Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


Countries That Have Eliminated
Tetraethyl Lead Use in Motor Vehicles
Albania
Antigua
Argentina
Austria
Bahrain
Bangladesh
Belgium
Belize
Bolivia
Brazil
Canada
Colombia
Costa Rica
Denmark
Dominican
Republic
TABLE 8.4
Ecuador
Egypt
El Salvador
Finland
Germany
Guatemala
Haiti
Honduras
Hungary
Iceland
India
Ireland
Jamaica
Japan
Luxembourg
Mexico
Netherlands
New Zealand
Nicaragua
Norway
Philippines
Saudi Arabia
Singapore
Slovakia
South Korea
Sweden
Switzerland
Taiwan
Thailand
United Kingdom
United States
Vietnam
The simplest example of a compound containing a covalent bond is hydrogen
gas (H
2
), used in the food industry to make partially saturated oils, which contain
mostly carbon–carbon and carbon–hydrogen single bonds, as well as in the man-
ufacture of ammonia intended for agricultural use as a fertilizer. The bond be-
tween the hydrogen atoms in H
2
results from the sharing of one electron from
each of the atoms. The atoms on either end of the bond have the same affinity for
electrons, so they must be shared if each hydrogen is to have the electron config-
uration of a noble gas. In other words, sharing the electrons between the two
atoms effectively gives each hydrogen a 1s
2
electron configuration. The sharing of
bonding electrons is the defining characteristic of a covalent bond. What holds
the two atoms together in a covalent bond? Each nucleus on either end of the
bond exerts a force of attraction on the pair of bonding electrons. This attractive
force pulls the nuclei close to form a bond. If we examine the electron cloud
around the two nuclei in molecular hydrogen, we note an interesting feature of
the covalent bond, which is shown in Figure 8.11. The density of electrons is con-
centrated between the two atoms, but a significant amount of electron density
surrounds each of the two nuclei. As shown in Figure 8.12, the picture of electron
density in HF, a compound used to etch glass, is remarkably different because the
318 Chapter 8 Bonding Basics
H(g)H(g)H
2
(g)
+
FIGURE 8.11
Electrons hold the nuclei together in this model of hydrogen
gas. Note that the electron density, shown in red, encircles
both nuclei.
H
2
HF
FIGURE 8.12
Compare the electron density
distribution in HF with that of H
2
.
Tetraethyl lead, Pb(C
2
H
5
)
4
, delivered from gas pumps similar to
today’s, has been banned as an additive to vehicular fuel in many
countries because of the harmful effects of lead on people and
the environment.
bonding electrons are not shared quite equally, although they are shared to an
important extent, and the bond is still considered to be covalent.
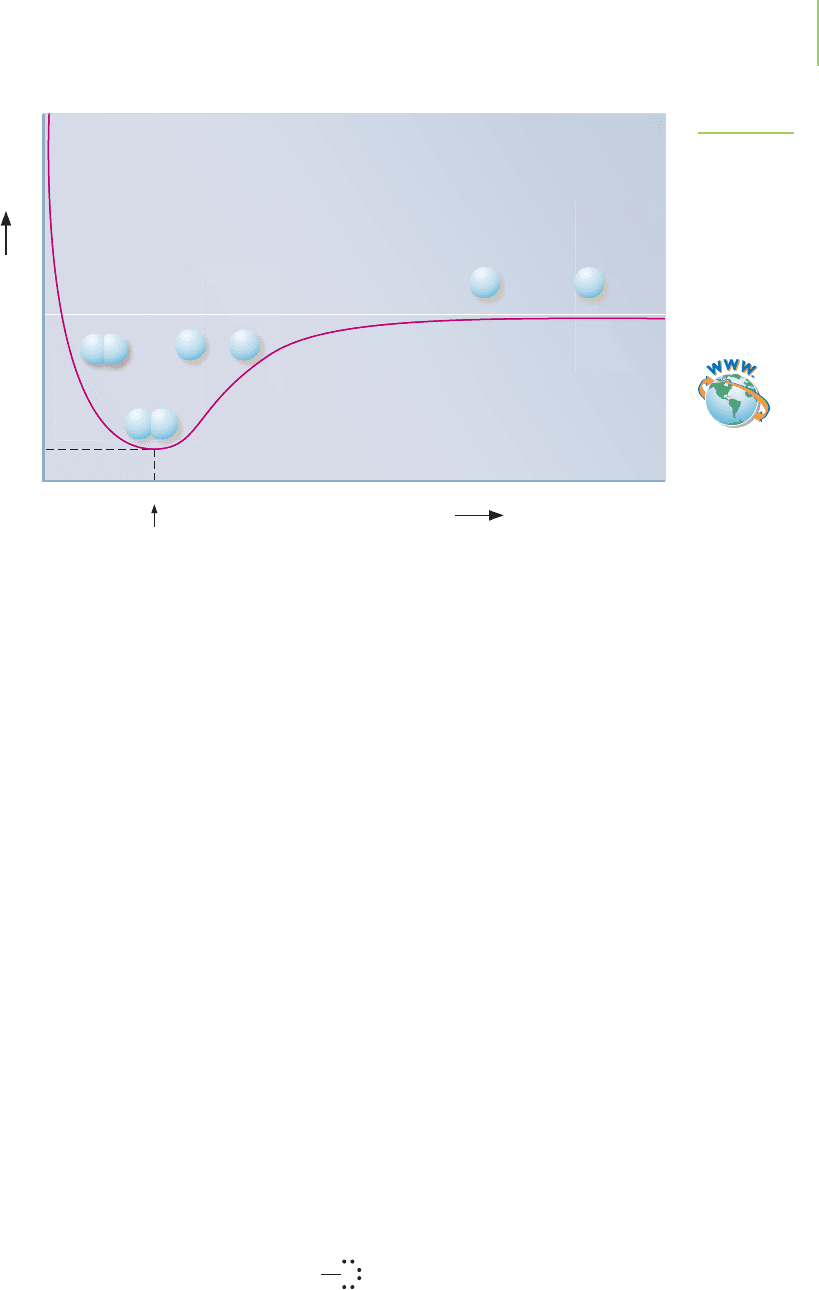
What keeps the nuclei from bumping into each other? While the attractive
force of the nuclei for the shared electrons pulls the atoms together, a repulsive
force of the like-charged nuclei pushes them apart. This repulsion keeps the nu-
clei from getting too close. It is the combination of attractive and repulsive forces
that holds the nuclei apart at a particular average distance that we refer to as the
bond length. A plot of the potential energy of two hydrogen atoms as a function
of their distance, shown in Figure 8.13, helps to illustrate this fact.
