Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

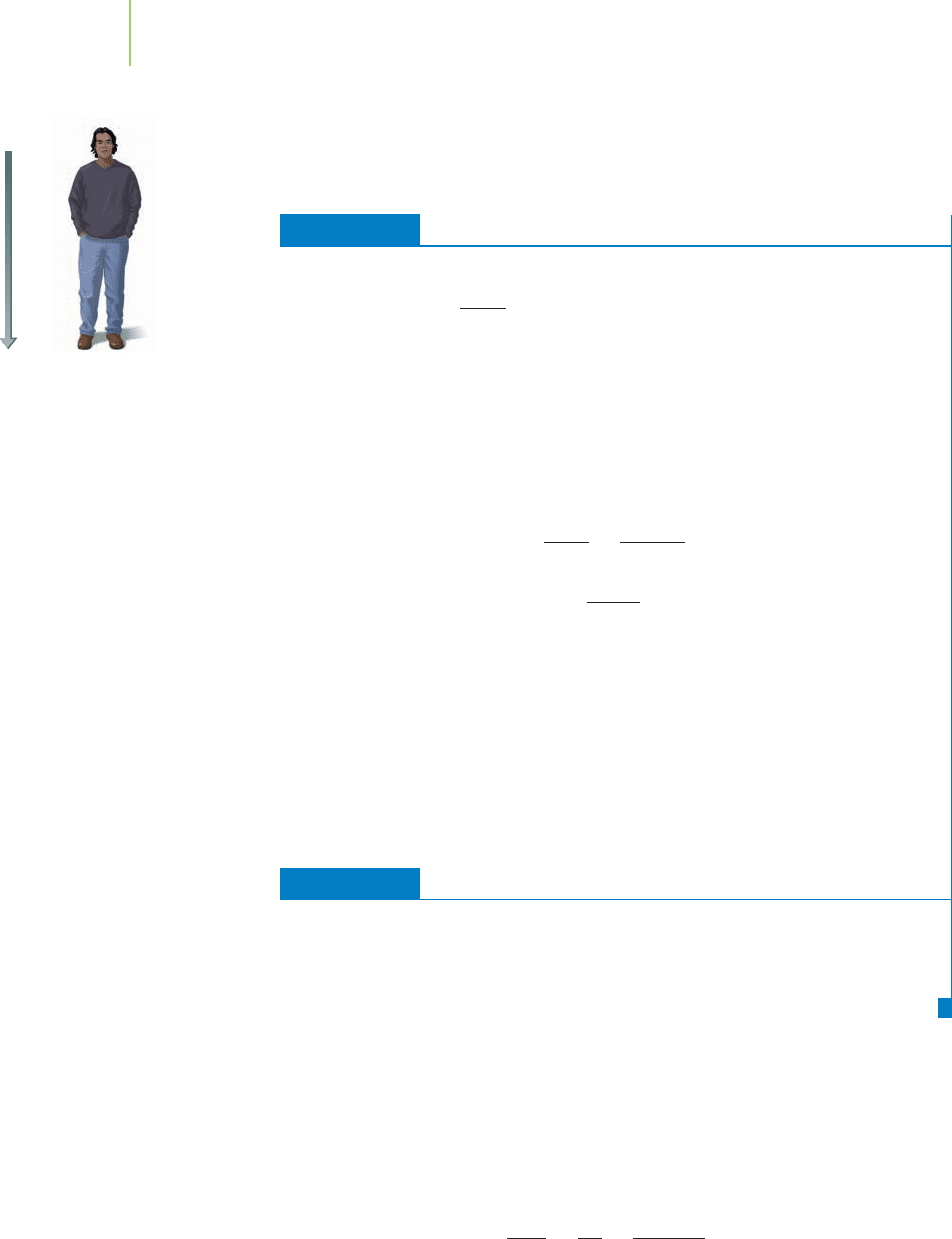
the floor upon which we are standing. In describing our own mass × ac-
celeration, we most often speak of force by using the familiar unit pounds, where
1 lb = 4.47 N.
EXERCISE 10.1 Calculating Force
Suppose that your mass is 55 kg and you are on the Moon, where the acceleration
due to gravity is about
1.6m
s
2
, roughly one-sixth that on Earth. What force would
you exert on a scale (“How much would you weigh”) in newtons and in pounds?
First Thoughts
Mass is a measure of the amount of substance, whereas force is the effect of acceleration,
in this case as gravity, on the mass. We would therefore expect the mass to remain the
same on the Moon (or on any other celestial body), whereas the force would change.
Solution
55 kg ×
1.6m
s
2
=
88 kg·m
s
2
= 88 N
88 N ×
1lb
4.47 N
= 20 lb
Further Insights
Jupiter and Mars reveal the effects of gravitational strength on planetary atmo-
spheres. Jupiter has a much greater gravitational pull than Earth because it is more
massive. This prevents light gases, such as hydrogen, from escaping, so Jupiter’s
atmosphere is largely hydrogen. Earth’s much lower gravitational pull cannot retain
hydrogen or helium, so our atmosphere is composed mostly of gases relatively
higher in molar mass, such as oxygen and nitrogen. Mars, which exerts an even
lower gravitational pull than Earth, has an atmosphere rich in carbon dioxide.
PRACTICE 10.1
A space traveler visiting the hypothetical planet X wishes to determine the gravita-
tional pull of that planet. A 10.0 kg block of lead has been found to weigh 15 lb on
planet X. What is the pull of gravity in m/s
2
on planet X?
See Problems 7, 8, 11, and 12.
