Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


348 Chapter 8 Bonding Basics
equatorial position The position of a portion of a mole-
cule when it is arranged along the x axis or y axis of
the molecule. (p. 340)
expanded octet The existence of more than eight elec-
trons in the valence shell of an atom. Atoms of
atomic number greater than 12 are capable of this
feat. In the third row of the periodic table, it typi-
cally occurs in sulfur and phosphorus. (p. 330)
formal charge The difference between the number of
valence electrons on the free atom and the number
of electrons assigned to that atom in the molecule.
(p. 324)
ion pair Ions of opposite charges that exist in solution
as an ionically bonded pair. (p. 308)
ionic bond A strong electrostatic attraction between
ions of opposite charges. (p. 304)
isoelectronic Having the same electron configuration.
(p. 305)
lattice energy The amount of energy released as a solid
ionic crystal is formed. (p. 314)
lattice enthalpy The amount of energy required to sep-
arate 1 mol of a solid ionic crystalline compound
into its gaseous ions. (p. 313)
Lewis dot structure A drawing convention used to
determine the arrangement of atoms in a molecule
or ion. (p. 305)
Lewis dot symbol A drawing convention used to deter-
mine the number of valence electrons on an atom
or monatomic ion. (p. 305)
lone pairs Pairs of electrons that are not involved in
bonding. (p. 322)
metallic bond A bond in which metal cations are
spaced throughout a sea of mobile electrons.
(p. 304)
molecular geometry The geometry of the atoms in a
molecule or ion. The molecular geometry comes
from the electron-group geometry but considers the
lone pairs “invisible.” (p. 337)
molecular models Models used by chemists to explain
the three-dimensional nature of molecules. Although
many can be made from plastic or wood, these
models can also be constructed using computers.
(p. 303)
nonpolar covalent bond A covalent bond with a very
small difference in electronegativity between the
bonded atoms. The result is minimal or no charge
separation in the bond. (p. 320)
nonpolar molecule A molecule in which there is no net
charge separation and no net dipole moment.
(p. 345)
octet rule The existence of eight electrons in the
valence shell of an atom, creating a stable atom or
ion. (p. 306)
pharmacognocist A person who studies natural drugs,
including their biological and chemical components,
botanical sources, and other characteristics. (p. 303)
polar covalent bond A covalent bond in which the atoms
differ in electronegativity, resulting in an unequal
sharing of electrons between two adjacent atoms.
(p. 320)
polar molecule A molecule in which there exists a
charge separation resulting in a net dipole moment.
(p. 345)
polarity The location of a compound on a scale from
polar to nonpolar. The dipole moment is a measure
of the polarity of a compound. (p. 345)
polarization A molecule or bond containing electrons
that are unequally distributed. (p. 319)
polarized A molecule or bond that contains an unequal
distribution of electrons. (p. 319)
radical A molecule that contains an unpaired non-
bonding electron. (p. 331)
resonance hybrid An equal or unequal (based on exper-
imental evidence) combination of all of the reso-
nance structures for a molecule. (p. 328)
resonance structure A model of a molecule in which the
positions of the electrons have changed, but the
positions of the atoms have remained fixed.
(p. 328)
triple bond A covalent bond in which three electron
pairs (six electrons) are shared between adjacent
atoms. (p. 326)
valence shell electron-pair repulsion model A model that
proposes the three-dimensional shapes of molecules
on the basis of the number of electron groups
attached to a central atom. Also known as VSEPR.
(p. 337)
VSEPR The valence shell electron-pair repulsion model.
(p. 337)
zeolite A porous solid with a well-defined structure,
typically made of aluminum and silicon. Zeolites are
capable of binding to ions of a specific radius based
on the relative size of its pores. (p. 310)
Focus Your Learning 349
The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
Section 8.1 Modeling Bonds
Skill Review
1. Use Lewis electron dot structures to answer these questions
about aluminum and nitrogen.
a. Which neutral atom, Al or N, has the greater number of
unpaired valence electrons?
b. Which neutral atom, Al or N, has the greater number of
valence electrons?
c. Which neutral atom, Al or N, is more likely to gain
electrons to form an octet?
2. Use Lewis electron dot structures to answer these questions
about carbon and sulfur.
a. Which neutral atom, C or S, has the greater number of
unpaired valence electrons?
b. Which neutral atom, C or S, has the greater number of
valence electrons?
c. Which neutral atom, C or S, is more likely to gain electrons
to form an octet?
3. Of the following, which, if any, would not have the same
configuration as a hydrogen atom with two electrons?
a. C
2+
b. B
3+
c. N
3–
d. H
+
4. Of the following, which, if any, would not have the same con-
figuration as a fluorine atom with eight valence electrons?
a. Ne b. Na
+
c. Br
−
d. S
2−
5. To which group does each of these ions belong?
a. Ion of an element with a –2 charge and a full octet
b. Ion of an element with a +2 charge and a full octet
c. Ion of an element with a –3 charge and a full octet
6. To which group does each of these ions belong?
a. Ion of an element with a +1 charge and a full octet
b. Ion of an element with a −1 charge and a full octet
c. Atom of an element with no charge and a full octet
7. The charges have been omitted from these Lewis structure
diagrams of ions. From your knowledge of the ground state
for atoms, determine the number of extra electrons that each
structure is showing, and provide the proper charge for each
ion.
a. b. c.
8. The charges have been omitted from these Lewis structure
diagrams of ions. From your knowledge of the ground state
for atoms, determine the number of extra electrons that each
structure is showing, and provide the proper charge for each
ion.
a. b. c.
9. Write Lewis electron dot diagrams for each of these ions.
a. Se
2–
b. I
–
c. Sr
2+
d. Sc
3+
e. Si
2+
10. Write Lewis electron dot diagrams for each of these ions.
a. S
2–
b. Br
–
c. Ti
2+
d. Ti
4+
e. B
3+
NBrO
ClPS
Focus Your Learning
11. Which of these ion(s) would have the same electron configu-
ration as the noble gas neon?
Cl
–
Na
+
F
–
C
2+
Al
3+
12. Which of these ion(s) would have the same electron configu-
ration as fluoride (F
−
)?
