Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

478 Chapter 11 The Chemistry of Water and the Nature of Liquids
Freezing-Point Depression
The same antifreeze solution that raises the boiling point does double duty,
because it also lowers the freezing point by about 18°C. This
freezing-point
depression
is another of the colligative properties and, like boiling point eleva-
tion, is approximately proportional to the molal concentration of the solute:
T
f
= iK
f
m
where T
f
= the change in freezing point in
◦
C
K
f
= the freezing-point depression constant, which depends on the solvent,
in units of °C/m
m = the molality of the solute in moles of solute per kilogram of solvent
i = the van’t Hoff factor
Raoult himself, in 1883, was the first to note that the lowering of the freezing
point was the same for any nonelectrolyte solute in a given solvent. Table 11.8 lists
freezing-point depression constants for several liquids. Water has a relatively low
value of K
f
; freezing-point depressions are much greater in other solvents. His-
torically, the freezing-point depression was used to determine the molar masses
of substances.
Why are freezing points depressed whereas boiling points are elevated? As
with all colligative properties, the key is the vapor pressure. In Figure 11.33, the
solution has a lower vapor pressure than the pure solvent. When the solvent
freezes, its vapor pressure must be lowered to equal that of the solution. In order
to reach that vapor pressure, the solution must be cooled below the freezing point
of the pure solvent. The result is a new freezing point that is lower than the freez-
ing point of the solvent.
One important consequence of freezing-point depression is the need to keep
food freezers well below 0
◦
C, because the food within contains all manner of
solutes, especially various salts and sugars, so the freezing points of products such
as meats and ice cream are significantly less than 0
◦
C.
Freezing-Point Depression
Constants for Several
Compounds
Solvent K
f
(
◦
C/m) T
f
(°C)
Benzene 5.1 5.5
Cyclohexane 20.0 6.5
Formic acid 2.8 8.4
Naphthalene 6.9 80.0
Phenol 7.4 43.0
Water 1.86 0
Pure solvent Solvent with
nonvolatile solute
FIGURE 11.33
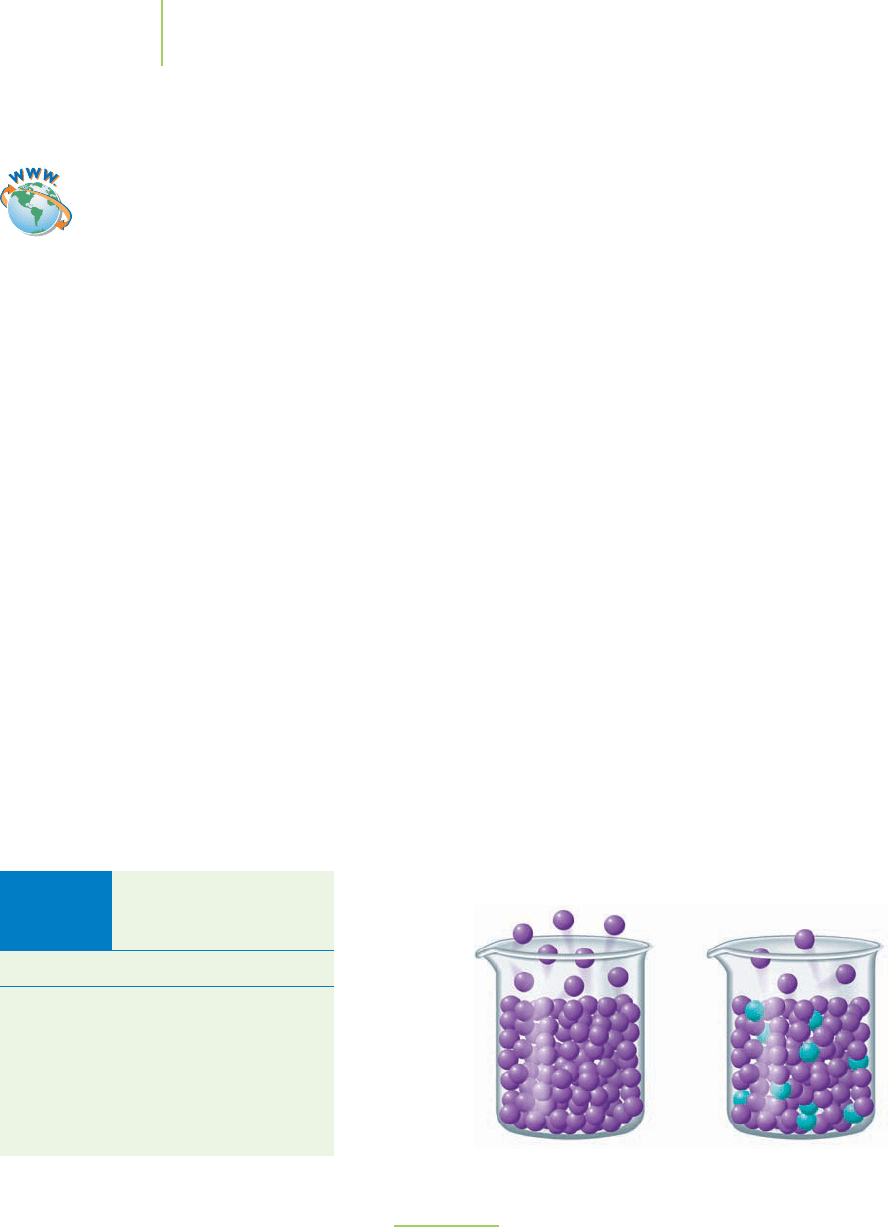
Note the differences between the two liquids in this figure. The more concentrated
solution on the right has a lower vapor pressure. We can understand this by spray-
ing a little perfume at the front of a room. In a short time, everyone in the room will
smell the perfume. Why is this so? There is a natural tendency for things to become
more dilute. The perfume vapors spread out across the room as they become more
dilute. The same situation occurs in vapor pressure lowering. If solvent molecules left
the container on the right, the solution would become more concentrated, which
would violate the natural tendency of the solution to become more dilute. Therefore,
fewer molecules leave the container on the right.
TABLE 11.8
Visualization: Freezing-Point
Depression: Solid/Liquid
Equilibrium
Visualization: Freezing-Point
Depression: Addition of a Solute
Visualization: Freezing-Point
Depression: Solid/Solution
Equilibrium
11.9 Colligative Properties 479
Osmosis
Our final colligative property is osmosis (from
the Greek osmos, meaning“to thrust”).Recalling the
question with which we opened the chapter (How
do we meet the long-term water needs of rapidly
growing communities?), we now have the payoff of
our discussion.
To answer the question, let’s set up the follow-
ing experiment in the same manner as experiments
that were done as early as the year 1748. A solution
of salt water is placed in a two-chambered con-
tainer separated by a
semipermeable membrane
from a sample of pure water, as shown in Fig-
ure 11.34. The membrane has very small holes in it
so that small water molecules can travel through,
but not larger solute particles. Why won’t small
ions, such as the potassium cation, travel through
the semipermeable membrane? Although the holes
are bigger than a potassium cation (so that the
water molecules can go through), they are smaller
than the entire hydrated ion of potassium. Remem-
ber that solutes dissolved in water contain a sphere
of hydration that surrounds the solute and helps
keep it dissolved. The sphere is typically much
larger than a hole the size of a water molecule.