The force that each molecule exerts on its container is very small, because the
mass of each molecule in kilograms is very small. But when large numbers of
molecules exert that force, as is common in the laboratory, the net result is read-
ily measurable. Scientifically, we say that the
pressure is the amount of force ap-
plied to a given area. If we substitute the SI base units for force and area, we see
that the pressure of a gas can be expressed in this way:
Pressure =
force
area
=
N
m
2
=
kg·m·s
−2
m
2
= kg·m
−1
·s
−2
The SI unit of pressure is the pascal (Pa) = 1 kg·m
−1
·s
−2
, named after the French
mathematician Blaise Pascal (1623–1662). Measuring pressure in pascals is
becoming ever more common, with kilopascals (kPa) used in maritime weather
reporting as well as on tire pumps.
What would happen to the force and pressure you exerted on the surface on
which you were standing in our previous exercise if you stood on one foot instead
of two? The force (88 N) would remain the same because your mass and the
398 Chapter 10 The Behavior and Applications of Gases
9.8 m/s
2
The force of gravity on a person.
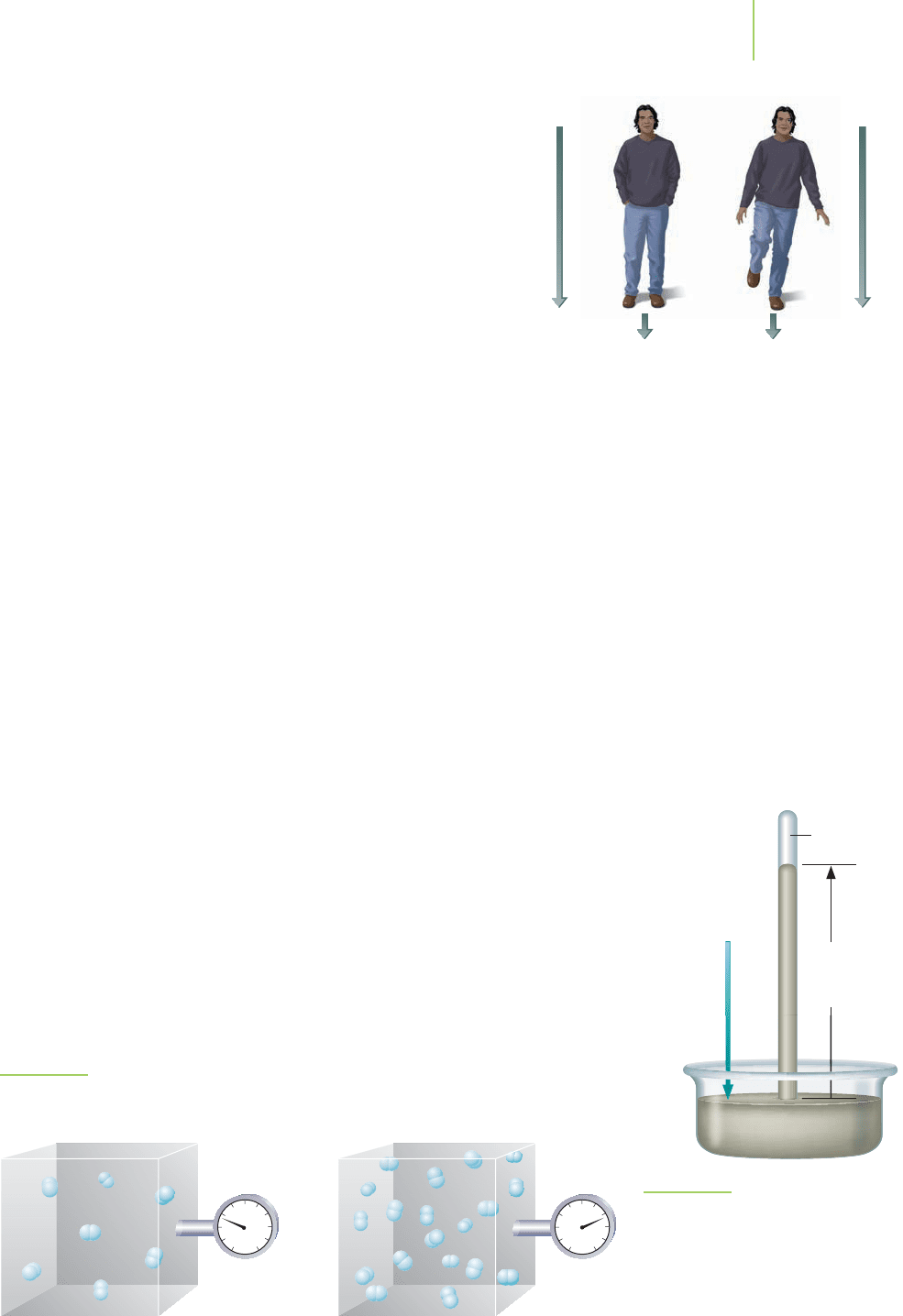
FIGURE 10.4
The pressure of the hydrogen container increases as more molecules enter the vessel.
The pressure is related to the force each molecule exerts on the walls of the container.
acceleration haven’t changed. However, the pressure would dou-
ble because the area on which you exerted your force would be
cut in half (one foot rather than two). In short, you can increase
pressure either by increasing force or by decreasing the area on
which that force is exerted.
We can extend this idea to hydrogen molecules being pro-
duced by electrolysis. As we increase the number of molecules
flowing into the collection vessel, more will collide in the given
area. Therefore, the force per unit area—the pressure—will in-
crease as shown in Figure 10.4. This suggests an important rela-
tionship that we will look at a bit later: At constant volume and
temperature, the pressure exerted by a gas is proportional to the
amount of gas present.