Ne
–
O
2−
NC
2+
H
+
13. Which of these, if any, are isoelectronic?
Ca
2+
Sc
+
SArCl
–
14. Which of these, if any, are isoelectronic?
Mg
2+
Na Be
2+
Ar F
−
15. Which neutral atoms could be represented by the Lewis dot
symbol shown below?
16. Which cations with a +1 charge could be represented by the
Lewis dot symbol shown below?
Chemical Applications and Practices
17. Lithium, sodium, and potassium are all very reactive alkali
metals.
a. Diagram the Lewis electron dot structure for each neutral
atom.
b. Predict the formula of the oxide compound of each.
18. Calcium, magnesium, and barium are members of the alka-
line earth metals.
a. Diagram the Lewis electron dot structure for each neutral
atom.
b. Predict the formula of the oxide compound of each.
Section 8.2 Ionic Bonding
Skill Review
19. Consider each statement below and determine whether it is
true or false for compounds with ionic bonding.
a. They are typically composed of a nonmetal and a metal.
b. Electrons are shared among atoms in the compound.
c. The metal typically becomes a cation.
d. Like charges repel each other.
20. Consider each statement below and determine whether it is
true or false for compounds with ionic bonding.
a. They require only one metal and one nonmetal.
b. The metal is always written first in the formula.
c. The nonmetal must have a –1 charge.
d. The metal and nonmetal must be in the same period.
21. Using the octet rule, explain why aluminum loses three elec-
trons in both aluminum oxide and aluminum chloride, yet
the ratio of aluminum to the nonmetal is 1:3 in aluminum
chloride and 2:3 in aluminum oxide.
22. Using the octet rule, explain why magnesium loses two elec-
trons in both magnesium oxide and magnesium chloride, yet
the ratio of magnesium to the nonmetal is 1:2 in magnesium
chloride and 1:1 in magnesium oxide.
X
X

350 Chapter 8 Bonding Basics
23. Within each of the series presented, rank the atoms or ions
from smallest to largest radius.
a. I
5+
II
–
c. C
+
CC
–
b. S
6+
S
4+
S
2–
d. Fe Fe
3+
Fe
2+
24. Within each of the series presented, rank the atoms or ions
from smallest to largest radius.
a. Cu
2+
Cu
+
Cu c. N
−
N
3−
N
+
b. Cr
6+
Cr
4+
Cr d. Pd Pd
4+
Pd
2+
25. Using Coulomb’s law, arrange these alkali halides from
strongest to weakest attraction of anion and cation: KCl, NaCl,
CsCl, LiCl, RbCl.
26. Using Coulomb’s law, arrange these alkali halides from
strongest to weakest attraction of anion and cation: LiCl, LiF,
LiBr, LiI.
Chemical Applications and Practices
27. Potassium chloride (KCl) has been used as a sodium substi-
tute in some commercial salt (NaCl) products. Show the for-
mation of this important ionic compound using the Lewis
dot symbol model.
28. When some metals oxidize, the product formed may be
detrimental to the strength of the metal structure. This is the
case when iron corrodes. However, aluminum forms an oxide
that resists further reaction and adheres to the metal, form-
ing a protective covering. Show the formation of this ionic
compound using the Lewis dot symbol model.
29. When placed in water, the oxide compounds of many metals
form alkaline, or basic, solutions. This takes place when the
metal oxide reacts with water to produce hydroxide ions. Di-
agram the Lewis dot structure of sodium oxide, and diagram
the structure of the compound that forms when sodium
oxide reacts with water.
30. Calcium oxide is often called lime. Lime has a number of
uses, including reacting with acidic gases from coal plants.
Diagram the Lewis dot structure for this compound, and di-
agram the structure of the hydroxide ion that forms when
lime is placed in water.
31. If Mn
5+
and Mn
4+
were in a solution together, which would
fit into a smaller zeolite hole?
32. Which of these copper ions would fit into a smaller zeolite
hole, Cu
+
or Cu
2+
?
33. The melting point of KBr is 734
o
C. The melting point of
CsBr is 640
o
C. Which of the two compounds do you predict
to have the higher lattice enthalpy? Explain your answer in
terms of Coulomb’s law.
34. Of NaCl, KCl, and MgCl
2
, which would you predict to have
the highest lattice enthalpy? Explain your answer in terms of
Coulomb’s law.
Section 8.3 Covalent Bonding
Skill Review
35. The terms electronegativity and electron affinity are both used
to explain some bonding concepts. Compare and contrast
these two important terms.
36. The expression covalent bond is actually used in three differ-
ent contexts within the chapter. Explain the differences
among nonpolar covalent bonds, polar covalent bonds, and
coordinate covalent bonds. Provide an example of a com-
pound that illustrates each of these three types of covalent
bonds.
37. Diagram the outline of a periodic table. Using arrows to
indicate a direction of increase, show the general periodic
trendfor electronegativity withina group and withina period.
38. Diagram the outline of a periodic table. Using arrows to in-
dicate a direction of increase, show the general periodic trend
for atomic size within a group and within a period.
39. Diagram the Lewis structure for each of these neutral com-
pounds. Identify any that contain an odd number of (radical)
electrons
H
2
ONO CO NO
2
HCl PCl
2
NBr
3
40. Diagram the Lewis structure for each of these neutral com-
pounds. Identify any that contain an odd number of (radical)
electrons.
CS
2
N
2
OPCl
3
SO
2
H
2
O
2
SeH
2
SF
4
41. Diagram the Lewis structure for each of these ions. Identify
any that contain an odd number of (radical) electrons.
OH
–
NO
2
−
Br
−
PO
4
3−
SO
3
2−
CO
3
2−
BrO
4
−
42. Diagram the Lewis structure for each of these ions. Identify
any that contain an odd number of (radical) electrons.
SH
–
NO
3
−
F
−
IO
4
−
BO
3
3−
CN
−
CrO
4
2−
43. Diagram the Lewis structure for each of these com-
pounds. Identify any that contain an odd number of (radical)
electrons.