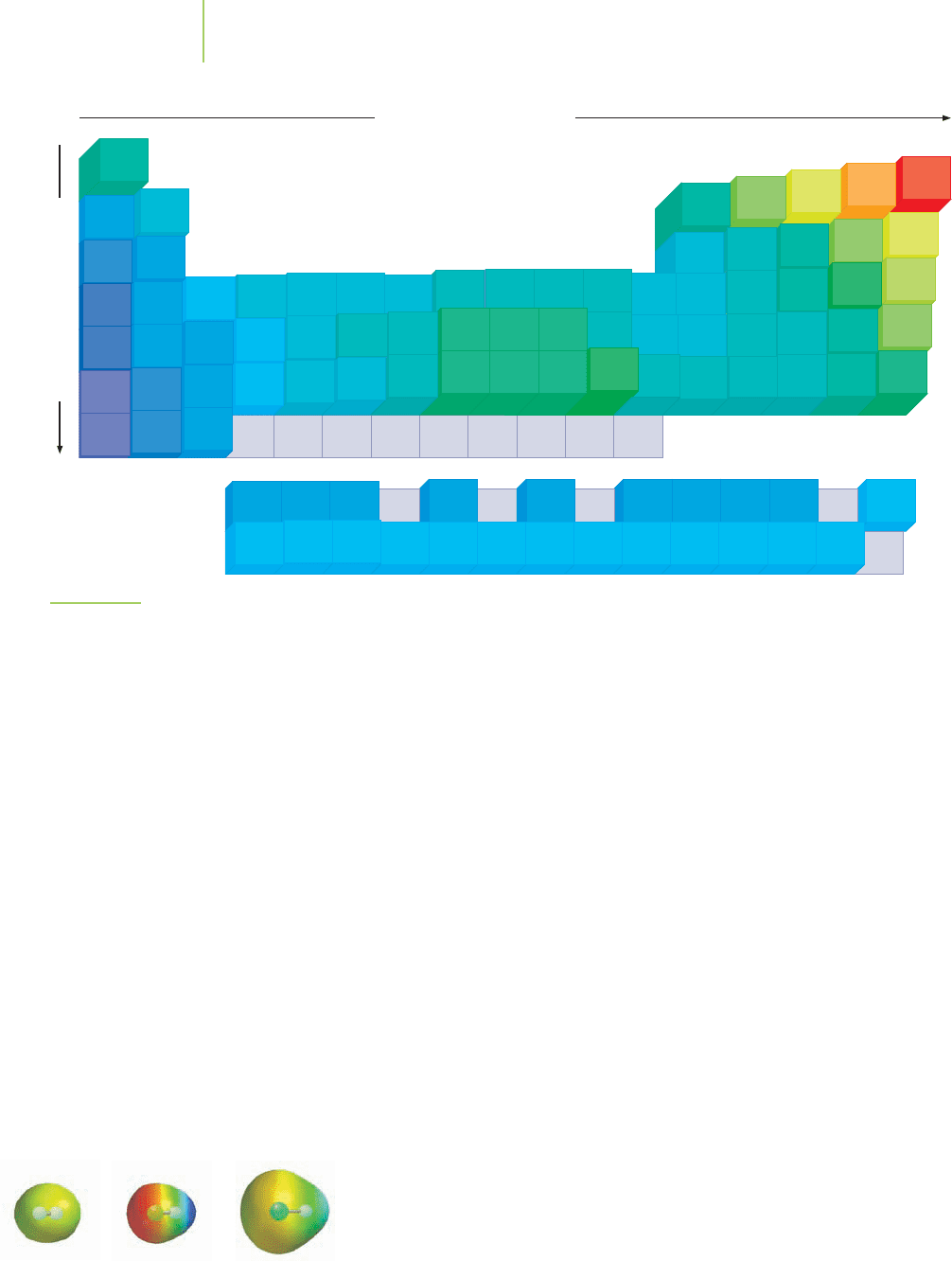
Electronegativity and the Covalent Bond
Why are electrons shared unequally in some covalent bonds such as HOF? Some
atoms have a greater attraction for shared electrons than do others. The electron
density in the covalent bond is pulled closer to the atom with the greater attrac-
tion for the bonding electrons. We say that some covalent bonds are
polarized
toward one of the atoms. In hydrogen fluoride, the attraction of the fluorine
atom for electrons causes the bonding electrons to spend more time at the fluo-
rine end of the molecule, resulting in a net
polarization of the bond. The lack of
electron density at the hydrogen end of the bond means that some of the total nu-
clear charge isn’t balanced on the atoms, so each of the atoms in the polar cova-
lent bond possesses a partial charge. We usually indicate the charge separation
with a lowercase Greek letter delta (δ) and a plus or minus sign. We’ll discuss how
to arrive at the bonding model for HF later, but the charge separation can be
represented like this:
Methods have been developed to attempt to identify the type of bond in a
molecule on the basis of the electronegativity of the bonded atoms.
