Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

Ozone
O
3
Oxygen gas
O
2
Oxygen atom
O
The reaction of ozone with
UV-B light reduces the intensity
of UV-B at the surface of the Earth.
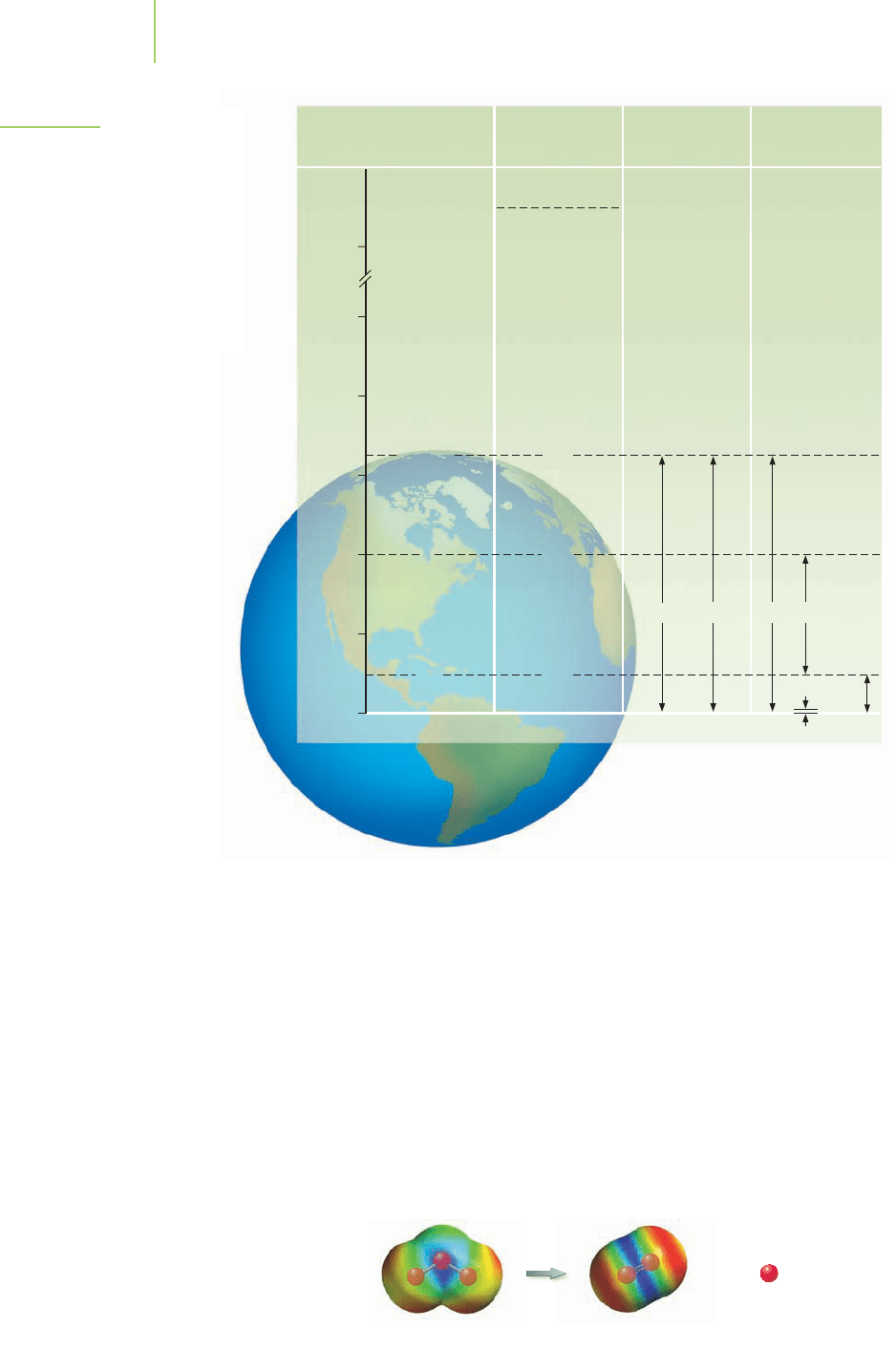
428 Chapter 10 The Behavior and Applications of Gases
Pressure
(torr)
Temperature
(K)
Major
Constituents
Minor
Constituents
125
500
Exosphere
H
He
N
2
, O
2
N
2
O
2
CO
2
O
3
O
3
H
2
O
OO
+
, NO
+
, O
2
+
,
N
2
+
, e
–
H
+
He
+
Thermosphere
180
280
218
1 × 10
–3
2
150
760
Mesosphere
Stratosphere
Troposphere
100
Altitude (km)
75
50
25
0
FIGURE 10.24
Two of the four layers of the
atmosphere contain ozone, O
3
.
band specifies radiation with a wavelength of 400 nm to 320 nm; UV-B indicates
wavelengths of 320 nm to 290 nm; and UV-C, the most energetic, is the classifi-
cation of wavelengths from 290 nm to 100 nm. The destruction of ozone occurs
by
photodissociation—that is, UV light of sufficient energy (UV-B) causes the
molecule to dissociate, forming oxygen gas and atomic oxygen:
UV
O
3
(g)
−→
O
2
(g) + O(g)
There is also a mechanism for the formation of ozone, which also contributes to
the filtering of UV radiation from the sun. Shorter-wavelength UV radiation
(UV-C) provides sufficient energy for the O
2
to separate into oxygen atoms:
UV
O
2
(g)
−→
2O(g)
These highly reactive individual atoms then recombine with O
2
to form ozone
along with the release of heat
O
2
(g) + O(g) n O
3
(g)
The resulting combination of these three equations gives rise to the Chapman
mechanism, a useful description of the chemistry involving oxygen and ozone in
the middle stratosphere.
