Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


4. Write out the ground-state configuration of aluminum.
According to the VSEPR model, how many bonds should one
atom of Al be able to form? Is your answer consistent with the
formula of AlCl
3
?
5. Write the ground-state electron configuration for each of the
following atoms. According to the VSEPR model, how many
bonds should each atom be able to form?
Cl Se B
6. Write the ground-state electron configuration for each of the
following atoms. According to the VSEPR model, how many
bonds should each atom be able to form?
Ge Ne Mg
Chemical Applications and Practices
7. Carbon tetrachloride (CCl
4
) is seldom used as a cleaning sol-
vent. It formerly saw widespread use, but more recently its ill
effects on human health have led to its restriction. What is
the ground-state designation for carbon’s valence electrons?
Explain why this configuration does not lend itself to form-
ing the four equal bonds found between carbon and chlorine
in this compound.
8. Tin can form compounds such as SnCl
2
and SnCl
4
. What is
the ground-state designation for tin’s valence electrons? Ex-
plain why this configuration does not lend itself to forming
the equal bonds found between tin and chlorine in one of
these compounds.
Section 9.2 Hybridization
Skill Building
9. a. Write out the electron configuration of hydrogen and
bromine.
b. What valence orbitals are involved in the overlap that
forms the bond between H and Br?
c. Compare the strength of this bond to the strength of the
HF bond described earlier in the chapter.
10. a. Write out the electron configuration of hydrogen and
chlorine.
b. What valence orbitals are involved in the overlap that
forms the bond between H and Cl?
c. Compare the strength of this bond to the strength of the
HF bond described earlier in the chapter.
11. Using valence bond theory, describe the orbital overlap in
Cl
2
.
12. Using valence bond theory, describe the orbital overlap in F
2
.
13. Using the concept of orbital overlap, predict in each pair of
compounds which bond will be longer:
H
2
or Cl
2
Br
2
or Cl
2
HCl or HBr
14. Using the concept of orbital overlap, predict in each pair of
compounds which bond will be longer:
H
2
or Li
2
F
2
or Cl
2
LiF or HF
15. Hypochlorous acid has excellent bleaching properties and
must be handled with care. Using valence bond theory, show
what orbitals are likely to be overlapped in HOCl. Which
bond would you predict to be longer, the bond between H
and O bond or the bond between O and Cl? Explain the basis
of your prediction.
388 Chapter 9 Advanced Models of Bonding
16. Look at the ground-state electron configurations of the
atoms in the HOCl compound mentioned in the previous
problem. Why do you think that the structure presented as
HOCl more logically represents the bonding than HClO?
17. Predict the formula of potassium hydride. Which hydride
would you predict to have the stonger bonds, potassium
hydride or cesium hydride? Explain the basis of your
prediction.
18. What would you predict as the formula of calcium hydride?
Which hydride would you predict to have the stronger bonds,
calcium hydride or magnesium hydride? Explain the basis of
your prediction.
19. When explaining and demonstrating orbital hybridization to
chemistry students, a teacher prepares “orbital omelets.” This
analogy involves mixing one, two, or three eggs with 100 mL
of milk. How could the three omelets be used to describe
three ways in which s and p orbitals can hybridize?
20. Develop an analogy similar to that in Problem 19 using an
“orbital milkshake.” Mix one, two, or three scoops of ice
cream with one glass of milk. How could the three resulting
milkshakes be used to describe three ways in which s and p
orbitals can hybridize?
21. In some situations d orbitals may become involved in hy-
bridization.
a. If an atom’s hybridization were designated sp
3
d
2
,how
many orbitals would be involved?
b. What would be the same and what would be different
about these orbitals compared to hybrid orbitals made
from only s and p orbitals?
22. In many sciences, including chemistry, we are sometimes
forced to use the same symbols to mean different things. In
each of the following examples, distinguish the meaning of
the two sets of similar symbols:
a. 2s
1
2p
1
and (2sp)
1
b. (2sp
3
) and 2p
3
23. a. Show the ground state for the valence electrons of silicon.
What type of hybridization would the orbitals of silicon
undergo to produce SiH
4
?
b. What would be the resulting geometric shape of a SiH
4
molecule?
24. BCl
3
and NH
3
have the same basic formula (one central atom
with three attached atoms), but they differ in shape. Using
the hybridization of their valence orbitals, explain the basis
for their different shapes.
25. The bonding in H
2
O, NH
3
, and CH
4
can be explained by
using sp
3
hybridization of the valence electrons in oxygen,
nitrogen, and carbon, respectively. However, all three mole-
cules have different shapes. What are the bond angles for the
three molecules and what is the basis for each?
26. SiCl
4
and SCl
4
both contain a central atom attached to four
other atoms. After examining the ground state of Si and S
and bonding four chlorine atoms to each, would you predict
the two molecules to have the same shape? Explain the basis
for your conclusion.
27. The carbon atom in both methane (CH
4
) and chloroform
(CHCl
3
) has undergone sp
3
hybridization. However, the two
molecules do not have exactly the same shape. Explain the
cause and result of any differences.
28. The oxygen atom in both H
2
O and HOF has undergone sp
3
hybridization. However, the two molecules do not have
exactly the same shape. Explain the cause and result of any
differences.
29. How many hybrid orbitals are formed from mixing an s
orbital with a p orbital?