Electronega-
tivity
was defined in Chapter 7 as the ability of an atom in a molecule to attract
shared electrons to itself (Figure 8.14). The atom with the stronger attraction for
the electrons pulls the bonding electrons closer to itself. By examining the differ-
ence in the electronegativity values of the two atoms involved in a bond, we can
H F
␦␦
8.3 Covalent Bonding 319
Energy (kJ/mol)
0
Internuclear distance (pm)
74 pm
–458
0
(Covalent bond
length for H—H)
HH
HH
HH
HH
FIGURE 8.13
The energy profile of a covalent bond in
H
2
as a function of distance. Note how
the attractive forces and repulsive forces
balance each other at a distance of
74 pm. At this distance the interaction
has the lowest energy. To break the
covalent bond in H
2
and separate the
atoms would require the addition of
458 kJ/mol.
Visualization: Bonding in H
2
assess the polarization of the bonding electrons (also known as the bonding elec-
tron pair). For example, fluorine is much more electronegative than hydrogen, so
the electrons spend more time on the fluorine end of the bond in HF.
The Pauling electronegativity scale is just one of the many attempts scientists
have made to develop a general trend in the polarization of electrons in bonds.
Pauling’s electronegativity scale, the most popular among several such scales, is
based on bond energies of diatomic molecules. Other scientists have developed
useful electronegativity trends based on ionization energies and electron affini-
ties (the Mulliken scale), atomic energies and covalent radii (the Allred–Rochow
scale), the “compactness of an atom’s electron cloud” (the Sanderson scale), di-
electric properties (the Phillips scale), and quantum defects (the St. John–Bloch
scale). All of these are based on the experimentally determined properties of
some standard compounds, and this makes the scales subject to some variation.
Even though all of these scales have different values for the electronegativity of
the elements, the general trends are identical among the different systems. Pauling’s
electronegativity scale, however, is the most important historically, it has proved
quite simple to use, and the conclusions drawn from it are reasonable. We will be
on safe ground using the Pauling scale.
Types of Covalent Bonding
How can we use electronegativity to determine the degree of polar character in a
bond?
In molecular hydrogen (H
2
), the difference in the Pauling values for elec-
tronegativity () between the two atoms is zero ( = 2.1 − 2.1 = 0). In a mole-
cule of HCl, the electronegativity difference is less than 1 electronegativity unit
( = 0.9). Between hydrogen and fluorine in HF, the difference is fairly large
( = 1.9). And in table salt (NaCl), the difference in electronegativity is 2.1.
Molecular hydrogen is an example of a molecule with a covalent bond that is
not polarized. We say that it contains a
nonpolar covalent bond ( 0.5).
Hydrogen chloride (HCl) and hydrogen fluoride (HF), on the other hand, con-
tain fairly polarized covalent bonds. We say that these are
polar covalent bonds
(0.5 2.0). If the difference in electronegativity is very large (2.0), the
320 Chapter 8 Bonding Basics
Nd
1.1
Pa
1.4
U
1.4
Lu
1.3
Yb
Tm
1.2
Er
1.2
Ho
1.2
Dy
1.2
Eu
Sm
1.2
Tb
Gd
1.2
H
2.1
Li
1.0
Be
1.5
Na
0.9
Mg
1.2
K
0.8
Ca
1.0
Rb
0.8
Sr
1.0
Cs
0.7
Ba
0.9
Fr
0.7
Ra
0.9
Sc
1.3
Y
1.2
La
1.1
Ac
1.1
Ti
1.5
Zr
1.4
Hf
1.3
Pm
Pr
1.1
Ce
1.1
Lr
No
1.3
Md
1.3
Fm
1.3
Es
1.3
Cf
1.3
Bk
1.3
Cm
1.3
Am
1.3
Pu
1.3
Np
1.3
Th
1.3
V
1.6
Nb
1.6
Ta
1.5
Cr
1.6
Mo
1.8
W
1.7
Mn
1.5
Tc
1.9
Re
1.9
Fe
1.8
Ru
2.2
Os
2.2
Co
1.9
Rh
2.2
Ir
2.2
Ni
1.9
Pd
2.2
Pt
2.2
Cu
1.9
Ag
1.9
Au
2.4
Zn
1.6
Cd
1.7
Hg
1.9
Ga
1.6
In
1.7
Tl
1.8
Al
1.5
B
2.0
Ge
1.8
Sn
1.8
Pb
1.9
Si
1.8
C
2.5
As
2.0
Sb
1.9
Bi
1.9
P
2.1
N
3.0
Se
2.4
Te
2.1
Po
2.0
S
2.5
O
3.5
Br
2.8
I
2.5
At
2.2
Cl
3.0
F
4.0
Increasing electronegativity
Decreasing electronegativity
Rf Db Sb Bh Hs Mt Uun Uuu Uub
FIGURE 8.14
Pauling’s electronegativity values for the
elements of the periodic table.
Compare the electron density
distributions of H
2
, HF, and HCl
with their electronegativity
values.

more electronegative atom’s ability to attract electrons in the bond overcomes the
tendency to share electrons, and we call this an ionic bond. The electronegativity
difference between sodium and chlorine in table salt ( =2.1) is characteristic of
an ionic bond. The difference in electronegativity can be useful in determining
the type of bond in a compound, but the values shown here are useful guidelines
only for the determination of the bonding pattern. We can say this in another
way: There is no fixed cutoff. For example, it is not chemically reasonable to say
that two bonds with electronegativity differences that are within, say, 0.1 of each
other are significantly different in bonding character. Electronegativity differences
describe a continuum, not a discrete “on-or-off,” as with a light switch.
