Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

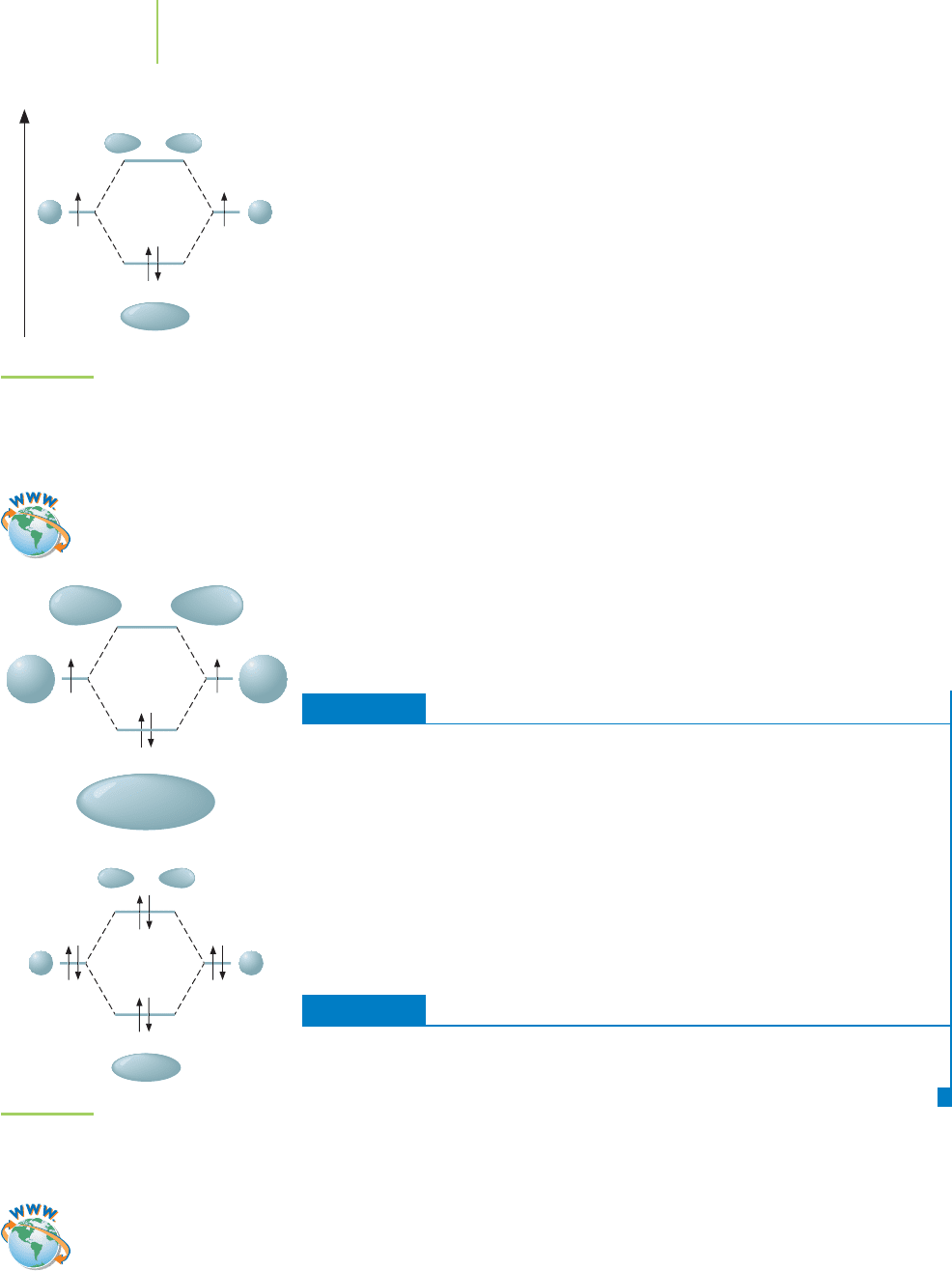
σ
1
s
σ
1
s
1
s
1
s
HO
M
O
LUM
O
E
ner
gy
H
H
*
FIGURE 9.23
MO diagram for H
2
. This diagram graph-
ically illustrates the energies and the
resulting orbital shapes of the different
molecular orbitals in the molecule.
*
σ
2
s
σ
2
s
2
s
2
s
H
O
M
O
L
U
M
O
σ
1
s
σ
1
s
1
s
1
s
Li
Li
*
FIGURE 9.24
Dilithium (Li
2
) MO diagram. The MO con-
figuration can also be written in short-
hand format as σ
1s
2
σ
*
1s
2
σ
2s
2
.
illustrate the energy of each of the bonding and antibonding orbitals and assist in
determination of the bond order for a molecule. Graphically, the shapes of each
of the molecular orbitals can be added to the diagram to get the overall picture of
the molecule.
We can draw the blueprint—the MO diagram—for molecular hydrogen (H
2
)
to illustrate how this is done. We show in Figure 9.23 that as the two hydrogen
atoms approach each other, their atomic 1s orbitals mix to provide two new mol-
ecular orbitals. The overlap of these orbitals possesses symmetry, which means
that they are equal in size and shape about the axis between the atoms (σ). One of
the molecular orbitals resulting from this overlap is lower in energy (the bonding
orbital, σ
1s
, read as “sigma one s”) and the other is higher in energy (the anti-
bonding orbital, σ
∗
1s
, read as “sigma one s star”). Placement of the two electrons
in H
2
follows the Pauli exclusion principle (two electrons with opposite spin per
orbital) and Hund’s rule (fill lowest-energy orbitals first) to fill the bonding
orbital. The antibonding molecular orbital is left empty. The
highest-energy occu-
pied molecular orbital (HOMO)
is the molecular orbital that contains electrons (in
this case, the bonding orbital). The antibonding orbital ends up as the
lowest-
energy unoccupied molecular orbital (LUMO)
. The bond order for hydrogen can be
calculated ((2 bonding electrons – 0 antibonding electrons)兾2) as 1. We therefore
predict that molecular hydrogen has a single bond between the two nuclei.
