Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

9.1 Valence Bond Theory
Just as light can be absorbed by the molecule known as retinal, it can also be
absorbed by the diatomic halogens. Chlorine (Cl
2
) and fluorine (F
2
) both absorb
light according to the following reactions:
Cl
2
+ light (h
ν
) n 2 Cl•
F
2
+ light (h
ν
) n 2 F•
The resulting very reactive atoms are called radicals (remember these from Chap-
ter 8) because they contain one unpaired electron. It is this reactivity that ac-
counts for chlorine’s effects on the ozone layer and fluorine’s ability to react with
normally unreactive atoms such as xenon.
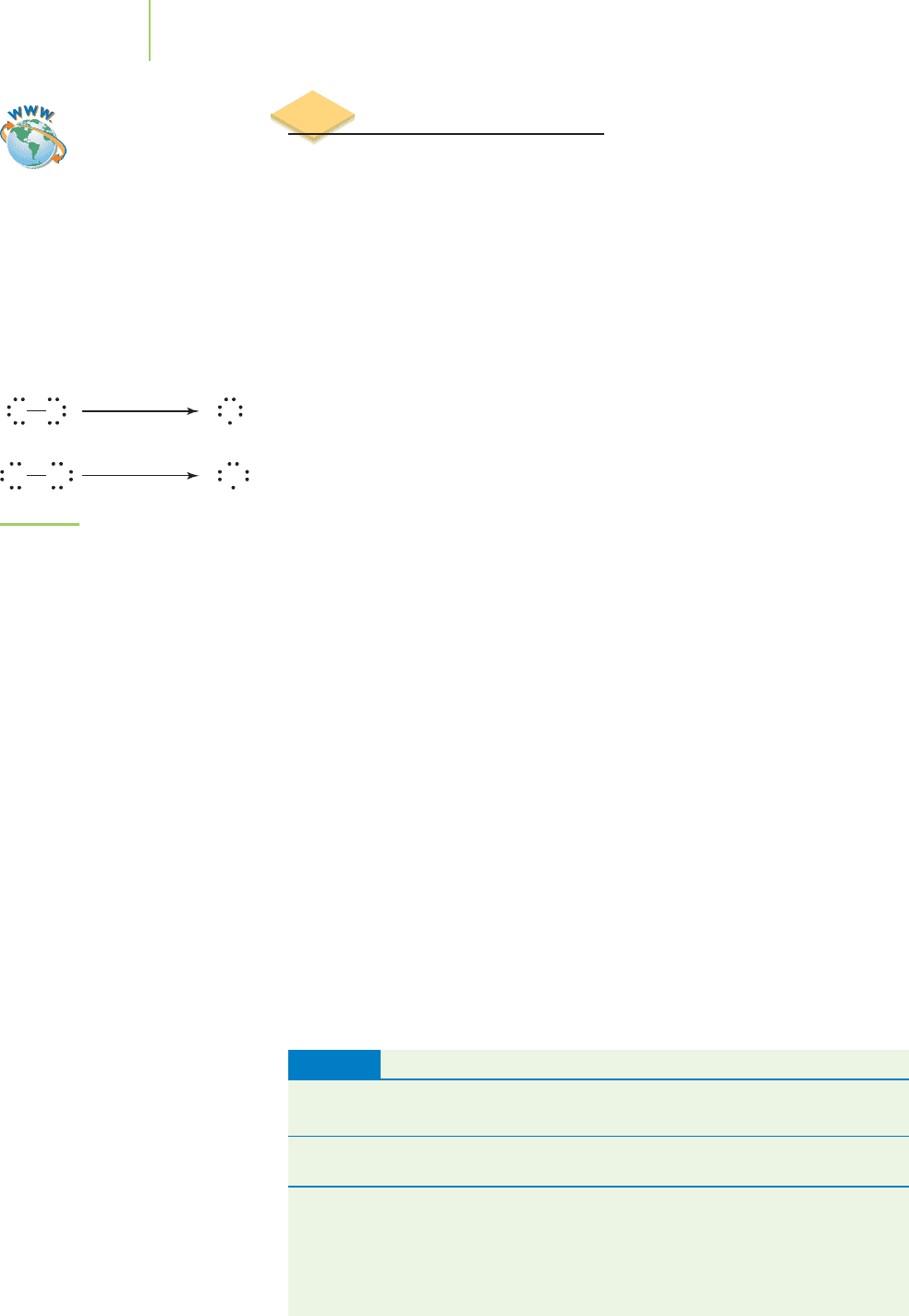
The Lewis dot structures of the halogens indicate that they contain nonpolar
covalent bonds and similar bond lengths. However, on the basis of the different
wavelengths of light (remember that energy is inversely proportional to wave-
length!) required to break the bond between these atoms (Figure 9.4), we can rea-
son that chlorine and fluorine must have different bond strengths. It appears that
they have some differences in their bonds that the Lewis dot structure model does
not identify.
Knowing the exact makeup of a bond can give us a good picture of its
strength. For instance, we discussed in Chapter 8 how bond strengths can be used
to arrive at a rough approximation of the enthalpy of a reaction. We also noted
that bond energies were averaged to get the values we saw in the table. The fact
that they were averaged implies that not all bonds between the same atoms have the
same energy. We can explore this idea by studying the combustion reactions of
both ethane (CH
3
CH
3
) and acetylene (CHqCH). Experimentally, as shown in
Table 9.1, the combustion of acetylene is accompanied by an enthalpy change of
–1300 kJ/mol, compared to the –1559 kJ/mol associated with the combustion of
ethane. Using the bond energy values from Chapter 8, we end up calculating
enthalpy changes that are different than these values. Even though the COC
bond in ethane (376.1 kJ/mol) is much weaker than the CqC bond of acetylene
(962 kJ/mol), this difference doesn’t account for the experimentally determined
difference in the enthalpy of combustion between ethane and acetylene.
Why are
our numbers so different?
Some of the missing energy is accounted for by the dif-
ferences in COH bond energy. Experimentally, researchers have determined that
the energy of the COH bond in ethane (410 kJ/mol) is much smaller than that
of the COH bond in acetylene (536 kJ/mol). However, according to Lewis dot
structures and to the VSEPR models, there should be no difference in energy
between the bonds because they are both COH bonds. We need to construct a
better model of these compounds that takes this difference into account.