What will happen in our experiment? Accord-
ing to Raoult’s law, the vapor pressure of the salt-
water solution will be lower than that of the pure
water. In an attempt to equalize the vapor pres-
sures, water will flow through the membrane to the
solution side, diluting it and making the vapor
pressures equal. In fact, water will keep flowing
until the weight of the solution (the solution’s
“hydrostatic pressure”) becomes so great that it
stops the flow (see Figure 11.34). Water can travel
through the membrane in both directions, but the
drive toward the most energetically stable equilib-
rium position makes the overall direction of flow
toward dilution. This process is known as
osmosis.
If we run the experiment again, but this time
apply pressure to the saltwater solution in an
amount that just prevents the osmosis, there will be no net change, as shown in
Figure 11.35. The pressure that must be applied on the solution side to prevent
osmosis of the solvent into it is called the
osmotic pressure, . In 1887, van’t Hoff
determined that osmotic pressure is proportional to the temperature and the
molarity of the solution:
= iMRT
where = osmotic pressure, in atmospheres
M = molarity
T = temperature in kelvins
i = the van’t Hoff factor
R = the universal gas constant, 0.08206 L·atm·mol
−1
·K
−1
Note that this colligative property uses molarity, not molality, to express the con-
centration of solute in the solution. Additionally, the gas constant R is the same
one we introduced in Chapter 10 when we discussed the behavior of gases.
Solute
Pisto
n
Pressure
Semipermeable
membrane
Semipermeable
membrane
Flow
Solute
Solvent
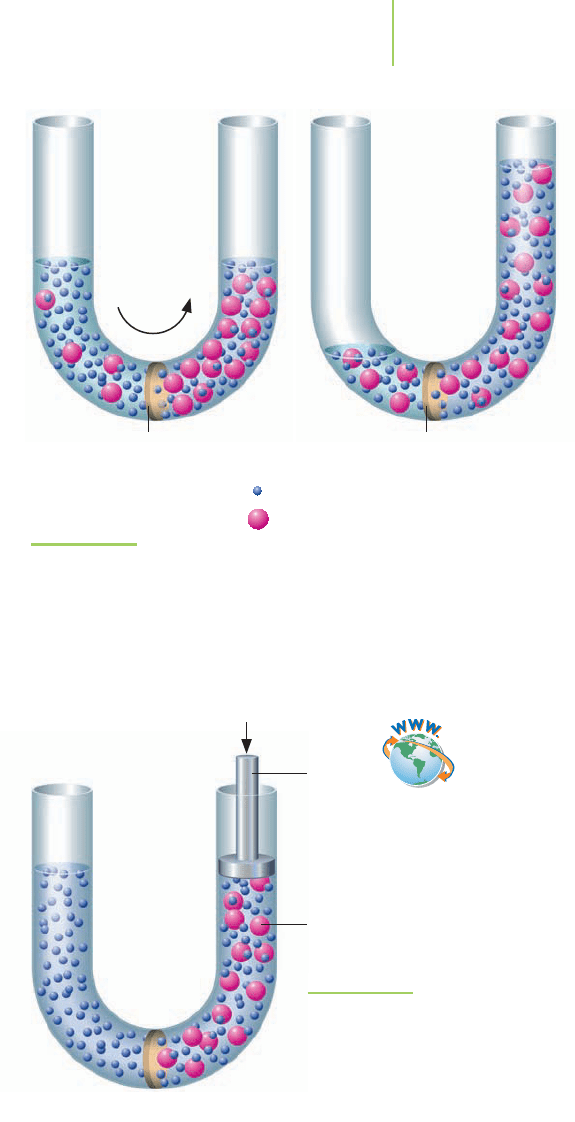
FIGURE 11.34
Salt water and water are separated by a semipermeable membrane. Water
molecules pass through, with more going from the purer water (on the
left) to the more concentrated solution (on the right) than the other way,
therefore raising the solution level. The pressure necessary to prevent this
shift in solution level via osmosis is called osmotic pressure.
FIGURE 11.35
Osmosis is prevented by a piston
applying just enough pressure,
called osmotic pressure, to the
solution.
Video Lesson:
Osmosis
Visualization:
Osmosis

The original measurements of osmotic pressure used animal membranes,
with limited success. More reliable membranes were developed in the 1860s, in-
corporating a film of copper(II) ferrocyanide, Cu
2
Fe(CN)
6
, on a porous tube, and
for decades, many quantitative measurements up to several hundred atmos-
pheres were made in this way. As with our other colligative properties, we are
mindful that the osmotic pressure equation holds well only for very dilute solu-
tions. But with a dilute solution, one can measure the molar mass of very large
polymers via the osmotic pressure experiment. Descriptive applications of this
process are also very meaningful.
One example relates to red blood cells, which have a salt concentration equal
to their surrounding fluid (which identifies the solutions as
isotonic). If an intra-
venous solution given to a patient has a higher solute concentration than blood
plasma (and is called a
hypertonic solution), water will leave the blood cell mem-
branes by osmosis. Hypertonic solutions are sometimes intentionally adminis-
tered to patients when they have too much water in their blood plasma, such as in
water intoxication, liver disease, or congestive heart failure. If the blood plasma is
too concentrated, as happens in severe diarrhea or excess sweating, a
hypotonic
solution (a solution that has a lower solute concentration than blood plasma) is
needed so that water will flow into the cells. Many intravenous solutions given to
patients in hospitals are isotonic with blood serum. Because these isotonic solu-
tions have the same solute concentration as blood serum, they will not cause cells
in the patient’s body to shrink or swell. A solution
isosmotic with blood plasma
(same osmotic pressure) is 0.154 M each in Na
+
and Cl
−
ions.
EXERCISE 11.13 Osmosis and You
What is the osmotic pressure of a solution at 25
◦
C that is isosmotic with blood
plasma, 0.154 M NaCl?
Solution
Sodium chloride dissociates into two ions, Na
+
and Cl
−
, when dissolved in water.
That means that the van’t Hoff factor for this solution should be equal to 2.0.
= iMRT
= 2.0 × 0.154 M × 0.08206 L·atm·mol
−1
·K
−1
× 298 K
= 7.532 atm = 7.53 atm
Osmosis is a fairly powerful property of solutions. If we placed this solution in an
osmotic pressure apparatus with pure water, we would need 7.53 atm (110 psi) of
pressure just to stop the osmosis.
PRACTICE 11.13
What is the osmotic pressure of each of the these solutions at 298 K?
a. 0.100 M sucrose b. 0.375 M CaCl
2
c. 1.250 M (NH
4
)
2
SO
4
See Problems 94, 101, and 102.
Reverse Osmosis
What would happen if our osmosis experiment were run in reverse? Instead of using
osmotic pressure merely to prevent the natural flow from the water to the saltwa-
ter side, let’s apply additional pressure to actually push water from the saltwater
solution through the semipermeable membrane, in opposition to the natural
tendency toward dilution by osmosis. What will happen? The solution is losing
solvent, so it will become more concentrated (saltier). Water is pushed through
the membrane, so the amount of pure water increases. We have used
reverse
osmosis
to desalinate water, producing more fresh water.