What would happen to the bond order for this molecule if one of the electrons
were promoted to the LUMO?
The bond order for such a molecule would be zero
((1 bonding electron – 1 antibonding electron)兾2). With no net bond between
the two atoms, the molecule would break into the individual hydrogen atoms.
EXERCISE 9.8 MO Theory: More Power, Scotty
We showed in Exercise 9.2 that dilithium (of Star Trek fame) can be explained by the
valence bond model. What does MO theory say about dilthium (Li
2
)?
Solution
The MO diagram for dilithium is shown in Figure 9.24. The HOMO is the σ
2s
orbital and the LUMO is the σ
∗
2s
orbital. The bond order for this molecule is
1 ((4 – 2)兾2), so MO theory suggests that Li
2
should exist as a molecule with one
bond between the two lithium atoms. The molecule does exist, but it quickly reacts
with other lithium atoms to make lithium metal. Because of this reactivity, it is not
possible to have a bottle of Li
2
.
PRACTICE 9.8
Use MO theory to determine whether He
2
is a stable molecule. What is the bond
order in this molecule?
See Problems 67, 68, and 71–74.
More About MO Diagrams
Lewis dot structures and valence bond theory agree that there are six bonding
electrons (that is, a triple bond) between the nitrogen atoms in gaseous nitrogen
(N
2
). The MO diagram for the molecule should verify that the theory also gives
rise to a bond order of 3. In molecules containing more than just the s orbitals,
the MO diagram becomes much more complicated. Figure 9.25 shows that the
overlap of the 2p
y
orbitals on each N and the 2p
z
orbitals on each N, produces
four orbitals: two π and two π
∗
. The overlap of the 2p
x
orbitals gives a sigma mol-
ecular orbital. If we now place the electrons in the diagram, we see that they fill
four bonding orbitals and one antibonding orbital. From the MO diagram, we
378 Chapter 9 Advanced Models of Bonding
Visualization: Sigma Bonding
and Antibonding Orbitals
Video Lesson: Applications of
the Molecular Orbital Theory
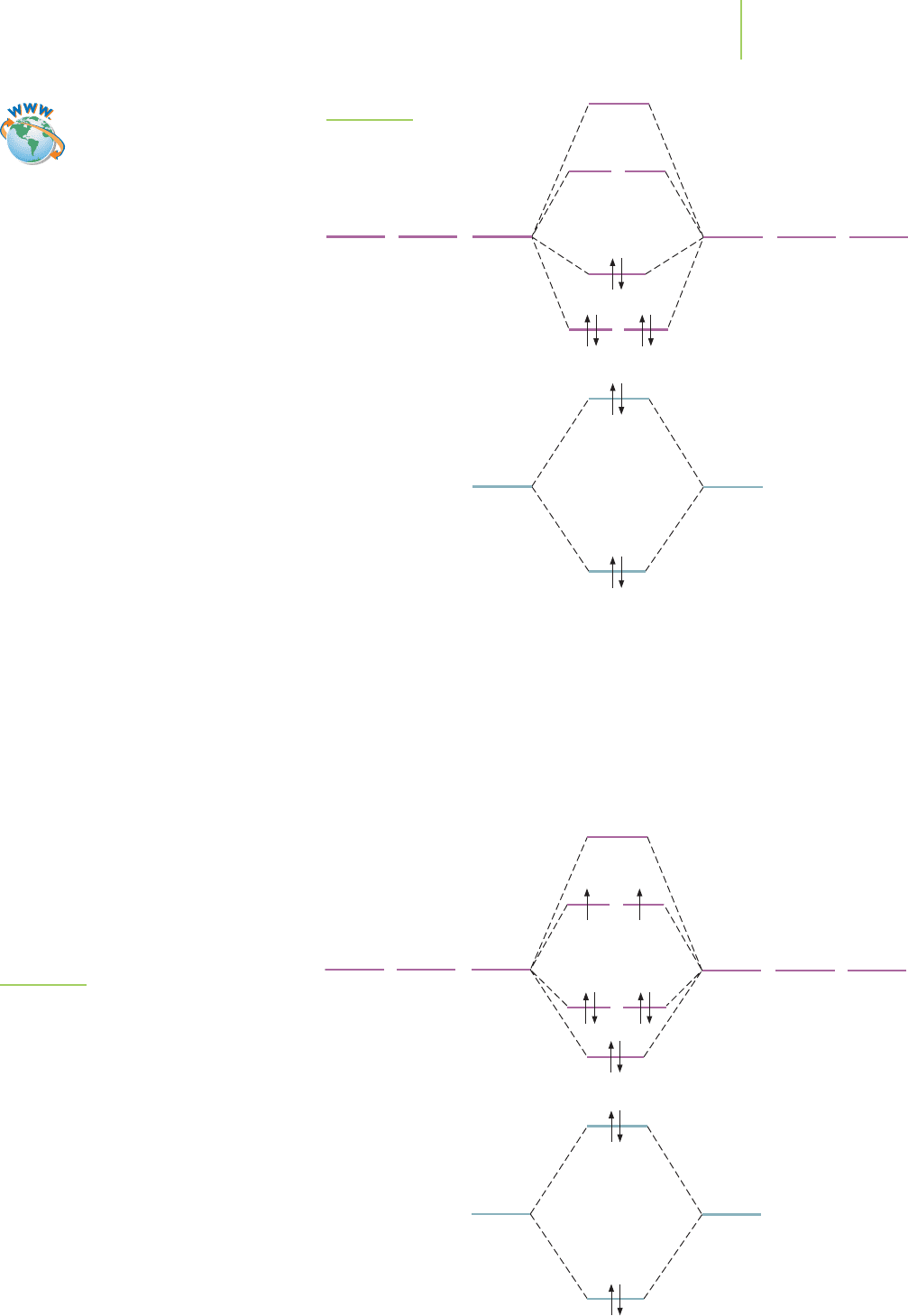
σ∗
2p
π
2p
σ
2p
π∗
2p
2p2p
σ∗
2s
σ
2s
2s 2s
σ∗
2s
σ
2s
2s 2s
σ∗
2p
σ
2p
π
2p
π∗
2p
2p2p
can calculate a bond order of 3 for molecular nitrogen, in
which the HOMO is the σ
2p
orbital and there are two
LUMOs (the degenerate π
2p
∗
orbitals).