We have noted that the pascal is the SI unit of pressure, but
other units of pressure are often used in scientific measurements
and in day-to-day conversations. For instance, we have discussed
previously the
atmosphere (atm), which is used extensively in scientific work. One
atmosphere is roughly equal to the pressure exerted by the earth’s atmosphere at
sea level on a typical day. Because scientific work requires a more precise stan-
dard, we can define a
standard atmosphere, exactly 1 atm, as the pressure that
supports a 760-mm column of mercury (
mm Hg) in a barometer—a device, like
that shown in Figure 10.5, that measures the pressure of the atmos-
phere. Measurements have indicated that the standard atmosphere is equal to
1.01325 × 10
5
Pa.
The first barometer was made by Evangelista Torricelli in 1643, who used it to
determine that atmospheric pressure changes with changes in the weather. In
recognition of his work, we say that 1 atm is equal to 760
torr. Therefore, one
standard atmosphere is equal to 760 mm Hg, to 760 torr, and to 1.01325 ×10
5
Pa.
Still other units are also used to describe pressure. For example, weather fore-
casters in some countries speak of atmospheric pressure in
bars. A bar is equal to
1×10
5
Pa, a little less than 1 atm. In the United States, however, weather forecast-
ers report atmospheric pressure in units of inches of mercury (which is abbrevi-
ated
in Hg). Another unit with which you may be familiar is used when we inflate
car or bicycle tires to their recommended pressure. That unit is known as
pounds
per square inch gauge (psig)
, or, commonly, gauge pressure. This is the pressure of
the gas in the tire in excess of the standard atmospheric pressure of 14.7
pounds
per square inch (psi)
. This means that a tire inflated to 32.0 psig really contains
air exerting a pressure of 46.7 psi (32.0 +14.7) on the inside walls of the tire. Table
10.4 gives the relationships among different units of pressure.
For many years, scientists defined a
standard pressure as 1 atm so that a
standard reference was available at a common pressure. In 1982, IUPAC
10.2 Production of Hydrogen and the Meaning of Pressure 399
Pressure = 44 N
per foot
Force = 88 N Force = 88 N
Pressure = 88 N
on the foot
Pressure and force are related.
Pressure
0
Pressure
0
Height of
mercury
column
Atmospheric
pressure
Vacuum
FIGURE 10.5
The pressure exerted by air at 1 atm
supports a column of mercury 760 mm
(29.9 inches) high. As the atmospheric
pressure changes, so does the height of
the mercury in the column. This is a
simple barometer.

400 Chapter 10 The Behavior and Applications of Gases
Pressure Unit Conversion Factors
1 standard atmosphere is equal to . . .
• 760 mm Hg (millimeters of mercury)
• 760 torr
• 14.7 psi (pounds per square inch)
• 101,325 Pa (pascals)
• 1.01325 bar
• 0.0 psig (pounds per square inch gauge)
• 29.921 in Hg (inches of mercury)
TABLE 10.4
recommended that standard pressure be defined as equal to exactly 1 bar. How-
ever, most chemists still commonly use 1 atm as the standard pressure, and we
will do so in this textbook.
Scientists also define a
standard temperature for gases as 0
◦
C, or 273 K. Gases
at these conditions are said to be at
standard temperature and pressure (STP).In-
creasingly, scientists are beginning to use
standard ambient temperature and pres-
sure (SATP)
, or 1 bar and 298 K, to discuss gases, although in this book we will
use STP as the standard. Why use either of these standards? Each is a convenient
reference point at which to compare the properties of gases. Did you note that
the standard temperature in STP conditions, 0
◦
C, is different from that often
used in thermodynamics, 25
◦
C? Keep this in mind as we learn more about
gases.
EXERCISE 10.2 Conversion Among Measures of Pressure
The gas in a volleyball is measured to have a gauge pressure of 8.0 psig. What is the
total pressure exerted by the gas in units of atmospheres and torr?
First Thoughts
In this problem, we are given the gauge pressure, which is the pressure in excess of
the atmospheric pressure, 14.7 pounds per square inch (psi). To determine the total
pressure of the gas in the volleyball, we need to add 14.7 psi to the gauge pressure.
P
gas
= P
gauge pressure
+ P
volleyball
= (8.0 + 14.7) psi = 22.7 psi
We can now do our unit conversions.
Solution
22.7 psi ×
1atm
14.7 psi
= 1.54 atm
(We retain an extra figure because we will use this value in the next calculation.)
1.54 atm ×
760 torr
1atm
= 1.2 × 10
3
torr
Further Insights
Airlines caution their maintenance staff to always use a pressure regulator, a device
that safely delivers gas at a desired pressure, when filling aircraft tires with nitrogen
gas. The servicing tanks contain nitrogen gas at pressures as high as 3000 psi
(200 atm). If the tank is hooked up directly to a tire that can tolerate only 200 psi
(14 atm), the tire may explode. Several workers have been killed or severely injured
in such accidents.

Ground turkey can be kept
longer if stored using MAP.
Food is easily spoiled by naturally occurring bacteria.
PRACTICE 10.2
A weather report provides the current barometric pressure as 29.30 in Hg. What is
this pressure in atm, torr, bar, and Pa?
See Problems 9, 10, 15, and 16.
10.3 Mixtures of Gases—
Dalton’s Law and Food Packaging
Using mixtures of industrial gases to prevent spoilage in food products, especially
poultry, is a rapidly growing segment of the food-processing industry. Microbes,
especially Pseudomonas bacteria, spoil the flavor and color of food and make it
unhealthful to eat. Carbon dioxide gas, which in small quantities is not particu-
larly harmful to the food or to humans, has been found to inhibit the growth of
many types of microbes. Food scientists discovered this fact and have used it to
develop
modified atmosphere packaging (MAP)—packaging the food item in an at-
mosphere that is different from air (recall Table 10.1). The results are impressive.