The natural balance between ozone photodissociation and ozone formation
keeps most harmful UV radiation away from the Earth’s surface. Within the last
30 years, however, activities related to industrialization have led to a significant
reduction in the stratospheric ozone layer over many parts of the planet, as
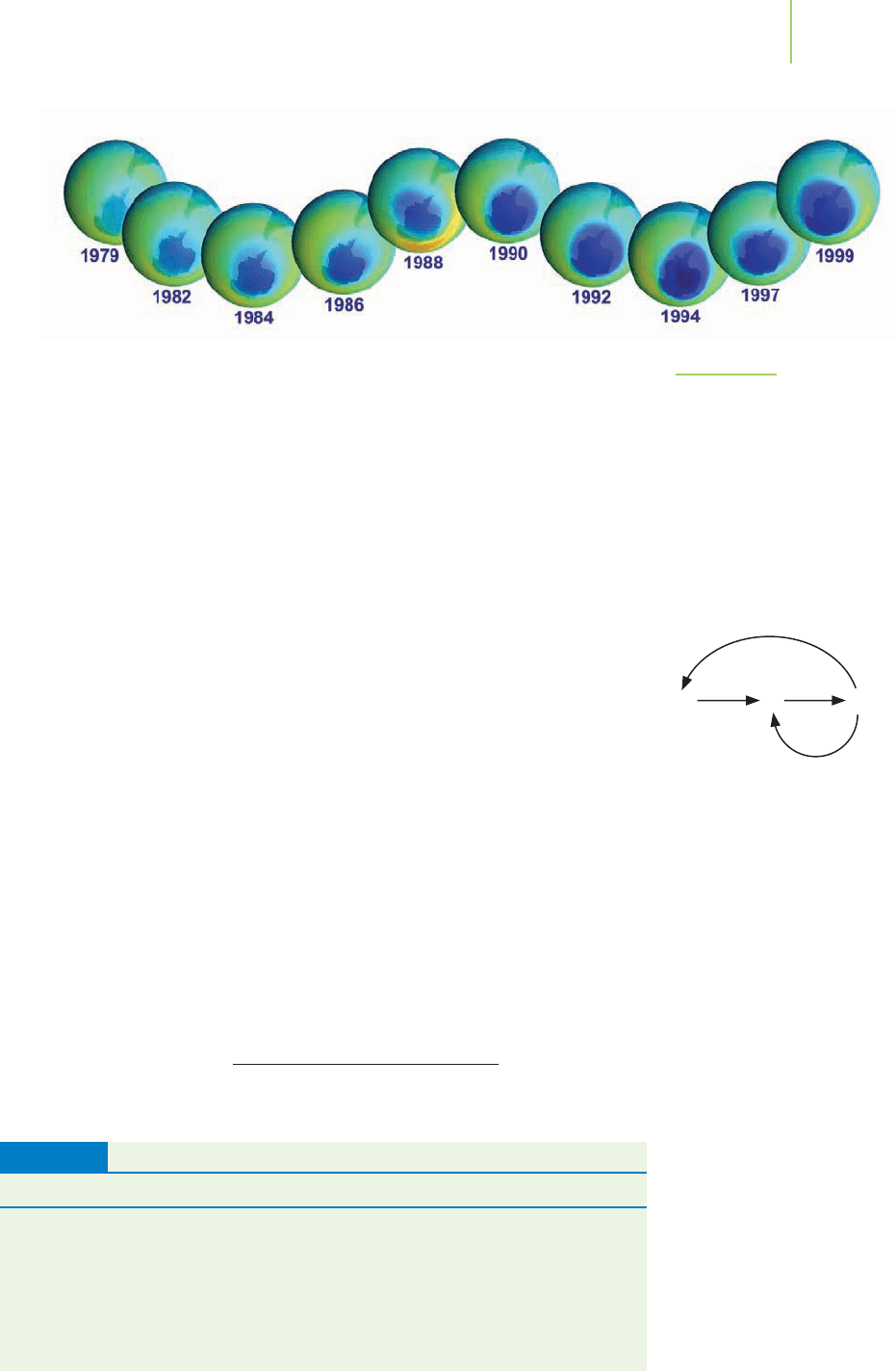
shown in Figure 10.25. Most notable is the discovery of a “hole”in the ozone layer
over Antarctica.
Sherwood Rowland and Mario Molina suggested in 1974 that
chlorofluorocar-
bons (CFCs)
, used in refrigeration and in the formation of polymer foams, were
the culprits in the destruction of the ozone layer. Table 10.7 lists common CFCs
and related compounds, called
halons, along with their uses.
CFCs were initially much sought after as refrigerants. This class of com-
pounds is chemically inert, nonflammable, inexpensive, and quite stable at the
Earth’s surface. These properties make them very suitable for use in homes and
automobiles. However, as they are released into the atmosphere, they travel up-
ward to the stratosphere without reacting with other compounds. Then, in the
stratosphere, they are bombarded with high-energy UV radiation and do react.
This produces highly reactive chlorine atoms that react with ozone as well as with
individual oxygen atoms that come from ozone.
Cl(g) + O
3
(g) n ClO(g) + O
2
(g)
ClO(g) + O(g) n Cl(g) + O
2
(g) .
overall reaction: O
3
(g) + O(g) n 2O
2
(g)
10.9 Industrialization: A Wonderful, Yet Cautionary, Tale 429
FIGURE 10.25
Total Ozone Mapping Spectrometer
(TOMS) data from satellites orbiting the
Earth give us a picture of the damage
done by ozone depleting substances.
These images from the period 1979 to
1999 show the development of a large
area of decreased ozone in the strato-
sphere over Antarctica.
O
2
O
UV-C + O
2
+ O
U
V-B
O
3
The Chapman mechanism.
Common Chlorofluorocarbons and Halons
Formula Common Symbol Major Uses
CCl
3
F CFC-11 Polymer foams, refrigeration, air conditioning
CCl
2
F
2
CFC-12 Polymer foams, refrigeration, air conditioning,
aerosols, food–freezing solvents
CCl
2
FCClF
2
CFC-113 Solvent
CBrClF
2
halon-1211 Portable fire extinguishers
C
2
Br
2
F
4
halon-2402 Fire extinguishers
TABLE 10.7

430 Chapter 10 The Behavior and Applications of Gases
As we monitor the decrease in stratospheric ozone with
growing concern, we also note the localized increase in
surface ozone, because this gas is an irritant to the eyes,
nose, and throat and damages cells in plants. Where does
tropospheric ozone come from? We typically note its
presence in the metropolitan areas of industrialized na-
tions. More specifically, the reaction mechanism for the
accumulation of ozone at the surface involves the release
of nitric oxide from automobiles without catalytic con-
verters. This is due to the unwanted combustion of ni-
trogen gas (present in the air) inside the combustion
chambers of automobiles:
N
2
(g) + O
2
(g) n 2NO(g)
Unfortunately, even automobiles with catalytic convert-
ers produce small amounts of NO. The released NO
reacts with oxygen to make nitrogen dioxide:
2NO(g) + O
2
(g) n 2NO
2
(g)
Then, when the Sun shines on a city filled with NO
2
,
the molecule decomposes to produce highly reactive
oxygen atoms:
sunlight
NO
2
(g)
−−−−−→
NO(g) + O(g)(reaction A)
The atomic oxygen combines with oxygen gas in the
same way that it does in the stratosphere to produce
ozone:
O
2
(g) + O(g) n O
3
(g) (reaction B)
Then ozone is consumed in this reaction:
NO(g) + O
3
(g) n NO
2
(g) + O
2
(g) (reaction C)
The resulting combination of reactions A, B, and C that
produce and consume ozone at ground level is known
as a null cycle. No net reaction occurs in a null cycle.