30. How many hybrid orbitals are formed from mixing an s
orbital with two p orbitals?
31. For each hybridization listed below, indicate the number of
bonds that could be formed by overlapping with the hy-
bridized orbitals.
sp sp
2
sp
3
32. For each type of hybridization listed below, indicate the ide-
alized geometric shape that would be produced around a
central atom having that type of hybridization.
sp sp
2
sp
3
33. What type of hybridization would be found in the central
atom of each of the following?
a. OF
2
b. CCl
4
c. BCl
3
d. BeCl
2
34. What type of hybridization would be found in the central
atom of each of the following?
a. CS
2
b. H
2
S c. CSCl
2
d. SO
2
35. Indicate what geometric shape is produced when the number
of orbitals around a central atom is:
a. 2 b. 3 c. 4 d. 5 e. 6
36. Indicate what bonding angle is produced when the number
of orbitals around a central atom is:
a. 2 b. 3 c. 4 d. 5 e. 6
37. Diagram the hybridization for the following three hydro-
carbons:
C
2
H
2
C
2
H
4
C
2
H
6
Energy is required to break chemical bonds. In which of
the three molecules would the least amount of energy be
required to break the carbon-to-carbon bond. Explain, or
justify your answer.
38. Diagram the hybridization of the carbon in each of the fol-
lowing compounds:
CO
2
CO CO
3
2−
Energy is required to break chemical bonds. In which of
the three molecules would the least amount of energy be
required to break the carbon-to-oxygen bond. Explain, or
justify your answer.
39. Sulfur is capable of many different oxidation states. In each of
the following, determine the hybridization of sulfur and the
resulting shape of the molecule or ion.
a. SO
3
b. SO
3
2−
c. SF
6
d. S
8
40. Nitrogen is capable of many different oxidation states. In
each of the following, determine the hybridization of nitro-
gen and the resulting shape of the molecule or ion.
a. NO
3
−
b. NO c. NO
2
d. N
2
41. Predict the hybridization of the underlined atom and the
overall shape of the following molecules.
a. N
H
4
+
b. XeF
4
c. SF
4
d. NO
2
−
42. Predict the hybridization of the underlined atom and the
overall shape of the following molecules.
a. HC
Nb.ClF
3
c. C
lF
5
d. I
Cl
3
43. Predict the hybridization of each of the atoms in the follow-
ing molecule.
44. Predict the hybridization of each of the atoms in the follow-
ing molecule.
Chemical Applications and Practices
45. In the realm of science fiction, as we noted earlier, dilithium
was used to propel the matter–antimatter drive of the star-
ship Enterprise. (Some of the details are still a bit elusive.)
Suppose a Klingon engineer suggests that dipotassium might
also be a possible fuel. Would you argue that K
2
would have a
stronger or a weaker bond than that found in Li
2
? What
would be the basis of your argument? (It will be best if you
can cite some sound basis in valence bond theory for your
argument. Klingons can be defensive when challenged.)
46. Several noble gas compounds have been synthesized under
controlled conditions. Explain, using ground-state electron
configurations and valence bond theory, why no diatomic
molecules such as He
2
,Ne
2
, and the like have been made.
47. One of the first synthesized“natural”organic compounds was
the nitrogen-based solid found in mammal waste: urea. From
examining the formula shown here, what would you predict
for the hybridization of the nitrogen atoms? What would be
the bond angle from hydrogen to nitrogen to carbon?
48. Three compounds composed of carbon and hydrogen can be
used to illustrate the importance of hybridization and molecu-
lar shape. Diagram the Lewis dot structure for each case shown
here. Then predict the hybridization in the carbon atoms, and
predict the bond angle from H to C to C in each structure.
a. C
2
H
2
(acetylene, used in high-temperature welding)
b. C
2
H
4
(ethylene, a plant hormone that hastens ripening)
c. C
2
H
6
(ethane,a heating fuel and an ingredient in polymers)
49. The structure of the artificial sweetener aspartame is shown
on the next page. How many pi bonds are shown in the struc-
ture? What would be the hybridization and bond angle
H
2
NC
O
NH
2
H
H
B
Cl
C
Cl
C
H
OH
OH
CC N
H
H
H
Focus Your Learning 389
around the carbon atom double-bonded to oxygen at the top
of the molecule?
50. The male sex hormone testosterone is synthesized from
cholesterol. Examine the structure shown here.
a. How many pi bonds are present in the molecule?
b. There are two carbon atoms that are bonded to oxygen.
Describe the hybridization present in these two carbon
atoms.
c. What are the bond angles around those two carbon atoms?
51. Antihistamines are often taken to counteract the effects of the
amino acid histamine that is released in allergic reactions. Ex-
amine the structure of histamine shown below. Compare the
two nitrogen atoms in the ring portion of the molecule on the
basis of lone-pair electrons, bond angle, and hybridization.
52. Vitamin B
6
(pyridoxine) is important in the metabolism of
carbohydrates, proteins, and fats because it enhances the ac-
tion of several enzymes. After examining the structure of this
important molecule, answer the following questions.
a. How many pi bonds are present in the structure?
b. Would the bond between the carbon atoms within the ring
be shorter or longer than the bond between a carbon atom
in the ring and one outside the ring? Explain.
c. Are the bond angles around the CH
3
group the same as or
different from the bond angles around the carbon in the
CH
2
OH group? Explain.