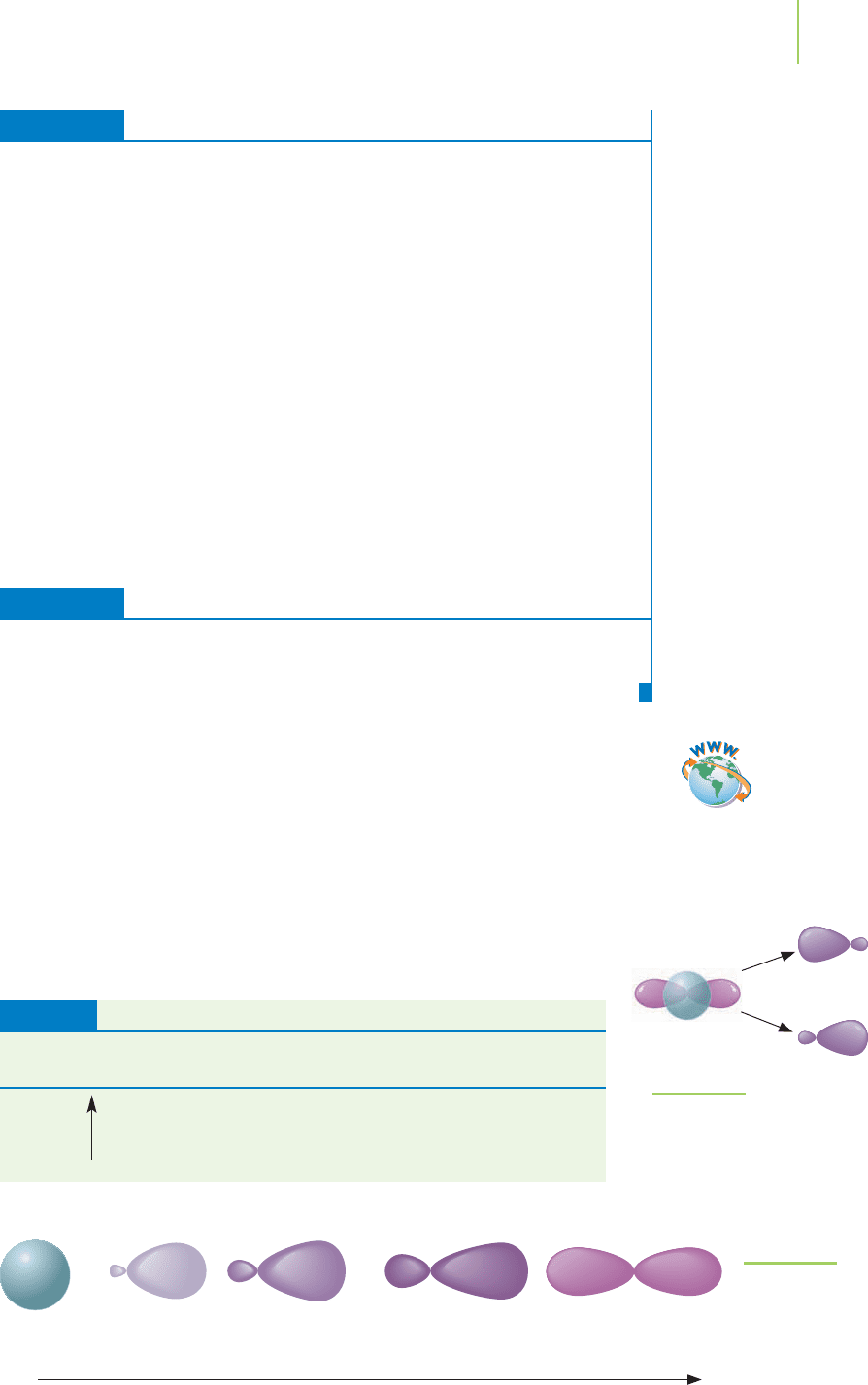
358 Chapter 9 Advanced Models of Bonding
F F F
λ 752 nm
Cl Cl Cl
λ 492 nm
2
2
FIGURE 9.4
The interaction of light with chlorine
and fluorine imply that these two
molecules possess different bond
strengths.
Calculated Versus Experimental ∆
c
H
The experimental and calculated ∆
c
H values for the fuels listed do not agree. The
temperature of the combustion is listed only for reference.
Flame ∆
c
H (kJ/mol) ∆
c
H (kJ/mol)
Compound Temperature (K) Experimental Calculated
Hydrogen (H
2
) 2490 –242 –242
Methane (CH
4
) 2285 –890 –803
Ethane (CH
3
CH
3
) 2338 –1559 –1429
Ethylene (CH
2
PCH
2
) 2643 –1411 –1324
Acetylene (CHqCH) 2859 –1300 –1257
TABLE 9.1
Video Lesson: Valence Bond
Theory
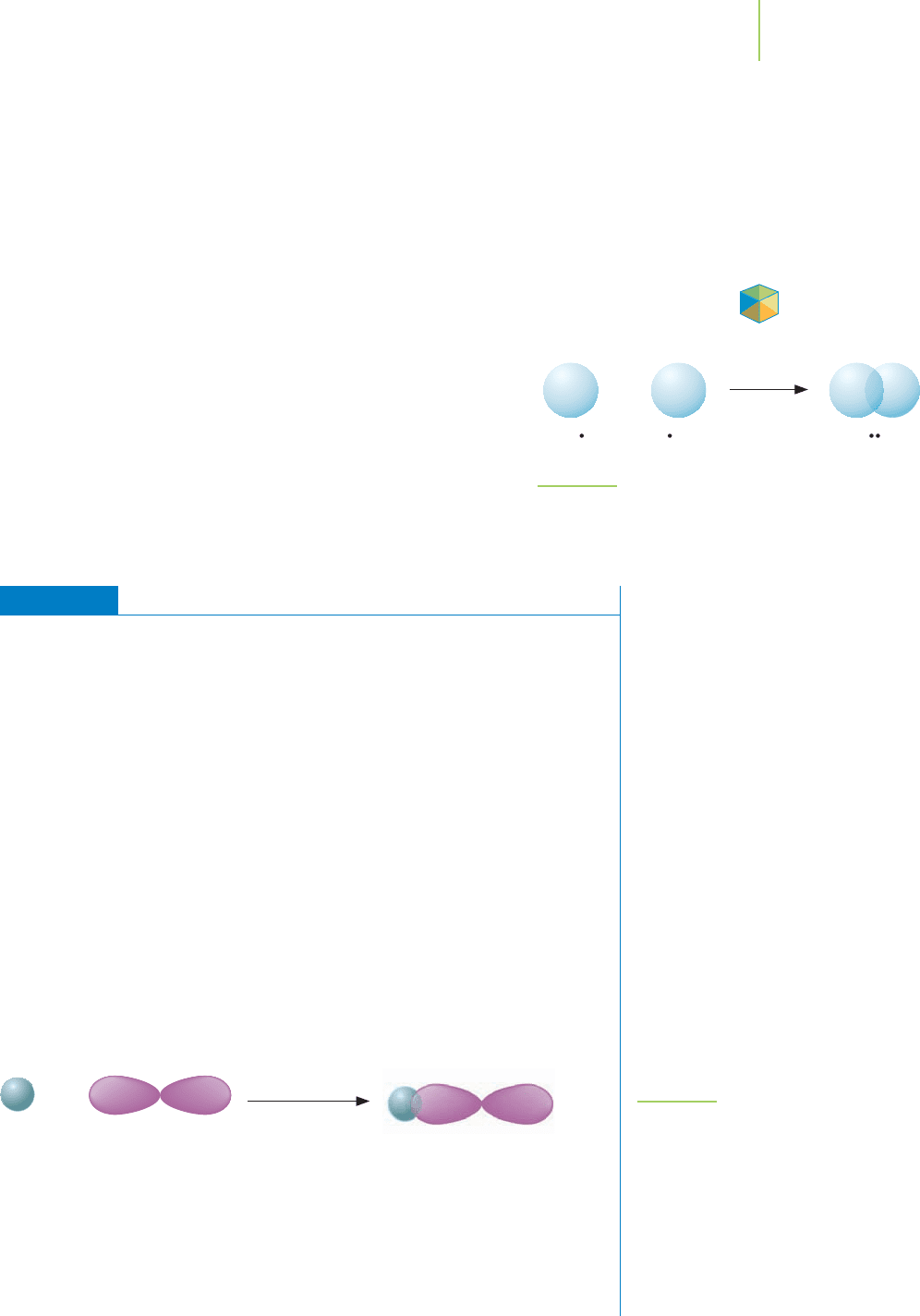
One such “better model” of a covalent bond is the valence bond model. In the
1930s, Linus Pauling (whom we remember from Chapters 7 and 8), devised va-
lence bond theory (VB theory) to address the inadequacy of the bonding models
of G. N. Lewis.