Instead, the reactions are governed specifically by the
relative concentrations of the individual reactants and
products. Especially important is the presence of or-
ganic (carbon-based) pollutants and heat, such as are
present on a hot summer day. These pollutants create
highly reactive organic compounds that react faster with
NO than does O
3
. In the presence of these organic pol-
lutants, the null cycle is modified and excess O
3
remains!
What is the effect of this “nanoworld” process on the
“macroworld”? This is the impact of heat and pollution
in urban areas. Ozone accumulates at the Earth’s sur-
face, especially in large cities that have lots of motor
vehicle traffic. Even smaller metropolitan areas, such as
the “Triad” cities in North Carolina shown in Figure
10.26, have experienced an increase in ozone concentra-
tions due to automobile exhaust.
NanoWorld / MacroWorld
The big effects of the very small:
Accumulation of ozone at the earth’s surface
Smog is a common annoyance—and often a hazard—in
industrialized cities. Its production is governed by a null
cycle that produces ozone in the presence of sunlight.
Air quality index (AQI)
Apr. 1
0
25
50
75
100
125
150
175
200
225
250
Apr. 15
Apr. 29
May 13
May 27
June 10
June 24
July 8
July 22
Aug. 5
Aug. 19
Sept. 2
Sept. 16
Sept. 30
Oct. 14
Oct. 28
2002 Date
Very unhealthful
Unhealthful
Unhealthful for sensitive groups
Moderate
Good
FIGURE 10.26
The ozone level in the “Triad” of cen-
tral North Carolina from April to Octo-
ber 2002. Even in this midsized region
(including the cities of Greensboro,
High Point, and Winston-Salem), air
quality is a significant concern.

10.9 Industrialization: A Wonderful, Yet Cautionary, Tale 431
The chlorine atom is a catalyst in the destruction of ozone. Each chlorine atom
can catalyze the breakdown of perhaps hundreds of thousands of ozone
molecules.
Rowland and Molina shared the 1995 Nobel Prize in chemistry for their
groundbreaking research and predictions about the fate of the ozone layer. Since
that time, substitute compounds called HCFCs have been developed, including
CHF
2
Cl and CF
3
CFH
2
. These react in the lower atmosphere to release HCl and
HF, never making it to the stratosphere. The loss of ozone in the stratosphere,
combined with the generation of unhealthful ozone levels in large cities, is a con-
tinued cause for concern and a focus of research. The very good news is that as a
result of worldwide action to reduce CFC emissions, we may have turned the cor-
ner. In 2005, scientists found a slight worldwide increase in the amount of ozone
in the stratosphere. Many decades of continued diligence will be required before
the damage to the ozone layer can be completely reversed, yet we have made an
important start.
The Greenhouse Effect
The greenhouse effect is caused by the accumulation, in the atmosphere, of gases
that permit light to enter but prevent some energy as heat from exiting, much like
a plant greenhouse. Methane and CFCs are examples of greenhouse gases, al-
though they are not present in sufficient concentration to be of real concern at
this time. A potent new greenhouse gas named trifluoromethyl sulfur pentafluo-
ride (SF
5
CF
3
) has recently been identified.
Carbon dioxide is entering the atmosphere in much greater amounts than are
being used up, and this makes CO
2
the most important greenhouse gas.
The
carbon cycle, discussed in Chapter 12, has historically kept the carbon in
the atmosphere, seas, and land in balance over the long term. Human razing of
forests has radically curtailed the number of plants that use up CO
2
in
photosynthesis:
6CO
2
(g) + 6H
2
O(g) n C
6
H
12
O
6
(s) + 6O
2
(g)
Burning carbon-based fuels, especially coal, oil, and natural gas, has added CO
2
to the environment, and the annual release of carbon into the atmosphere con-
tinues to rise (see Figure 10.27). There is general agreement among scientists
The greenhouse effect keeps the Earth warm. The gases in the atmosphere absorb infrared energy emitted
from the Earth and reemit it to the Earth, much as the panes of glass keep heat in a greenhouse.
Application
C
HEMICAL ENCOUNTERS:
The Greenhouse
Effect
432 Chapter 10 The Behavior and Applications of Gases
that the 100-year increase in the Earth’s average surface temperature, shown in
Figure 10.28, is at least partially caused by the greenhouse effect.
It is often said that the problems caused by the use of chemistry can also be
fixed by the use of chemistry. Some good signs are on the horizon. As shown in
Figure 10.29, the fraction of global energy consumption derived from the burn-
ing of coal and wood is declining.
The interaction among gases in the atmosphere is so complex that we cannot
confidently predict the long-term atmospheric effects of industrialization. The
best models indicate that as we produce gases that meet the needs and the wants
of the nearly 7 billion people on Earth, we must be mindful of the possible impact
of our current consumption. We are, after all, the keepers of the global commons.
800 1000 1200
Ice-core data
Atmospheric measurements
1400 1600 1800 2000
260
280
300
320
340
360
380
Carbon dioxide concentration (parts per million)
FIGURE 10.27
The amount of carbon released as CO
2
into the atmosphere continues to rise
along with our seemingly insatiable
appetite for fossil fuels.
GREENHOUSE GASES
Atmospheric carbon dioxide in parts per million
1880: 290.7
1999: 368.4
200
250
300
350
400
1900 1920 1940 1960 1980 19991880
Data prior to 1959 is derived from ice core samples.