C
2
H
6
C
3
H
6
C
2
H
4
C
3
H
8
C
4
H
10
44. Diagram the Lewis structure for each of these com-
pounds. Identify any that contain an odd number of (radical)
electrons.
C
2
H
2
C
3
H
4
C
3
H
8
OC
2
H
6
O
CH
2
Cl
2
45. It is possible to diagram three resonance structures for the ni-
trate (NO
3
–
) ion. Show, by giving formal charges, that each
contributes equally to the overall hybrid structure that
depicts bonds of equal strength between the three oxygen
atoms and one nitrogen atom.
46. It is possible to diagram many resonance structures for the
phosphate (PO
4
3−
) ion. Show, by giving formal charges, that
each contributes equally to the overall hybrid structure that
depicts bonds of equal strength between the four oxygen
atoms and one phosphorus atom.
47. Under the proper conditions, carbon atoms can bond to
form closed ring structures when the carbon atoms are
bonded to each other. These are called cyclic compounds.
Three four-carbon ring compounds (C
4
H
4
,C
4
H
6
, and C
4
H
8
)
are known to exist. Diagram the Lewis dot structure of each
and predict which, if any, may have a resonance structure.
Diagram the resonance structures of any possible results.

Focus Your Learning 351
48. Three straight-chain four-carbon compounds that are not
cyclic (C
4
H
10
,C
4
H
6
, and C
4
H
8
) are known to exist. Diagram
the Lewis dot structure of each and predict which, if any, may
have a resonance structure. Diagram the resonance struc-
tures of any possible results.
49. The compounds N
2
O, N
2
, and N
2
H
4
all contain nitrogen to
nitrogen bonds. Diagram the Lewis electron structure of
each. Then match these bond energies for the nitrogen-to-
nitrogen bond in each. (946 kJ/mol, 160 kJ/mol, 418 kJ/mol)
50. In which structure would you expect to find the shorter
carbon-to-oxygen bond, carbon monoxide or carbon diox-
ide? Explain the basis of your choice. In which would you
expect to find the higher value for bond energy? Explain the
basis of your choice.
51. Each of these compounds contains both ionic and covalent
bonding. Using their Lewis dot structures, indicate where
each type of bonding occurs.
a. Na
3
PO
4
b. CaCO
3
c. Fe(NO
3
)
2
52. Each of these compounds contains both ionic and covalent
bonding. Using their Lewis dot structures, indicate where
each type of bonding occurs.
a. NaOH b. NaHCO
3
c. K
2
CrO
4
53. This list gives bonds that could form in compounds. Arrange
them in order from least polar bond to most polar bond.
CaONHOCCaOH
COCNOO
54. This list gives bonds that could form in compounds. Arrange
them in order from least polar bond to most polar bond.
HOFOOPLiOF
SOFNOS
55. a. Calculate the formal charges on the carbon atoms in
ethane (C
2
H
6
).
b. If one of the hydrogen atoms is replaced with an OH
group, the compound becomes ethanol. What if any
changes in the formal charge of the carbon atoms takes
place when this conversion is made?
c. What is the formal charge on the oxygen atom in ethanol?
d. If the hydrogen atom were then removed from the oxygen
atom, with no other atom taking its place, what would you
calculate as the formal charge for oxygen?
56. Diagram the Lewis dot structure of the sulfate ion (SO
4
2−
)
without an expanded octet and with an expanded octet.
According to the reasoning about formal charges suggested
in the chapter, which of the two structures is more likely to
represent the actual structure of this important ion?
Chemical Applications and Practices
57. Ammonium chloride (NH
4
Cl) is used in the manufacture of
some dry cell batteries. Show the structure of this important
ionic battery component using the Lewis dot symbol model.
58. One of the major manufactured compounds containing
iodine is hydrogen iodide. It is used to produce other com-
pounds that contain the iodide anion. Diagram the Lewis dot
structure of this compound.
59. Diagram the Lewis structure for the air pollutant nitrogen
dioxide (NO
2
). Determine the formal charges on each atom
in the structure. Circle and label any electrons that would be
considered bonding, lone-pair, or radical.
60. Many states are blending ethanol with gasoline to produce
“gasohol” products to use in automobiles. This mixture al-
lows us to extend our dwindling gasoline supplies and im-
prove the octane rating of the gasoline. Diagram the Lewis
structure for this renewable energy extender (C
2
H
5
OH). De-
termine the formal charges on each atom in the structure.
Circle and label any electrons that would be considered
bonding, lone-pair, or radical.
61. The cyanide ion (CN
–
) plays a critical role in reacting metals
from ores in the process of producing pure metals. Diagram
the Lewis structure of this important ion, and determine the
formal charges on both the carbon and nitrogen atoms.
When the ion reacts with H
+
, hydrocyanic acid is formed. On
the basis of your calculations of formal charges, would it be
more likely for the H
+
to attach to the carbon side or the
nitrogen side of the ion? Explain your choice.
62. Hypochlorous acid is the acid that can be used to make the
salt sodium hypochlorite that is found in most commercial
bleach preparations. Hypochlorous acid consists of one atom
each of hydrogen, chlorine, and oxygen. Diagram three dif-
ferent arrangements of the atoms in the molecule, and use
formal charge considerations to predict which is most likely.
63. Sulfurous acid (H
2
SO
3
) is one of the molecules that con-
tributes to acid rain. The molecule has three oxygen atoms
attached to the central sulfur atom. Diagram the Lewis struc-
ture for the acid, and predict the formal charge on the sulfur
atom. Is the formal charge the same as the oxidation number?
Explain any differences.
64. Dimethyl sulfoxide is a solvent used in some veterinary ap-
plications. The molecule consists of a central sulfur atom
bonded to an oxygen atom and two carbon atoms. Each car-
bon atom has three bonded hydrogen atoms attached. Each
atom follows the Lewis electron dot rules. Diagram the Lewis
structure and determine the formal charge on the sulfur
atom.
65. The concentrations of both nitrite ion (NO
2
–
) and nitrate ion
(NO
3
–
) must be monitored in well water. Both can be quite
harmful for humans. Diagram the Lewis dot structures of
both (the nitrogen atom is in the center of both, and there are
no oxygen-to-oxygen bonds), and assign a formal charge to
the nitrogen atom in each.