480 Chapter 11 The Chemistry of Water and the Nature of Liquids
Many different ions are dissolved in
blood serum, the light yellow solution
that can be isolated by centrifuging
whole blood.
Application
Application
C
HEMICAL
ENCOUNTERS:
Meeting
Municipal
Water Needs
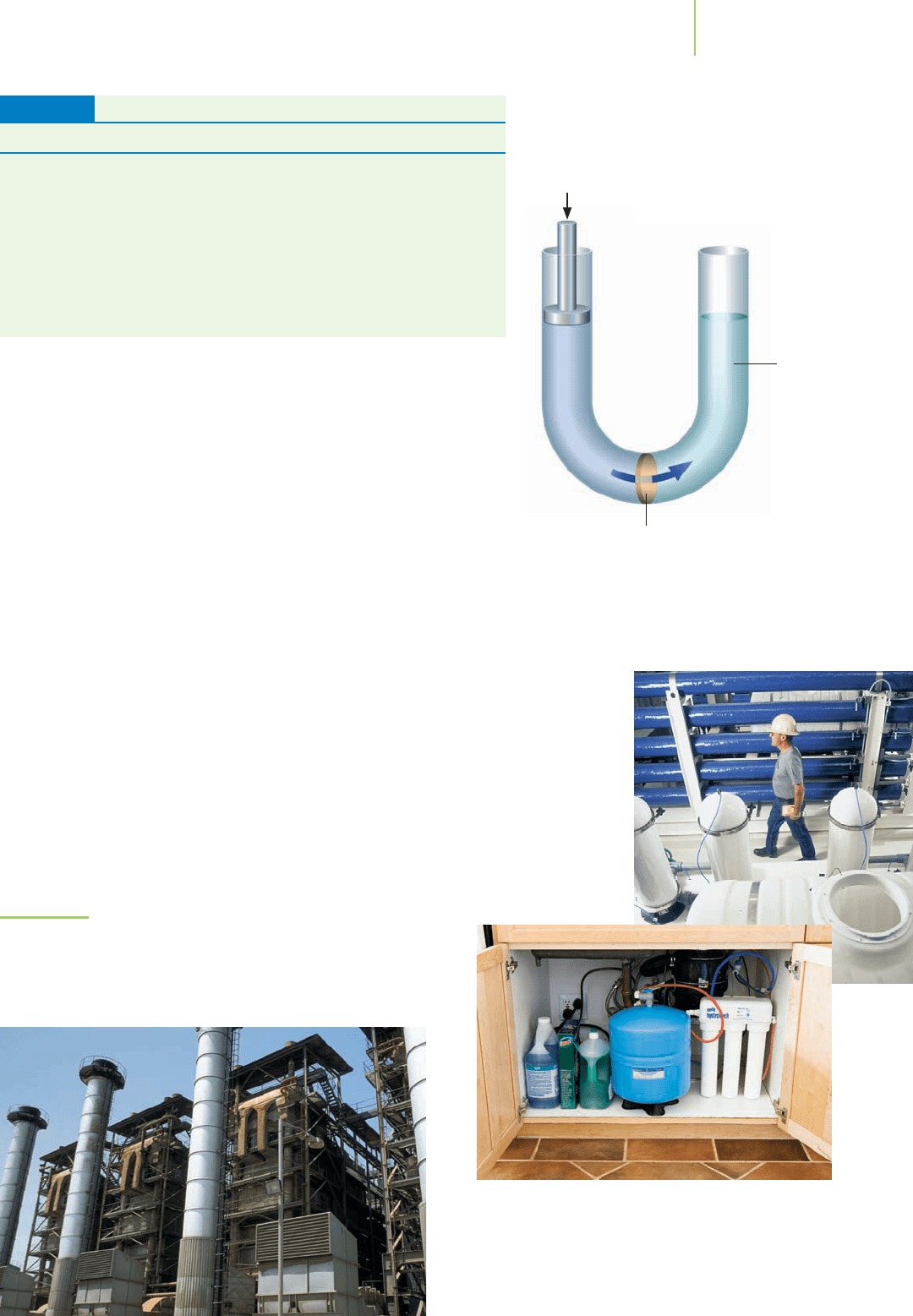
FIGURE 11.36
Reverse osmosis units can filter up to 120,000 gal of salt water per day.
Such units are likely to play an increasingly important role in supplying
growing communities with fresh water. This plant in Saudi Arabia con-
tains many reverse osmosis units and produces 210 million gal of fresh
water each day for the kingdom’s capital city of Riyadh.
Back to the Future
After quite a long discussion on many aspects of water as a liquid and a
solvent, we now answer the third and final question with which we
started the chapter: “How can we use these properties to help in the
search for clean water in places like Tampa Bay?” An important part of
the answer is “by reverse osmosis.” This will help meet Tampa Bay’s bur-
geoning water demand by supplying nearly 25 million gallons per day
(mgd) of desalinated water, according to the “Master Water Plan” ap-
proved by the Tampa Bay Water Authority. The desalinating plant
opened in January 2003, although its operation has been sporadic and
not without controversy, including the debated hazard of putting water with a
relatively high salt concentration back into Tampa Bay. (Food for thought:
Will it
harm the ecosystem?
) To date, other U.S. municipal desalination plants have been
used only in water emergencies. They include a 2-mgd plant in Key West, Florida;
a 1-mgd facility on Marathon Key, Florida; and a 6.7-mgd plant in Santa Barbara,
California.
But the process doesn’t have to be used on such a large scale in order to be
useful. Reverse osmosis units find applications in manufacturing, food process-
ing, printing, and even car washes, as shown in Table 11.9. The use of long-lasting
nylon or polyamide semipermeable membranes gives the factories, such as the
one shown in Figure 11.36, the ability to filter up to 120,000 gals per day. Usable
water, this precious resource, is becoming ever more scarce. It is through our un-
derstanding of its structure and its properties that we can create safe, long-term
solutions that will keep fresh water flowing.
11.9 Colligative Properties 481
Water flows through
semipermeable membrane
Apply force
to saltwater
Quantity of
pure water
increases
If a force greater than the
osmotic pressure is applied
to the saltwater, pure water will
flow into the side containing
the pure water. The saltwater
will become more concentrated.
Drinking water can be purified by reverse osmosis.
Applications of Reverse Osmosis
Industry Application
Cosmetics Product preparation
Desalination Potable water, beverage preparation
Drinking water Mineral removal
Electronics Water low in impurities for manufacturing processes
Food Low sodium and organic chemicals in food preparation
Laboratories Rinsing glassware
Pharmaceutical Pure water for large-scale production
Restaurants Spot-free rinses, drinking water
TABLE 11.9
A technician inspects the reverse
osmosis equipment at a desalina-
tion plant in Catalina, California.