For instance, meat and poultry stored in air lasts only a few days, but food stored
under MAP can have a shelf life as long as a month, with proper refrigeration.
Nitrogen and argon gases are also used in meat-based MAP, and sulfur diox-
ide gas inhibits the growth of microbes in some beverages. Typical MAP includes
mixtures containing a mole ratio of 30–35% CO
2
and 65–70% N
2
for meats. This
mixture brings up an interesting and useful point. If the total pressure of the gas
mixture is equal to 0.97 atm, how much does each gas contribute to the pressure?
JohnDalton(remember his work from Chapter 2) conducted experiments that
led to what we now know as
Dalton’s law of partial pressures. This law states that for
a mixture of gases in a container, the total pressure is equal to the sum of the pres-
sures that each gas would exert if it were alone. Each individual gas within a mix-
ture contributes only a part of the total pressure, so we say that each gas exerts a
partial pressure. Dalton’s law can be summarized by the following equation:
P
total
= P
1
+ P
2
+···+P
n
where P
total
= the total pressure exerted by a mixture of gases
P
1
= the partial pressure exerted by gas 1
P
2
= the partial pressure exerted by gas 2
P
n
= the partial pressure exerted by gas n
For example, let’s assume that the total pressure of the MAP gas that sur-
rounds cuts of chicken in a package is 0.97 atm and that the gas is composed of
35% CO
2
and 65% N
2
by volume. Using Dalton’s law of partial pressures, we see
that 35% of the pressure, or 0.34 atm (0.35 × 0.97 atm) is exerted by CO
2
and
0.63 atm (0.65 × 0.97 atm) is exerted by the N
2
gas. The calculation of the partial
10.3 Mixtures of Gases—Dalton’s Law and Food Packaging 401
Application
CHEMICAL ENCOUNTERS:
Dalton’s Law and
Food Packaging
Video Lesson: Partial Pressure
and Dalton’s Law
pressures is straightforward, but Dalton’s law is truly correct only
for ideal gases. The actual pressures of the gases are a bit different
from what we calculate because N
2
and CO
2
are not ideal gases; we
will discuss corrections for nonideal behavior in Section 10.5. A key
point for our current discussion is that the closer gases come to ideal
behavior, the more closely they follow Dalton’s law of partial pressures,
and deviations occur when gases are at high pressure, are at low tem-
perature, or are otherwise concentrated enough to exhibit intermolecu-
lar interactions with each other.
We can apply our understanding of partial pressures to the pro-
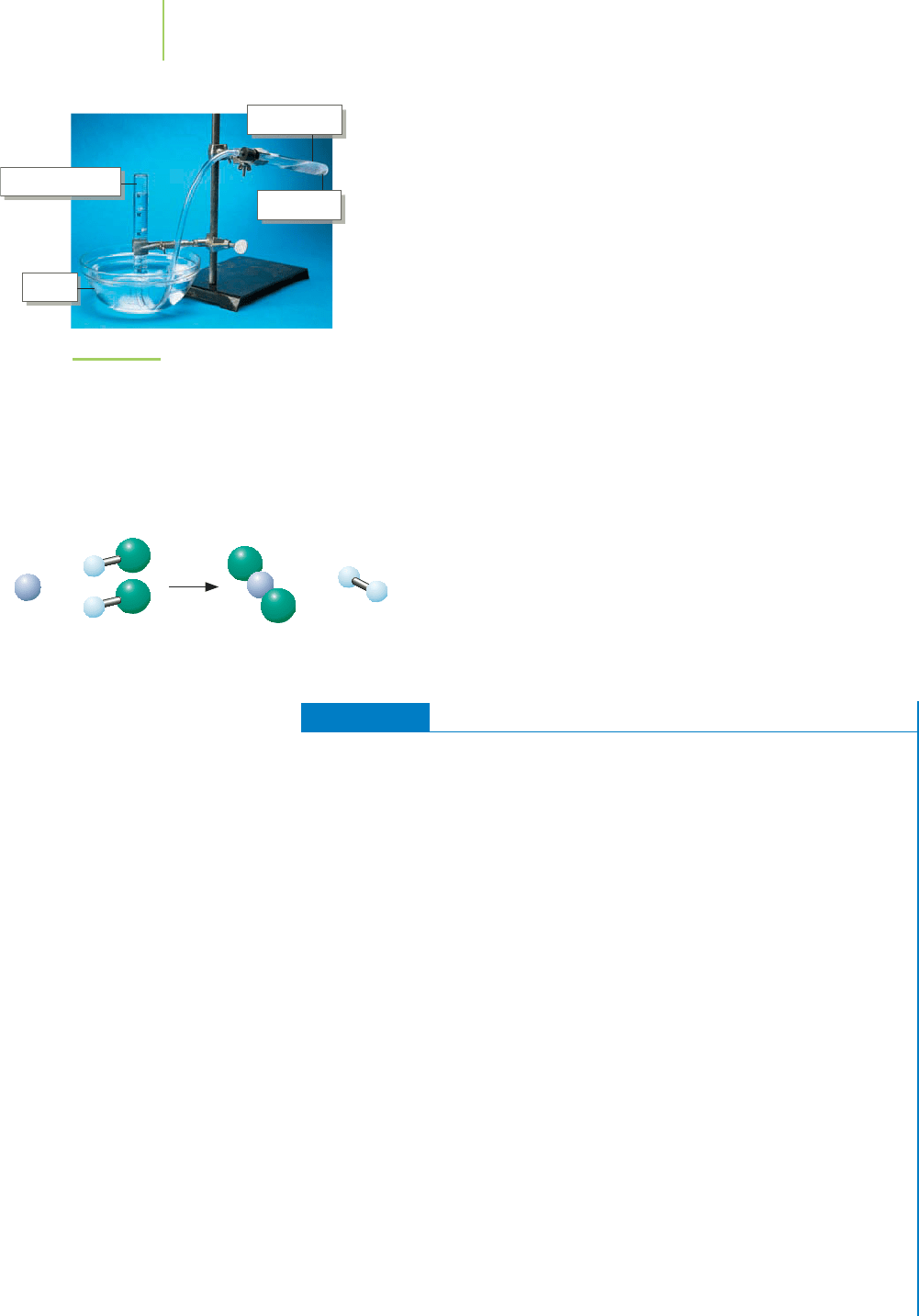
duction of hydrogen gas, our focus at the opening of Section 10.2. A
convenient way to produce small amounts of hydrogen gas is by the
reaction of zinc with hydrochloric acid. The hydrogen gas produced
by this reaction can be collected over water, as illustrated in Fig-
ure 10.6. As we’ll discover in Chapter 11, a sample of water is accom-
panied by a certain amount of gaseous water—water vapor—above
it. When we collect a gas by bubbling it through water or by leaving it
in contact with moisture, the total pressure above the liquid is the
sum of the partial pressure of the water vapor and the partial pressure
of the gas.At 20
◦
C, the vapor pressure of water is 17.5 torr. There-