The energy of the molecular orbitals in N
2
results in
the ordering shown in Figure 9.25. This is the same order-
ing that is observed in most of the diatomic molecules of
the second period of the periodic table. However, a differ-
ent ordering of the molecular orbitals is seen in O
2
,F
2
,
and Ne
2
, as shown in Figure 9.26. The change in the order
of the molecular orbitals arises because the difference in
energy between the 2s and 2p atomic orbitals on O, F, and
Ne is relatively greater than the other elements in the sec-
ond row (see Figure 9.7). Note that the two electrons in
the HOMO are not paired in O
2
. This allows us to ratio-
nalize a property of molecular oxygen that is unexplain-
able by other bonding theories. The other models of
molecular oxygen correctly assign two bonds between the oxygen atoms, but
oxygen possesses properties that we would not predict on the basis of those
models. Oxygen, shown in Figure 9.27, is an example of a paramagnetic mole-
cule. The term
paramagnetism refers to the ability of a substance to be attracted
into a magnetic field. This attraction arises because of the presence of unpaired
electrons within the molecule. A special case of this is
ferromagnetism (so
named because the effect is especially strong in iron), in which the paramag-
netic atoms are close enough together that they reinforce their attraction to the
9.3 Molecular Orbital Theory 379
FIGURE 9.25
MO diagram for N
2
.
FIGURE 9.26
MO diagram for O
2
. Note that molecular oxygen
has a different order for its molecular orbitals.
There are also two unpaired electrons in the MO
diagram. What is the bond order for O
2
?
Visualization: Molecular Orbital
Diagram (N
2
)

Liquid oxygen Liquid nitrogen
NS NS
(a) (b)
Energy
σ
2p
σ
2p
σ
2p
σ
2s
σ
2s
σ
2s
σ
2s
F–F FF
*
**
σ
2p
*
π
2p
*
π
2p
π
2p
π
2p
*
FIGURE 9.28
MO diagram of F
2
. The promotion of an electron from a π
MO to the σ
∗
MO reduces the bond order from 1 to 0. When
this happens, there is no net bond remaining between the
atoms. Addition of a photon of light to the molecule with a
wavelength of 754 nm causes the molecule to break in two.
magnetic field, so the whole is, in effect, greater than the sum of its parts. The
opposite of paramagnetism is
diamagnetism. A diamagnetic molecule’s electrons
are paired, resulting in the molecule being repelled from a magnetic field. Ni-
trogen (N
2
) is an example of a diamagnetic molecule because all of its electrons
are paired (see Figure 9.25).
Constructing models of molecules made with more than two atoms or
molecules made from two different atoms (heteronuclear diatomic molecules)
complicates the molecular orbital diagram because similar atomic orbitals on
each atom have different energy levels. A further complication is added because
the orbitals encompass the entire molecule and therefore must be constructed
from the linear combination of the atomic orbitals on every atom in the mole-
cule. For a 10-atom molecule, that would mean that each molecular orbital
would arise from the combination of 10 different atomic orbitals. Such a diagram
would be very complex indeed!
EXERCISE 9.9 MO Theory: The Power of a Photon
The halogens (F
2
,Cl
2
,Br
2
, and so on) are extremely reactive compounds. In fact,
their reaction can be initiated by a single photon of light, as we note at the start of
this chapter when we discussed 11-cis-retinal in the eye. Given that the photon will
promote one electron from a bonding orbital to an antibonding orbital of similar
symmetry, illustrate the reaction of F
2
with that photon using MO diagrams. The
MO diagram for F
2
is similar to that for O
2
.
Solution
The MO diagram for F
2
is shown in Figure 9.28. Promotion of an electron from the
π
2p
orbital to an MO of the same symmetry, the σ
∗
2p
orbital, would cause the bond
order for F
2
to become zero. The photon would effectively break the bond between
the fluorine atoms. The reaction of photons with chlorofluorocarbons in the ozone
layer similarly breaks COCl bonds. The resulting chlorine atoms are extremely
reactive toward ozone (O
3
).
380 Chapter 9 Advanced Models of Bonding
FIGURE 9.27
The results of paramagnetism and dia-
magnetism. Molecular oxygen is para-
magnetic. Because of this, liquid oxygen
is attracted to a magnetic field (a). Liquid
nitrogen is diamagnetic and is repelled
from the magnetic field (b).