What Is a Valence Bond?
Pauling’s valence bond theory envisions a bond as the overlap of atomic orbitals
on adjacent atoms. Because two electrons interact in a bond, overlapping two
half-filled orbitals supplies these electrons, which are covalently shared. If all of
the bonds were treated this way, an atom would be able to satisfy the octet (or
duet, for hydrogen) rule.
Molecular hydrogen’s use in industry to make such products as
the hydrogenated vegetable oils used in processed foods and its
possible use as an alternative to gasoline in motor vehicles makes it
a meaningful compound to study. The electron configuration for a
hydrogen atom is 1s
1
. When the 1s orbital on the hydrogen atoms
overlap, a bond results. Because the electrons are shared by both
orbitals, each of the hydrogen atoms possesses the 1s
2
electron
configuration, as shown in Figure 9.5, which is a full 1s orbital (the
duet rule is satisfied for both hydrogen atoms).
EXERCISE 9.1 Modifications to the Structure of HF
We noted in Chapter 8 that hydrogen fluoride is often used by master glassworkers
as they etch a design into a piece of art. Some of the world-famous Steuben art has
been made via this etching technique. Using valence bond theory, describe which
orbitals overlap to form a covalent bond between the hydrogen and fluorine atoms
in HF.
First Thoughts
Our first thoughts bring to mind the Lewis dot structure of HF. We note that the
structure includes three lone pairs around the fluorine atom and the one bonding
pair of electrons between the two atoms. To construct the valence bond model for
HF, we need to consider the electron configurations of these atoms. How many
p orbitals are there in the second principal energy level of fluorine? Which type of
orbital on fluorine might overlap with the hydrogen 1s to share a needed electron?
Solution
The configuration of the valence electrons in fluorine is 2s
2
2p
5
. Overlap of the
hydrogen 1s orbital with one of the 2p orbitals allows the electrons to be shared
between the two atoms. The valence bond model of HF in Figure 9.6 shows a 1s–2p
orbital overlap that constitutes the covalent bond.
9.1 Valence Bond Theory 359
Application
+
HH HH
FIGURE 9.5
Overlap of orbitals makes the bond in H
2
. As two hydrogen
atoms approach, their 1s orbitals overlap to form a bond. The
overlap allows both hydrogen atoms to satisfy the duet rule.
H F H-F
FIGURE 9.6
Orbital overlap in HF.
Further Insights
Does the valence bond model we constructed imply anything about the properties
of the bond? What is the strength of the bond? Does the s–s orbital overlap in H
2
result in a stronger bond than the s–p orbital overlap in HF? Does the overlap of the
s orbital in the first principal energy level and the p orbital in the second principal
energy level indicate anything about the bond? We answer these questions next.
PRACTICE 9.1
Using valence bond theory, describe the orbital overlap in F
2
, a very reactive mole-
cule used to manufacture Teflon ((C
2
F
4
)
n
) and other fluorinated compounds.
See Problems 2, 9, and 10.
Application of Valence Bond Theory
Because the degree of electron sharing is related to the strength of a bond, orbitals