OH
C
CH
2
OH
C
HOCH
2
CHC
C
N
CH
3
C
CH
2
CH OHH
2
NC
O
CH
N
NHHC
H
3
C
O
CH
3
C
C
CC
C
C
C
C
C
C
C
C
C
C
H
H
H
H
H
H
OH
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
C
C
C
C
HN
H
C
HC
C
C
H
H
C
HH
C
HH
C
C
C
CH
3
O
O
O
C
O
H
H
OHC
HNH
2
C
390 Chapter 9 Advanced Models of Bonding
53. One form of the molecule POCl
3
is shown below. What
hybridization would be found in the phosphorus atom?
What are the bond angles around the phosphorus?
54. Biacetyl is one of the additives included in margarine to
enhance the butter-like taste. The diagram of biacetyl shown
here does not accurately depict the bond angles. What would
be the correct bond angles around the two central carbon
atoms? Redraw the structure to show those angles.
55. Capsaicin is one of the key ingredients in chili peppers that
produce the spicy taste sensation sought by chefs. Examine
its structure and answer the following questions.
a. Which carbon atoms have sp
3
hybridization?
b. How many H atoms would have to be removed to produce
a double bond between the two carbon atoms that are
shown farthest to the right?
c. How many pi bonds are shown in the molecule?
d. How many lone pairs of electrons are needed around the
oxygen atom that is double-bonded to the carbon atom?
56. Estradiol is the active steroid used in estrogen replacement
therapy.
a. Which carbon atoms have sp
2
hybridization?
b. How many H atoms would have to be removed to produce
a double bond between the oxygen and carbon atoms that
are shown farthest to the right?
c. How many pi bonds are shown in the molecule?
d. How many lone pairs of electrons are needed around each
oxygen atom?
C
HO
C
C
H
H
H
C
H
H
H
H
C
C
H
C
C
H
C
H
CH
3
OH
C
C
C
H
H
C
C
C
H
H
H
H
H
C
C
H
H
C
CH
3
O
HO
C
C
C
H
C
H
CH
C
C
HH
C
HH
HHHH
N
H
C
O
C
C
C
HH
C
H
C
H
CH
3
CH
3
CH
H
3
CCH
3
C
O
C
O
Cl P
Cl
Cl
O
Section 9.3 Molecular Orbital Theory
Skill Building
57. Working from left to right, create a table in which the name
of each model is correctly aligned with the scientist who de-
vised it and a general summary of the model.
58. The development of molecular orbital theory gave chemists
some advantages in explaining chemical bonding.
a. What is the theoretical basis of MO theory?
b. What main advantage over VSEPR does this treatment of
bonding offer?
c. What is a disadvantage of MO theory?
59. Oxygen and sulfur are both found in Group VIA on the peri-
odic table. Consequently, they share many properties, includ-
ing some bonding characteristics. However, the larger size of
the p orbitals in sulfur prevents them from overlapping in an
effective way to form pi bonds. Use orbital diagrams to show
how this can be used to explain why O
2
is more stable than S
2
.
60. Nitrogen and phosphorus are both found in Group VA on
the periodic table. Consequently, they share many properties,
including some bonding characteristics. However, the larger
size of the p orbitals in phosphorus prevents them from over-
lapping in an effective way to form pi bonds. Use orbital dia-
grams to show how this can be used to explain why N
2
is
more stable than P
2
.
61. Cite two differences between bonding and antibonding
orbitals.
62. Cite two similarities between bonding and antibonding
orbitals.
63. Most sciences have their share of acronyms. In this chapter
you have encountered several important ones related to
chemical bonding. Supply the full name and meaning of each
of the following:
a. LCAO b. HOMO c. LUMO
64. In this chapter you have encountered several important sym-
bols related to chemical bonding. Supply the full name and
meaning of each of the following:
a. π b. π
∗
c. σ
65. Removing or adding electrons to molecules can change sev-
eral properties of the bonding within the molecule. Provide
complete MO diagrams for N
2
−
,N
2
, and N
2
+
.
a. Calculate the bond order for each.
b. Indicate which, if any, are paramagnetic.
c. Rank the three nitrogen species in order from shortest to
longest nitrogen-to-nitrogen distance.
Model Scientist General summary
VB G. Lewis Subtract and add overlap to
create π and σ bonds.
MO L. Pauling Distribute bonding and
nonbonding electron pairs.
VSEPR E. Schrödinger Overlap and hybridization
create new orbitals.
Focus Your Learning 391
66. Removing or adding electrons to molecules can change sev-
eral properties of the bonding within the molecule. Provide
complete MO diagrams for O
2
−
,O
2
, and O
2
+
.
a. Calculate the bond order for each.
b. Indicate which, if any, are paramagnetic.
c. Rank the three oxygen species in order from shortest to
longest oxygen-to-oxygen distance.
67. A diatomic homonuclear +3 ion has the following molecular
orbital configuration:
(σ
1s
)
2
(σ
1s
∗
)
2
(σ
2s
)
2
(σ
∗
2s
)
2
(σ
2p
)
2
(π
2p
)
4
(π
2p
∗
)
3
a. What is the identity of the element used to make this ion?
b. Is the ion paramagnetic or diamagnetic?
c. What is the bond order of the ion?