Visualization: Magnetic
Properties of Liquid Nitrogen
and Oxygen
Video Lesson: CIA
Demonstration: The
Paramagnetism of Oxygen
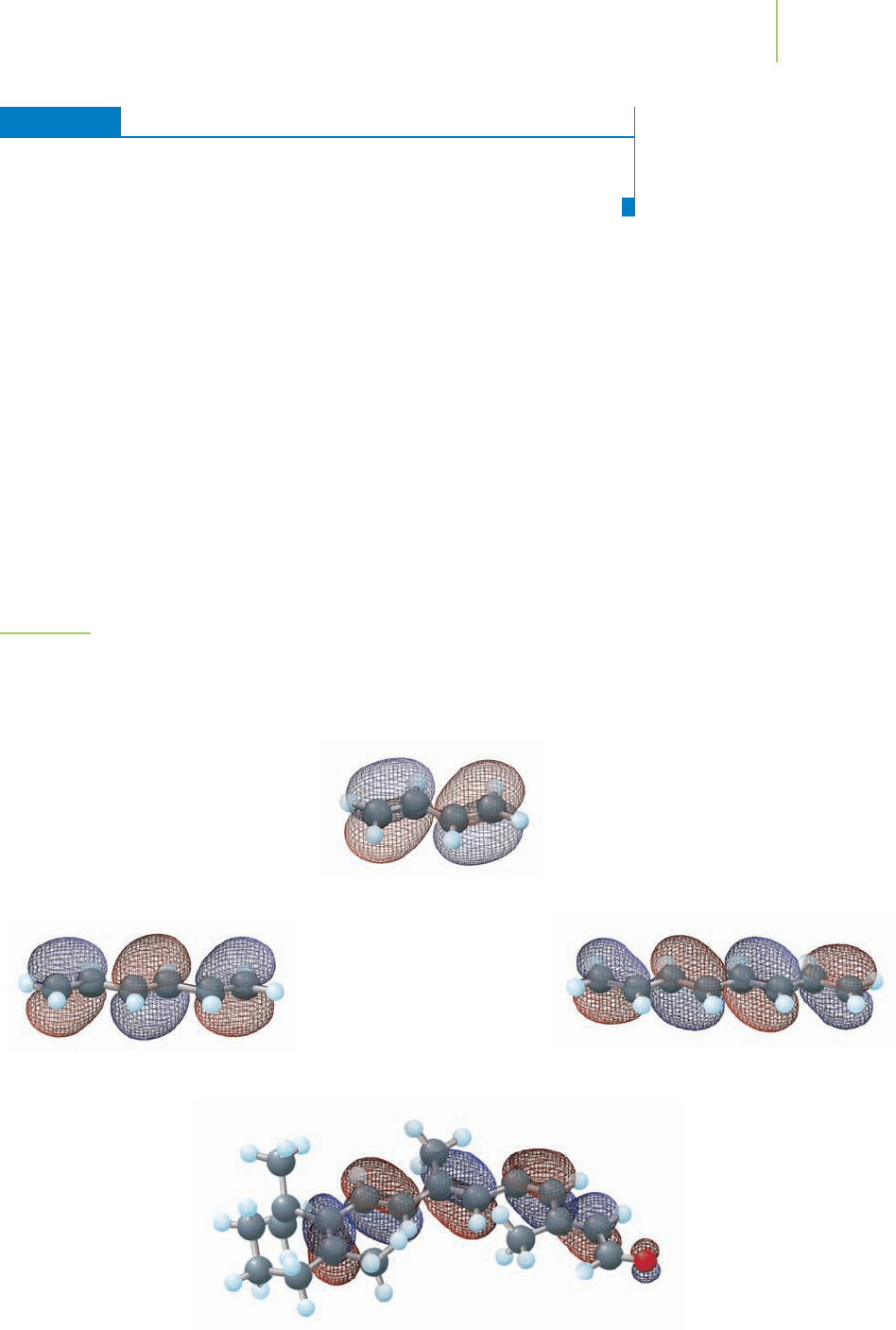
FIGURE 9.29
The MOs of conjugated systems. As the length of the conjugated π system increases, the energy
required to promote an electron decreases. Evidence of this is observed in the wavelength of
light absorbed by the molecules. Longer
π systems require less energy and a larger wavelength
(energy and wavelength are inversely proportional, E
=
hc
__
λ
).
PRACTICE 9.9
Illustrate the interaction of H
2
and O
2
with a single photon of light. After the pro-
motion of an electron, which molecule has the larger bond order?
See Problems 65 and 66.
Looking back to 11-cis-retinal, we can produce an MO diagram that
illustrates the location of the electrons in the conjugated π system. Although the
diagram is unusually complex, the energy levels of the conjugated π system are
more closely spaced as the π system increases in length. Promotion of an elec-
tron requires less and less energy as the π system lengthens, as indicated by the in-
creasing wavelength of absorbed light in the molecules of Figure 9.29. This occurs
because it takes less energy to promote an electron to a closer molecular orbital.
We pointed out earlier that this phenomenon is used by the photographic indus-
try in the development of color print film. Here’s how. By constructing a
molecule that has a conjugated π system of just the right length, photo manufac-
turers can make molecules that absorb exactly the color needed to make your
pictures really stand out. We develop this idea further in the accompanying
NanoWorld/MacroWorld feature.