that exhibit more overlap result in stronger covalent bonds. What determines the
amount of overlap? The keys are the relative energy, as shown in Figure 9.7, and
the size of the atomic orbitals. More specifically:
■
Smaller orbitals overlap more than larger orbitals.
■
Orbitals with similar sizes overlap more than orbitals with mismatched sizes.
■
Orbitals with similar energies overlap more than orbitals with very different
energies.
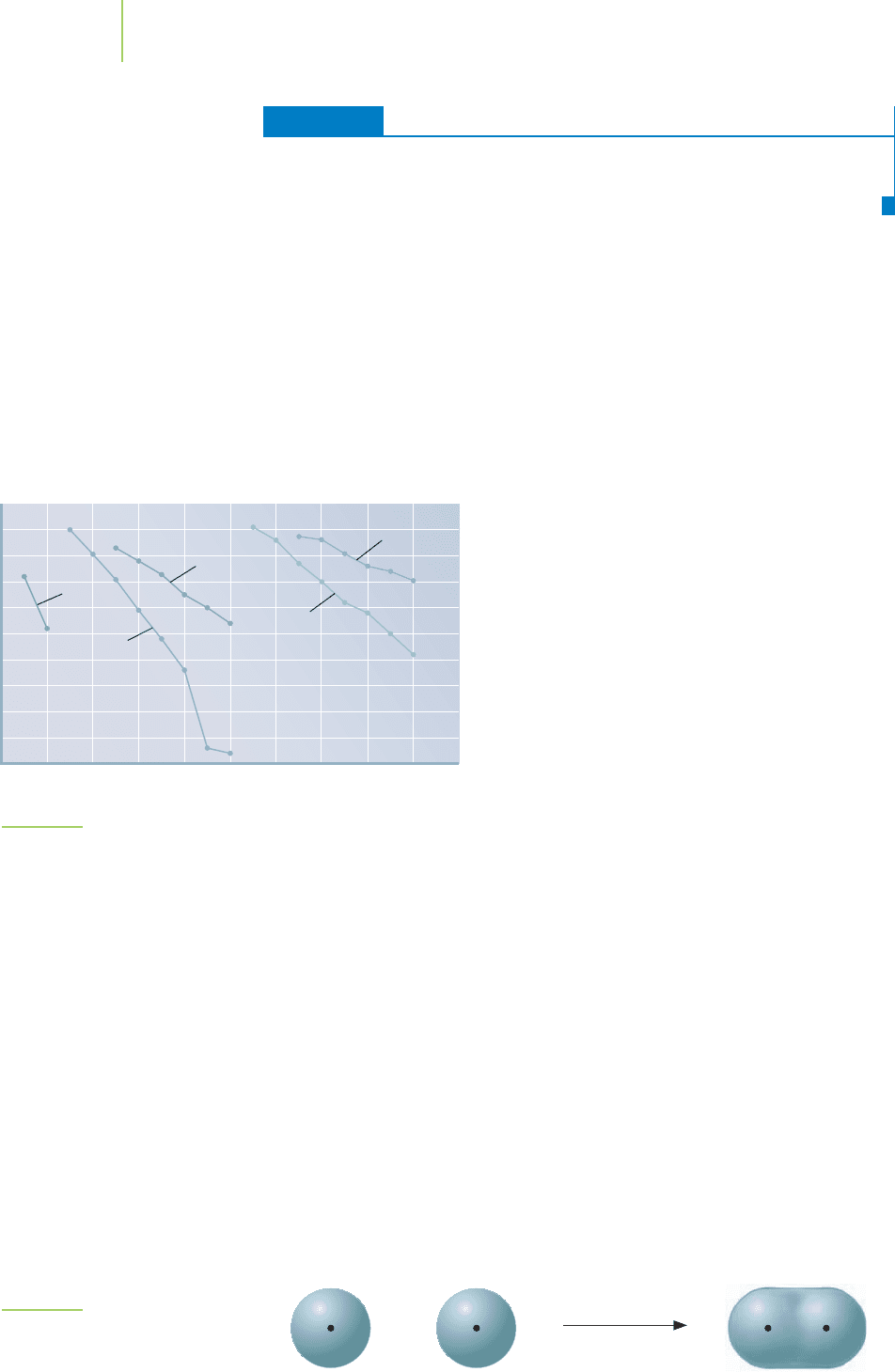
The hydrides LiH, NaH, and KH—used as
bases in chemical reactions, in the removal of oxide
coatings on metals, and in processes to make puri-
fied hydrogen gas—make excellent case studies. In
each of these substances, the bonds between adja-
cent atoms result in covalent overlap of a 1s orbital
of the hydrogen atom and the ns valence orbital of
the metal atom. The valence bond in LiH results
from a 2s–1s orbital overlap. The valence bond in
NaH results from the overlap of a 3s (Na) and a 1s
(H) orbital. Similarly, overlap in KH results from a
4s orbital and a 1s orbital. Because the energy and
the size of the metal’s s orbital are dramatically
greater in potassium than in lithium, the overlap of
the potassium 4s and hydrogen 1s orbitals isn’t well
matched (see Figure 9.8). We would therefore pre-
dict the bond in LiH to be much stronger (better
overlap) than the bond in KH.
Table 9.2 lists the bond energies for LiH, NaH,
and KH as 238 kJ/mol, 185.7 kJ/mol, and 174.6 kJ/
mol, respectively. Note in the table the relatively low FOF bond energy. Although
the 2p–2p orbital overlap is expected to be quite good, the electronegativity of
each halogen atom competes with the orbital overlap. The electrons that partici-
pate in the bond between the two fluorine atoms are held more tightly to the
atoms. This results in a decreased electron density between the atoms in F
2
—and
an unusually low bond energy.
Valence bond theory also addresses any misconceptions reflected in the Lewis
dot structure and VSEPR models about the lengths of bonds. There doesn’t
appear to be any difference in the bond length for H
2
compared to F
2
if we use
only Lewis dot structures as our model. Experimentally, however, we know that the
bond lengths are different. Which is longer, the bond in the hydrogen molecule or
that in fluorine? The difference in bond lengths results from a difference in the
orbitals that overlap to form the covalent bond. Orbitals that extend farther from
the nucleus result in bonds that are longer. The key question then, is which orbital
360 Chapter 9 Advanced Models of Bonding
Atomic number
Potential energy (eV)
–50
2468101214161820
–45
–40
–35
–30
–25
–20
–15
–10
–5
0
Ar
Cl
B
C
N
O
F
Ne
Al
Si
P
S
Cl
Ar
S
P
Si
Al
Mg
Na
Ne
F
O
N
C
B
Be
Li
He
1s
2s
H
3s
2p
3p
FIGURE 9.7
Relative energy of the atomic orbitals. The energy level of the atomic
orbitals decreases with increasing nuclear charge, and the atomic
orbital becomes more stable because it has a lower potential energy.
FIGURE 9.8
Overlap of atomic s orbitals.
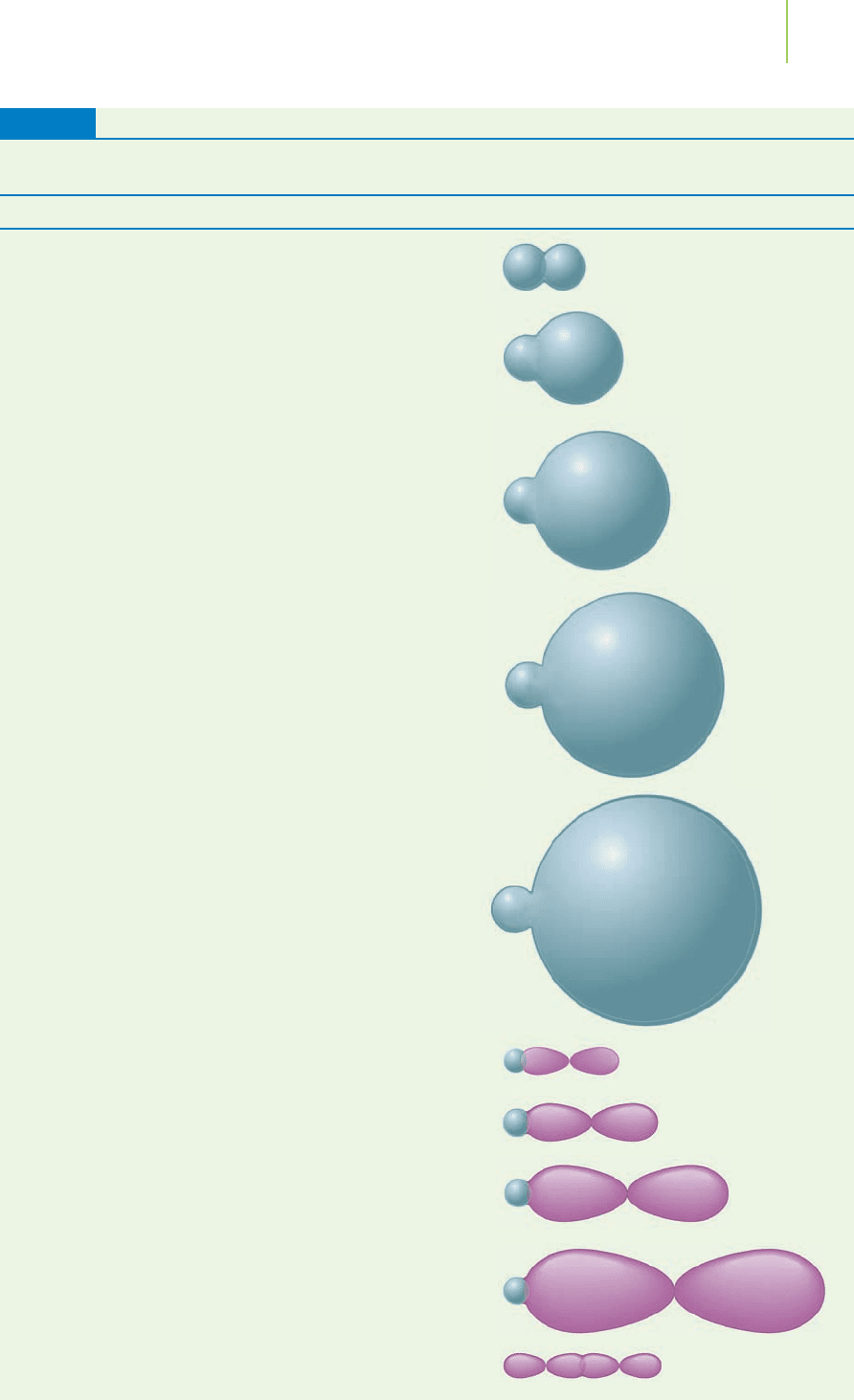
9.1 Valence Bond Theory 361
Bond Energies Resulting from Orbital Overlap
As the difference in size of the overlapping orbitals increases, the strength of the resulting bond decreases.