68. A diatomic homonuclear +2 ion has the following molecular
orbital configuration:
(σ
1s
)
2
(σ
∗
1s
)
2
(σ
2s
)
2
(σ
∗
2s
)
2
(σ
2p
)
2
(π
2p
)
2
a. What is the identity of the element used to make this ion?
b. Is the ion paramagnetic or diamagnetic?
c. What is the bond order of the ion?
69. Indicate the total number of sigma and pi bonds in the fol-
lowing molecule.
70. Indicate the total number of sigma and pi bonds in the
following molecule.
71. What is the bond order between the indicated atoms in the
following molecule?
72. What is the bond order between the indicated atoms in the
following molecule?
H
C
H
C
C
H
H
C
OH
H
c.
a.
b.
H
H
C
C
H
H
C
C
O
H
C
C C
H
C
H
H
b.
c.
a.
HCC
H
H
H
H
C
CH
2
OH
C
H
OH
HCC F

Chemical Applications and Practices
73. Typically phosphorus may be found in nature as P
4
(among
other forms). However, P
2
is also known to exist at suffi-
ciently lower temperatures. Using the outer valence elec-
trons, produce the MO diagram for P
2
. (You may assume the
same sequence of orbitals as found in N
2
.)
a. What is the bond order of P
2
?
b. How many electrons would be found in the highest π
∗
?
74. Br
2
is a liquid at room temperature. Using the outer valence
electrons, produce the MO diagram for Br
2
. (You may as-
sume the same sequence of orbitals as found in F
2
.)
a. What is the bond order of Br
2
?
b. How many electrons would be found in the highest π?
Section 9.4 Putting It All Together
Skill Building
75. a. The term orbital overlap was used throughout this chapter.
Describe the meaning of this important term. What ex-
actly is being “overlapped”?
b. Diagram the orbital overlap typical of an s–s, s–p, and
p–p overlap.
76. a. The term hybrid was used throughout this chapter.
Describe the meaning of this important term.
b. Draw the shapes of at least three different hybrid orbitals.
77. a. Why is it necessary to draw resonant hybrids when repre-
senting the structure of ions and molecules that have delo-
calized electrons?
b. Using the diagram of acetylsalicylic acid shown in
Problem 87 as one example, show another example of a
resonance hybrid of the molecule.
78. Using the diagram of the steroid illustrated in Problem 56,
show another example of a resonance hybrid of the molecule.
Use the compound shown below to answer Problems 79–82.
79. Numbering the carbon atoms from right to left, what would
be the hybridization for the second carbon?
80. Using the same numbering system, between which three
atoms would you predict the bond angle to be largest?
81. How many pi bonds are in the molecule? How many sigma
bonds are present?
82. Bonds between atoms can rotate if they lack a nodal plane
aligned along the bond’s axis. Using the same right-to-left
numbering system, which carbon atom(s) would be free to
rotate without affecting the movement of any other carbon
atoms?
Use the compound shown below to answer Problems 83–86.
HCC
H
H
H
C
O
C
H
C
H
H
H
HCC
H
H
H
C
H
C C C
H
H
H
392 Chapter 9 Advanced Models of Bonding
83. Numbering the carbon atoms from right to left, what would
be the hybridization for the second carbon?
84. Using the same numbering system, between which three
atoms would you predict the bond angle to be largest?
85. How many pi bonds are in the molecule? How many sigma
bonds are present?
86. Bonds between atoms can rotate if they lack a nodal plane
aligned along the bond’s axis. Using the same right-to-left
numbering system, which carbon atom(s) would be free to
rotate without affecting the movement of any other carbon
atoms?
Chemical Applications and Practices
87. The structure for the common pain reliever acetylsalicylic
acid (found in aspirin products) is shown below.
a. Indicate which carbon atoms have associated delocalized
electrons.
b. What type of hybridization do these carbon atoms have?
c. What types of orbitals are directly involved with the π
bonding?
88. The versatile carbonate ion, found in seashells, in chalk, and
as part of our blood buffering system, is made from three
oxygen atoms bonded to a central carbon atom and two ad-
ditional electrons. Diagram three resonance structures for
the ion, and show the resonance hybrid. What is the approx-
imate bond order for a carbon-to-oxygen bond in the ion?
89. Naphthalene (C
10
H
8
) is the active ingredient in moth balls,
giving them their characteristic odor. Naphthalene contains
two equal-sized rings of carbon atoms that share a common
side. Every carbon atom is part of a ring. Diagram the struc-
ture of this molecule and indicate the hybridization of each
of the atoms.
90. Draw two resonance structures for naphthalene; see Prob-
lem 89. Then indicate the resonance hybrid for the molecule.
What is the approximate bond order for a COC bond in
naphthalene?
Comprehensive Problems
91. How many unpaired electrons would be found in one atom
of carbon in each of the following conditions?
a. ground state c. sp
2
hybridization
b. sp hybridization d. sp
3
hybridization
92. Magnesium hydride is one of the few covalent metallic
hydrides. Write out the ground-state electron configuration
for magnesium.
a. What type of hybridization would be necessary to form
equal bonds to the two hydrogen atoms?
b. What orbitals would overlap to form the bonds?
c. What shape would this molecule have?