9.3 Molecular Orbital Theory 381
1,3-Butadiene
λ = 217 nm
1,3,5,7-Octatetraene
λ = 304 nm
cis-Retinal
λ = 325 nm
1,3,5-Hexatriene
λ = 268 nm

382 Chapter 9 Advanced Models of Bonding
“Smile, you’re on candid camera!” Everyone hopes never
to be ambushed with those words. In the nineteenth cen-
tury, when photography was in its infancy, candid pho-
tographs were not possible. In order to create a visual
memory of an event, you had to visit the photographer
and sit for a picture.“Sitting” meant holding a pose for
up to 30 seconds. Any movement would show as a blur
in the final picture. However, the idea that an inexpen-
sive, yet realistic, picture could be created in such a short
time brought people to the photographer’s studio in
droves.
Scientists in the early 1800s were intrigued by the
idea of painting portraits without the artist’s pen. In the
summer of 1827, Joseph Nicéphore Niépce, using mater-
ial that hardened on exposure to light exposed film for
eight hours in order to get a picture. Because no one
wanted to sit still that long, most of his pictures were
landscapes. In 1839, however, two other photographic
processes were developed, the daguerreotype (perfected
by Louis Daguerre) and the calotype (Henry Fox Talbot).
Although these techniques allowed pictures to be created
with much shorter exposures, they still produced a
black-and-white image (see Figure 9.30). This didn’t
matter much to the general public, and photographic
portrait studios seemed to pop up on every corner across
the globe. Despite some marginal success in producing
faint color images during the 1850s and 1890s, the ad-
vent of the color photograph as an inexpensive, repro-
ducible and stable process didn’t occur until the 1920s.
Researchers at Kodak Research Laboratories in 1935
finally hit upon the process that would bring the color
photograph to the world. Kodachrome®, their color
photography process, introduces color to a picture
by separating the three primary colors into specific
emulsion layers. How does it work? The emulsion layers
contain a compound that reacts when light strikes it. A
quick look at the chemistry behind the black-and-white
photograph will give us some insight.
In black-and-white photography, a piece of transpar-
ent plastic is coated with silver bromide crystals (see Fig-
ure 9.31) and a sensitizer. The plastic sheet is then placed
in a camera, and when the shutter is opened, the silver
bromide absorbs photons of light. In the presence of the
sensitizer, the silver cation is reduced by the sensitizer
(gains an electron) and becomes silver metal:
Ag
+
(s) + sensitizer n Ag°(s) + sensitizer
+
Then the film is wound back into its container and
sent to be developed. The technician rinses the plastic
film to remove unreacted silver bromide. The silver
metal remains in place on the plastic. The result is an in-
verted image called a negative. Next the technician shines
light through the negative onto another silver bromide/
sensitizer-coated support (typically paper this time) and
rinses off the unreacted silver bromide. Violà! We have a
positive image called a photograph.
Why is the film coated with silver bromide instead of
silver chloride? We can answer this by examining the
wavelength of light that is absorbed; see Figure 9.32. Com-
parison of the two shows that silver bromide absorbs a
large amount of the visible light that enters our camera.
To get the best picture, we want to absorb as much light
as we can.
NanoWorld / MacroWorld
Big effects of the very small:
Color photography and the tricolor process
FIGURE 9.30
A daguerreotype of Michael Faraday
(see Chapter 19) taken sometime
between 1844 and 1860 at Mathew
Brady’s studio in New York City.
Because the process required the
subject to remain completely
motionless for up to 10 seconds,
most daguerreotypes were taken
in well-lit studios. Even though
the early daguerreotypes cost one
month’s wages for the average
person, they were much cheaper
than a painted portrait. This made
them an instant success.
FIGURE 9.31
A magnified image of silver bromide crystals used in the photographic
industry. Note that the crystals are mostly hexagonal in shape and flat
so that more surface area is exposed to incoming photons.
9.3 Molecular Orbital Theory 383
Silver bromide, though, doesn’t absorb the entire
spectrum of visible light. What color of light does silver
bromide absorb? Figure 9.32 shows that it absorbs blue
light. In order for us to make a full-color photograph, we
need to make some of the silver bromide on the plastic
absorb yellow light and some absorb red light. By mixing
these primary colors (blue, yellow, and red), we can recre-
ate the entire spectrum of colors. This is what happens in
the tricolor film process. How, then, can we make silver
bromide absorb a different color of light?
The process requires coupling the silver bromide
with a dye molecule. These molecules are designed to ab-
sorb different colors by varying the length of their conju-
gated pi bonds. When the photon is absorbed into this
system, an electron in a π MO is promoted to a π
∗
MO,
and the dye molecule becomes excited. As with all
excited molecules, many fates exist for the pro-
moted electron. First, the molecule could release
a photon and return the promoted electron to
the
π MO, which would result in the original dye
molecule. The released photon would have the
same energy as the absorbed photon. Second,
the electron could collapse back to the π MO
through thermal excitation. In this process, the
dye molecule would release the energy by vibrat-
ing. Third, the electron could transfer to another
MO on another molecule. This is the fate that
gives rise to the photographic reaction.
The excited electron transfers from the dye
molecule to an antibonding orbital on the silver
bromide. And as it does so, our silver cation is re-
duced to silver metal. Even though the silver bro-
mide was unable to absorb the non-blue photon
of light, it still ended up as silver metal. Therefore,
all we have to do to make a color photograph is
find two dye molecules that can transfer electrons
to the silver bromide. One should absorb yellow
light and the other should absorb red light. The exact
structures of these molecules are a closely guarded secret
in the photography industry, but the compounds shown
in Figure 9.33 have been used to do this in the past. Each
has an extended pi system that allows the molecules to
absorb light in the visible region of the spectrum.
Current advances in the photography industry in-
clude the development of four-color processes. These
processes make possible better definition of objects and
a more brilliant picture. Development of better ways
to coat the silver bromide evenly on plastic and the
preparation of silver bromide crystals that give the best
interaction with light are at the forefront of current re-
search. One day these developments may be able to com-
pletely eliminate “red eye” and make it easier to tolerate
being the subject of a candid shot.