SURFACE TEMPERATURES
Expressed as departures from the 1880–1998 average
1880 1900 1920 1940 1960 1980 1999
1.6
°
1.1
°
.54
°
-.54
°
0
°
Farenheit
-1.1
°
FIGURE 10.28
There is general, though not universal,
agreement among scientists that the
100-year increase in the Earth’s average
surface temperature, shown here, is at
least partially caused by the greenhouse
effect.
0
20
40
60
80
100
1855
Source: International Institute for Applied Systems Analysis.
1875 1895 1915 1935 1955 1975 1995
Ye a r
Wood
Share of global
energy consumption (%)
Coal
Oil
Natural gas
Hydropower
Nuclear
FIGURE 10.29
Advances in chemistry have changed
the way in which energy is produced
and consumed.
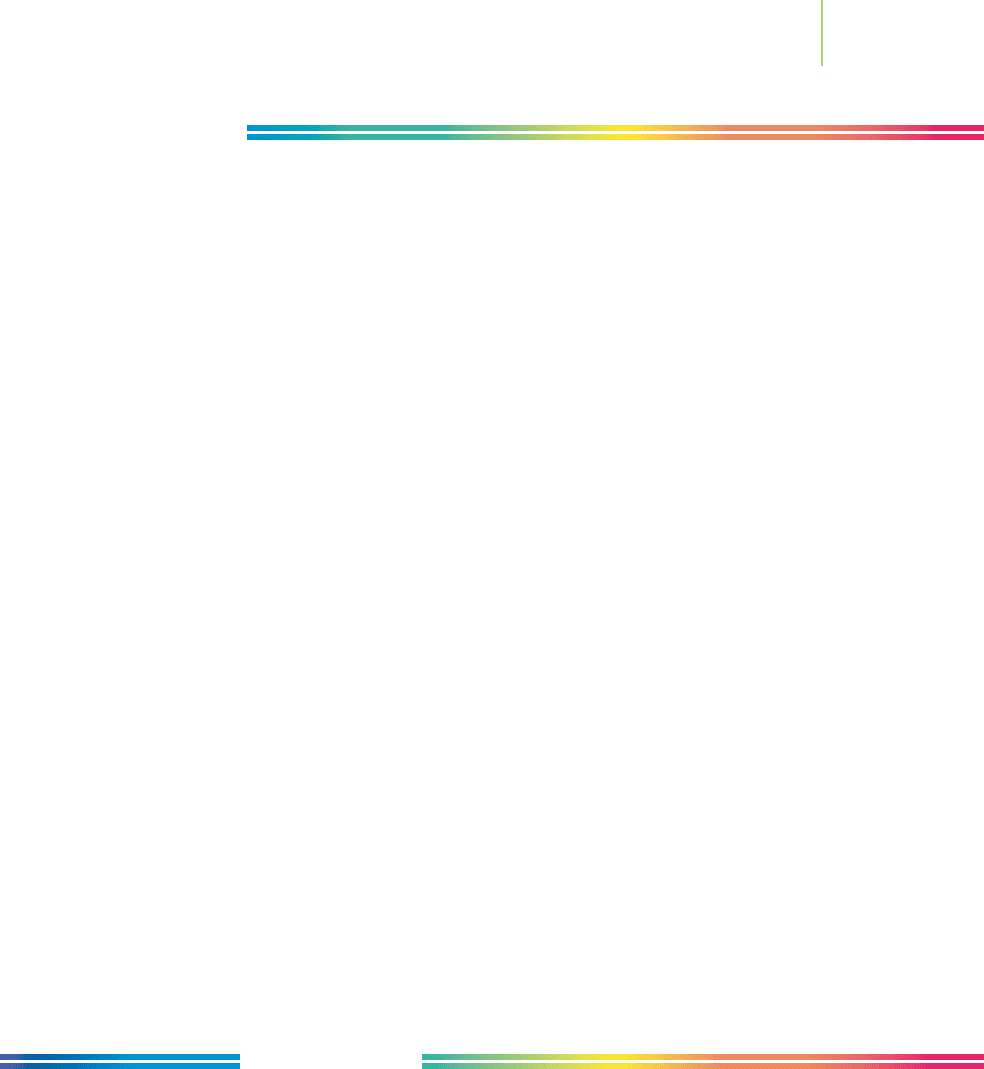
Key Words 433
The Bottom Line
■
Industrial gases are used in manufacturing, medi-
cine, and other industries. (Section 10.1)
■
Gases present at low density all behave in about the
same way. This permits us to generalize about their
behavior in a manner that is not possible with solids
and liquids. (Section 10.1)
■
Gases that behave as though each particle has no
interactions with any other are called ideal gases.
(Section 10.1)
■
High pressure, low temperature, and intermolecular
forces of attraction between gas molecules cause
gases to deviate from ideal behavior. (Section 10.1)
■
Real gases deviate from ideal behavior, especially at
low temperatures and high pressures. (Section 10.1)
■
Pressure is a measure of force per unit area. Pres-
sure is the result of collisions of gas molecules with
the walls of a container. (Section 10.2)
■
Several units of pressure exist, including the pascal
(the SI unit), atmosphere, mm Hg, in Hg, torr, bar,
psi, and psig. (Section 10.2)
■
In current usage, the standard atmosphere (1 atm)
is different from standard pressure (1 bar, or about
0.987 atm). (Section 10.2)
■
Dalton’s law of partial pressures is concerned with
the contribution of each gas to the pressure of the
entire gas mixture. (Section 10.3)
■
Avogadro’s law deals with the relationship between
the volume and number of moles of ideal gases, at
constant temperature and pressure. (Section 10.4)
■
Boyle’s law expresses the relationship between vol-
ume and pressure of an ideal gas, at constant num-
ber of moles and temperature. (Section 10.4)
■
Charles’s law expresses the relationship between the
volume and temperature of an ideal gas, at constant
number of moles and pressure. (Section 10.4)
■
The ideal gas equation combines the gas laws to
interrelate the pressure, volume, amount, and tem-
perature of an ideal gas. (Section 10.5)
■
The ideal gas equation can be applied to find the
molar mass and the density of an ideal gas.