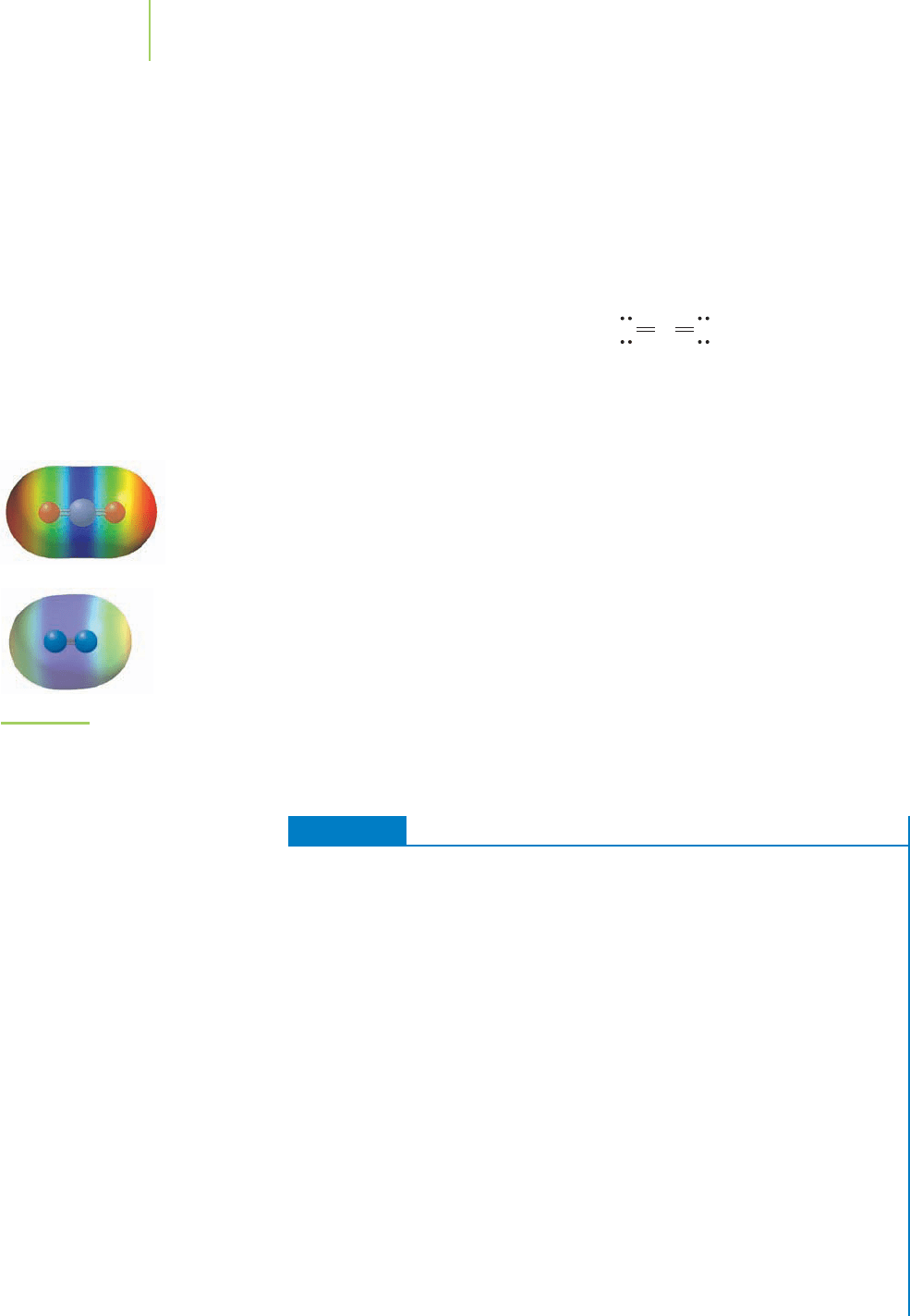
Let’s revisit our example of a covalent bond between a metal and a nonmetal
in tetraethyl lead. Does the electronegativity difference between the lead and car-
bon atoms indicate that the bond should be covalent? Examination of the table of
electronegativity values indicates that the difference is only 0.6 electronegativity
unit. On the basis of this information, we can say that the PbOC bonds in
tetraethyl lead are polar covalent bonds and that these bonds are not as polarized
as the bond in HF or HCl. We’ll discuss the implications of this fact in the next
section.
EXERCISE 8.5 What Type of Bond Is It?
For each of these compounds, indicate whether the bond is an ionic bond, a polar
covalent bond, or a nonpolar covalent bond.
a. KCl (a replacement for table salt in low-sodium diets)
b. H
2
O (water)
c. Br
2
(used in the manufacture of brominated vegetable oils)
First Thoughts
To answer this question, we need to consider the electronegativity difference
between the bonded atoms or ions.
Solution
a. We’d predict an ionic bond between potassium and chloride. The two atoms
differ by 2.2 electronegativity units.
b. A polar covalent bonding pattern is predicted (the difference is 1.4
electronegativity units). Hydrogen is less electronegative than oxygen, so the
majority of the electron density in the bonds lies closer to the oxygen.
c. A nonpolar covalent bond is indicated here (no difference in electronegativity).
Further Insights
Differences in electronegativity can be used to identify the type of bond in a com-
pound. However, is it correct to consider that NaCl ( = 2.1) contains a purely
ionic bond and that NaBr ( =1.9) contains a polar covalent bond? What do we say
about the bond in LiCl ( =2.0)? Questions such as these help illustrate that bond-
ing is a spectrum, from the “purely”ionic to the “purely” nonpolar covalent. Almost
all bonds have some ionic and some covalent character. We’d surmise that the bond
in NaCl is more ionic than the bond in either LiCl or NaBr, but we’d realize that it,
too, has some covalent character.
