 kg of ice? The calculation shown below
indicates that we need to add 3340 kJ to the system.
q = mass × ∆
fus
H
10,000 g ice ×
334 J
gice
= 3,340,000 J = 3340 kJ
This is about the amount of heat supplied by burning about 60 g of methane in
air.
Change 3: Heating the Liquid
The heat going into the (pure liquid) water increases molecular motion, raising
the temperature of the liquid (Figure 11.16). The heat needed to raise the tem-
perature of our 10.0-kg water sample from 0.0°C to 100.0°C can be calculated
using the specific heat of water, 4.184 J/g·°C.
q = specific heat × mass × change in temperature
4.184J
g·
◦
C
× 10,000 g × (100.0°C − 0.0°C)
= 4,180,000 J = 4180 kJ
The total of 4180 kJ can be supplied by about 75 g of methane burning in air.
Change 4: The Boiling Point
As we continue to heat the water, it boils as the liquid is converted to vapor at
constant temperature (Figure 11.16). Analogous to the heat of fusion for melting
is the
heat of vaporization (
vap
H) for converting the liquid water to vapor at the
normal boiling point. The value of
2.44 kJ
g water
is over 7 times that of the heat of
fusion
0.334 kJ
gice
. This indicates that a lot more energy is required to overcome
11.3 Impact of Intermolecular Forces on the Physical Properties of Water, I 457
FIGURE 11.16
As water is heated, it goes through changes from solid
to liquid to gas. Why are some regions sloped and oth-
ers flat? Why are areas of constant temperature of
different lengths?
Visualization: Changes of State
Video Lesson: CIA
Demonstration: Boiling Water in
a Paper Cup