The data shown here are for diatomic molecules.
Bond ∆
diss
H (kJ/mol) Orbital Overlap Representation of Orbital Overlap
HOH 435.8 1s–1s
LiOH 238.0 1s–2s
NaOH 185.7 1s–3s
KOH 174.6 1s–4s
RbOH 167.0 1s–5s
HOF 569.9 1s–2p
HOCl 431.9 1s–3p
HOBr 366.3 1s–4p
HOI 298.4 1s–5p
FOF 158.3 2p–2p
TABLE 9.2
ula_09_06
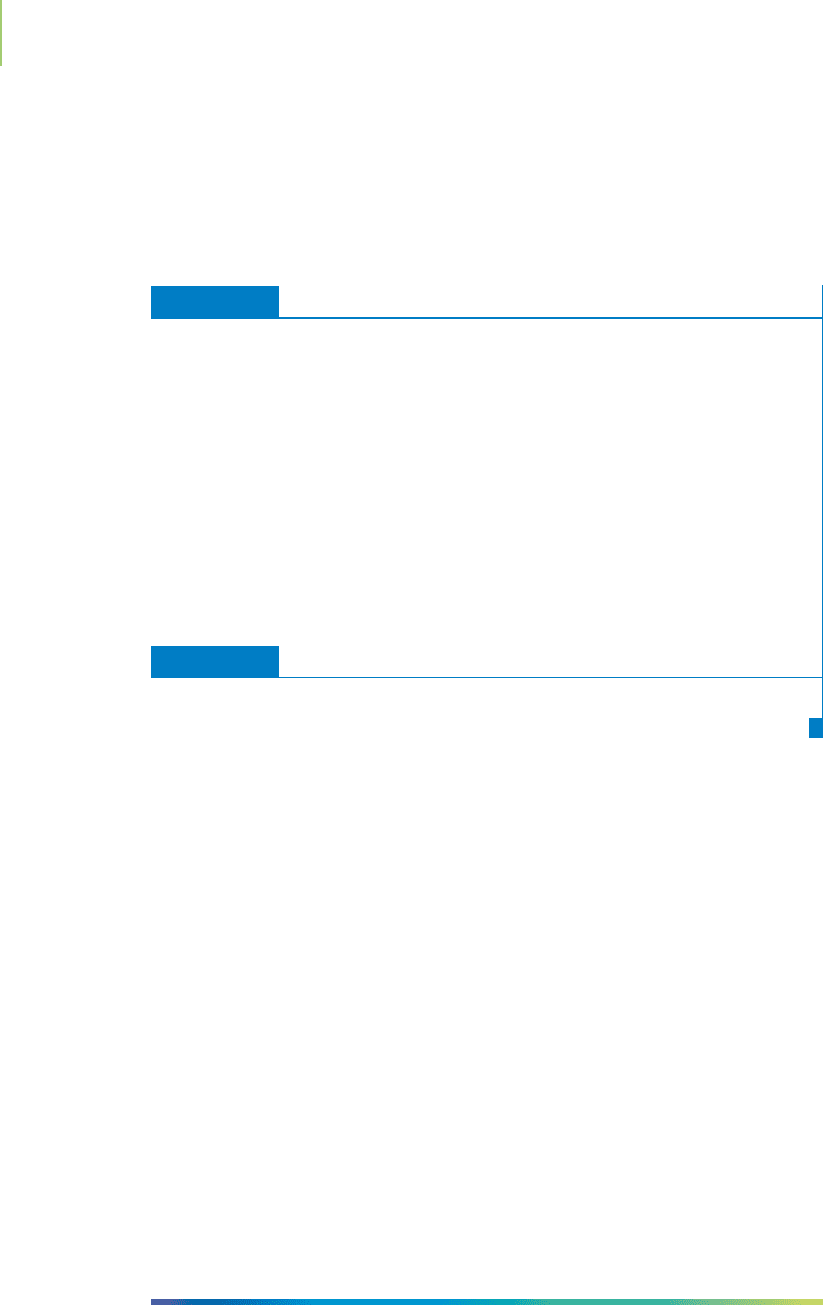
reaches farther from the nucleus, an s or a p? The end-on overlap of two p orbitals
makes a longer bond than the overlap of two s orbitals, because the average elec-
tron density lies farther from the nucleus of the atom. For this reason, the bond
in F
2
is longer (141.7 pm) than the bond in H
2
(74.6 pm). Overlap of two 1s or-
bitals makes a shorter bond than the overlap of two 2s orbitals for the same rea-
son. We’ll discuss this in greater detail in Section 9.2.
EXERCISE 9.2 Using Valence Bond Theory: Beam Me Up, Scotty
According to Star Trek fans, dilithium crystals (Li
2
) power much of the universe of
the future. Judging on the basis of valence bond theory, does this compound actu-
ally exist? Does Na
2
exist? Which would have a longer bond?
Solution
Because each of the lithium atoms has a half-filled 2s orbital, we’d predict that their
overlap should provide a stable dilithium molecule. Dilithium does exist, but be-
cause the formation of metallic lithium is so favorable, dilithium can be observed
only as a gas at high temperatures. Two sodium atoms can have overlap of their
3s orbitals to make a bond. So, theoretically, disodium should also exist. Because
the disodium molecule is made up of bigger orbitals, we’d also predict it to have a
longer bond than dilithium.
PRACTICE 9.2
Which of these molecules has the longest bond: Cl
2
,HCl,or F
2
?
See Problems 13–15.
What’s Wrong with This Model?
Methane (CH
4
) is the major component of natural gas. Piped into our homes, it
undergoes combustion to heat our water, cook our food, and warm our rooms.
Let’s build a valence bond model of methane. We start by writing the valence elec-
tron configurations of the atoms in methane. Immediately, we note that the va-
lence electron configurations of carbon (2s
2
2p
2
) and hydrogen (1s
1
) indicate a
problem. The valence shell of carbon contains a completely full 2s orbital, two
partially filled p orbitals, and one completely unfilled p orbital. Valence bond
theory indicates that we should be able to make only two bonds to the hydrogen
atoms resulting from the overlap of the two partially filled p orbitals with the
hydrogen 1s orbitals, as shown in Figure 9.9. Something is wrong here.