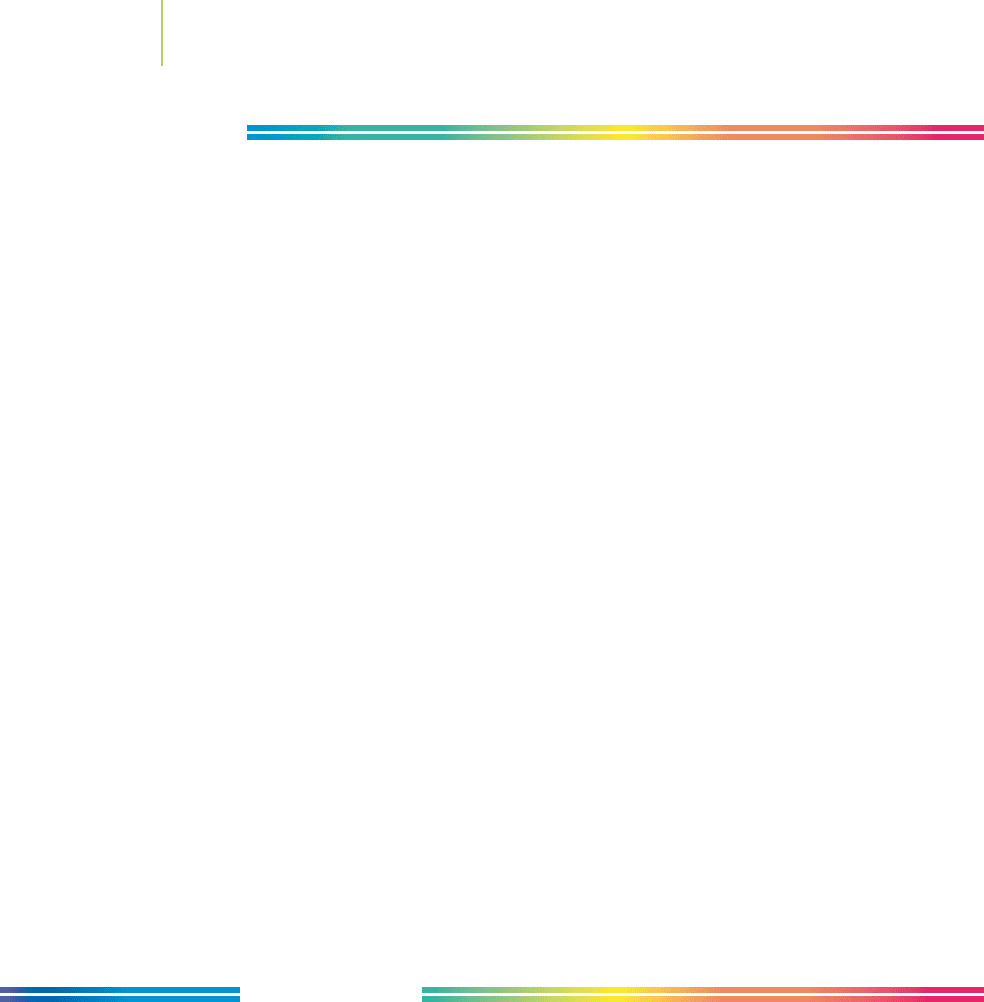
482 Chapter 11 The Chemistry of Water and the Nature of Liquids
The Bottom Line
■
Water and other liquids have in common intermol-
ecular forces called van der Waals forces that hold
their molecules together. These forces are weaker
than covalent or ionic bonds. (Section 11.2)
■
Among these forces is dipole–dipole interactions.
(Section 11.2)
■
A hydrogen bond is an especially strong interaction
that occurs when a hydrogen attached to an oxygen,
nitrogen, or fluorine atom is close to a different
atom of oxygen, nitrogen, or fluorine. (Section 11.2)
■
Weaker, but collectively important intermolecular
interactions in large molecules are known as
London forces. (Section 11.2)
■
Water is special among liquids because of intermol-
ecular hydrogen bonds. (Section 11.3)
■
Intermolecular interactions can be used to explain
many physical properties of water, including its vis-
cosity, surface tension, and capillary action. (Sec-
tion 11.5)
■
The pressure exerted by the evaporation of a liquid
in equilibrium with its surroundings is called its
vapor pressure. The boiling point is reached when
the vapor pressure is equal to the surrounding pres-
sure. (Section 11.3)
■
A heating curve describes the changes in phase and
temperature that occur as a substance is heated at
constant pressure. (Section 11.3)
■
A phase diagram describes the changes in phase as
pressure and temperature are changed. (Section
11.4)
■
Water is known as the universal solvent because of
its ability to form solutions with many substances.
(Section 11.6)
■
Solution formation can be understood in terms of
specific types of energy changes. (Section 11.6)
■
Solution concentration can be expressed in a vari-
ety of units, including molarity, molality, and parts
per million. (Section 11.7)
■
Raoult’s law describes the change in vapor pressure
of a solvent as solute is added to a solution. (Sec-
tion 11.9)
■
Solubility is affected by pressure and temperature
in, as a first approximation, predictable ways. (Sec-
tion 11.8)
■
Colligative properties are based on the number of
particles in a solution and can be understood in
terms of Raoult’s law. (Section 11.9)
■
Colligative properties include vapor pressure lower-
ing, boiling-point elevation, freezing-point depres-
sion, and osmosis. (Section 11.9)
■
It is possible that reverse osmosis will become a
vital process for supplying clean water to large
cities. (Section 11.9)
Key Words
adhesion The interaction of a compound with the sur-
face of another compound, such as the interaction
of water with the surface of a drinking glass.
(p. 463)
aqueous solution A homogeneous mixture of a solute
dissolved in the solvent water. (p. 465)
boiling The process that occurs when the vapor pres-
sure of a liquid is equal to the external vapor
pressure. (p. 455)
boiling point The temperature at which the pressure of
a liquid’s vapor is equal to the surrounding pressure.
(p. 455)
boiling-point elevation The increase in the boiling point
due to the presence of a dissolved solute. (p. 475)
buret A piece of laboratory glassware used to measure
volume and to add discrete amounts of solution in a
repetitive fashion. (p. 463)
capillary action The effect seen when a liquid rises
within a narrow tube as a consequence of adhesive
forces being stronger than cohesive forces. (p. 463)
cohesive forces The collection of intermolecular forces
that hold a compound within its particular phase.
(p. 463)
colligative properties Physical properties of a solution
that depend only on the amount of the solute and
not on its identity, including boiling-point elevation,
freezing-point depression, vapor pressure lowering,
and osmotic pressure. (p. 474)
condensation The process of a gas undergoing a phase
transition to a liquid; the opposite of evaporation.
(p. 452)
cooling tower A large industrial apparatus used to con-
dense gaseous vapor into liquid or to cool water that
has been heated before it is discharged. (p. 471)

Key Words 483
critical point The temperature and pressure at which
the physical properties of the liquid phase and vapor
phase of a substance become indistinguishable.
(p. 460)
critical pressure The highest pressure at which a liquid
and gas coexist in equilibrium. (p. 460)
critical temperature The highest temperature at which a
liquid and a gas coexist in equilibrium. (p. 460)
deposition The process of a gas undergoing a phase
transition to a solid; the opposite of sublimation.
(p. 452)
dynamic equilibrium A term sometimes used in chem-
istry as synonymous with equilibrium to emphasize
that molecular-level equilibrium is not static.