C
C
C
C
O
H
H
C
O
C
O
H
H
C
H
O
C
C
H
H
H

93. Using the designated lone pairs and bonded pairs to de-
scribe electrons around a molecule’s central atom, predict
the shape that would be produced in each of the following:
a. Three bonded pairs and one lone pair
b. Six bonded pairs and no lone pairs
c. Two bonded pairs and two lone pairs
d. Five bonded pairs and no lone pairs
94. Nitrogen and phosphorus are both found in Group VA on
the periodic table. As you would expect, they have some
reactions and properties in common. For example, both ni-
trogen and phosphorus form trihalides such as NCl
3
and
PCl
3
. However, phosphorus also forms PCl
5
but nitrogen
does not. Explain the reason behind this difference.
95. The nitrate ion (NO
3
−
) and the nitrite ion (NO
2
−
) can both
be drawn showing a double bond between one oxygen atom
and the central nitrogen atom. However, the bond angle is
not the same in the two compounds. Which would have the
smaller angle? Explain the basis for your choice.
96. The air you inhale is mostly nitrogen gas (N
2
). The air
you exhale is mostly the unchanged N
2
that you just inhaled.
Use ground-state electron configurations and valence bond
theory to show why N
2
is so stable that your biochemical
processes cannot change its structure.
97. Two molecules found in petroleum oil are the straight-
chain octane and the highly branched compound 2,2,3,3-
tetramethylbutane. The structures of both are shown here.
From what you can deduce about the three-dimensional
shape of these structures, decide which one is most likely be
able to associate closely with more molecules of itself and to
have a higher boiling point. Explain the basis of your answer.
CH
3
OCH
2
OCH
2
OCH
2
OCH
2
OCH
2
OCH
2
OCH
3
(octane)
(CH
3
)
3
COC(CH
3
)
3
(2,2,3,3-tetramethylbutane)
98. Examine the structure of oleic acid shown below (H atoms
are omitted from the structure). You may have read the
label on a food product that described “hydrogenated” or
“partially hydrogenated” oils as an ingredient. These terms
refer to the addition of hydrogen atoms to the area of the
double bond(s) in an oil. How many hydrogen atoms have
been left off the oleic acid structure? How many more
hydrogen atoms would have to be added in order to fully
hydrogenate oleic acid and remove the double bond?
Focus Your Learning
393
100. Dinitrogen monoxide, also known as nitrous oxide (N
2
O),
has had some use as an anesthetic and, because of some side
effects, is occasionally called “laughing gas.” Diagram two
suitable Lewis dot structures for the compound, and state
the hybridization of the nitrogen atoms in each.
101. In the opening discussion of the chapter, the molecule
retinal is introduced. This molecule contains a series of
conjugated double bonds—double bonds that are separated
by single bonds.
a. Which is a longer bond in the molecule, the CPC bond
in the ring, or the COC bond on the opposite side of
the ring?
b. This molecule absorbs light at about 500.0 nm. What
is the energy of a single photon of light with this
wavelength?
c. What is the energy of a mole of photons of this
wavelength?
d. To what color does this wavelength correspond?
102. The text of the chapter indicates that the chlorine–chlorine
bond can be cleaved with 492 nm light.
a. What is the energy of a mole of photons of this
wavelength?
b. Using Lewis dot diagrams, show how the chlorine
molecule breaks apart into two chlorine atoms.
c. What is the difference between a chlorine atom and a
chloride ion?
d. What is the hybridization of the chlorine atom in a
molecule of chlorine?
103. Oleic acid, Exercise 9.7, can be treated with hydrogen gas to
make stearic acid.
a. Write the balanced molecular equation for this process.
b. How many grams of hydrogen are needed to prepare 1.0
lb stearic acid?
c. Stearic acid can form a film on the surface of water. In
doing so, the molecule stands straight up—one end
points toward the water and one end points toward the
sky. Which end of stearic acid would you expect to point
toward the water? Explain.
Thinking Beyond the Calculation
104. Hydrazine (N
2
H
4
) has many uses. For example, it is em-
ployed as a rocket fuel and as a starting material in the
production of fungicides.
a. Draw the Lewis dot structure for hydrazine.
b. What is the VSEPR shape of the nitrogens in this
molecule?
c. What is the hybridization of each nitrogen atom?
d. Would you expect this molecule to have a color that we
can see?
e. Hydrazine can be used as rocket fuel. The products of
combustion are nitrogen gas and water vapor. Write a
balanced equation showing this reaction, and calculate
the enthalpy of combustion for this reaction using the
bond energies from Chapter 8.
COCOCOCOCOCOCOCOCPCOCOCOCOCOCOCOCOCOOH
(oleic acid)
99. The compound formamide (CH
3
NO) has one hydrogen
atom, the nitrogen, and the oxygen attached to the carbon.
In addition, the remaining two hydrogen atoms are attached
to the nitrogen. Every atom satisfies the octet rule. Diagram
the structure of this molecule, and explain the hybridization
of both the carbon and nitrogen atoms. If you built a model
of this molecule, would it lie flat on a table (planar) or have
a “puckered” structure?

The
Behavior and
Applications
of Gases
Earth’s atmosphere is a very thin layer of
gases (only 560 km thick) that surrounds
the planet. Although the atmosphere
makes up only a small part of our planet,
it is vital to our survival.