fore, for example, if the total pressure of the gases is 750 torr, then
the partial pressure of the H
2
can be calculated as follows:
P
total
=
P
H
2
O
+
P
H
2
750 torr = 17.5 torr +
P
H
2
732 torr =
P
H
2
EXERCISE 10.3 Partial Pressure of Gases in the Atmosphere
A jet is cruising at 11,500 ft (3500 m) above sea level, where the atmospheric
pressure outside the plane is 493 torr (0.649 atm). The plane, normally pressurized
to about 650 torr (0.85 atm), suddenly has a loss of pressure until the cabin pres-
sure equals the pressure outside. What is the partial pressure of oxygen gas
(see Table 10.1) when the pressure of the gas in the plane is lowered? Most people
unaccustomed to low-oxygen environments will lapse into unconsciousness, and
eventually die, if the partial pressure of oxygen falls below 30 torr. With that figure
in mind, if the pressure in the plane isn’t quickly restored, can the passengers survive
this accident?
First Thoughts
We can use the law of partial pressures to determine the partial pressure of oxygen
gas in air at 3500 m above sea level. Oxygen gas makes up about 21% of air. There-
fore, we multiply the air pressure by 0.21 to obtain the partial pressure due to O
2
.
Solution
P
O
2
= P
air
× 0.21 = 493 torr × 0.21 = 1.0 × 10
2
torr
The partial pressure of O
2
at this altitude, about 100 torr, is less than the sea-level O
2
pressure of about 160 torr and the plane’s normal O
2
pressure of about 140 torr, but
it is still easily sufficient for survival.
Further Insights
The partial pressure of O
2
falls below 30 torr at an altitude of about 12,000 m, or
39,000 ft. This is higher than Mount Everest (8848 meters, or 29,028 ft), which ex-
plains why climbers without oxygen supplies can survive on the top of the world’s
highest peak (
P
O
2
= 60 torr). In fact, a stowaway is known to have survived an
402 Chapter 10 The Behavior and Applications of Gases
P
T
= P
H
2
+ P
H
2
O
HCl solution
Water
Mossy zinc
FIGURE 10.6
Hydrochloric acid solution can be mixed
with pieces of zinc metal to produce hydro-
gen gas. The gas is collected over water, so
the total pressure, P
T
, of the collected gas
is equal to the sum of the partial pressures
of the H
2
and the water vapor.
++
→
Zn(s) + 2HCl(aq) ZnCl
2
(aq) + H
2
(g)
airplane flight in the wheel well of a jumbo jet that flew at 38,000 ft (11,600 m),
where
P
O
2
= 32 torr.
PRACTICE 10.3
What is the partial pressure of nitrogen gas under the same conditions that the
stowaway mentioned above endured?
See Problems 17–24.
HERE’S WHAT WE KNOW SO FAR
■
Gases exert a force on the system that holds them. That force is known as
pressure.
■
Pressure is force distributed over an area. There are many units that describe
the pressure of a system.
■
A barometer is used to measure the pressure of the atmosphere.
■
STP is the abbreviation for standard pressure and temperature. For chemists,
this means exactly 1 atm pressure and 0
◦
C.
■
The total pressure of a system is the sum of all of the individual pressures of
the component gases. This is Dalton’s law of partial pressures.
■
An ideal gas is a hypothetical gas with no intermolecular forces of attraction,
where the particles of the gas have no volume. No gases are ideal, but they
approach ideal behavior at low pressure and high temperature.