(Section 10.5)
■
Several scientists have made mathematical models
to account for nonideal behavior. J. D. van der
Waals’s model is the most commonly used because
it is relatively simple and takes into account correc-
tions for pressure and volume. (Section 10.5)
■
The molar mass and the density of an ideal gas are
directly proportional. (Section 10.6)
■
The kinetic-molecular theory shows how it is possi-
ble to start from some elementary constructs about
gas behavior and derive the gas laws, therefore
showing the consistency between theory and exper-
iment. (Section 10.7)
■
Graham’s law of effusion shows the inverse rela-
tionship between the speed of a gas and its molar
mass. (Section 10.8)
■
The effects of industrialization on the atmosphere
are currently being debated. Ozone levels and the
greenhouse effect are two issues of greatest social
concern. (Section 10.9)
Key Words
absolute zero The temperature obtained by extrapola-
tion of a plot of gas volume versus temperature to
the “zero volume” point. The lowest temperature
possible, which is 0 K, or –273.15°C. (p. 409)
atmosphere (atm) A unit used to measure pressure.
1 atm = 760 mm Hg. (p. 399)
average speed The sum of the molecular speeds of
each molecule divided by the number of molecules.
(p. 424)
Avogadro’s law Equal amounts of gases occupy the
same volume at constant temperature and pressure.
(p. 404)
bar A unit used to measure pressure. 1.01325 bar =
1 atmosphere. (p. 399)
Boyle’s law The volume of a fixed amount of gas is
inversely proportional to its pressure at constant
temperature. (p. 406)
carbon cycle The natural process that describes how
carbon atoms are moved among the land, sea, and
atmosphere. (p. 431)
Charles’s law The volume of a fixed amount of gas is
directly proportional to its temperature in kelvins at
a constant pressure. (p. 408)
chlorofluorocarbons (CFCs) Compounds containing only
carbon, chlorine, and fluorine. CFCs are typically
used as solvents and refrigerants and are known to
be harmful to the ozone layer. (p. 429)
combined gas equation The combination of Avogadro’s
law, Boyle’s law, and Charles’s law. (p. 411)

434 Chapter 10 The Behavior and Applications of Gases
Dalton’s law of partial pressures The total pressure of a
mixture of gases is the simple sum of the individual
pressures of all the gaseous components. (p. 401)
diffusion The process by which two or more substances
mix. (p. 426)
effusion The process by which a gas escapes through a
small hole. (p. 425)
electrolysis A process in which an electric current pass-
ing through a solution causes a chemical reaction.
(p. 397)
force The mass of an object multiplied by its accelera-
tion. (p. 397)
Graham’s law of effusion The rates of the effusion of
gases are inversely proportional to the square roots
of their molar masses. (p. 426)
greenhouse effect The re-radiation of energy as heat
from gases in the atmosphere back toward Earth. An
increase in the greenhouse effect leads to an increase
in global temperatures. (p. 431)
halons Compounds containing carbon and halogens.
Halons are typically used in fire extinguishing appli-
cations but are considered harmful to the ozone
layer. (p. 429)
ideal gases Gases that behave as though each particle
has no interactions with any other particle and
occupies no molar volume. (p. 396)
ideal gas constant R, the constant used in the ideal gas
equation. R = 0.08206 L·atm/mol·K. (p. 414)
ideal gas equation The equation relating the pressure,
volume, moles, and temperature of an ideal gas.
(p. 414)
in Hg A unit used to measure pressure. 29.921 in Hg
equals 1 atmosphere. (p. 399)
industrial gases Gases used in industrial or commercial
applications. (p. 395)
kinetic-molecular theory A theory explaining the rela-
tionship between kinetic energy and gas behavior.
This theory makes possible derivation of the ideal
gas laws. (p. 422)
mm Hg A unit used to measure pressure. 760 mm Hg
represents the height of a column of mercury that
can be supported by 1 atm pressure. (p. 399)
modified atmosphere packaging (MAP) The replacement
of the air within food packaging with gases in order
to prolong the shelf life of the food. (p. 401)
most probable speed The most likely speed of a mole-
cule from among a group of molecules. (p. 424)
newton (N) The basic SI unit of force.
1 N = 1 kg·m·s
−2
.(p. 397)
partial pressure The pressure exerted by a single com-
ponent of a gaseous mixture. (p. 401)
pascal (Pa) The basic SI unit of pressure.
1 Pa = 1 N·m
−2
.(p. 398)
photodissociation A chemical reaction where light, of
sufficient energy, causes the cleavage of a bond in a
molecule. (p. 428)
pounds per square inch (psi) A unit of pressure.
14.7 psi = 1 atm. (p. 399)
pounds per square inch gauge (psig) A unit of pressure
expressing the pressure of a gas in excess of the
standard atmospheric pressure. Also known as gauge
pressure. 14.7 psig = 2 atm. (p. 399)
pressure The force per unit area exerted by an object
on another. (p. 398)
root-mean-square (rms) speed The square root of the
sum of the squares of the individual speeds of parti-
cles divided by the number of those particles.
(p. 424)
standard atmosphere Exactly 1 atm, the pressure that
supports 760 mm of mercury. (p. 399)
standard pressure Defined as 1 bar, although chemists
typically use 1 atm. (p. 399)
standard temperature For gases, defined to be 0°C, or
273 K. (p. 400)
standard temperature and pressure (STP) 1 atm and 273 K.
(p. 400)
standard ambient temperature and pressure (SATP) 1 bar
and 298 K. (p. 400)
torr A unit used to measure the pressure of gases.
1torr = 1 mm Hg. (p. 399)
van der Waals equation The equation that corrects the
gas laws for gases that deviate from ideal behavior.
(p. 417)
Focus Your Learning
The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
10.1 The Nature of Gases
Skill Review
1. Define the intermolecular forces typically experienced by
molecules in the gaseous state.
2. Explain why we never see signs on delivery trucks that read
“Danger—Compressed Liquid.”
3. What conditions tend to favor ideal behavior in gases?
4. What are some characteristics common in nonideal gases?
10.2 Production of Hydrogen and the Meaning
of Pressure
Skill Review
5. If your chemistry textbook had a mass of 0.89 kg, how much
force, in newtons, would the textbook exert on a desk in your
classroom?

6. If your textbook (mass = 0.89 kg) measured 27 cm × 23 cm,
what pressure, in pascals, would the book exert on the desk?
7. How much force, in newtons, would an 86-kg man exert on
the ground?