66. Phosphoric acid (H
3
PO
4
) is used in the production of phos-
phate fertilizers and is often found in soft drinks (check the
labels). In the molecule, phosphorus is at the center, and the
hydrogen atoms are attached to the oxygen atoms. Phospho-
rous acid (H
3
PO
3
) differs slightly in that one of the hydrogen
atoms is attached to the central phosphorus atom. Diagram
the Lewis electron dot structure of both, and compare the
formal charges on the phosphorus atoms.
67. Of the approximately 20 naturally occurring amino acids,
glycine, the principal component in silk, has the simplest
structure. Show the Lewis dot structure of glycine. (It is com-
posed of two atoms of carbon, one of which is bonded to two
hydrogen atoms, and a nitrogen, which is also bonded to
two hydrogen atoms. The other carbon atom is bonded
to two oxygen atoms, one of which is bonded to an atom of
hydrogen. Examine the structure. Is there a possible reso-
nance structure to draw for the one you have shown? If so,
diagram the other resonance form.
352 Chapter 8 Bonding Basics
68. Oxalic acid (C
2
O
4
H
2
) is a toxin found in rhubarb leaves. It
can also be used in general chemistry labs, in its pure form, as
a standard acid with which to react unknown base solutions.
In the structure, the two carbons are connected by a single
bond. Each carbon is connected to two oxygens (one with a
double bond and one with a single bond). The hydrogens are
on opposite sides of the structure. Diagram the structure and
determine whether a possible resonance structure exists for
the compound. If so, draw all of the possible resonance
structures.
69. The three simplest two-carbon hydrocarbon compounds
are the heating fuel ethane (C
2
H
6
), the plant hormone ethene
(C
2
H
4
), and the welding gas ethyne (C
2
H
2
). Diagram the
Lewis structure of each, and predict which would have
the highest bond energy. Which would have the longest
carbon-to-carbon bond?
70. Absorbed light can be sufficient to break chemical bonds.
Compare the bonding of Cl
2
and O
2
. In which would the
light absorbed have to be greater in energy? Use Planck’s con-
stant to calculate the frequency of the energy needed to break
apart the molecule you selected. What would the energy need
to be in order to break 1 mol of those bonds?
71. Butane (C
4
H
10
) is the fuel typically used in small, hand-held
lighters to provide heat for other reactions through combus-
tion. Use bond energies to determine the heat of the reaction
between butane and oxygen to produce water and carbon
dioxide. When another determination for the heat of this
reaction was made, using a calorimeter rather than tabular
values, the answer was slightly different. Explain why the two
values may not agree, even though they were obtained for the
same combustion reaction.
72. One way to produce ethanol (CH
3
CH
2
OH) is by the reaction
of ethene (C
2
H
4
) and water. If the heat of this reaction was
–37 kJ/mol, what would you calculate as the bond energy for
the carbon double bond in ethene? How does this compare
with the tabular value for the CPC?
73. Using hydrogen gas and oxygen gas, calculate the heat of re-
action both for water and for the bleaching agent hydrogen
peroxide (H
2
O
2
). Judging on the basis of your calculation,
which of the two should be more stable? Explain the basis of
your choice.
74. Small portable tanks often contain propane (C
3
H
8
) as the
fuel to provide heat for camping trips. Use bond energies and
the balanced chemical equation for the combustion of
propane to determine the heat of the reaction between oxy-
gen and propane to produce carbon dioxide and water. On a
per-gram basis, which fuel provides more heat, propane or
butane (C
4
H
10
)?
Section 8.4 VSEPR Theory—A Better Model
Skill Review
75. For those compounds listed in Problem 39 that contain more
than two atoms, determine the electron group and molecular
geometry of the central atoms.
76. For those compounds listed in Problem 40 that contain more
than two atoms, determine the electron group and molecular
geometry of the central atoms.
77. For those compounds listed in Problem 41 that contain more
than two atoms, determine the geometry of the central
atoms.
78. For those compounds listed in Problem 42 that contain more
than two atoms, determine the geometry of the central
atoms.
79. For those compounds listed in Problem 43, determine the
geometry of each carbon atom.
80. For those compounds listed in Problem 44, determine the
geometry of each carbon atom.
81. Use Lewis dot structures and the VSEPR theory to explain the
bond angles in ClO
2
.
82. Use Lewis dot structures and the VSEPR theory to explain the
bond angles in SF
2
.
83. Use VSEPR modeling to arrange this list in order from largest
to smallest Cl-to-element-to-Cl bond angle:
BeCl
2
AlCl
3
CCl
4
XeCl
4
NCl
3
84. Use VSEPR modeling to arrange this list in order from largest
to smallest F-to-element-to-F bond angle:
MgF
2
NF
3
CF
4
PF
6
IF
3
Chemical Applications and Practices
85. Carbon tetrachloride follows the “octet rule” and has tetrahe-
dral geometry. However, another tetrachloride compound,
SeCl
4
, has a different geometry and does not follow the octet
rule. Predict the shape of the second compound, and
diagram its Lewis dot structure.
86. Noble gases already have an octet of valence electrons. There-
fore, any combination with another atom is likely to produce
an exception to the octet rule. Predict the shape of the com-
pound XeF
2
, and diagram its Lewis dot structure.
87. The compound N
2
H
2
has a double bond between the two
nitrogen atoms. Use the VSEPR model to predict the
HONON bond angle in the molecule. If N
2
H
2
2+
had a
triple bond between the two nitrogen atoms, how would
that affect the same bond angle? Explain the basis of your
prediction.
88. The bicarbonate ion (HCO
3
–
) is essential as a buffer
(discussed in Chapter 18) in your blood.
a. Diagram the Lewis dot structure for this important ion.
b. Is there a possibility for resonance in the structure? If so,
show the example(s).
c. In VSEPR modeling, what shape would this ion have?
Section 8.5 Properties of
Ionic Compounds and Molecules
Skill Review
89. Examine each of the bonds depicted as dashes between atoms
here. Rank them from least polar to most polar. In addition,
rewrite the bond, showing an arrow in the direction of the
more negative atom.