PRACTICE 8.5
Predict the type of bond (covalent, polar covalent, or ionic) present in each of these
binary compounds: NO, F
2
,MgO.
See Problems 51 and 52.
8.3 Covalent Bonding 321
Here is the electron density distribution
in tetraethyl lead. How does it compare
to those of other covalent molecules?
Modeling a Covalent Bond—Lewis Structures
Felix Hoffman, a chemist hired by the Friedrich Bayer & Co. fabric dye plant, was
charged in the 1890s with finding some better products that could be made by the
company. One of the ideas that came to Hoffman was related to his father’s
rheumatism. At the time, this ailment and other pains were treated with large
doses of salicylic acid, found in the inner bark of several types of willow trees.
Unfortunately, Hoffman’s father was unable to take this pain medication because
its acidity irritated his stomach and throat. In 1897, Hoffman set about trying
to reduce the acidity of the medicine. To do this, he needed to know how the
arrangement of the atoms made the compound acidic. After some experimenta-
tion, Hoffman was able to produce a compound that reduced this acidity. His
product, acetylsalicylic acid (C
9
H
8
O
4
) later named aspirin, reduced the harmful
irritation by chemically modifying one of the acidic parts of salicylic acid to make
it less acidic. Knowing how the atoms are attached, and what kind of bonds link
the atoms, is important to understanding how compounds react and interact in
the body. One of the ways to illustrate how atoms are attached in covalent bonds
is to build a model of the molecule using Lewis dot structures. After we discuss
the basic ideas, we will draw the Lewis dot structure of aspirin.
Rules describing how the atoms and electrons are placed in a Lewis dot struc-
ture enable us to draw compounds in a systematic way. These rules, found in
Table 8.5, include drawing a skeletal picture of the molecule and then placing
extra valence electrons in the skeleton until the model of the compound is com-
plete. We will use these rules as we draw a Lewis dot structure of molecular
hydrogen (H
2
).
According to Table 8.5, the first step is to count all of the valence electrons on
the atoms in the molecule. Because valence electrons are involved in bonding,
knowing the total number of these electrons helps us determine the number of
bonding pairs (electrons involved in bonding) and the number of lone pairs (elec-
trons not involved in bonding). In hydrogen, there are two total valence electrons
(one from each atom). In step 2 (Table 8.5), we draw a skeleton for the molecule.
The guidelines (steps 2a–e) for drawing a skeletal picture of a molecule are based
on preferences that atoms have for particular locations in a molecule. There are
only two atoms in the molecule, so the skeleton of H
2
is
HH
322 Chapter 8 Bonding Basics
Application
A Set of Rules for Drawing Lewis Dot Structures
1. Determine the total number of valence electrons.
2. Determine the skeletal structure.
a. Hydrogen atoms are on the edges of the molecule.
b. The central atom has the lowest electronegativity. (There are many exceptions.)
c. In oxoacids, hydrogens are usually on the oxygens.
d. Think compact and symmetric.
e. Use intuition.
3. Draw the Lewis dot structure.
a. Draw the bonds connecting the atoms.
b. Determine the number of electrons remaining.
c. Place the remaining electrons as lone pairs, beginning on the most
electronegative atoms, until each atom has an octet.
d. All remaining lone pairs go on the central atom.
e. Assign formal charges, and redraw bonding electrons if necessary.
TABLE 8.5
Video Lesson: Lewis Dot
Structures for Covalent Bonds
Video Lesson: Predicting Lewis
Dot Structures
In step 3a, we place pairs of electrons to indicate bonding pairs between each
atom. For molecular hydrogen, the electrons are placed as follows;
H • • H
Most often, we use shorthand to show bonding pairs of electrons by replacing the
bonding electrons with a line. We draw the line to show that the atoms are chem-
ically bonded together in the molecule.
HOH
In steps 3b and 3c, we place all remaining electrons around the more electroneg-
ative atom first until the octet rule (or duet rule, for hydrogen) is satisfied.
Because we do not have any remaining electrons, and because the duet rule is
satisfied for both atoms, we are finished.