We know that the Lewis dot structure model of methane correctly shows
four bonds. VSEPR models represent the molecule as a tetrahedral structure
with four equal bonds. Therefore, each of the COH bonds in methane must be
made up of the same types of orbitals. But unless we modify the valence bond
theory to account for our experimental evidence, we’re sure to build a structure
that will fail. In the next section, we will describe a modification that enables us
to correct this discrepancy.
HERE’S WHAT WE KNOW SO FAR
■
Lewis dot structure and the VSEPR model do not properly explain such prop-
erties of molecules as bond lengths and bond energies. Valence bond theory
(VB theory) was introduced by Linus Pauling to better explain molecular
properties.
■
A valence bond is seen as the overlap of atomic orbitals on adjacent atoms.
362 Chapter 9 Advanced Models of Bonding
■
In VB theory, smaller orbitals overlap more than larger orbitals.
■
In VB theory, orbitals similar in size overlap more than orbitals with mis-
matched sizes.
■
In VB theory, orbitals with similar energies overlap more than orbitals with
very different energies.
■
Bond length and bond energy in simple molecules can be explained by valence
bond theory.
■
VB theory does not properly explain bond angles in molecules, so we must
modify it to make a better model.
9.2 Hybridization
Linus Pauling (we have already discussed his contributions in the areas of elec-
tronegativity and valence bond theory) advanced the theory of hybridization to
address the problems associated with VB theory. The result fixes the problems
with the valence bond approach to model construction. In 1954, Pauling was
awarded the Nobel Prize in chemistry for his years of research into the nature of
the chemical bond.
Hybridization Defined
A hybrid is a mixture of two species. Hybrid roses, tulips, marigolds, and lilies add
beauty to the garden. Much as red roses and white roses can be interbred to give
pink hybrids, we can mathematically combine two or more orbitals to provide
new orbitals of equal energy in the process of
hybridization.
9.2 Hybridization 363
1s
XX
Atomic orbitals on four hydrogen atoms
Atomic orbitals on carbon atom
1s 1s 1s
2s1s 2p 2p 2p
FIGURE 9.9
Valence bond model of methane. Atomic orbitals that
contain only one electron can overlap to form a bond.
These are shown with a dotted line. The orbitals that
cannot overlap to form a bond (either too many elec-
trons or not enough electrons) have an “
X” on the
dotted line. The model needs some corrections because
there aren’t enough orbitals to make four bonds.
Just as gardeners combine red and white
roses to obtain pink hybrids, chemists
mathematically combine two or more
orbitals to represent new orbitals of
equal energy in the process of hybridiza-
tion. The Pinocchio rose and the Crimson
Glory rose can be combined to give the
Fashion rose.
Pinocchio rose Crimson Glory rose Fashion rose
Tutorial: Hybridization

Recall from Chapter 6 that orbitals with the same energy are known as degen-
erate. What types of orbitals can be mixed together? The degree to which an or-
bital mixes with another is directly related to the difference in energy of the two
orbitals. Typically, we hybridize only orbitals of the same subshell, such as a hy-
brid made using 2s and 2p orbitals, resulting in a set of new orbitals that have
the properties of all the orbitals from which they were mixed. The preparation
of pink paint offers an analogy to hybridization. To make a good pink paint, we
must mix red and white paint that have the same base (i.e., latex or oil). We
don’t get a good mixture by combining one can of latex paint and one can of oil
paint.
How many orbitals do we make when we hybridize atomic orbitals? Just as if
we were to mix one can of red paint and one can of white paint to get two cans of
pink paint, we should expect to get two hybridized orbitals if we mix two atomic
orbitals. The number of orbitals that are hybridized determines the number of
new orbitals that are made. The orbitals that result from this mixing will be de-
generate (have the same energy) and will have the same shape, but they will be
oriented in different directions.
What is the energy of the resulting hybridized
orbitals?
Consider our paint example again. We expect the color of the mixed
paint to be the weighted average of the colors that we added. Similarly, the energy
of the resulting hybridized orbitals should be the weighted average of the energies
of the atomic orbitals that were mixed.
sp, sp
2
, and sp
3
Orbitals
To determine the hybridized orbitals used by an atom, we follow a brief series of
steps that convert atomic orbitals into hybridized orbitals. These steps are out-
lined in Table 9.3, and we will show in detail how the process works for methane
(CH
4
). The Lewis dot structure of methane indicates that we should have one
bond from each hydrogen atom to the carbon atom.
Step 1: Write the electron configuration of the carbon atom. The electron con-
figuration of carbon (1s
2
2s
2
2p
2
) indicates that there are only two half-filled
orbitals.
Step 2: In order to place four hydrogen atoms in degenerate bonds around the
carbon atom, we need to make four orbitals on carbon that will be able to in-
teract with the four hydrogen 1s orbitals. How do we show that the carbon
makes four bonds?
Step 3: We hybridize the existing orbitals to make four orbitals. Knowing that we
need to mix four orbitals to make four orbitals, we take the available 2s orbital
and the three 2p orbitals and mix them to make four new degenerate orbitals, as
shown in Figure 9.10.
364 Chapter 9 Advanced Models of Bonding
To make pink paint, we can mix red
paint and white paint. The resulting
paint is a hybrid—a product that has
characteristics of both paints.
Algorithm for Hybridizing Atomic Orbitals
Step 1: Electron Configuration Write the electron configuration for the atom that will be hybridized.
Step 2: Bonds Needed Note the number of attached atoms and lone pairs, and select the orbitals to be
hybridized.
Step 3: Hybridize Mix the orbitals to make an identical number of new orbitals. Note the energy of
the hybridized orbitals.
Step 4: Rewrite Electron Configuration Redraw the electron configuration and include the hybridized orbitals. Place the
electrons in the new orbitals according to the Aufbau principle, Hund’s rule, and
the Pauli exclusion principle.
Step 5: Form Valence Bonds Identify which orbitals will overlap to form a bond.
Step 6: Examine Structure Describe the geometry and bond lengths for the new bond.