Wavelength (nm)
Absorption coefficient (cm
–1
)
10
–2
260220 300 340 380 420 460 500 540 580
2.1 eV2.52.93.33.7
10
–1
10
0
10
1
10
2
10
3
10
4
10
5
10
6
AgCl
AgBr
FIGURE 9.32
Spectrum of silver bromide and silver chloride. Silver bromide absorbs more
of the visible spectrum than silver chloride. What color is silver bromide?
Basic Red 9, a magenta dye
NH
2
H
2
NNH
2
Cl
A cyan dye
H
3
C
C
H
C
H
I
C
C
H
C
H
C
H
C
H
C
H
C
S
HC
C
H
C
N
CH
3
C
H
C
H
C
C
S
CH
C
H
C
N
FIGURE 9.33
Dyes used in the photographic industry. Note the extended pi
bond networks in these molecules. The length of the conjuga-
tion is related to the wavelength of light that these molecules
can absorb.
OOH
OH
OH
O
Purpurin, a magenta dye
9.4 Putting It All Together
The models of each of the bonding theories provide increasingly better cor-
relation with the observed properties of many covalent compounds. Valence
bond theory works well at showing how the electrons in sigma bonds come to-
gether to create the skeletal framework of a molecule. At the same time, the local-
ized bonding picture described by valence bond theory conveniently determines,
among other things, the lengths of bonds in a molecule. The rules of VSEPR
modeling help identify the three-dimensional shape of the molecule, and Lewis
dot structures rapidly reveal the connectivity of atoms in a molecule. However,
molecular orbital theory works best at describing the behavior of electrons lo-
cated in the bonds.An approach to drawing a molecule that uses all of these mod-
els is often followed. We can show this approach using benzene (C
6
H
6
).
Benzene is a carcinogenic compound that is widely used in industry as a non-
polar solvent and as a starting compound in the manufacture of other useful
products such as phenol (an antiseptic compound) and nylon. Experiments have
revealed that benzene is a hexagon of six carbon atoms, each bonded to one hy-
drogen atom. It has also been observed experimentally that the distances between
carbon atoms in the molecule are identical. In the laboratory, benzene reacts as
though it has CPC bonds at each position in the molecule.
No single Lewis dot structure of benzene can be drawn to illustrate the exper-
imentally determined bond lengths and reactivity in the molecule. Considering
that benzene is best shown as a hybrid of two resonance structures, we can ad-
dress the experimental observation that all COC bonds in benzene are the same
length as shown in Figure 9.34. Valence bond theory can
also be used to indicate that each carbon is sp
2
hy-
bridized. The sigma bond network that makes up the
molecule defines 120° bond angles at each flat sp
2
hy-
bridized carbon. Bonds between carbon atoms result
from the overlap of a 2sp
2
hybrid orbital with another
2sp
2
hybrid orbital. Each COC bond is 140 pm in length.
Bonds between the carbon and hydrogen atoms are the
result of 2sp
2
–1s orbital overlap. In addition, each carbon
atom contains a half-filled 2p orbital perpendicular to the
plane of the molecule.
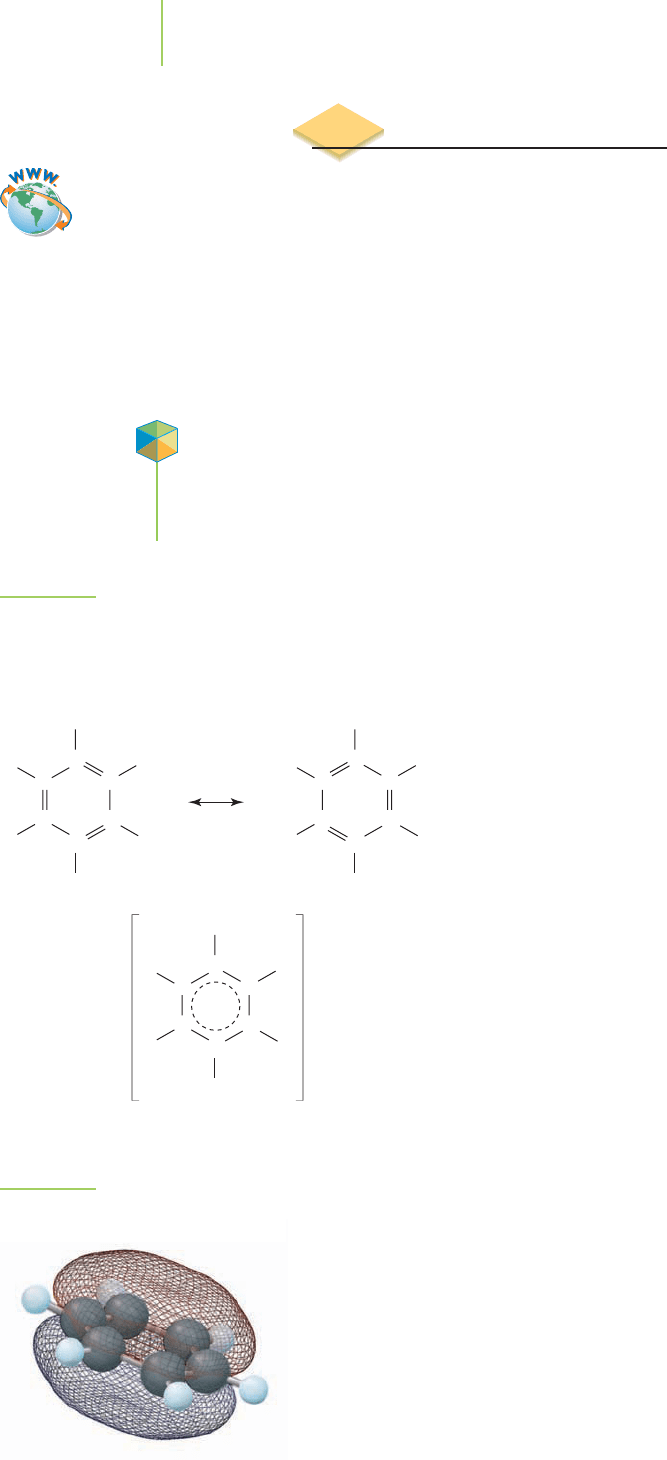
However, the six half-filled 2p orbitals in benzene are
best handled by molecular orbital theory. The σ bond
network is separated from the π bond network in this ap-
proach. Doing so makes preparing a MO diagram a more
manageable task. The six 2p orbitals on the carbon atoms
in benzene have the same symmetry and energy, so they
can be mixed to provide six new π MOs. Three bonding
MOs and three antibonding MOs result (see Figure 9.35). We place the six elec-
trons into the new MOs, filling the three bonding MOs. The electrons in the MO
diagram occupy a π bond at every carbon atom, implying that there isn’t any dif-
ference in the carbons of benzene. Each COC bond has a bond order of 1.5. The
approach we have taken to draw the best model of benzene has provided us with
the best (and quickest) way to represent the experimentally determined proper-
ties of benzene (such as the COC bond lengths and the reactivity of benzene). We
no longer have to imagine different structures (arrived at from different models)