394
Contents and Selected Applications
10.1 The Nature of Gases
10.2 Production of Hydrogen and the Meaning of Pressure
10.3 Mixtures of Gases—Dalton’s Law and Food Packaging
Chemical Encounters: Dalton’s Law and Food Packaging
10.4 The Gas Laws—Relating the Behavior of Gases
to Key Properties
Chemical Encounters: Balloons and Ozone Analysis
10.5 The Ideal Gas Equation
10.6 Applications of the Ideal Gas Equation
Chemical Encounters: Automobile Air Bags
Chemical Encounters: Acetylene
10.7 Kinetic-Molecular Theory
10.8 Effusion and Diffusion
10.9 Industrialization: A Wonderful, Yet Cautionary, Tale
Chemical Encounters: Ozone
Chemical Encounters: The Greenhouse Effect
Go to college.hmco.com/pic/kelterMEE for online learning resources.
Our planet is surrounded by a relatively thin
layer known as an atmosphere. It supplies all living
organisms on Earth with breathable air, serves as the
vehicle to deliver rain to our crops, and protects us from
harmful ultraviolet rays from the Sun.
Although it does contain solid particles, such
as dust and smoke, and liquid droplets, such as sea
spray and clouds, much of the atmosphere we live in is
actually a mixture of the gases shown in Table 10.1. These
gases, and the rest of the components in the atmosphere of
planet Earth, are vital to our survival.
In addition to forming the atmosphere surrounding
planet Earth, gases are vital to our society in many other
ways. Gases that are used in manufacturing, medicine, or
anything else related to the economy are called
industrial
gases
, shown in Table 10.2. Some, such as nitrogen and oxy-
gen, can simply be separated from the air. Others, including
sulfur dioxide, hydrogen, and chlorine, are produced by
chemical reactions. No matter what uses we make of them,
nearly all of these common gases behave in about the same
way when present at low density. The fact that almost all
gases share this characteristic enables us to work with them
predictably and successfully.
395
Composition of Dry Air
Percent Parts per Million
Component by Volume by Volume
N
2
78.08 780,800
O
2
20.95 209,500
Ar 0.934 9,340
CO
2
0.033 330
Ne 0.00182 18.2
He 0.000524 5.24
CH
4
0.0002 2
Kr 0.000114 1.14
H
2
0.00005 0.5
N
2
O 0.00005 0.5
Xe 0.0000087 0.087
O
3
<1 × 10
−5
<0.1
CO <1 ×10
−6
<0.01
NO <1 × 10
−6
<0.01
Source: Chemical Rubber Company. Handbook of Chemistry
and Physics, 70th ed. CRC Press: Boca Raton, FL, 1990.
TABLE 10.1
Industrial Uses of Some Common Gases
U.S. Production (2004) in Metric
Gas Use Tons (1 metric ton = 1000 kg)
Cl
2
Preparation of bleaches and cleansing agents, 12.2 million
purification of water, preparation of pesticides
CO
2
Refrigeration, preparation of beverages, inert 8.1 million (estimated)
atmosphere in chemical reactions and food
packaging, fire extinguishers
H
2
Hydrogenation of oils in food and other 17.7 bcm (gas + liquid)
unsaturated organic molecules, energy source (bcm = billion m
3
)
in vehicles, petroleum-refining reactions,
manufacturing of resins used in plastics
NH
3
Fertilizer and preparation of other fertilizers 10.8 million
N
2
Inert atmosphere in chemical reactions and food 30.3 million (not incl. NH
3
)
packaging, electronics, and metalwork; manufacture
of ammonia; refrigerant
O
2
Oxidizer in many chemical processes, such as 25.5 million
production of steel and acetylene; oxidizer in reaction
of fuels in rockets; in hospitals (breathing); pulp and
paper industries for bleaching
SO
2
Production of sulfuric acid, bleaching agent in food 300,000
and textile industries, controls fermentation in wines
Source: Chemical and Engineering News, July 11, 2005.
TABLE 10.2

FIGURE 10.1
Gases can be significantly compressed, which makes it possible to ship large
quantities of industrial gases via truck in compressed gas cylinders.
10.1 The Nature of Gases
Many of the chemists in the eighteenth century became aware of a very interest-
ing phenomenon as they worked with gases. They noted that dissimilar gases
appeared to behave in relatively similar ways.
Why is this possible? It all comes
down to the common features of gases. Under ambient conditions, electrostatic
interactions make water a liquid and sodium chloride a solid. Gases have few such
interactions, and when they occur, they are often weak and fleeting. In fact, the
atoms or molecules of gases most often behave as individual units. Gases that behave
as though each particle has no interactions with any other particles are called
ideal gases; their properties are listed in Table 10.3. Most gases behave nearly
ideally under normal conditions, so unless otherwise noted, the quantitative rela-
tionships we will discuss assume ideal behavior. Notably, deviations from ideal
behavior occur when gases are examined under conditions that encourage the in-
teractions between molecules of a gas: high pressures and low temperatures.
Gases have an exceedingly low density—that is, the particles are relatively far
apart. For example, at 25°C, liquid water has a density of 1.00 g/mL, whereas dry
air at sea level has a density of 0.00118 g/mL. The low density of gases means that
they can be significantly compressed in order to save space during shipping, as
shown in Figure 10.1.