TABLE 9.3
Video Lesson: An Introduction to
Hybrid Orbitals
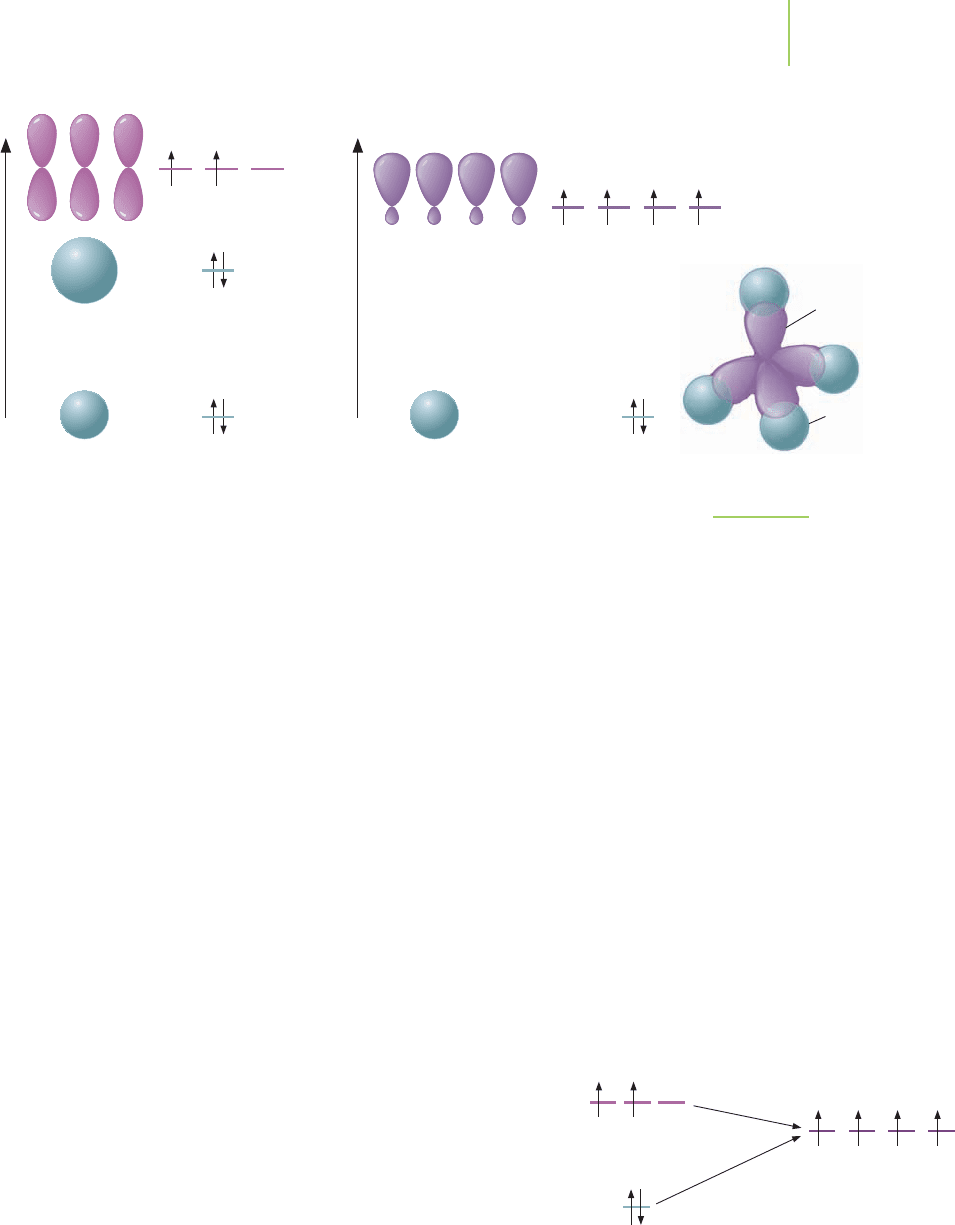
The new orbitals are given a name in order to distinguish them from the 2s and
2p orbitals, but to show their relationship to these orbitals, we call them the
2sp
3
orbitals. The name of the new orbitals illustrates that one 2s orbital and
three 2p orbitals are mixed to make four new orbitals.
Step 4: The next step in Table 9.3 is to show the electron configuration of our
hybridized carbon atom. We can write 1s
2
(2sp
3
)
4
to show the hybrid orbitals.
Carefully observe how this is written because the notation is tricky. Each of the
four 2sp
3
orbitals on our carbon atom contains only one electron and is able
to participate in bonding.
Step 5: The new shape of the orbitals (large at one end and small at the other) is
shown in Figure 9.10. Does the geometry of this hybridized carbon atom—that
is, with what are now four equivalent pairs surrounding it—agree with that
obtained from the VSEPR model? In other words,
does our answer make sense?
Step 6: Let’s look at the energy changes that occurred with this hybridization.
Energy was added to two 2s electrons on carbon in order to promote them to
the new 2sp
3
orbitals. A small amount of energy was removed when two 2p
electrons moved to the new orbitals. The net result is that the carbon atom
did increase its energy in making the new hybrid orbitals. However,
when the hybridized carbon combines with the four hydrogen atoms,
a large amount of energy is released as the new C—H bonds are
formed. Although the hybridization process itself is energetically up-
hill, the end result (four equal bonds) is still energetically downhill
(and quite favorable). Why? What reduces the energy? In methane,
the s–sp
3
overlap between each hydrogen and the central carbon atom
forms the four bonds (Figure 9.10). The outcome of the resulting va-
lence bond model now agrees with the Lewis dot structure and the
VSEPR model. Moreover, we also have an idea of the relative bond
lengths and strengths in methane.
Boron trifluoride (BF
3
) was mentioned in Chapter 8. This compound is par-
ticularly useful in enhancing the reactivity of molecules during the synthesis of
new pharmaceutical agents. Using the ideas of hybridization and valence bond
theory, what is the structure of BF
3
? The electron configuration of boron is
1s
2
2s
2
2p
1
. The Lewis dot structure of boron trifluoride indicates that we should
have three single bonds, so we must hybridize the orbitals on boron to make three
9.2 Hybridization 365
2s
1s
1s
2p
Atomic orbitals on carbon Hybrid orbitals on carbon
Note: Only two bonds are possible. Note: Four bonds are possible
with the new hybridized orbitals.
2p 2p
Energy
1s
2sp
3
2sp
3
2sp
3
2sp
3
2sp
3
Energy
FIGURE 9.10
Hybridization to give four orbitals.