10.4 The Gas Laws—Relating the
Behavior of Gases to Key Properties
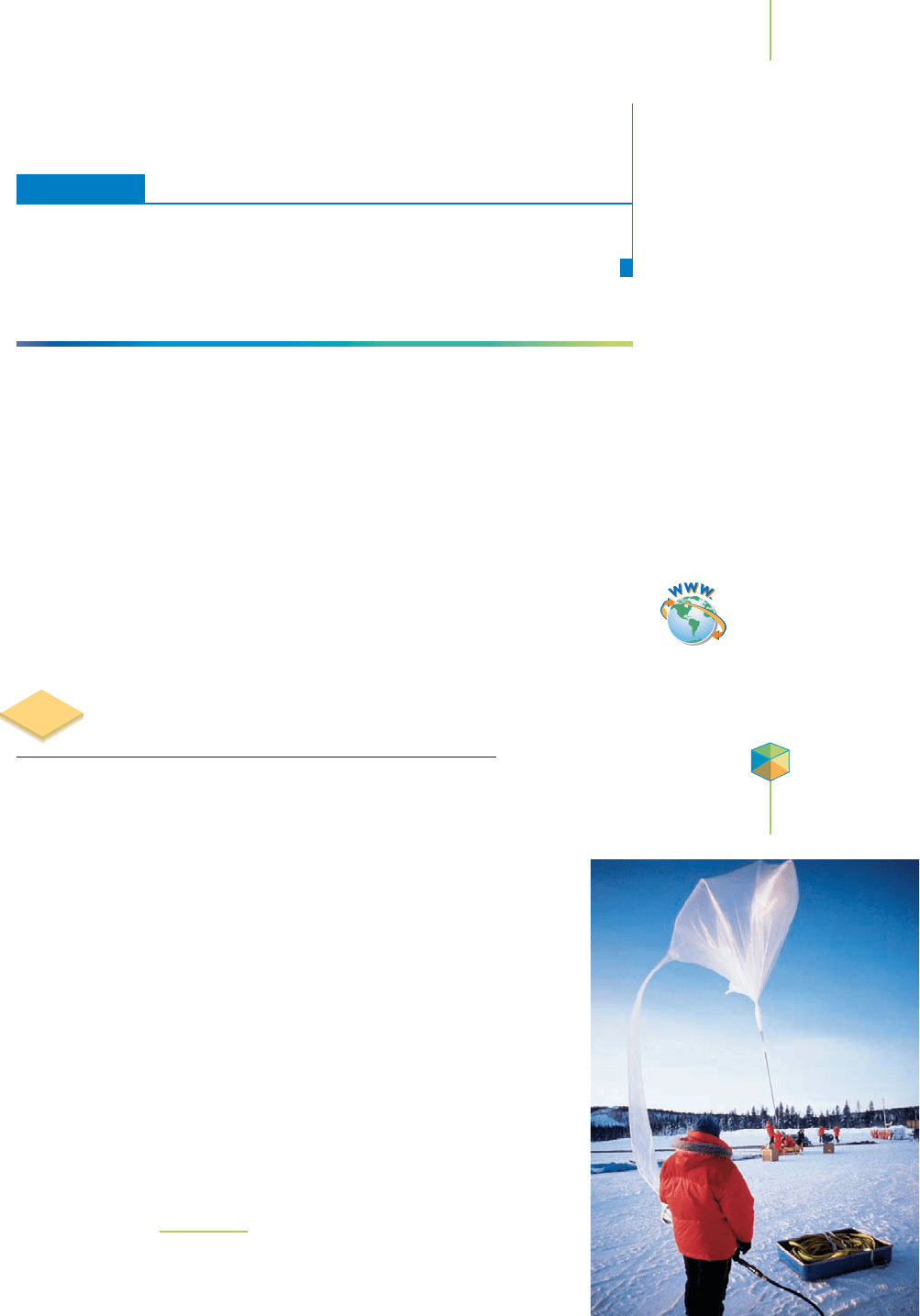
Atmospheric scientists at the South Pole routinely measure the ozone (O
3
) con-
tent in our atmosphere as a function of altitude. To complete this task, they
employ a helium-filled balloon, such as that shown in Figure 10.7, to carry
sophisticated instruments 35 km up into the atmosphere. The balloon
rises because the density of the helium gas it contains is much less than
that of the air outside. As the balloon climbs, the pressure and tempera-
ture of the surrounding atmosphere change, and the balloon expands.
Eventually, the balloon expands so much that it bursts and falls back to
Earth, allowing the scientists to recover the scientific instruments.
However, in order to have a successful flight, the balloon must be filled
with just the right amount of helium. Moreover, the scientists need to
know how the helium gas will be affected by changes in temperature and
pressure as the balloon rises through the atmosphere, so that the bal-
loon can carry the instruments up to where they are needed. Too much
gas and the balloon will burst before it reaches 35 km; too little gas and
the balloon may never burst. To make their decisions, atmospheric
scientists rely on some of the most fundamental properties of gases,
which investigators have been aware of for hundreds of years. These
10.4 The Gas Laws—Relating the Behavior of Gases to Key Properties 403
Application
C
HEMICAL ENCOUNTERS:
Balloons and Ozone
Analysis
FIGURE 10.7
A helium-filled balloon is used to carry sophisticated instru-
ments into the atmosphere to monitor the levels of atmos-
pheric ozone (O
3
). The balloon rises because the helium gas
it contains is much less dense than the air outside.
Video Lesson: Application of the
Gas Laws

FIGURE 10.10
Mylar balloons of O
2
and a balloon of H
2
, containing
0.050 mol of gas, each has the same volume.
FIGURE 10.9
At a volume of 1.2 L, the balloon on the left holds 0.050 mol of H
2
.If
we double the number of moles of the gas to 0.10, the volume of the
balloon (shown on the right) also doubles, to 2.4 L.
long-established laws describing the behavior of ideal gases—the “gas laws”—
help us to study modern concerns about gases, such as the level and fate of ozone
in our atmosphere.
Avogadro’s Law
A fundamental relationship that atmospheric scientists understand addresses the
relationship between the amount of a gas and its volume. Let’s explore this con-
nection by taking an empty balloon and attaching it to a cylinder containing hy-
drogen gas like that shown in Figure 10.8. The gas, which is stored inside the
cylinder at a pressure of 14 MPa, or about 140 atm, can be dispensed safely into
the balloon witha pressureregulator.The temperature of the roomis 25°C (298 K),
and the pressure is 1.0 atm.
As we begin to fill the balloon, it expands because of the addition of the gas.