COCl CONCOO
COHCOBCOMg
Focus Your Learning 353
that would allow this variety in bonding? Diagram the
structure of the N
2
molecule.
99. When you inhale a breath of air, you are taking in oxygen
and nitrogen molecules. Examine the Lewis dot structure of
an N
2
molecule (see Problem 98). Suggest a reason why the
N
2
that you inhale is unaffected and is soon exhaled as the
same N
2
that you inhaled.
100. Prepare a diagram, using circles with charges inside, show-
ing a relative size comparison between magnesium oxide
(used as an abrasive) and calcium oxide (used in some plas-
ter formulations). On the basis of your representations, ex-
plain how Coulomb’s potential could be used to predict
which compound contains the larger lattice energy.
101. These circles represent cations and anions. From the choices
provided, select the cation and anion combination that
would have the lowest lattice energy. Explain your choice.
Electrical
Solubility In Conductivity As
Melting
Compound Point Water Hexane Solid Molten
A Very high Yes No No Yes
B Very low No Yes No No
C Medium Yes No No No
94. Refer to the clues in the previous problem and match these
substances as possible identities with A, B, and C.
C
3
H
8
KCl
citric acid, C
3
H
5
O(COOH)
3
95. The disinfectant hydrogen peroxide (H
2
O
2
) has a dipole mo-
ment greater than zero. Explain why this information sup-
ports suggesting that the molecule is not linear. If the molecule
were linear, what would you predict about its dipole moment?
96. The formula C
2
H
2
Cl
2
can be drawn many different ways. Pro-
vide the Lewis dot structure that would give a molecule with
the largest dipole moment. Provide the Lewis dot structure
that would give a molecule that did not have a dipole moment.
Comprehensive Problems
97. These diagrams represent a hydrogen atom with different
numbers of electrons. Determine which diagram represents
hydride (H
−
), which hydrogen (H), and which a proton (H
+
).
+1 +3 –2
102. a. In your own words explain the lattice energy formula.
b. Lithium forms many useful salts. For example, lithium
fluoride is used extensively in industry to assist in
purifying aluminum. Lithium bromide has applications
in the air–conditioning industry. Explain which of the
two compounds has the stronger lattice energy.
103. On the basis of your arrangement of the ionic compounds
in Problem 101, which compound would you predict to
have the highest lattice enthalpy?
104. a. What is the qualitative relationship between lattice
enthalpy and melting point?
b. On the basis of that relationship, which would you
predict to have a higher melting point, MgF
2
(lattice
enthalpy = 2913 kJ/mol) or NaCl (lattice enthalpy =
787 kJ/mol)? Use another reference such as the Merck
Index or the Chemical Rubber Company’s Handbook of
Chemistry and Physics to obtain the melting points of
the two compounds to check your assumptions.
105. Most ionic compounds have very high melting points.
a. Use Coulomb’s law to explain this property.
b. Using Table 8.2, select the ionic combination that would
be likely to have the highest melting point and the one
that would be likely to have the lowest melting point.
Explain the basis of both selections.
106. Magnesium metal will burn so vigorously in air that it will
even combine with nitrogen.
a. What is the formula of magnesium nitride?
b. Would the compound be ionic or covalent?
c. Diagram the Lewis dot structure of the compound.
107. a. Explain why the bond energy listed in tables is only an
approximation, based on averages, of the bond energy
for a bond within a molecule.
90. Examine each of the bonds depicted as dashes between atoms
here. Rank the bonds from least polar to most polar. In
addition, rewrite the bond, using the δ+ and δ– symbols to
indicate the polarity of the bond.
HOOHOCHOF
HONHONa HOH
91. Diagram the Lewis structures of both SF
5
and SF
6
. Use the
VSEPR model to predict the shape and the resulting polarity
of each.
92. Diagram the Lewis structures of both PBr
5
and PBr
3
. Use the
VSEPR model to predict the shape and the resulting polarity
of each.
Chemical Applications and Practices
93. This table illustrates some properties of three unknown sub-
stances. On the basis of the clues provided, decide whether
each compound listed in the table is an ionic compound, a
nonpolar molecule, or a polar molecule.
98. In various compounds, nitrogen can be shown to form sin-
gle, double, or even triple bonds. Diagram the Lewis dot
structure of a nitrogen atom. What arrangement do you see

354 Chapter 8 Bonding Basics
b. In which case would the carbon-to-carbon bond be
expected to deviate more from the average, H
3
COCH
3
or H
3
COCF
3
? Explain the basis for your reasoning.
108. The environment of an electron in a bond varies consider-
ably depending on the type of bonding arrangement in
which the electron finds itself. Electrons are attracted to the
positive nucleus of an atom or ion. Using the examples of
covalent, polar covalent, ionic, and metallic bonds, describe
the degree of attraction of an electron to its “original” atom
once it has become part of a bond with another atom.
109. Iron metal can react with oxygen gas to make iron(III)
oxide.
a. Write the balanced equation for this reaction.
b. How many unpaired electrons are in an isolated atom of
iron(III)?
c. What is the mass percent of iron in this compound?
110. People on low-sodium diets sometimes use a salt substitute
for their meals. Suppose the salt substitute contains potas-
sium chloride.
a. What is the electron configuration of potassium ions?
b. Would you expect potassium chloride to taste similar to
sodium chloride? Explain.
c. Which is larger, the potassium cation or the sodium
cation?
d. How many potassium cations are there in 1.00 g of
potassium chloride?
111. Calcium carbonate is a very common mineral found in
nature.
a. Is this compound soluble in water?
b. What is the mass percent carbon in this compound?
c. When hydrochloric acid is added to this compound,
calcium chloride and water are formed.What is the Lewis
dot structure of the other product?
112. A fertilizer salesperson remarks that potassium nitrate pro-
vides more nitrogen per pound than calcium nitrate.
a. Based on the mass percent nitrogen, is the salesperson
correct?
b. How many grams of nitrogen would be present in 1.00 lb
of the potassium nitrate fertilizer?
c. Write the Lewis dot structure for the nitrate ion.