of benzene to account for the observed properties.
If we more closely examine the molecular orbital model of benzene, we find
that the electrons in the π orbitals are spread out over the six atoms in the struc-
ture. This conjugated π system is said to be
delocalized. In fact, delocalized sys-
tems occur any time the electron density in a molecule can be distributed among
384 Chapter 9 Advanced Models of Bonding
Application
C
HEMICAL
ENCOUNTERS:
Benzene, Stability,
and MO Theory
H
HC
H
H
C
H
CC
C
C
H
H
HC
H
H
C
H
CC
C
C
H
H
HC
H
H
C
H
CC
C
C
H
Resonance hybrid
FIGURE 9.34
Delocalized π bonding in benzene.
Lewis dot structures and valence bond
models do a good job of describing
the sigma bond network in benzene.
FIGURE 9.35
Delocalized π bonding in benzene accord-
ing to MO theory.
Visualization: Delocalized
Pi Bonding in the Nitrate Ion
more than two atoms. Delocalization also can be seen in the carbonate ion
(CO
3
2–
) and in 11-cis-retinal. However, in the case of benzene (C
6
H
6
) and related
compounds, the delocalization of the electron density imparts a particular stabil-
ity to the molecule (more so than the delocalization of electrons in the carbonate
ion and 11-cis-retinal). The stability is so important to the molecule that it is
often written as shown in Figure 9.36, with a circle to illustrate the delocalization
of three π bonds in the molecule. Benzene’s stability makes it a good choice for
use as a reaction solvent. Unfortunately, this stability also makes it difficult to me-
tabolize if accidentally ingested. One of the major problems with using benzene
is that it causes cancer in humans. Table 9.6 lists certain properties of benzene
and of some alternative solvents that have been used in place of it. Their proper-
ties aren’t identical to those of benzene, but their substitution for benzene has
done a great deal to safeguard the health and lives of industrial chemists.
9.4 Putting It All Together 385
π∗
π
π
π∗
π
π∗
Energy
Molecular orbital diagram for benzene.
H
H
C
H
H
C
H
CC
C
C
H
FIGURE 9.36
Shorthand notations for benzene.
Common Solvents
Solubility
Solvent Structure Boiling Point Dipole Moment in Water
Benzene C
6
H
6
80.1°C 0 Very slight
Hexane C
6
H
14
68.7°C 0 Insoluble
Dichloromethane CH
2
Cl
2
40°C 1.60 Slight
Te t r a h y d r o f u r a n C
4
H
8
O 66°C 1.63 Miscible
To l u e n e C
7
H
8
111°C 0.36 Very slight
TABLE 9.6
Application

386 Chapter 9 Advanced Models of Bonding
9.5 Molecular Models in the Chemist’s Toolbox
Just as the best models in civil engineering give the best information on the out-
come of a construction project, our happiness with a particular molecular model
is based on our satisfaction with the information that the model provides. The
more rigorous models provide better information, but at a cost in time and
calculations that we may not be willing to accept. Valence bond theory quickly
determines the skeletal framework of a molecule by considering separate parts of
the molecule. It does not explain the molecule as a whole, nor does it consider
how electrons can be delocalized between more than two atoms. Molecular or-
bital theory, on the other hand, considers the molecule as a whole. It explains the
bonding patterns in detail, providing information about the energy of the elec-
trons in a particular orbital and the properties of the molecule. However, detailed
molecular orbital theory is difficult to solve mathematically. Using approxima-
tions of molecular orbital theory, such as the LCAO–MO model, considerably
reduces the amount of time involved in determining the model, but at the ex-
pense of some of the detail provided by the theory. All of the models we have
discussed—Lewis dot structures, VSEPR, valence bond theory, and molecular
orbital theory—have a place in the toolbox of the chemist.
The Bottom Line
■
The molecular models that chemists construct
increase in complexity from Lewis dot structures,
to the VSEPR model, to valence bond theory, to
MO theory. This increase in complexity is accom-
panied by an increase in satisfactory agreement
with observed properties for the molecule.