Solids and liquids are nearly incompressible and have volumes that change
very little with temperature and pressure. This difference, too, is related to the
nature of gases. Because gas molecules are far apart compared to molecules
within a liquid or solid, the volume of a gas can be greatly affected by changes
in temperature and pressure. As we will discuss later, we make use of the
compressibility of gases, and the energy exchanges that accompany it, in applica-
tions from refrigeration to rocketry.
396 Chapter 10 The Behavior and Applications of Gases
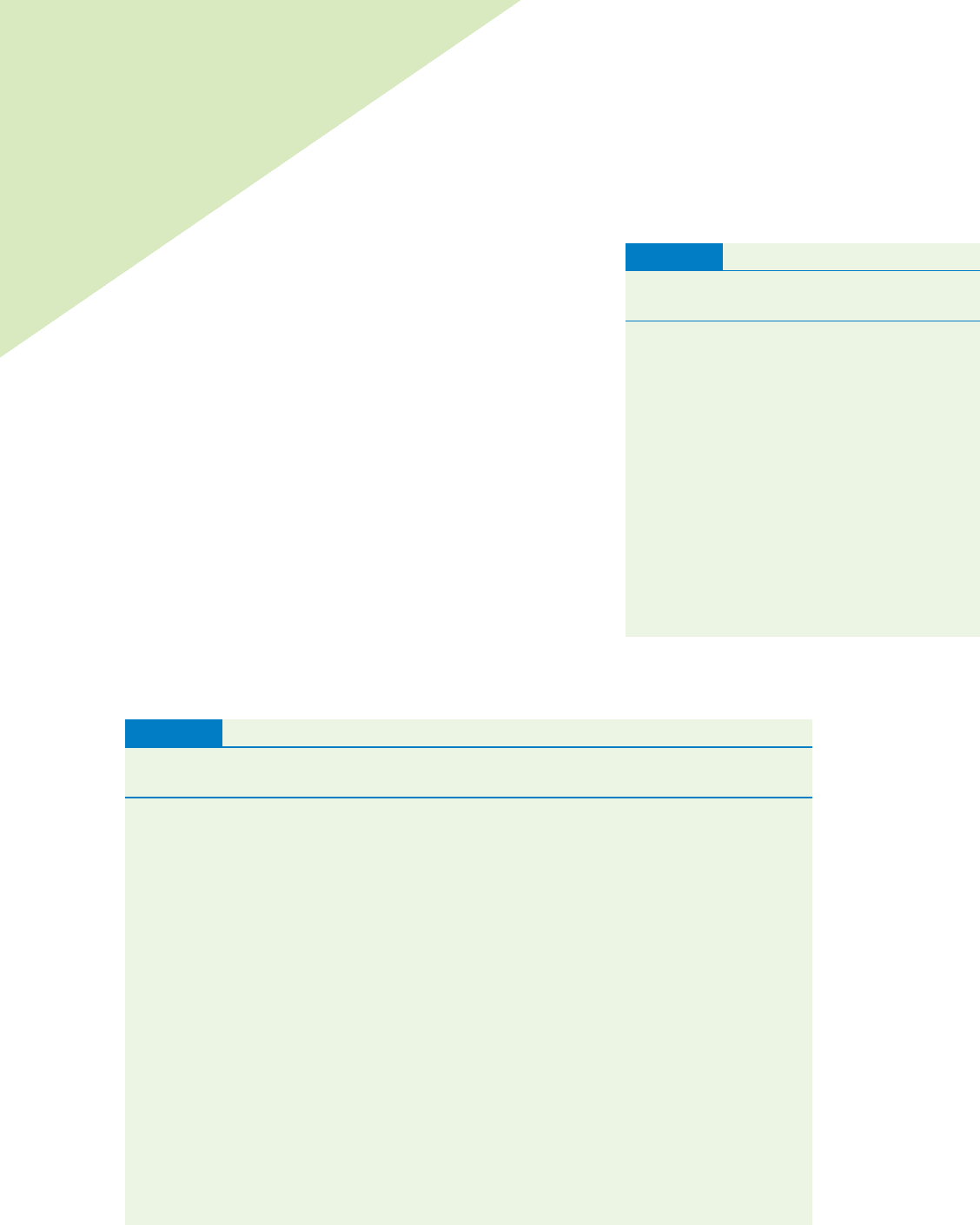
Smaller volume
more dense
GasLiquid
Larger volume
less dense
Gas molecules occupy a much larger volume than the
same number of molecules of a liquid. This results in
a much lower density for gases than for liquids.