The Mylar balloon has relatively little tension until it is nearly full, so the balloon
skin doesn’t significantly affect the pressure of the gas within. We find that at a
volume of 1.2 L, the balloon holds 0.050 mol of H
2
. If we double the number of
moles of the gas to 0.10, the volume of the balloon also doubles, to 2.4 L, as
shown in Figure 10.9.
Now let’s take another balloon and hook it up to a cylinder containing oxygen
gas, also at a pressure of 140 atm. If we add 0.050 mol of O
2
we find, as with H
2
,
that the volume of the balloon is 1.2 L, also shown in Figure 10.10. Double the
number of moles, and the volume doubles to 2.4 L, just as in the hydrogen-filled
balloon. This linear relationship holds irrespective of the nature of the gas,
assuming ideal behavior.
Avogadro’s law, named after its discoverer Amadeo
Avogadro (1776–1856), states that equal numbers of molecules are contained in
equal volumes of all dilute gases under the same conditions. An implication of
this law is that volume is directly proportional to the amount of a gas expressed in
moles, at constant temperature and pressure. We represent this as follows:
V = kn
which can be rewritten as
V
n
= k
(at constant T, P)
where V= volume of a gas
n = amount of the gas expressed in moles
k = a constant
404 Chapter 10 The Behavior and Applications of Gases
FIGURE 10.8
A Mylar balloon can be filled with gas
from a cylinder.
O
2
H
2
Video Lesson: Avogadro’s Law
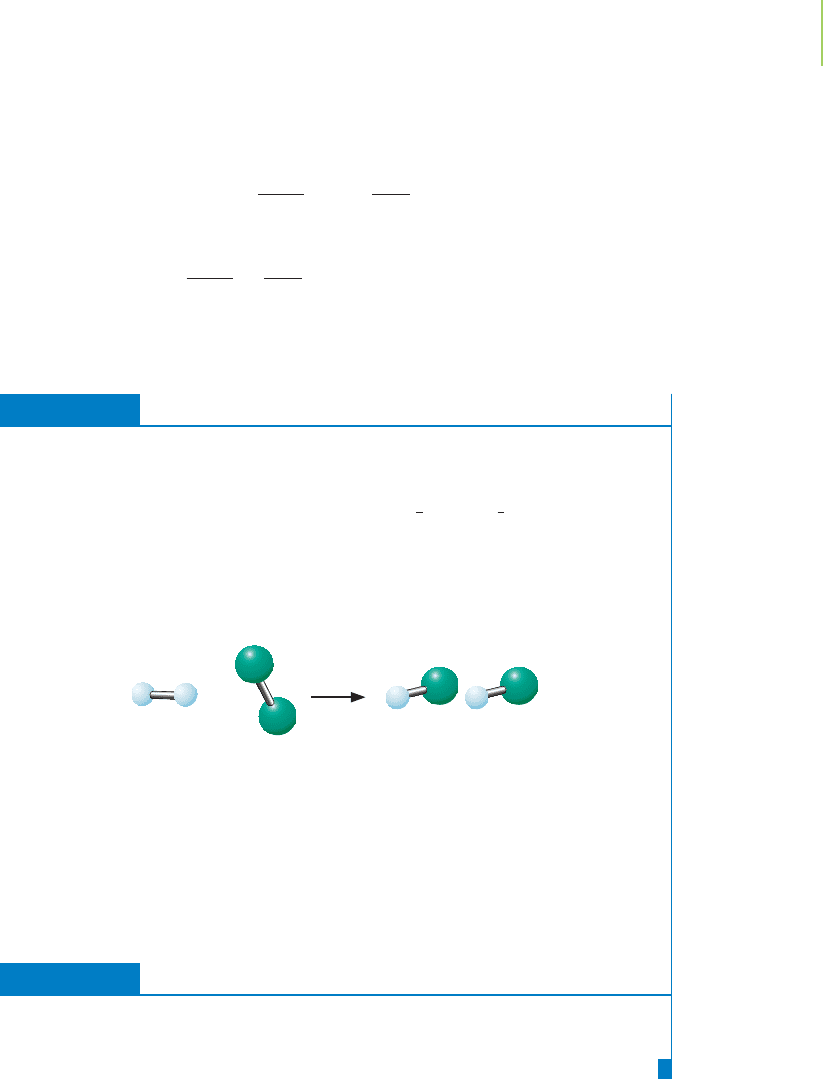
Because the constant k is the same value for a given temperature and pressure, we
can write
V
initial
n
initial
= k =
V
final
n
final
or
V
initial
n
initial
=
V
final
n
final
(at constant T, P)
The conclusion seems to make sense, because our experience tells is that the
more gas we add to a balloon, the larger it gets, until its skin resists.
EXERCISE 10.4 Avogadro’s Law
Much of the chlorine gas produced industrially is manufactured from the electroly-
sis of aqueous Group IA chlorides, such as sodium chloride. The reaction is
NaCl(aq) + H
2
O(l) n NaOH(aq) +
1
2
H
2
(g) +
1
2
Cl
2
(g)
Sodium hydroxide is formed along with hydrogen gas at one electrode, and chlorine
gas is formed at the other electrode. If after such a reaction, 22.4 L each of hydrogen
and chlorine gases, equaling 1.0 mol of each gas, is collected at STP, and then reacted
to form hydrogen chloride gas, what would be the final volume of HCl gas at STP?
10.4 The Gas Laws—Relating the Behavior of Gases to Key Properties 405
+
→
2HCl(g)H
2
(g) + Cl
2
(g)
Solution
According to Avogadro’s law, if 1.0 mol of each gas occupies 22.4 L at STP, then the
2.0 mol of reactants have a total volume of 44.8 L. Because 2.0 mol of gas product is
produced from 2.0 mol of reactants, the product would occupy 44.8 L at STP. The
numerical value would change only if the number of moles of gaseous products
differed from the number of moles of reactants.