(p. 453)
equilibrium In a reaction or process, a condition
wherein the rates of the forward and backward reac-
tions are equal. The amounts of the reactants and
products do not change, but the forward and back-
ward reactions are still proceeding. (p. 453)
equilibrium vapor pressure The pressure exerted by the
vapor of a liquid or solid under equilibrium condi-
tions. (p. 453)
evaporation The process of a liquid undergoing a
phase transition to a gas; the opposite of condensa-
tion. (p. 452)
freezing-point depression The lowering of the melting
point of a compound due to the presence of a dis-
solved solute. (p. 478)
gas The phase of a substance characterized by widely
spaced components exhibiting low density, ease of
flow, and the ability to occupy an enclosed space in
its entirety. (p. 443)
heat of fusion (∆
fus
H) The enthalpy change associated
with the phase transition from liquid to solid.
(p. 457)
heat of solution (∆
sol
H) The enthalpy change associated
with the dissolution of a solute into a solvent.
(p. 466)
heat of vaporization (∆
vap
H) The enthalpy change asso-
ciated with the phase transition from a liquid to a
gas. (p. 457)
heating curve A plot of the temperature of a com-
pound versus time or energy as heat is added at
constant pressure. The plot indicates the specific
temperature ranges for solid, liquid, and gas phases.
(p. 456)
Henry’s law The solubility of a gas is directly propor-
tional to the pressure that the gas exerts above the
solution. (p. 472)
hydration The interaction of water (as the solvent)
with dissolved ions. (p. 466)
hydrogen bond A particularly strong intermolecular
force of attraction between F, O, and/or N and a
hydrogen atom. (p. 448)
hypertonic Containing a concentration of ions greater
than that to which it is judged against. (p. 480)
hypotonic Containing a concentration of ions lower
than that to which it is judged against. (p. 480)
ideal solution A solution in which the properties of the
solute and solvent are not changed by dilution. This
means that other than being diluted, combining
solute and solvent in an ideal solution does not
release or absorb heat, and the total volume in the
solution is the sum of the volumes of the solute and
solvent. (p. 474)
induced dipole A dipole produced in a compound as a
consequence of its interaction with an adjacent
dipole. (p. 446)
intermolecular force A force of attraction between two
molecules. (p. 444)
ion–dipole interaction An attraction between an ion and
a compound with a dipole. (p. 466)
isotonic Containing the same concentration of ions.
(p. 480)
isosmotic Possessing the same osmotic pressure.
(p. 480)
liquid A phase of a substance characterized by closely
held components. The properties of a liquid include
a medium density, the ability to flow, and the ability
to take the shape of the container that holds it by
filling from the bottom up. (p. 443)
London forces The weakest of the intermolecular forces
of attraction, characterized by the interaction of
induced dipoles. (p. 446)
maximum contaminant level (MCL) The highest acceptable
level of a contaminant in a particular solution, ac-
cording to the Environmental Protection Agency.
(p. 470)
melting point The temperature of the phase transition
as a compound changes from a solid to liquid.
(p. 456)
meniscus The concave or convex shape assumed by the
surface of a liquid as it interacts with its container.
(p. 464)
miscible Mixable. Two solvents that are miscible dis-
solve in each other completely. (p. 466)
molality A concentration measure defined as moles of
solute per kilogram of solvent. (p. 468)
molarity A concentration measure defined as moles of
solute per liter of solution. (p. 468)
mole fraction A concentration measure defined as
moles of solute per total moles of solution. (p. 468)
natural gas A complex mixture of gases extracted from
the Earth. The major component in many cases is
methane. (p. 443)
normal boiling point The temperature at which a liquid
boils when the pressure is 1 atm. (p. 455)
osmosis The flow of solvent into a solution through a
semipermeable membrane. (p. 479)

osmotic pressure The pressure required to prevent the
flow of a solvent into a solution through a semiper-
meable membrane. (p. 479)
parts per billion A concentration defined as the mass of
a solute per billion times that mass of solution.
(p. 470)
parts per million A concentration defined as the mass of
a solute per million times that mass of solution.
(p. 470)
parts per trillion A concentration defined as the mass of
a solute per trillion times that mass of solution.
(p. 470)
phase boundary On a phase diagram, a set of specific
temperatures and pressures at which a compound
undergoes a phase transition. (p. 460)
phase diagram A plot of the phases of a compound as
a function of temperature and pressure. (p. 459)
polarizability The extent to which electrons can be
shifted in their location by an electric field. (p. 446)
pressure The force exerted per unit area by a gas
within a closed system. (p. 452)
Raoult’s law Describes the change in vapor pressure of
a solvent as solute is added to a solution. (p. 474)
reverse osmosis A purification process wherein pure
solvent is forced (with pressure) to flow through a
semipermeable membrane away from a solution.
(p. 480)
saturated When considering a pure substance—the
surrounding atmosphere contains the maximum
possible amount of vapor, expressed as the vapor
pressure. When considering a solution—the solution
contains the maximum concentration of dissolved
solute. (p. 452, 467)
semipermeable membrane A thin membrane (typically
made from a polymer) with pores big enough to
allow solvent to pass through, but small enough
to restrict the flow of dissolved solute particles.
(p. 479)
solid A phase of a substance characterized by high
density, rigid shape, and the inability to flow.
(p. 443)
484 Chapter 11 The Chemistry of Water and the Nature of Liquids
solubility A property of a solute; the mass of a solute
that can dissolve in a given volume (typically
100 mL) of solvent. (p. 467)
solute The minor component in a solution. (p. 465)
solution A homogeneous mixture of two or more sub-
stances. (p. 465)
solvation The interaction of a solvent with its dissolved
solute. (p. 466)
specific heat The heat needed to warm 1 g of a sub-
stance so that its temperature rises 1.0
◦
C. (p. 456)
sublimation The process of a solid undergoing a phase
transition to a gas; the opposite of deposition.
(p. 452)
supercritical fluid This phase is seen only when the pres-
sure and temperature of the substance are greater
than the critical pressure and critical temperature. It
is characterized with a density midway between that
of a liquid and a gas, a viscosity similar to a gas, and
the ability to completely fill the volume of a closed
container. (p. 460)
surface tension A measure of the energy per unit area
on the surface of a liquid. (p. 494)
thermal pollution An artificial increase in the tempera-
ture of water. (p. 471)
triple point The temperature and pressure at which the
gas, liquid, and solid phases of a substance are in
equilibrium. (p. 460)
universal solvent Water is often called the universal sol-
vent because of its ability to dissolve so many chem-
icals to form homogeneous mixtures. (p. 465)
van der Waals forces The intermolecular forces of
attraction that result in associations of adjacent sub-
stances. (p. 445)
van’t Hoff factor A factor that modifies the colligative
properties on the basis of their ability to dissociate
in solution. (p. 476)
vapor pressure The pressure of vapor above a liquid in
a closed system. (p. 453)
viscosity A measure of resistance to flow. (p. 494)
weight percent A concentration unit based on the mass
of the solute per total mass of solution, reported in
percent. (p. 470)
Focus Your Learning
The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
Section 11.1 The Structure of Water:
An Introduction to Intermolecular Forces
Skill Review
1. Describe the differences between intermolecular and intra-
molecular forces.
2. Describe the similarities between intermolecular and in-
tramolecular forces.