The Ideal Gas
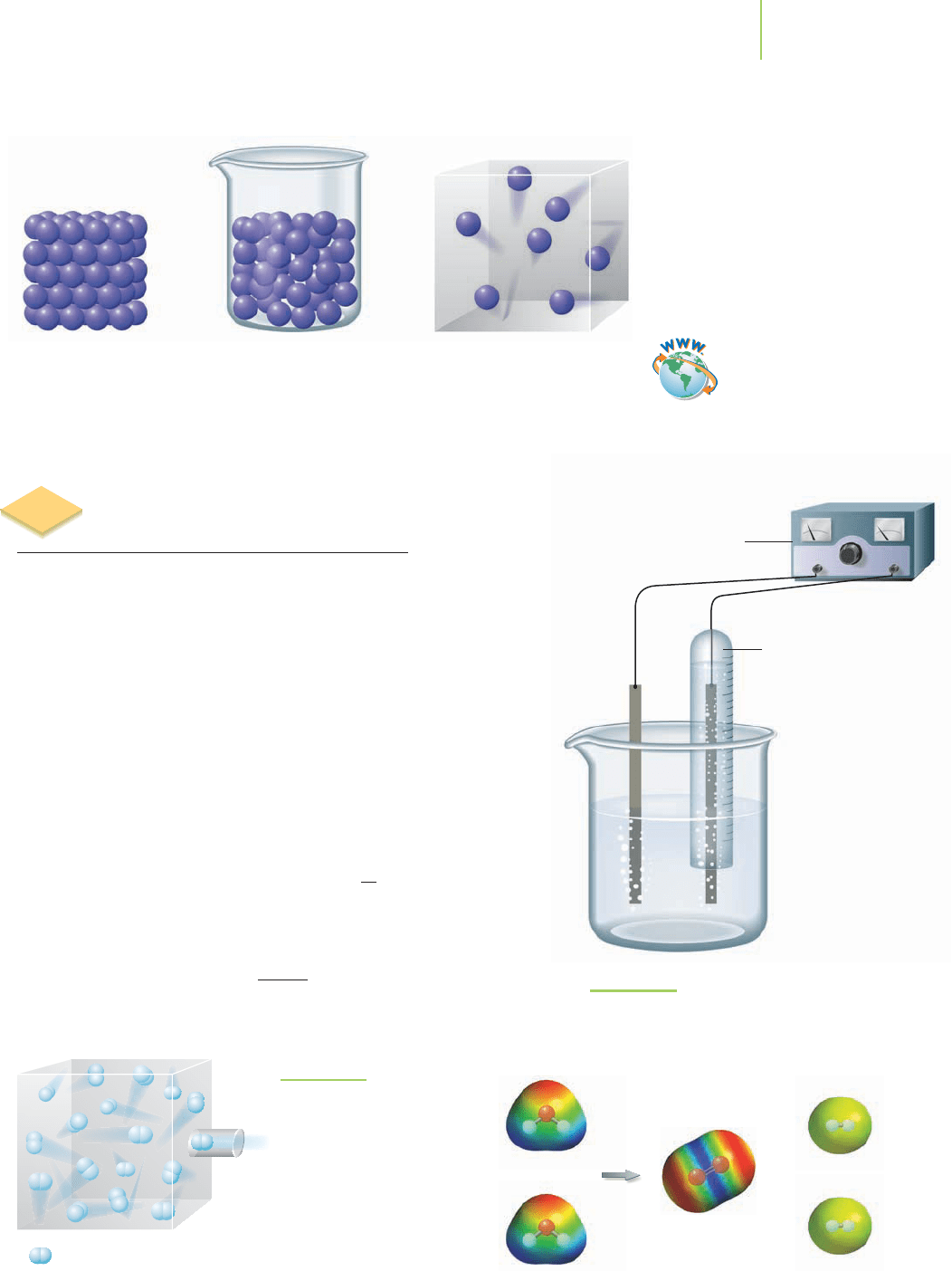
The ideal gas is one that follows each of these rules:
• The individual gas particles do not interact with each other.
• The individual gas particles are assumed to have no volume.
• The gas strictly obeys all of the simple gas laws.
• The gas has a molar volume of 22.414 L at 1 atm of pressure and 273.15 K.
TABLE 10.3
Video Lesson: Properties of
Gases
10.2 Production of Hydrogen and the Meaning of Pressure 397
10.2 Production of Hydrogen
and the Meaning of Pressure
Nearly 18 billion cubic meters of hydrogen gas were produced in the
United States in 2004. Because hydrogen gas is a very minor compo-
nentof air (see Table10.1),it must be generatedviachemical processes.
One method is the decomposition of water by
electrolysis,inwhichan
electric current passing through a solution causes a chemical reaction.
The resulting hydrogengas can be collectedseparately from the oxygen
gas. Let’s assume that the process takes place at constant temperature
and that the collection vessel,which has a constant volume,is empty as
hydrogen begins to enter, as shown in Figure 10.2.
What happens as H
2
flows in? The molecules begin to collide
with the walls of the container and with each other, as shown in Fig-
ure 10.3. The
force that each molecule applies during these collisions
is equal to the mass of the particle times its acceleration:
Force = mass × acceleration =
kg ×
m
s
2
= kg·m·s
−2
The SI unit of force is the newton (N), which is equal to 1 kg·m·s
−2
.
Because each of us has mass and is accelerated toward the center of
the Earth by the pull of gravity
9.81 m
s
2
,eachofusexertsaforceon
= H
2
molecul
e
Liquid GasSolid
Loosely packed
slightly compressible
Widely spaced
highly compressible
Tightly packed
least compressible
+
Hydrogen and oxygen can be produced by the electolysis of water.
Gases are highly compressible compared to liquids and solids.
FIGURE 10.2
Our hypothetical reaction set-up for the electrolysis
of water. The hydrogen gas can be isolated from
the reaction and sent to the collection chamber.
FIGURE 10.3
Molecules entering a
vacant container collide
with each other and with
the walls of the container.
The molecules have mass,
acceleration, and a
resulting force.
Power supply
Hydrogen
gas
Visualization: Electrolysis of
Water