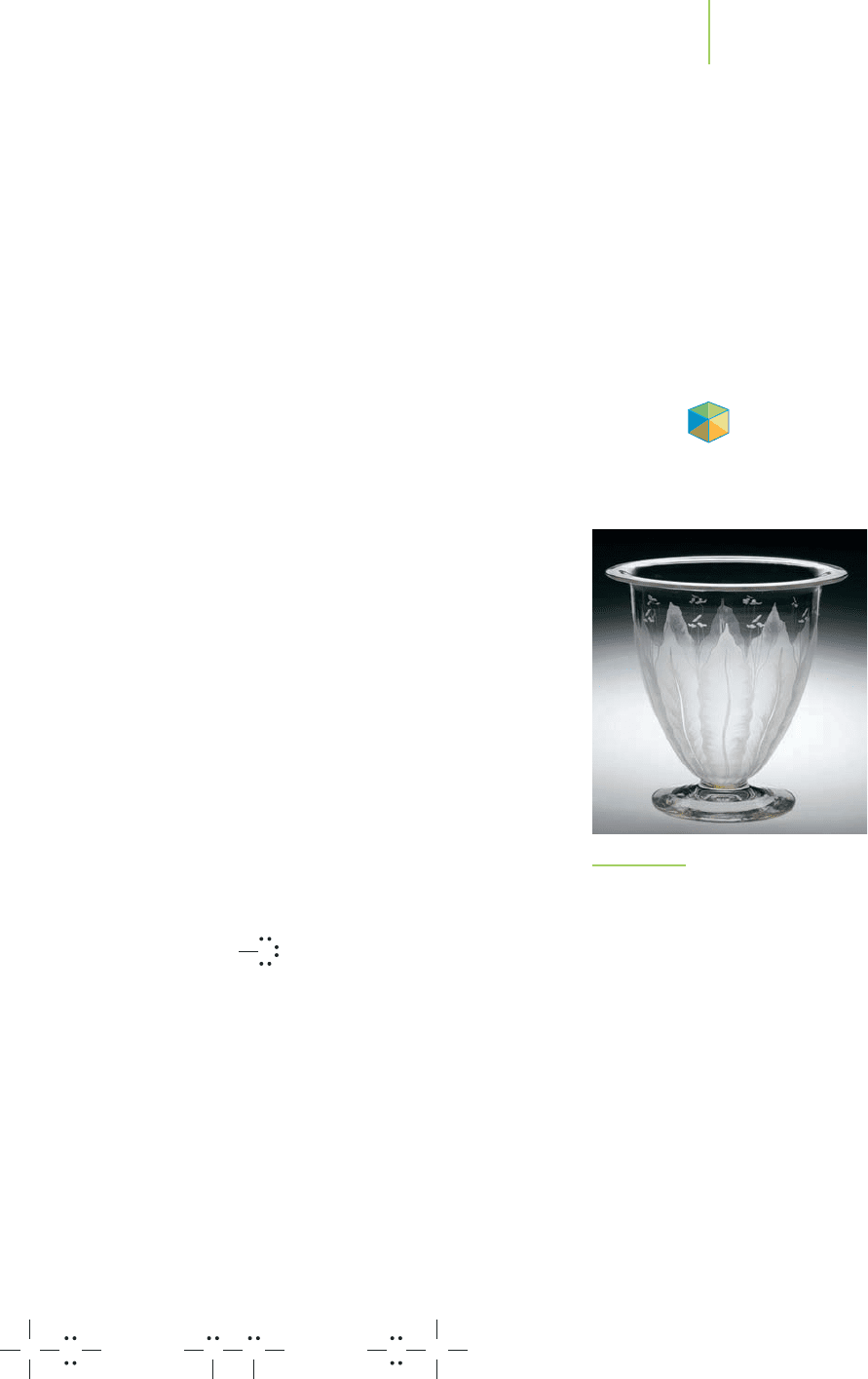
Hydrogen fluoride is either a fuming gas or a liquid, depending on the
temperature of use (its boiling point is 19.5
o
C, about 67
o
F), and has a host of
industrial applications. For example, it is used as a raw material in the production
of chlorofluorohydrocarbons (CFCs), insecticides, and fertilizers; as a catalyst in
the production of pharmaceuticals; in the manufacture of semiconductors; and
in etching glass (see Figure 8.15). Let’s use the rules in Table 8.5 to prepare a Lewis
dot structure for HF. Step 1 requires us to count the valence electrons on all the
atoms. Hydrogen has one valence electron, and fluorine has seven, so we have a
total of eight valence electrons with which to work. Next, in step 2, we draw the
best skeletal structure of the molecule:
HF
There are only two atoms in the molecule, so we’ll draw them next to each other.
Our bonding pair of electrons is represented as a line connecting the two atoms
(step 3a).
HOF
We’ve used two electrons in our skeleton to represent the bond between the two
atoms, so we have six electrons remaining (step 3b). These are placed in pairs
around the more electronegative atom until the octet rule (or duet rule) is satis-
fied (step 3c). If any electrons remain (step 3d), the pairs are placed around the
other atoms.
Because we used all of the electrons to fulfill the octet rule around fluorine, and
because both atoms now satisfy the octet rule (or duet rule), we have completed
the structure. Both fluorine and hydrogen in HF have electron configurations of
a noble gas.
Formal Charges
Methanol (also known as methyl alcohol and wood alcohol, CH
4
O) is a com-
pound being advanced as a potential substitute for gasoline, because it has a high-
octane-rating equivalent. Methanol is also a renewable resource obtained from
the fermentation of cellulose-containing materials (such as wood). The following
three structures satisfy the octet rule (or duet rule) for every atom. Only one
of them is methanol.
C
H
H
H
H
O
CH
H
H
O
C
H
H
H H
H
O
H F
8.3 Covalent Bonding 323
Application
FIGURE 8.15
This punch bowl (American, 1918–1919)
was made from blown glass. The pattern
was applied by etching the glass using a
dilute hydrogen fluoride solution.

Which structure is it? Chemists use formal charges on atoms as one important
piece of evidence in determining the most reasonable structure for a compound
in which more than one structure might be possible.
What is a formal charge (see step 3e in Table 8.5) and how does it help us to
select the most reasonable structure? The
formal charge on an atom is the differ-
ence between the number of valence electrons on the free atom and the number
of electrons assigned to the atom when it is part of a molecule. The use of formal
charges on atoms indicates that the atoms exhibit an imbalance between the
number of electrons and the number of protons on the atom. Although this im-
balance can be estimated by assigning an oxidation number to each of the atoms
(see Chapter 4), the method of calculating formal charges lends us additional
guidance. Oxidation numbers provide an indication of the charge an atom would
have if it were completely ionic. Formal charges, on the other hand, provide the
charge on an atom assuming there is no difference in electronegativity among
the atoms in a structure. In other words, formal charges assume that electrons
are shared equally, as is the case in the covalent bonding pattern.
Formal charge = valence electrons − # bonds − # nonbonded electrons
Mathematically, the formal charge equals the number of valence electrons on
the free atom minus the number of bonds to that atom minus the number of
nonbonded electrons. As a check of our math, the sum of the formal charges on
all the atoms should equal the total charge of the molecule (0) or ion (+ or −).