3. Explain how intermolecular forces between molecules arise.
4. How is the strength of an intermolecular force related to the
phase of matter?
5. Using electronegativity differences, rank these bonds from
least to most polar:
O—H C—H S—H N—H
6. Using electronegativity differences, rank these bonds from
least to most polar:
O—C Cl—C F—H P—O
7. In each of these statements, insert the term intermolecular or
intramolecular to describe the process taking place.
a. Water freezes when attractions are forming.
b. The production of carbon and sulfur from CS
2
indicates
that bonds have been broken.
c. When dry ice sublimes, bonds are broken.
d. In general, bonds are stronger than
bonds.
8. The terms polar and polarizable have both been used in dis-
cussions of intermolecular forces.
a. Distinguish between these two important terms.
b. Which of these contains the more polar bond, CO or NO?
c. Which of these is the more polarizable atom, He or Ne?
Chemical Applications and Practices
9. In a sample of water there are two types of hydrogen-to-
oxygen attractions taking place. In one attraction the average
distance between the hydrogen and oxygen is 0.101 nm. In
the other, the distance is 0.175 nm.Which distance represents
an intermolecular attraction, and which represents an in-
tramolecular attraction? Which of the two is the stronger
attraction?
10. Carbon dioxide is generated as a result of metabolism and as
a result of the combustion of carbon-based materials. This
substance is a gas under “normal” conditions. Explain why
this is so.
Section 11.2 A Closer Look at Intermolecular Forces
Skill Review
11. In each of these pairs of organic compounds, select the one
that would have the higher boiling point. Then, using inter-
molecular forces, describe the basis for your selection.
a. Pentane or 2,2-dimethylpropane
b. Hexane or cyclohexane
c. Pentane or hexane
d. Pentane or water
e. Pentane or methane
13. In these pairs, compare the polarity of the bonds shown.
Decide which bond in each pair is more polar, and show in
which direction the electron density would be shifted.
a. B—O and B—S
b. P—S and P—Cl
c. O—H and O—C
14. In these pairs, compare the polarity of the bonds shown.
Decide which bond in each pair is more polar, and show in
which direction the electron density would be shifted.
a. C—O and C—S
b. N—S and N—H
c. C—H and C—Cl
15. Name the type of intermolecular forces that must be over-
come in order to change each of these from the liquid state to
the gas state.
a. CH
3
CH
2
OH c. SF
6
b. CO
2
d. HF
16. Name the type of intermolecular forces that must be over-
come in order to change each of these from the liquid state to
the gas state.
a. CH
3
OH c. CCl
4
b. CS
2
d. COS
17. Select the molecule in each pair that is less polarizable.
a. CF
4
or CCl
4
b. H
2
O or H
2
Se
18. Select the molecule in each pair that is less polarizable.
a. HI or HF
b. CH
4
or COS
19. Which of these compounds are likely to participate in hydro-
gen bonding? For those that do not qualify, explain what is
lacking.
a. CH
3
OH (methanol)
b. NH
3
(ammonia)
c. C
6
H
6
(benzene)
d. C
2
H
5
OC
2
H
5
(diethyl ether) Diethyl ether
20. Which of these compounds are likely to participate in hydro-
gen bonding? For those that do not qualify, explain what is
lacking.
a. H
2
S c. B
2
H
6
b. NCl
3
d. CH
3
NH
2
Chemical Applications and Practices
21. “Wake up and smell the coffee!” is a phrase that you may
have used to catch someone’s attention. Perhaps less com-
pelling, but more accurate, would be “Wake up and smell the
2-methylfuran.” This compound is one of several that are
typically responsible for the aroma of coffee. Its structure is
shown here.
a. Which bond in the molecule do you think is the most
polar?
b. What intermolecular forces exist between the molecules of
2-methylfuran?
HC
HC
CH
CH
3
C
O
CH
2
CH
3
CH
2
CH
3
O
O
Focus Your Learning 485
Pentane
CH
2
CH
3
CH
2
CH
2
CH
3
Hexane
CH
2
CH
3
CH
2
CH
2
CH
3
CH
2
Cyclohexane
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
2,2-Dimethylpropane
CH
3
C
CH
3
CH
3
CH
3
12. In each of these pairs of organic compounds, select the one
that would have the higher boiling point. Then, using inter-
molecular forces, describe the basis for your selection.
a. Methanol (CH
3
OH) or ethanol (CH
3
CH
2
OH)
b. Water or hydrogen sulfide
c. Pentane or ethanol
d. Hydrogen sulfide or hydrogen fluoride
e. Ammonia (NH
3
) or methanol
200 210 220 230 240 250 260 270
1.0
2.0
3.0
4.0
5.0
Temperature (K)
Pressure (atm)
486 Chapter 11 The Chemistry of Water and the Nature of Liquids
22. Answer these questions on the basis of your answer to Prob-
lem 21.
a. Would you predict that this compound would have a high
or a low normal boiling point?
b. Would you predict that this compound would be a solid, a
liquid, or a gas at 1 atm and 25
◦
C? Explain.
23. Suppose a small lab accident involved spilling the same
amounts of hexane and hexanol. Which compound would
you expect to evaporate first? Using intermolecular forces,
explain the rationale behind your answer.
24. Would you predict a cup of water or a cup of Everclear (pure
ethanol) to evaporate more rapidly? Using intermolecular
forces, explain the rationale behind your answer.
25. Under the proper conditions, London forces are sufficient to
liquefy the noble gases. Of Ne, Ar, and Kr, which would pos-
sess the stronger London forces? Explain the basis of your
selection.
26. Which of these compounds would you predict to have the
greatest intermolecular forces, F
2
,Cl
2
,Br
2
,or I
2
?
27. The Group V elements form compounds with hydrogen as
follows:
NH
3
PH
3
AsH
3
SbH
3
BiH
3
Boiling point: −33
◦
C −88
◦
C −63
◦
C −17
◦
C16
o
C
a. Temporarily excluding NH
3
, explain the general trend
shown here.
b. Explain why NH
3
deviates from the general trend.
28. HF and H
2
O are both compounds that exhibit hydrogen
bonding. The hydrogen bonding that takes place between HF
molecules is stronger than those in H
2
O, yet H
2
O has the
higher boiling point. Explain why.
Section 11.3 Impact of Intermolecular
Forces on the Physical Properties of Water, I
Skill Review
29. Explain why the heat of vaporization of water is so much
larger than the heat of vaporization of methane.
30. Would you expect the heat of vaporization of CO to be closer
to that of water or of CH
4
? Explain.
Chemical Applications and Practices
31. Water’s relatively high specific heat (4.184 J/g
◦
C) makes it an
ideal coolant for automobile engines. How much heat from
an engine would be absorbed if 12.0 kg of cooling system
water were heated from 25
◦
C to 75
◦
C?
32. Ethanol (C
2
H
5
OH) is the alcohol found in intoxicating
beverages. It has a heat of vaporization of approximately
39.0 kJ/mol. How many joules would be released when 42.0 g
of ethanol vapor condensed into the liquid phase?