113. The structure of nifedipine, Figure 8.18, contains four
different types of atoms.
a. What is the molar mass of this useful drug?
b. Draw at least one other resonance structure for this
molecule.
c. When this molecule is treated with an acid, a proton
(H
+
) is added to one of the nitrogen atoms in the
structure. To which nitrogen does that proton bond?
Thinking Beyond the Calculation
114. Someone proposes the use of dichloroethane (C
2
H
4
Cl
2
) as a
solvent to remove small wax (nonpolar hydrocarbon)
buildups.
a. Diagram a way to depict the structure that possesses an
essentially zero dipole moment.
b. Diagram another structure that would yield a dipole
moment.
c. Which of the compounds, that in part a or that in part b,
would be best suited to dissolve wax?
d. Dichloroethane can be prepared by adding chlorine (Cl
2
)
to ethene (C
2
H
4
). Draw a Lewis dot structure for ethene.
e. Would you predict a nonzero dipole moment for ethene?
f. If 2.0 g of dichloroethane was prepared using the method
in part d, how many grams of ethene was consumed in
the reaction?
355
Contents and Selected Applications
Chemical Encounters: Molecular Structure and Eyesight
9.1 Valence Bond Theory
9.2 Hybridization
9.3 Molecular Orbital Theory
9.4 Putting It All Together
Chemical Encounters: Benzene, Stability, and MO Theory
9.5 Molecular Models in the Chemist’s Toolbox
Advanced
Models of
Bonding
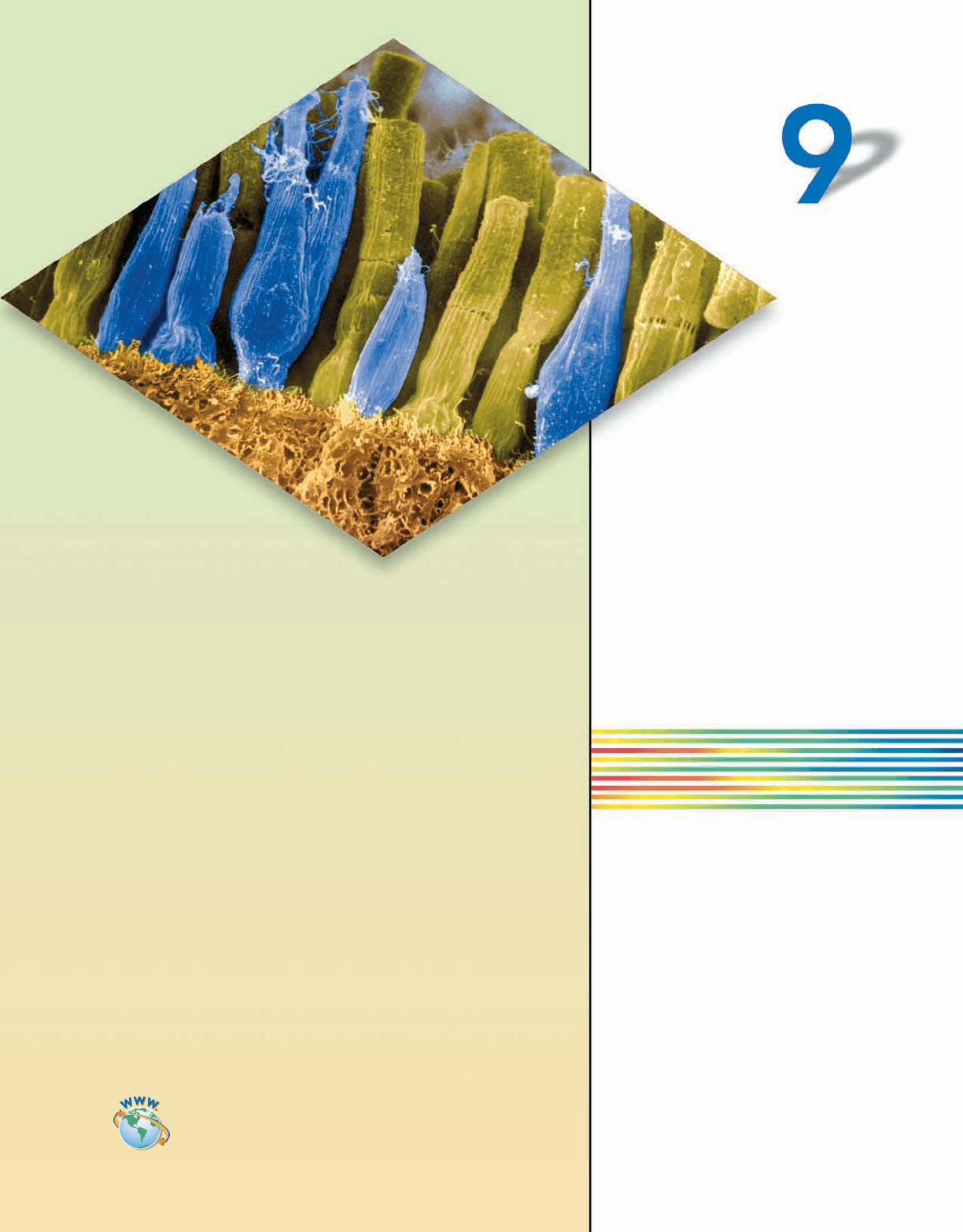
At the back of the human eye are mil-
lions of photoreceptor cells known as
rods and cones. These cells are home
to rhodopsin, a molecule that uses
11-cis-retinal to catch light and initiate a
nerve impulse. The double bonds in
11-cis-retinal enable it to interact with
light in the visible region of the electro-
magnetic spectrum. This chapter will
focus on the construction of models
that help explain this property.
Go to college.hmco.com/pic/kelterMEE for online learning resources.
356
We begin our study of advanced models
of bonding by peering down into a most
remarkable organ, the human eye. Unlike our
hands, our eyes enable us to interact with our environ-
ment without direct contact. Light reflected from other
objects enters the eye through a small opening called the iris.