8. A 115-lb flight executive wears spike heels every day to work.
If each heel has an area of 4.0 cm
2
, what is the pressure
exerted by each heel in pascals? (Assume all of the woman’s
weight is placed on the heels of her shoes.)
9. The pressure of a gas is measured to be 797 mm Hg. Convert
this pressure into units of Pa, kPa, atm, torr, bar, and in Hg.
10. The pressure of a gas is measured to be 0.750 atm. Convert
this pressure into units of Pa, kPa, torr, bar, and mm Hg.
11. An astronaut weighs 145 lb on the planet Earth, where the
gravitational pull is 9.8 m/s
2
. She is sent on a mission to a
planet whose gravitational pull is only 0.33 m/s
2
. What would
a scale on this planet indicate that the astronaut weighs?
12. What is the mass in kg and weight in lb of a person who
exerts a force of 375 N on a scale?
13. The air in a car tire exerts a pressure of 22.3 psig. What is
the force exerted by the air if the total area inside the tire is
567 cm
2
?
14. A full book bag that exerts a force of 12 N on a table takes up
an area that measures 25 cm × 36 cm. Calculate the amount
of pressure exerted by the bag.
Chemical Applications and Practice
15. Small inflatable sleeping mattresses make camping enjoyable
for many outdoor enthusiasts. If the air pressure of a com-
fortable mat is measured as 17.5 psig, what is the actual
pressure that the air is exerting on the inside walls of the mat?
What is the pressure if reported in units of atmospheres?
16. As the altitude increases, the air pressure decreases. To prove
this fact, a mountain climbing group measured the air pres-
sure on Mount Everest at 0.67 atm. What would this pressure
be if it were reported in units of kPa, bar, mm Hg, and psi?
10.3 Mixtures of Gases—Dalton’s Law and Food Packaging
Skill Review
17. The partial pressure of O
2
in a sample of air on a mountain-
top is 115 mm Hg. The oxygen makes up 21% of the gas
in the atmosphere. What is the total atmospheric pressure on
the mountaintop?
18. A gaseous mixture contains 54% N
2
, 39% O
2
, and 7% CO
2
by
volume. If the total pressure is 813 mm Hg at STP, what is the
partial pressure of each of the gases?
19. A total of 12.4 g of N
2
and 12.4 g of O
2
are combined in a
mixture that exerts a pressure of 1.23 atm. Calculate the
partial pressure of N
2
and that of O
2
in the mixture.
20. A mixture of gases contains CO
2
and O
2
in a 3-to-1 ratio. If
the partial pressure of CO
2
in the mixture is 2.34 atm, what is
the partial pressure of O
2
? What is the total pressure of the
mixture?
Chemical Applications and Practices
21. A common way to generate small amounts of oxygen in the
lab is by heating potassium chlorate in the presence of a
catalyst and collecting the oxygen by bubbling it through
water. If the total pressure of the collected gas was 785 mm Hg
at 27°C, what is the partial pressure of the collected oxygen?
The pressure of gaseous water at 27°C is 27 torr.
22. Producing hydrogen gas is becoming a very important tech-
nical and economic concern for food processing, and poten-
tially as a fuel for future transportation needs. If a chemical
engineer were developing a model for this purpose and col-
lected 4.25 g of H
2
over water at 25°C that had a total pressure
of 1.15 atm, what would be the partial pressure of the H
2
collected? The pressure of gaseous water at 25°C is 23.8 torr.
23. In a helium–neon laser, a mixture of helium and neon gas is
contained in a small tube used to generate coherent light. In
some applications, this type of laser is used to scan price codes
at the grocery store. If the internal gas mixture is 8.95% Ne
and 91.15% He by volume and the total pressure is main-
tained at 3.42 mm Hg, what are the partial pressures of the gas
components?
24. A balloon is filled with H
2
and O
2
for a demonstration. The
gases are added such that they will react completely when a
flame is brought in contact with the balloon. Write the equa-
tion describing this reaction. If the total pressure of the bal-
loon is 3.4 atm, what would the partial pressure of each gas
need to be in order for the reaction to go to completion?
10.4 The Gas Laws—Relating the Behavior of Gases
to Key Properties
Skill Review
25. At STP, a 2.5-L sample of gas contains 4.5 mol of Ne. How
many moles would the sample contain if the volume in-
creased to 5.0 L? . . . if the volume decreased to 1.0 L?
26. A balloon containing 0.50 mol of Heis found to have a volume
of 1.75 L.Whatvolumewould the balloon haveif anadditional
0.15 mol of He were added? What volume would the balloon
haveif 0.23 molof Hewereremovedfromthe balloon?Assume
that the temperature and pressure remain constant.
27. A container holds 4.70 L of air at a pressure of 861 torr. If the
gas in the container were lowered to standard pressure, what
would be its volume? If the pressure of the gas were reduced
to 400 torr, what would be its volume? Assume the tempera-
ture remains constant.
28. A balloon holds 2.50 L of air at a pressure of 19.5 psi. If the
balloon were squeezed to a volume of 1.00 L, what would be
the new pressure of the gas? If the balloon were stretched to a
volume of 5.00 L, what would be the new pressure of the gas?
Assume the temperature remains constant.
29. A balloon containing 1.00 L of CO
2
at 72°F is placed in a
freezer at −10°F. What is the new volume of the balloon? If
the balloon is placed in the oven at 250°F, what is the new vol-
ume of the balloon? Assume the pressure remains constant.
30. The volume of a fixed quantity of gas at 25°C is changed from
0.750 L to 5.57 L. What is the resulting temperature of the gas
if the pressure remains constant? If the volume of the origi-
nal sample were reduced to 0.150 L, what would be the re-
sulting temperature? Assume the pressure remains constant.
31. A 0.47-mol sample of gas at 37°C occupies 3.20 L at 2839 mm
Hg. What volume would the same gas occupy at STP?