Hexanol
CH
2
CH
3
CH
2
CH
2
CH
2
CH
2
OH
Hexane
CH
2
CH
3
CH
2
CH
2
CH
3
CH
2
33. Propane (CH
3
CH
2
CH
3
) is a fuel typically used to operate our
outdoor barbecue grills. This compound is often found in-
side pressurized cylinders.
a. What type of intermolecular forces of attraction would
you predict to exist in propane?
b. Given this information, would you predict propane to
exist as a gas or as a liquid at room temperature and
1.0 atm pressure?
c. Why is propane often found in liquid form (as it is inside
the barbecue grill cylinder)?
34. Chlorine exists, at typical room conditions, as a diatomic gas
(Cl
2
).However,under higher pressures(greater than 250 kPa),
the gas can be converted to a liquid. Chlorine has many uses,
chief of which is to produce bleaching agents.
a. What type of bond exists between two chlorine atoms
in Cl
2
?
b. What type of intermolecular force(s) are responsible for
the gas molecules entering the liquid phase?
35. Oxygen and tellurium both form dihydrogen compounds
(H
2
O and H
2
Te.) Explain why water molecules participate in
hydrogen bonding, whereas H
2
Te does not.
36. a. Commercial rubbing alcohol is typically sold as a 70%
solution of 2-propanol (CH
3
CHOHCH
3
). Explain why,
when it is in contact with your skin, you soon feel a cool-
ing sensation.
b. The heat of vaporization of 2-propanol is approximately
42 kJ/mol. How much heat would be required to evaporate
5.0 g of 2-propanol?
37. Using the values given in the chapter, calculate the amount
of heat needed to change a 15.0-g ice cube at −5.0
◦
C to 15.0 g
of steam at 100.0
◦
C.
38. Using the values given in the chapter, calculate the amount
of heat that is liberated when a 1.0-kg bucket of steam at
100.0
◦
C is cooled to give a 1.0-kg bucket of water at 25.0
◦
C.
Section 11.4 Phase Diagrams
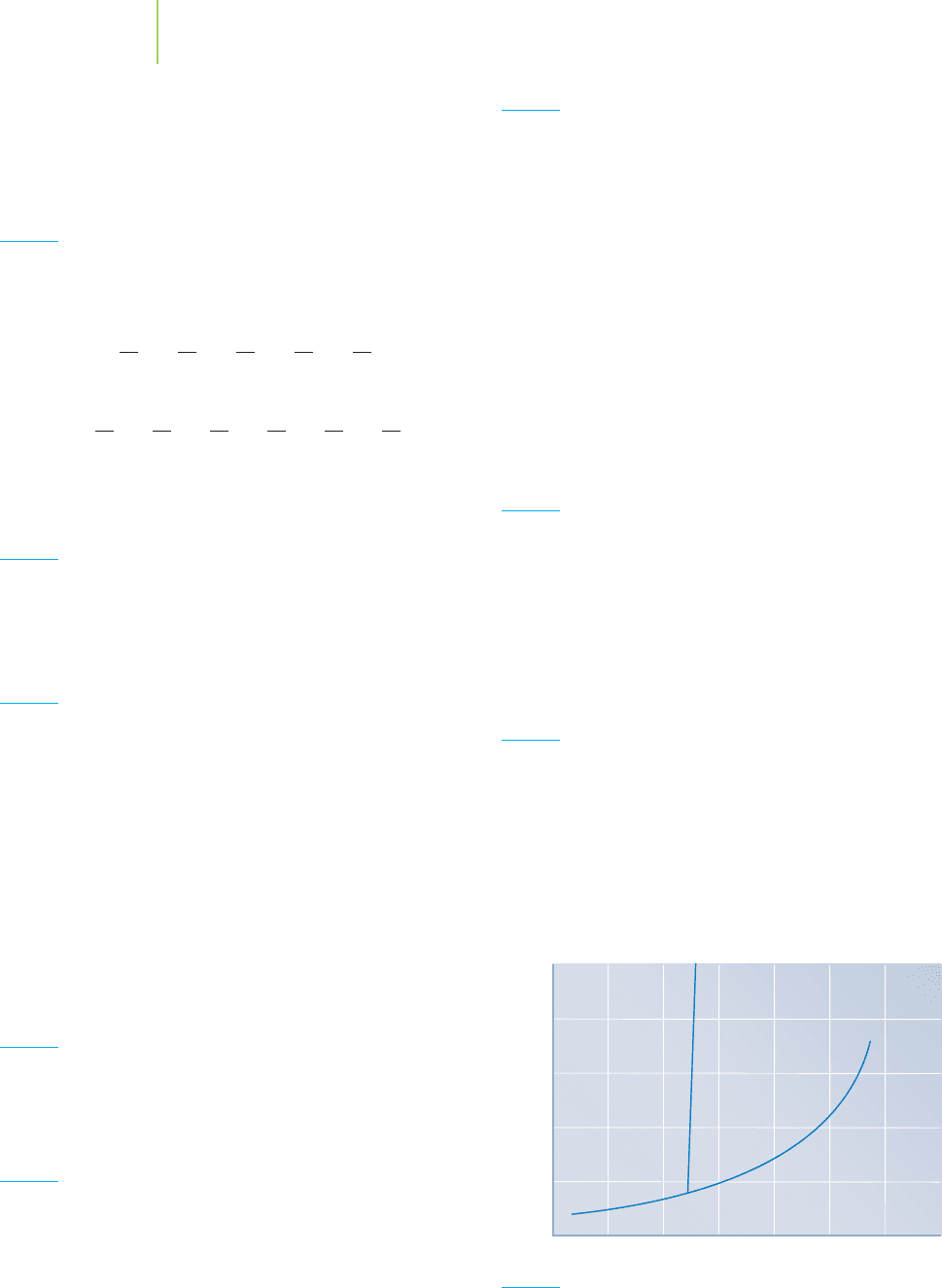
Skill Review
39. Using the phase diagram above, determine the following:
a. The normal boiling point
b. The triple point
c. In what phase would you find the compound at −25
◦
C
and 1 atm?