Once there, the photons hit the retina, where they are collected,
converted into electrical impulses, and sent to the brain for processing
(Figure 9.1). The retina contains two types of photoreceptor cells (rods and
cones) that collect the light. The number and type of rods and cones distinguish
the human eye from that of other species.
Over 120 million rods occupy the human retina. These cells are extremely sensi-
tive to light but do not distinguish color. It is these rods that allow humans some
limited nighttime vision. The 7 million cones, which are much less sensitive to
light than the rods, allow humans to perceive color.
How are rods and cones related to molecular structure and advanced bonding
models? It has to do with the way they absorb light. Located in each rod and cone
is a protein called opsin. The protein itself doesn’t absorb visible light. However,
when a molecule known as 11-cis-retinal (see Figure 9.2) is attached to the opsin,
the resulting protein, rhodopsin, becomes able to absorb wavelengths near 500 nm.
A photon that strikes the rhodopsin causes a reaction that converts 11-cis-retinal to
the more stable all-trans-retinal. This results in a change in the position of the atoms
in the retinal molecule, causing the rhodopsin to alter its shape. This change assists
in the generation of a nerve impulse and the release of trans-retinal from the protein.
After the impulse, the trans-retinal is converted back into 11-cis-retinal and attached
to another opsin—ready for the next photon of light to begin the process again (see
Figure 9.3).
What is it about rhodopsin that makes it capable of absorbing photons? Why
does 11-cis-retinal absorb light near 500 nm and not near, say, 850 nm?
Application
C
HEMICAL
ENCOUNTERS:
Molecular Structure
and Eyesight
Cornea
Pupil
Lens
Iris
Aqueous
humor
Suspensory
ligament
Ciliary
muscles
Vitreous
body
Retina
Fovea
Blind spot
Optic
nerve
Hyaloid
canal
Choroid
Retinal
artery
and vein
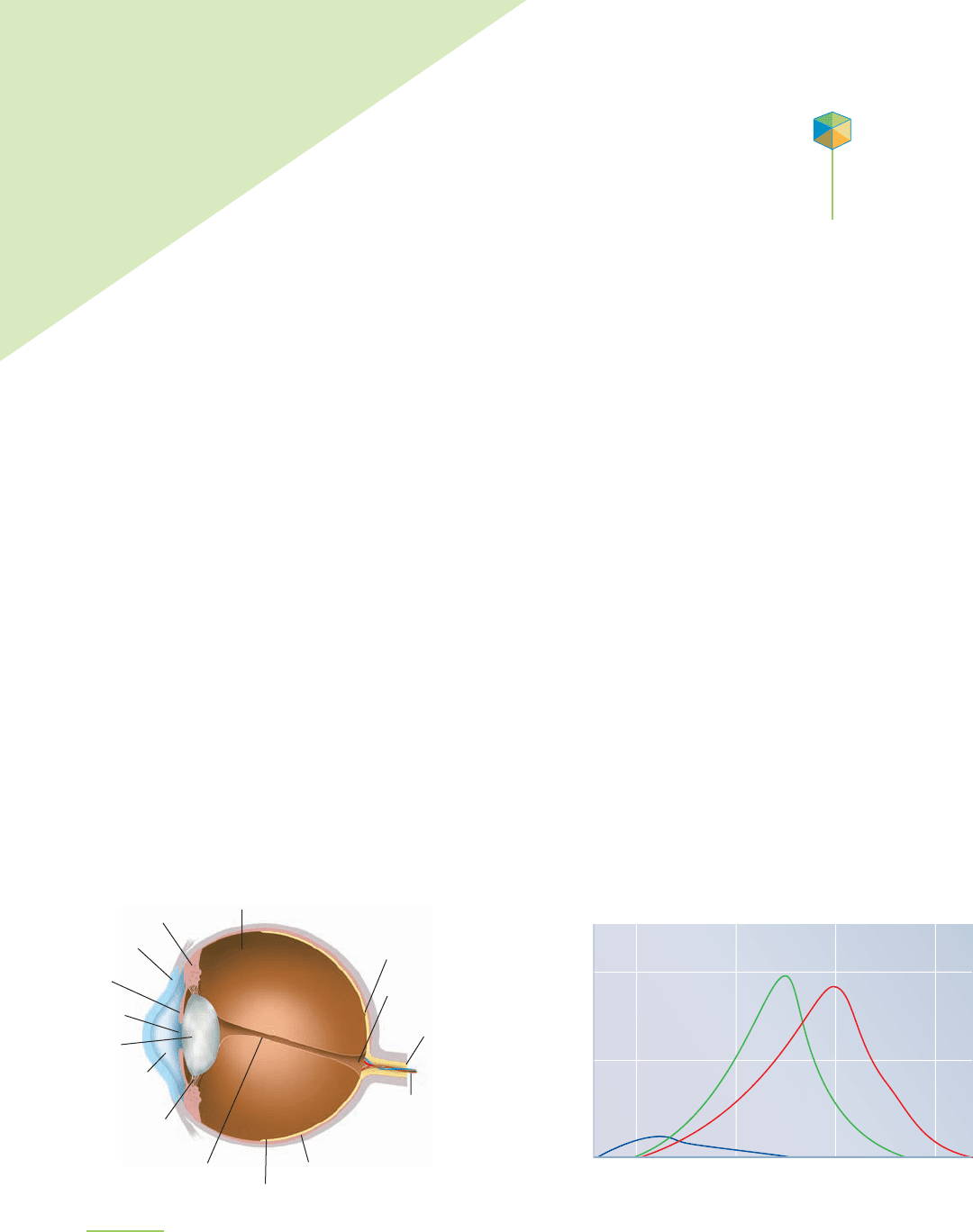
FIGURE 9.1
The human eye is a complex organ. Light enters the front
of the eye through the cornea and is focused by the lens
on the retina at the back of the eye.
Wavelength (nm)
Light fraction absorbed
0
400 500 600 700
1
2
S
M
L
There are three kinds of cones in the human eye (they are
known as S, M, and L). About 64% are sensitive to light in the
red region of the visible spectrum, 32% are green-sensitive,
and 2% respond best to blue light.