Focus Your Learning
435
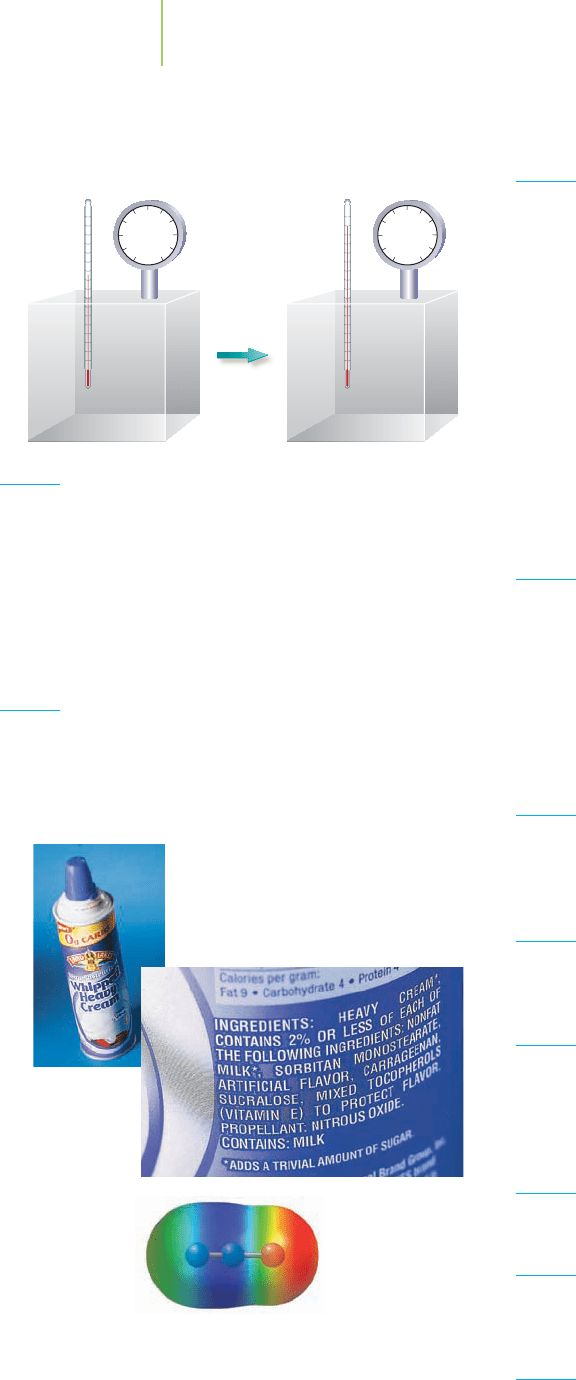
Whipped cream in a bottle uses N
2
O
as the propellant. Does the electro-
static potential map for N
2
O tell us
anything about how to draw the
Lewis dot structure?
436 Chapter 10 The Behavior and Applications of Gases
32. Consider this experiment, wherein a gas is heated in a sealed
chamber of fixed volume. What is the new pressure of the
gas?
33. A balloon containing 0.15 g of H
2
at STP is pressurized to
32.0 psi. What is the new temperature of the gas inside the
balloon? Assume the volume remains constant.
34. A CO gas cylinder possesses a volume of 45.0 L of gas at
2.20 × 10
3
psi. If the valve breaks and the gas is immediately
released from the cylinder, what is the new volume of the gas?
Assume the temperature remains constant and standard
pressure is attained.
Chemical Applications and Practice
35. Pressurized N
2
O gas can be used to provide the “inflating”
power for canned whipped cream. If such a can that is used to
provide the topping for a sundae contains 0.58 g of N
2
O and
corresponds to 0.217 L, how many liters of N
2
O will 0.33 g of
N
2
O fill at the same temperature and pressure?
pressure is 0.988 atm, what pressure will be required to com-
press the gas mixture to 50.0 mL? Assume the temperature
remains constant.
37. The molecule nitrogen monoxide (NO) plays several vital
roles in animal physiology. Nitrogen monoxide helps regu-
late blood pressure, influences blood clotting, and also influ-
ences the immune system. A researcher studying this impor-
tant gas isolates a small, 0.150-mL sample at 37°C. If this
sample is cooled to room temperature (25°C), what volume
will the sample have if the pressure remains constant?
38. Because ozone (O
3
) has beneficial properties at high altitudes
and harmful properties at low altitudes, atmospheric scien-
tists often study it. If a 50.0-mL sample of ozone collected at
high altitude has a temperature of −25°C is brought back to
the lab to study (where the temperature is 25°C), what will
the new volume of the gas be? Assume the pressure remains
constant.
10.5 and 10.6 The Ideal Gas Equation and Its Applications
Skill Review
39. Determine the value of the ideal gas constant, R, to four sig-
nificant figures if 1.00 mol of gas at 1.00 atm and 273 K oc-
cupies 22.4 L. Repeat the calculation, but use each of the fol-
lowing as the label for the equivalent amount of pressure.
a. Pa b. mm Hg c. psi
40. A researcher explores the ideal gas equation. He measures the
pressure of a system at different temperatures (keeping the
volume and moles of the gas constant) and creates a plot of
P versus T. What is the slope of the line that results if the vol-
ume is 1.0 L when 1.0 mol of gas is used in the exploration?
41. When inflated with 6.0 ×10
6
g of helium, a weather balloon
has a pressure of 0.955 atm at 22.6°C. What volume does the
balloon occupy?
42. What would be the pressure of H
2
in a tank that has a volume
of 25 L, if it contained 45 g of H
2
at 25°C?
43. What is the volume of 44.0 g of CO
2
at STP?...at 39°C and
0.500 atm?
44. What is the temperature of 85.0 g of N
2
if the volume is
7.49 L at 850 mm Hg?
45. How many moles of Ar would be found in a 4.33-L balloon
at STP?
46. A gas is trapped in a 1.00-L flask at 27°C and has a pressure of
0.955 atm. The mass of the gas is measured at 1.95 g. Of
these, which is most likely to be the identity of the gas: NO,
NO
2
, or N
2
O
5
?
47. An unknown gas has a density of 0.600 g/L at 743 mm Hg
and 66°C. What is the molar mass of the unknown gas?
48. What is the density of CO
2
at STP? ...ofN
2
at STP?
49. At STP, the density of methane (CH
4
) is 0.714 g/L. What is
the density of methane at 25°C and 1.15 atm?
50. If a 500.0-mL sample of air at 26.5°C and 698 mm Hg has a
mass of 0.543 g, what is the average molar mass of air?
51. A typical breath may have a volume of 450 mL. If the air you
breathed were 21% oxygen gas, how many molecules of
oxygen would you inhale at 37°C and 0.922 atm?