(+Energy) + (–Energy) = net increase in energy
–Energy
+Energy
2s
2p
2p 2p
2sp
3
2sp
3
2sp
3
2sp
3
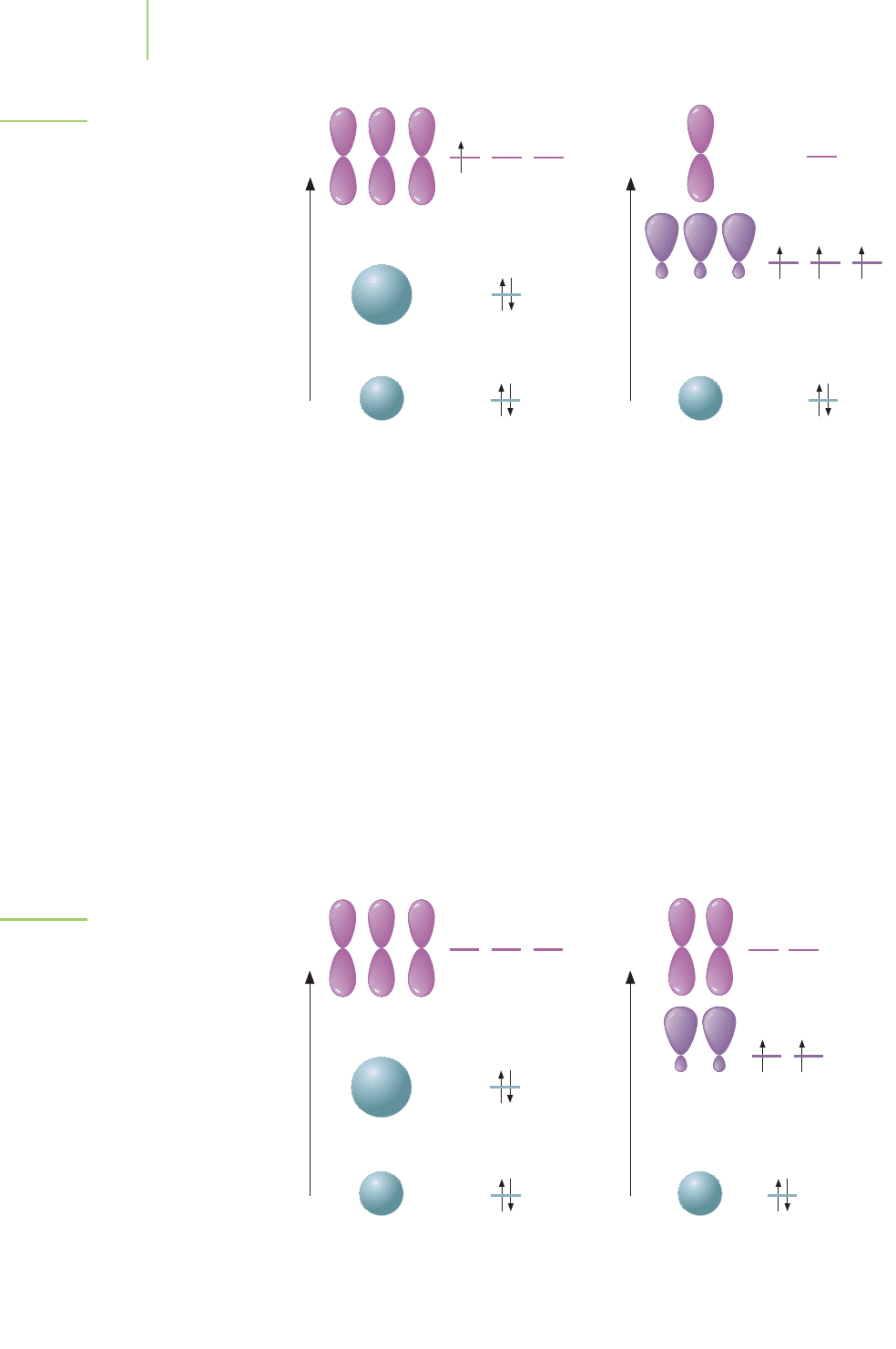
366 Chapter 9 Advanced Models of Bonding
2s
1s
2p
Atomic orbitals on boron Hybrid orbitals on boron
2p 2p
Energy
1s
2sp
2
2p
2sp
2
2sp
2
Energy
1s
Atomic orbitals on boron Hybrid orbitals on boron
Note: Only one bond is possible. Note: Three bonds are possible
with the new hybridized orbitals.
1s
FIGURE 9.11
Hybridization to give three orbitals in BF
3
.
2s
1s
2p
Atomic orbitals on beryllium Hybrid orbitals on beryllium
2p 2p
Energy
1s
2sp
2p 2p
2sp
Energy
1s
Atomic orbitals on beryllium Hybrid orbitals on beryllium
Note: No bonds are possible. Note: Two bonds are possible
with hybridized orbitals.
1s
FIGURE 9.12
Hybridization to give two orbitals in BeH
2
.
orbitals. Following the steps in Table 9.3, we mix the 2s orbital and two of the 2p
orbitals (all from boron) to make the new orbitals. The three resulting orbitals
have the designation 2sp
2
, as shown in Figure 9.11. We didn’t hybridize one of the
2p orbitals because it wasn’t needed to make a bond (i.e., the empty unhybridized
2p orbital is still available on the boron atom). We rewrite the electron configura-
tion for boron trifluoride to end up with three orbitals that are available to bond
with the half-filled 2p orbital on each unhybridized fluorine atom (p–sp
2
overlap).
We can build a model of beryllium hydride (BeH
2
) showing covalent bonds
between the beryllium (1s
2
2s
2
) and hydrogen atoms. There are no electrons in
the atomic 2p orbitals. Because we know that there are two bonds in BeH
2
,we hy-
bridize two orbitals to make two new 2sp orbitals, as shown in Figure 9.12.
Rewriting the electron configuration shows that we can now make bonds to the
hydrogen atoms, resulting in an overlap of the 1s and 2sp orbitals. In beryllium
hydride, there are two empty, unchanged 2p orbitals.
Selected Hybrid Orbitals
COH Bond COC Bond
Hybrid Example Length (pm) Angle Length (pm)
sp
3
CH
3
OCH
3
109 109.5° 154
Orbital
sp
2
CH
2
PCH
2
108 120° 134
Energy
sp CHqCH 106 180° 120
TABLE 9.4
Subtraction
Addition
p
s
FIGURE 9.13
Mixing an s and a p orbital. Addition and
subtraction of the s and p orbitals gives
two sp orbitals.
sp
180°
s
—
p
90°
sp
2
120°
sp
3
109.5°
Increasing length of orbital
9.2 Hybridization
367
EXERCISE 9.3 Hybridization in Common Molecules
Describe the hybridization of the central atom in each of these molecules:
a. H
2
Ob.NH
3
c. AlH
3
Solution
a. Lewis dot structure models for water indicate that the central oxygen has two
bonds and two lone pairs. Mixing the four orbitals (one 2s and three 2p) on
the oxygen atom allows us to have two lone pairs of equal energy and two
equal bonds to the adjacent hydrogen atoms. The oxygen atom possesses 2sp
3
hybridized orbitals.
b. In a similar fashion, the nitrogen atom requires four things (three bonds and
one lone pair) in ammonia. Mixing the four orbitals in its valence shell gives
us three bonds to the hydrogen atoms and one lone pair (all degenerate in en-
ergy). The nitrogen atom is 2sp
3
hybridized.
c. Aluminum has only three electrons in its valence shell. On the basis of its
electron configuration, only one bond would be allowed. Because three points
of attachment are needed (one to each hydrogen), we mix the 3s and two of
the 3p orbitals to make an 3sp
2
hybridized aluminum.
PRACTICE 9.3
What is the hybridization of the central atom in each of the following substances?
a. OF
2
b. H
2
Sc.NH
4
+
See Problems 23, 24, 33, 34, 43, and 44.
Shapes of the Hybrids
Hybrid orbitals have a shape that results from the mixing of the corresponding
atomic orbitals. If we add an s orbital and a p orbital together, the result is one of
the two hybrid sp orbitals. If we subtract the s orbital from the p orbital, we get the
other hybrid sp orbital as shown in Figure 9.13. The angles between the resulting
orbitals agree with what we expect from VSEPR rules. The sp hybridized atom has
bonds with 180° angles, the sp
2
hybridized atom forms bonds with 120° angles,
and the sp
3
hybridized atom contains bonding angles of 109.5° (see Figure 9.14;
see also Table 9.4).
FIGURE 9.14
Shapes of the hybrid orbitals.
Visualization: Hybridization:
sp
Visualization: Hybridization:
sp
2
Visualization: Hybridization:
sp
3