We can use this information to calculate the formal charge on each atom in a sim-
ple molecule, our Lewis dot structure of HF. The fluorine atom has seven valence
electrons (in Group VIIA, noted from the periodic table). Subtracting the num-
ber of bonds in the structure (one) and the number of nonbonded electrons (six)
from this number gives a formal charge of zero for the atom. Similar calculations
can be done with the hydrogen atom, which also has a formal charge of zero.
When we draw Lewis dot structures, the best structure is one that satisfies the
largest number of formal charge rules. The best structure:
■
has the smallest magnitude for all of the formal charges
■
places negative formal charges on the more electronegative atoms
■
has the smallest number of nonzero formal charges
We can now consider the second part of our section-opening question:
Why is
it useful to know the formal charges on atoms within a particular Lewis dot struc-
ture?
Let’s return to our discussion of methanol. Calculating the formal charges
on the central atoms in each of the possible structures enables us to choose the
one on the left as the correct structure. Every atom in both structures has an octet
of electrons, but only the structure on the left shows a formal charge of zero on
each atom. This is one piece of evidence that the structure on the left is likely to
be the most energetically stable structure of the three.
All zero formal charges
Methanol
Formal charge on C 1
Formal charge on O 1
Not methanol
Formal charge on C 2
Formal charge on O 2
Not methanol
C
H
H
H
H
O
CH
H
H
O
H
C
H
H
H
H
O
324 Chapter 8 Bonding Basics
Video Lesson: Formal Charge
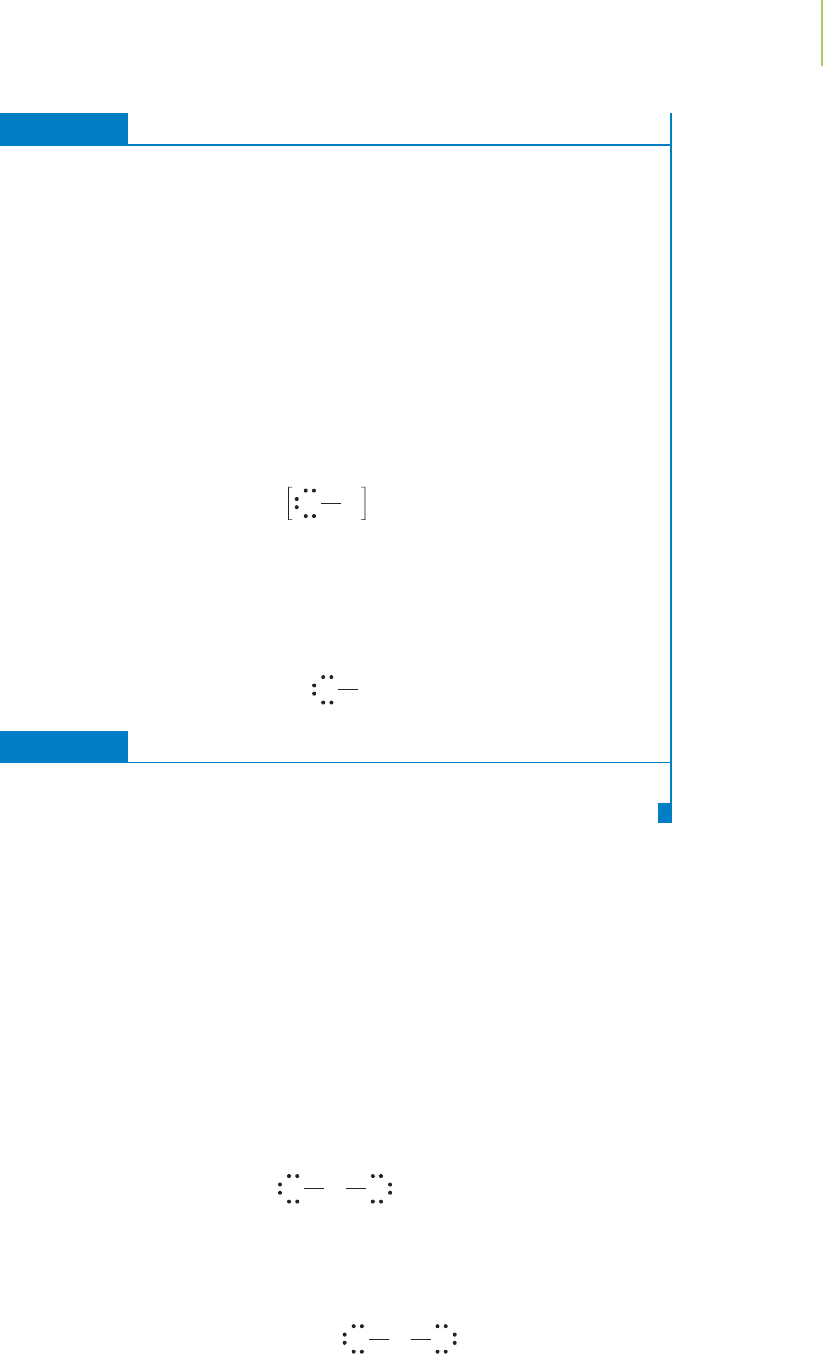
EXERCISE 8.6 Return to Basics
Pioneers in the American West made most of their everyday items from natural
sources. On the treeless plains of the Midwest they built homes of sod, burned dried
buffalo dung in the stove for heat, and made lye soap. Lye (a mixture of sodium hy-
droxide, NaOH, and potassium hydroxide, KOH) obtained from fireplace ashes was
used to make soap from animal fat. What is the Lewis dot structure model for the
hydroxide ion (OH
−
)? On which atom in lye does the nonzero formal charge reside?