PRACTICE 10.4
If a 12.8-L sample of He gas contains 6.4 mol of He, how many moles would there
be in a 1.5-L sample of He at the same temperature and pressure?
See Problems 25, 26, and 35.
Boyle’s Law
Two long-time friends, one of whom teaches at a U.S. college located at sea level
(air pressure = 1.00 atm) and the other at a university in Mexico City (7340 ft
above sea level, air pressure =0.764 atm), prepare to show their students the link
between moles of hydrogen and oxygen gas and the volume they occupy. Each of
them adds 0.050 mol of hydrogen at 25
◦
C to a balloon. The U.S. professor notes
that his balloon has a volume of 1.2 L. His colleague in Mexico City measures the
volume of his balloon as 1.6 L. Except for the pressure of the gas in each balloon,
which equals the atmospheric pressure in each city, the conditions are identical. The
Mexico City balloon contains gas at lower pressure and, as a result, larger volume
than the balloon at sea level in the United States. Take this to extremes and the in-
crease in volume can cause a balloon to burst, as we described at the beginning of
this section.

This inverse relationship between pressure and volume of a given amount of gas
at constant temperature has been understood since 1662, when Robert Boyle
(1627–1691; Figure 10.11) demonstrated what is now known as
Boyle’s law. This
law can be summarized as follows:
PV = k´ (at constant n, T)
where P = pressure of the gas
V = the volume occupied by the gas
k´ =a constant (different from the constant in Avogadro’s law)
Using the same logic as we did for Avogadro’s law, we can rewrite Boyle’s law to
relate the pressure and volume of the same gas under two different conditions.
For a change in either volume or (as in the case of the two balloons) pressure, we
can say,
P
initial
V
initial
= P
final
V
final
(at constant n, T)
Boyle investigated the relationship between pressure and volume using the
apparatus shown in Figure 10.12. Mercury was added to the open end of a
U-shaped tube so that air was trapped between the mercury and the closed end.
The height of the trapped air was indicative of the volume, V, of the gas. During
his studies, Boyle noted that the difference in the heights of the mercury on both
sides of the U-tube, plus the height of mercury at atmospheric pressure, 29
1
⁄8 in.
406 Chapter 10 The Behavior and Applications of Gases
FIGURE 10.11
Robert Boyle (1627–1691) was the
youngest of fourteen children born to a
wealthy Irish family. He was interested
in advancing his understanding of the
world at a very early age. He kept very
accurate observations about his experi-
ments and was one of the first to build
and use a vacuum pump.
4
3
2
1
0
1
2
h
3
4
FIGURE 10.12
Robert Boyle investigated the relationship between pressure and volume
using the apparatus shown. Mercury was added to the open end of a
U-shaped tube so that air was trapped between the mercury and the
closed end. The height (h) of the trapped air was indicative of the vol-
ume, V, of the gas. The difference between the heights of the mercury
on the two sides of the U-tube, plus the height of mercury at atmos-
pheric pressure, 29
1
⁄8 in. (740 mm), was indicative of the pressure, P.
Atmospheric pressure causes changes in the volume of these balloons.
Visualization: Boyle’s Law: A
Graphical View
Visualization: Boyle’s Law: A
Molecular-Level View
Video Lesson: Boyle’s Law

(740 mm), was indicative of the pressure, P. A plot of the pressure–volume rela-
tionship using Boyle’s actual data for air is shown in Figure 10.13. The plot shows
that the product P × V is fairly constant at these pressures, which are not too dif-
ferent from normal.
We began Section 10.3 by using food packaging to illustrate Dalton’s law of
partial pressures. Food packagers also acknowledge Boyle’s law when they allow
for the effect of higher altitudes (lower atmospheric pressures) on food packages.
In fact, the food packagers adjust the pressure in their packages for the specific
destination of the food items. For example, Figure 10.14 shows a bag of potato
chips in Estes Park Colorado, at an altitude of 2440 m (8000 ft). The bag, bought
in a store near sea level, is nearly ready to burst!
EXERCISE 10.5 Boyle’s Law
A teacher wants to know what volume her balloon will need to be if it is to hold
0.050 mol of hydrogen at 25
◦
C (as with the previous balloons) at a pressure of
1.3 atm. Using data for either sea level or Mexico City (provided in the main text),
what is the minimum volume her balloon must hold?
First Thoughts
In solving the problem, the key questions to consider are “What do we expect to
happen to the volume of the balloon as a result of increasing the pressure? Is it likely
to increase or decrease?” Framing good answers to these questions before we proceed
will give us an indication of whether our answer makes sense.
Solution
Using the Mexico City data, we get
P
initial
= 0.764 atm V
initial
= 1.6 L
P
final
= 1.3 atm V
final
= ??? L
P
initial
V
initial
= P
final
V
final
0.764 atm(1.6 L) = 1.3 atm(V
final
)
V
final
= 0.94 L
10.4 The Gas Laws—Relating the Behavior of Gases to Key Properties 407
0 204060
P (inches Hg)
80 100 120 140
50
100
150
200
250
300
350
400
0
PV
FIGURE 10.13
A plot of the pressure–volume relationship using
Boyle’s actual data for air. The fact that the product
P × V is fairly constant at these pressures testifies to
the care with which Boyle did these experiments. The
pressure was measured as the height of a mercury
column in inches, which is proportional to the pressure
of the system. The volume was measured as the height
of a column of air in inches, which is directly
proportional to the volume of the system.
FIGURE 10.14
This bag of potato chips is a lot bigger in
Estes Park, Colorado, at 2440 m, than at
sea level. This is one reason why food
manufacturers express the amount of
product that their packages contain in
terms of weight, rather than volume.
Application