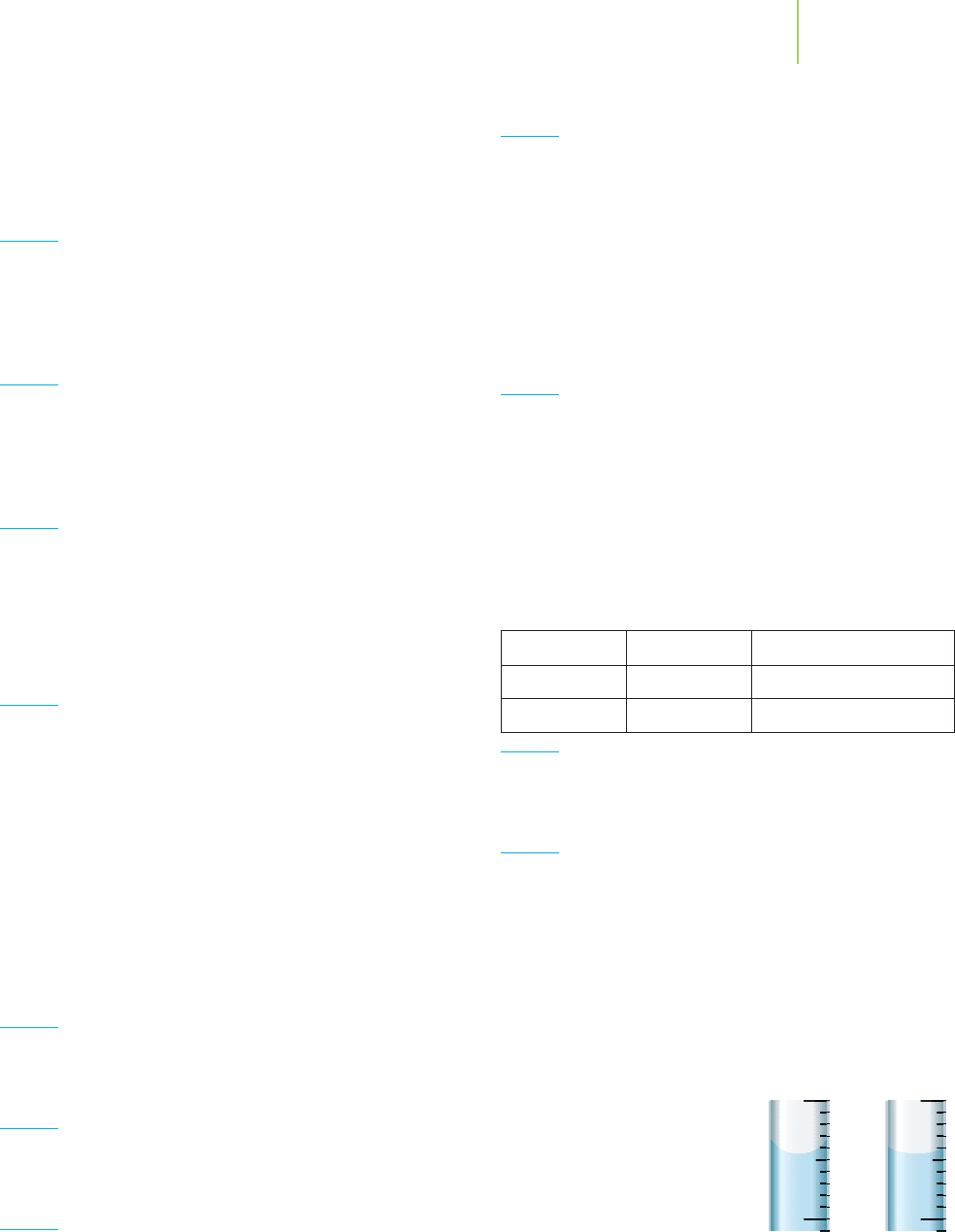
Focus Your Learning 487
40. Using the phase diagram shown on page 486, determine the
following:
a. The critical pressure
b. The critical temperature
c. In what phase would you find the compound at −63
◦
C
and 3 atm?
41. Using the phase diagram shown on page 486, indicate what
phase transition(s) occur as the compound goes from 220 K
and 0.5 atm to 220 K and 3 atm.
42. Using the phase diagram shown on page 486, indicate what
phase transition(s) occur as the compound goes from 220 K
and 1.0 atm to 240 K and 1.0 atm.
43. Using the phase diagram shown on page 486, indicate what
phase change will occur when the compound is heated from
−45
◦
C to −53
◦
C at 1 atm.
44. Using the phase diagram shown on page 486, indicate what
phase change, if any, will occur when the pressure is adjusted
from 2.0 atm to 1.0 atm at constant temperature of −33
◦
C.
45. Using the phase diagram shown on page 486, is it possible to
liquefy a gas sample of this compound at −33
◦
C using in-
creasing pressure? Explain why or why not.
46. Using the phase diagram shown on page 486, is it possible to
form a solid from a liquid sample when the temperature is
kept constant at −53
◦
C? Explain your answer.
Chemical Applications and Practices
47. Ethylene (C
2
H
4
) is one of the most widely used compounds
in the manufacture of synthetic materials. From the follow-
ing data, construct a labeled phase diagram similar to the one
shown for Problems 39–46. The normal boiling point is ap-
proximately −104
◦
C. The critical point, at 50 atm, is approx-
imately 9.8
◦
C. The triple point is −169
◦
C and 0.0012 atm.
The normal melting point is −169
◦
C.
48. Using the diagram you constructed in Problem 47, indicate
the phase in which ethylene would be found at typical room
conditions.
Section 11.5 Impact of Intermolecular
Forces on the Physical Properties of Water, II
Skill Review
49. Explain in your own words the differences among surface
tension, viscosity, and capillary action.
50. Would you predict there to be a relationship among surface
tension, viscosity, and capillary action?
51. Draw a diagram of a meniscus that would form from a buret
filled with mercury.
52. Draw a diagram of a meniscus that would form from a buret
filled with honey.
53. Arrange these substances in order of their increasing viscos-
ity. Explain your ordering.
honey water gasoline
54. Which of these compounds do you predict will rise highest
within a capillary tube: water, hexane, or ethanol? Explain
your prediction.
Chemical Applications and Practices
55. Lubricating motor oils are rated on the basis of their viscos-
ity. The oils in these lubricants are nonpolar molecules. Ex-
plain why these hydrocarbon-based oils have a relatively high
viscosity.
56. Honey has a very high viscosity. It contains (in large part)
many different carbohydrates similar in molecular structure.
What specific atoms would you predict to exist in a carbohy-
drate? Explain your reasoning.
Section 11.6 Water: The Universal Solvent
Skill Review
57. The solubility of NH
4
Cl at 20
◦
C is approximately 39 g per
100 g of water. At 80
◦
C, 68 g per 100 g of water dissolves.
a. Is the value for the enthalpy of solution of NH
4
Cl positive
or negative?
b. Assuming a near linear relationship over the temperature
range, if you saturated a solution at 40
o
C, how many
grams of NH
4
Cl would be dissolved?
58. Organic alcohols have varying solubilities in water. Examine
the following list, and explain the cause of the trend you
observe.
59. Water is often considered the universal solvent. Explain this
designation in your own words.
60. What properties would you associate with a solvent that was
considered to be universal?
61. a. Is it possible for a compound to have a
soln
H = 0?
b. Why would a solute and solvent form a solution if the
value for
soln
H were 0?
62. Cyclohexane (C
6
H
12
) is often used as a nonpolar solvent.
Of the following solutes, which would best dissolve in cyclo-
hexane, and which would best dissolve in water?
NaCl C
6
H
6
CH
3
OH C
6
H
12
O
6
CH
3
(CH
2
)
16
COOH
Salt Benzene Methanol Dextrose Stearic acid
Chemical Applications and Practices
63. Two burets are filled with
different liquids. Which
meniscus is more likely
to be representative of
heptane (C
7
H
16
) and which
of water?
64. If you were to extract caffeine from some cola beans, you
would find that you could extract much more using hot
water than using cold water. Caffeine is more soluble in
hot water than in cold. Explain what this fact tells you about
the heat of solution for caffeine.
1–Butanol C
4
H
9
OH 79 g/1000 mL of water
1–Pentanol C
5
H
11
OH 27 g/1000 mL of water
1–Hexanol C
6
H
13
OH 5.9 g/1000 mL of water
(
a
)(
b
)