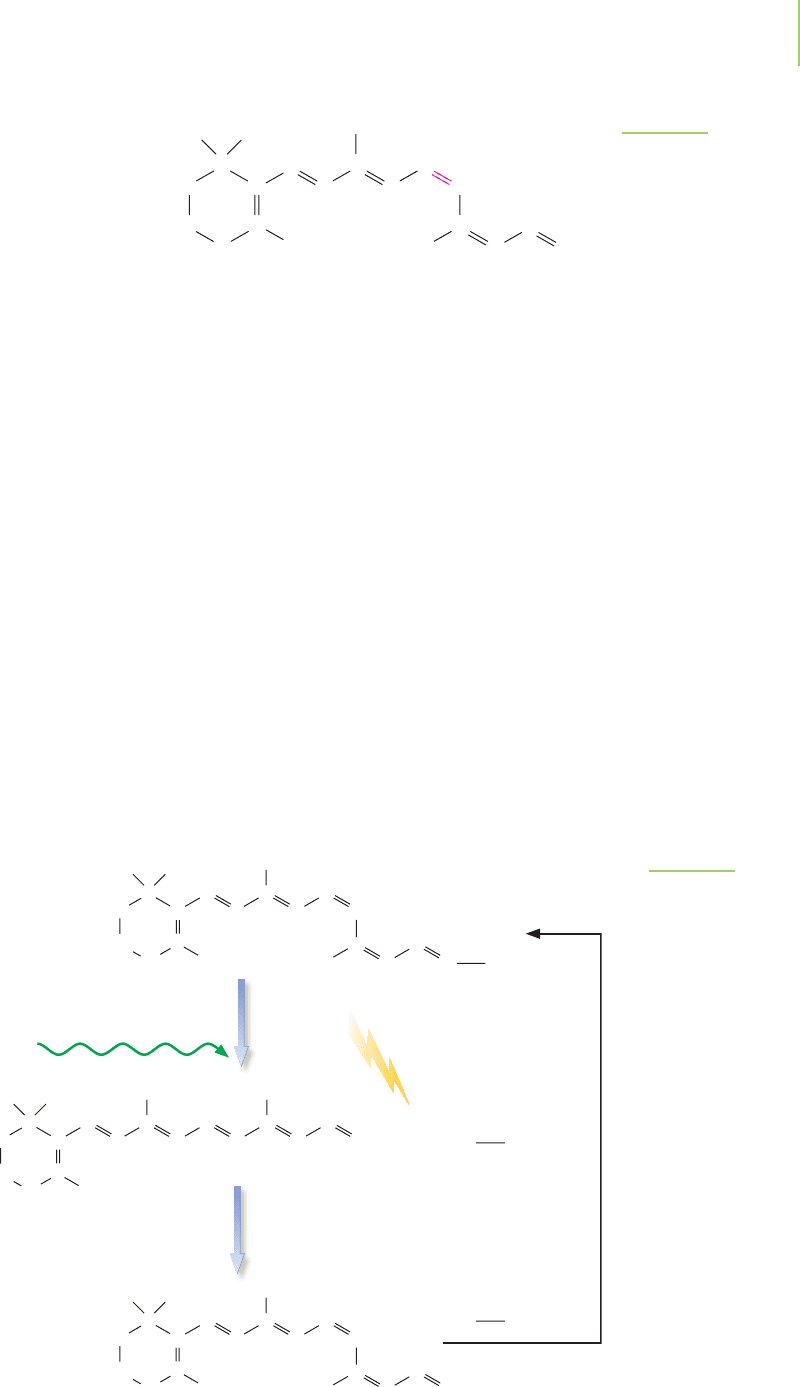
The answer to these questions largely lies in the structure of the retinal mole-
cule. However, this is best understood when we can examine more advanced
models of bonding. Although the VSEPR model and Lewis dot structures are
useful for determining the shape and connectivity of the atoms within a molecule,
they do not adequately predict many of its properties. For instance, the VSEPR
model of water does predict that the HOOOH bond angle should be less than
109.5°. However, Lewis dot structure and VSEPR models do not properly explain
why the COC bond in ethane (CH
3
OCH
3
) is longer than the COC bond in
butadiene (CH
2
PCHOCHPCH
2
). Moreover, VSEPR doesn’t explain how we can
end up with equally spaced bonds. For example, in methane (CH
4
), the bonding
electrons occupy s and p orbitals. VSEPR doesn’t even hint at why electrons in
p orbitals (90° apart) would, in reality, form 109.5° bond angles. In order to
explain the reasons behind the observed bond angles, and to more accurately
assess and predict the properties of molecules, we need to make better models.
Our goal in this chapter is to introduce you to some of the better models.
Advanced Models of Bonding 357
H
H
CH
2
CH
3
CH
3
CH
3
CH
3
CH
2
C
CC
H
C
H
CC
C
C
CH
2
C
H
C
H
C N Opsin
H
2
N Opsin
H
2
N Opsin
500 nm
Nerve impulse
Rhodopsin
trans-Retinal
CH
H
H
CH
2
CH
3
CH
3
CH
3
CH
2
C
CC
H
CC
CH
3
C
C
CH
2
CH
3
CH
3
H
H
CH
2
CH
3
CH
3
CH
3
CH
3
CH
2
C
CC
H
C
H
CC
C
C
CH
2
C
H
C
H
CO
11-cis-Retinal
CH
CH
3
CO
H
C
H
C
HH
CC
FIGURE 9.3
Transformation of 11-cis-retinal to trans-
retinal. After the absorption of light and
the reorganization of the double bond,
the rhodopsin molecule undergoes a confor-
mational change that brings about a nerve
impulse. The trans-retinal is recycled by con-
version back to 11-cis-retinal and reattached
to an opsin molecule.
C
C
HH
H
C
H
C
C
H
H
2
C
H
2
C
C
H
3
C
H
2
C
H
3
C
C
CH
3
C
CH
3
C
CH
3
CH
C
H
C
O
C
11-cis-Retinal
FIGURE 9.2
11-cis-Retinal absorbs light with wavelengths
near 500 nm. The absorption of light causes an
electron in the double bonds to become excited,
allowing the molecule to rotate about the double
bond in red.