1 atm
273 K
5.44 mol of gas 5.44 mol of gas
? atm
500°C
36. Inside the cylinder of an automobile engine, a mixture of
gasoline vapor and oxygen is combusted by a spark. However,
the gaseous mixture is first pressurized by the action of a
moving piston that decreases the volume of the gas. If the ini-
tial volume of the cylinder is approximately 485 mL and the

52. If you were able to inhale 2.50 L of air at STP, how many
moles of N
2
would you be breathing? Assume that air is com-
posed of 79% nitrogen and 21% oxygen.
53. A gas at 301 K and 0.97 atm has a density of 4.48 g/L. What is
the molar mass of the gas?
54. At 27.0°C and 802 torr, a gas has a molar mass of 62.0 g/mol.
What is the density of this gas? What would be the density of
the gas if the pressure were changed to 1.00 atm?
55. A 5.00-g sample of a gas has a volume of 85.0 mL at 20.0°C
and 1.00 atm. What is the molar mass of this gas? Would
the molar mass change if the temperature were increased to
23°C?
56. What is the density of CO
2
at 50°C and 0.44 atm?
Chemical Applications and Practice
57. A student working in the lab forgets to record the tempera-
ture at which she obtained 0.675 g of O
2
(g). She did, how-
ever, report the pressure as 745 mm Hg and the volume as
478 mL. What would have been the temperature at those
conditions?
58. Ozone (O
3
) in the stratosphere helps reduce the amount
of ultraviolet radiation reaching the surface of the Earth. As-
suming a temperature of −25°C and a partial pressure due
to ozone of 1.2 ×10
−7
atm, how many ozone molecules
would be present in 1.00 L of air in the stratosphere?
59. The metabolism of glucose is the main source of energy for
humans. This equation shows the overall reaction for the
process:
C
6
H
12
O
6
(s) + 6O
2
(g) n 6CO
2
(g) + 6H
2
O(g)
Calculate the volume of CO
2
produced at 37°C and 1.00 atm
when 10.0 g glucose is oxidized.
60. Small portable lighters use compressed butane (C
4
H
10
)as
theirfuel.Whenbutane iscombusted,this reactiontakesplace:
2C
4
H
10
(g) + 13O
2
(g) n 8CO
2
(g) + 10H
2
O(g)
a. At 27.0°C and 0.888 atm, how many liters of oxygen are
required for every 1.00 g of butane to react completely?
b. If the system is compressed to 3.50 atm, keeping the
temperature at 27.0°C, how many liters of oxygen will be
required to react completely with 1.00 g of butane?
61. An industrial process can be used to change ethylene into
ethanol. The process uses a catalyst, high pressures (6.8 MPa),
and temperatures of 3.00 × 10
3
K. What is the density of
ethanol vapor under these conditions?
62. The familiar aroma of garlic comes mainly from the com-
pound diallyl disulfide. Based on these measurements, deter-
mine the molar mass of this pungent compound. At 298 K,
a 105-mL container holds 0.618 g of diallyl disulfide with a
pressure of 0.987 atm.
63. Argon gas is being used in some incandescent light bulbs to
extend the life of the tungsten filament. If a light bulb has a
volume of 200.0 mL and contains 0.100 g of Ar at 25°C, what
is the pressure inside the bulb?
64. An aerosol can contains carbon dioxide as its propellant. If
the can has an internal pressure of 1.3 atm at 25.0°C, what
Focus Your Learning 437
pressure would it have if the temperature were accidentally
raised to 450°C? (Assume that the can has not yet burst,
although this would probably be a very close call.)
65. The Haber process is used to produce ammonia (NH
3
) for
use in fertilizing corn fields in the Midwest. The reaction is
N
2
(g) + 3H
2
(g) n 2NH
3
(g)
a. Which, if either, is the limiting reagent when 2.00 L of N
2
at STP is prepared to react with 2.00 L of H
2
at 1120 mm Hg
and 21.0°C?
b. If 2.00 L of the gases, both at STP, reacted via the Haber
process, how many liters of ammonia would be produced?
66. Much of the electricity in the United States is produced from
the combustion of coal. However, some of the coal deposits
contain FeS
2
(iron pyrite) as a contaminant. During the
combustion of coal, this impurity also burns and produces
harmful sulfur dioxide gas (SO
2
). How many liters of SO
2
are
produced from the burning of 1.00 kg of FeS
2
at 758 torr
and 275°C?
FeS
2
(s) + 2O
2
(g) n Fe(s) + 2SO
2
(g)
67. A gaseous sulfur compound causes the pungent aroma of
rotten eggs. If 1.60 g of the gas were collected in a 1.00-L ves-
sel at 1065 torr and 89.0°C, what would be the molar mass of
the trapped gas?
68. One common way to obtain metal samples from their im-
pure oxide ores is to react the oxide with carbon. Generically,
the equation can be written this way:
2MO(s) + C(s) n 2M(s) + CO
2
(g)
If 5.00 g of an unknown metal oxide (MO) reacted with
excess carbon and formed 0.738 L of CO
2
at 200.0°C and
0.978 atm, what is the identity of the metal?
69. The halogen gases are caustic and toxic. Therefore, they must
be handled with extreme care. Which halogen gas has a den-
sity of approximately 1.70 g/L at STP?
70. Hydrogen has an isotope known as deuterium (D) that is
made up of a proton, a neutron, and an electron. Deuterium
is present in measurable amounts in all hydrogen-containing
substances. At STP, what are the densities of the three com-
pounds that could exist in a hydrogen gas sample: H
2
,HD,
and D
2
?
71. Neon lights actually do contain neon gas. But from where do
we get neon? Very small amounts of Ne are present in air, less
than 2.0 × 10
−3
% by volume. However, when air is liquefied,
the sample can carefully be separated on the basis of boiling
points. This must be controlled, but because N
2
,O
2
, and Ar
boil away in the range from 77 to 97 K, neon (with a boiling
point of 27 K) can be separated. If a manufacturer collected
1.00 lb of neon at 25°C and 789 torr, what volume would the
Ne occupy?
72. Assume you are pumping air into the tires of your mountain
bike before a ride. If the tire volume is 1.50 L at 28.5°C and
6.55 atm, how many moles of air have you put inside the tire?
How many molecules of air are in the tire? (Refer to Problem
50 for the average molar mass of air.)