(Section 9.1)
■
Valence bond theory, originated by Linus Pauling,
defines bonds as the overlap of atomic orbitals.
Sigma bonds result from end-on overlap of orbitals;
pi bonds result from side-to-side overlap of orbi-
tals. (Section 9.1)
■
Hybridization of atomic orbitals gives rise to new
orbitals that help explain bonding. Hybridization
also gives structures consistent with the VSEPR
model. (Section 9.2)
■
Mixing n atomic orbitals gives n hybridized orbitals.
(Section 9.2)
■
Hybridization can be used to explain the existence
of sigma (σ) and pi (π) bonds in molecules. (Sec-
tion 9.2)
■
Molecular orbital theory defines bonding with
orbitals that are not confined to a single atom.
Bonds result from molecular orbitals in a molecule
that encompass the atoms. (Section 9.3)
■
Mixing n atomic orbitals gives n molecular orbitals.
One-half n of these are bonding; the other half are
antibonding. Bonding MOs are lower in energy
than the atomic orbitals from which they are con-
structed. Antibonding MOs are higher in energy
than the atomic orbitals from which they are con-
structed. (Section 9.3)
■
The Pauli exclusion principle and Hund’s rule must
be obeyed when placing electrons in the new MOs.
(Section 9.3)
■
MO diagrams can be used to identify the number
of bonds between atoms. (Section 9.3)
■
Paramagnetism results from unpaired electrons in
a molecule. Diamagnetism results from complete
pairing of all electrons in a molecule. (Section 9.3)
■
Electrons in conjugated π orbitals are said to be
delocalized, because the electron density can be
distributed among more than two atoms.
(Section 9.4)
Focus Your Learning 387
antibonding orbital An orbital that indicates a lack of
electron density between adjacent nuclei (no bond
exists when the orbital is occupied). The antibonding
orbital arises from the subtraction of two overlapping
atomic orbitals. (p. 375)
bond order The number of electrons in bonding orbitals
minus the number of electrons in antibonding or-
bitals, divided by 2. The bond order indicates the de-
gree of constructive overlap between two atoms.
(p. 377)
bonding orbital An orbital that indicates the presence
of electron density between adjacent nuclei (a bond
exists when the orbital is occupied). The bonding or-
bital arises from the addition of two overlapping
atomic orbitals. (p. 375)
conjugated π bonds An extended series of alternating
single and double bonds. (p. 374)
conjugation The presence of conjugated π bonds—that
is, a series of at least two double bonds alternating
with single bonds. (p. 374)
delocalized Term used to describe a π system wherein
the electron density in a molecule can be distributed
among more than two atoms. (p. 384)
diamagnetism The ability of a substance to be repelled
from a magnetic field. This property arises because all
of the electrons in the molecule are paired.
(p. 380)
ferromagnetism A property of a compound that occurs
when paramagnetic atoms are close enough to each
other (such as in iron) that they reinforce their at-
traction to the magnetic field, such that the whole is,
in effect, greater than the sum of its parts. (p. 379)
highest-energy occupied molecular orbital (HOMO) The
most energetic molecular orbital that contains at least
one electron. (p. 378)
hybridization The mathematical combination of two or
more orbitals to provide new orbitals of equal energy.
(p. 363)
linear combination of atomic orbitals–molecular orbitals
(LCAO–MO) theory
An approximation of molecular
orbital theory wherein atomic orbitals are added
together (both constructively and destructively) to
make molecular orbitals. (p. 375)
lowest-energy unoccupied molecular orbital (LUMO) The
least energetic molecular orbital that contains no
electrons. (p. 378)
molecular orbital (MO) diagram A diagram used to illus-
trate the different molecular orbitals available on a
molecule. (p. 377)
molecular orbital (MO) theory A theory that mathemati-
cally describes the orbitals on a molecule by treating
each electron as a wave instead of as a particle.
(p. 375)
nodal planes Flat, imaginary planes passing through
bonded atoms where an orbital does not exist.
(p. 370)
paramagnetism The ability of a substance to be attracted
into a magnetic field. This attraction arises because of
the presence of unpaired electrons within the mole-
cule. (p. 379)
pi bond (π bond) A covalent bond resulting from side-
to-side overlap of orbitals. The pi bond possesses
a single nodal plane along the axis of the bond.
(p. 370)
sigma bond (σ bond) A covalent bond resulting from
end-on overlap of orbitals. The sigma bond does not
possess a nodal plane along the axis of the bond.
(p. 369)
symmetry The property associated with orbitals that
have similar size and shape. (p. 376)
valence bond theory The theory that all covalent bonds
in a molecule arise from overlap of individual valence
atomic orbitals. A modification of this theory allows
valence atomic orbitals to include hybridized orbitals.
(p. 359)
Focus Your Learning
The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
Section 9.1 Valence Bond Theory
Skill Building
1. a. Professional scientific journal articles, textbooks, and
popular writing have all depicted chemical bonds as a
typed dash between two atomic symbols. Although this
does communicate that the atoms are associated with
each other, cite one reason why it does not accurately
describe a chemical bond.
b. Why do chemists seek to provide better models to describe
the makeup of a chemical bond?
2. The VSEPR model of chemical bonding has been very suc-
cessful for some descriptions of chemical bonding, but what
is the major weakness of this popular model?
3. Write out the ground-state configuration of silicon. Accord-
ing to the VSEPR model, how many bonds should one atom
of Si be able to form? Is your answer consistent with the for-
mula of SiCl
4
(a compound used in the formulation of some
smoke screens)?
Key Words