Solution
The hydroxide ion can best be modeled by drawing the skeleton of the molecule
with a single bond between the oxygen and the hydrogen. Placing the remaining
electrons in pairs around the most electronegative element gives the structure
shown below. The brackets indicate that the negative charge has not been assigned
to a particular atom.
Formal charge (F.C.) calculations indicate that the charge must reside on the oxygen
(F.C. = 6 − 1 − 6 =−1) and not on the hydrogen (F.C. = 1 −1 − 0 = 0), which
makes sense because the oxygen atom has a much higher electronegativity (3.5)
than the hydrogen atom (2.1). Our structure can be drawn as follows:
PRACTICE 8.6
What is the formal charge on each of the atoms in OCl
−
? in H
3
CONH
2
?
See Problems 55 and 56.
Multiple Bonds
We often discuss carbon dioxide in this text because it is important to life in pho-
tosynthesis and cell respiration, and it is one product of combustion. The Pauling
electronegativity values of the atoms indicate that CO
2
probably contains polar
covalent bonds. Following our guidelines for constructing a Lewis dot structure
of CO
2
, we count the valence electrons in all atoms (4 in carbon and 6 in each of
the two oxygen atoms = 16 valence electrons) and build the skeleton. The less
electronegative carbon atom is the central atom in this molecule, and the oxygen
atoms are symmetrically placed about the carbon. Next, the bonding pairs of
electrons are placed between the atoms. The remaining 12 electrons are then
placed on the more electronegative oxygen atoms to fill their octets. The result is
shown below. Is this a satisfactory Lewis dot structure?
No. Both oxygen atoms have octets of electrons, but the carbon has only four
electrons (all bonding electrons) around it. What are the formal charges on each
atom in the structure?
C
1Formal charges
Formal charge (O) 6 1 6 1
Formal charge (C) 4 2 0 2
12
O O
OCO
OH
OH
8.3 Covalent Bonding 325
Our calculations reveal a lot of nonzero formal charges. Although the formal
charges add up to zero, the size and number of formal charges indicate that some-
thing may be amiss. Because energy is required to cause a separation of charges
(as indicated by the presence of nonzero formal charges in a molecule), the bet-
ter structure is generally that which minimizes formal charges. You also might
have noted that the octet rule is disobeyed for carbon in the structure. To elimi-
nate the charge on the oxygen atoms, let’s move a lone pair from each oxygen and
place them as a bond between the oxygen and the adjacent carbon. These are
called double bonds.
The resulting model has no nonzero formal charges and allows each atom to
satisfy the octet rule. Overall, the molecule is neutral. How is this model different
from the structures we discussed previously? Does the existence of more than one
pair of electrons between two atoms indicate something about the properties of
this molecule? We’ll discuss the answers to these questions in the next section.
The best sign that we’ve constructed a good model is its agreement with observed
properties for the compound. In the case of CO
2
, the Lewis dot structure model
does agree with the experimentally determined shape and polarity of carbon
dioxide. Based on differences in electronegativity, we observe that the electrons
are polarized toward each end of the molecule.
Multiple covalent bonds occur in many molecules. Carbon dioxide has two
double bonds. Each oxygen atom shares four bonding electrons with a carbon
atom. In a molecule of nitrogen, three pairs of electrons are used to satisfy the
octet rule for each of the atoms. The resulting molecule contains two nitrogen
atoms that share six electrons. A
triple bond links the atoms. Nitrogen, which
makes up nearly 80% of the air we breathe, is a very stable molecule that contains
a particularly strong triple bond, with the Lewis structure shown in Figure 8.16.
EXERCISE 8.7 Chemical Warfare and Bonding
Phosgene (COCl
2
), a highly toxic gas, was used in World War I and several subse-
quent wars to kill soldiers who were hiding in places that bombs couldn’t penetrate.
The gas, now used in industry to make polymers, pharmaceuticals, herbicides,
and other useful compounds, reacts with water and other electron-rich molecules
(molecules with lone pairs of electrons). Draw the best Lewis dot structure for this
molecule.
First Thoughts
All of the bonds in phosgene are covalent (COCl
2
contains only nonmetals), but a
Lewis dot structure model of the compound may help explain why phosgene reacts
with electron-rich molecules.
Solution
The skeletal picture of the molecule places the carbon in the center (least elec-
tronegative). The other atoms are placed symmetrically about the carbon. Of the
24 total electrons, 6 (three pairs) are used to connect the atoms. The remaining
18 electrons (nine pairs) are placed as lone pairs around the more electronegative
atoms. The result is that all of the atoms, except the carbon, have a full octet. We
need to share a lone pair of electrons with carbon to satisfy the octet rule on each
atom. Calculation of the formal charges on each of the atoms indicates that the oxy-
gen and the carbon atoms should share another pair of electrons.
C
0Formal charges 00
O O
326 Chapter 8 Bonding Basics
FIGURE 8.16
(Top) The double bonds in carbon
dioxide (CO
2
). (Bottom) The triple
bond in nitrogen (N
2
).
The best model of this molecule therefore shows a double bond between the carbon
and the oxygen atoms and single bonds between the carbon and the chlorine atoms.
We could have drawn a Lewis dot structure that placed a double bond between the
carbon and one of the chlorine atoms. This would have changed the formal charge
on carbon to zero, but the formal charge on the chlorine atom would become
+1,
and the formal charge on oxygen would remain −1. The resulting structure would
not be the best Lewis structure because of the existence of nonzero formal charges.
Further Insights
Does this explain why phosgene is so reactive? By noting how the bonds in the mol-
ecule are polarized, we can identify why this molecule reacts with electron-rich
compounds. According to the electronegativity values of the atoms, each of the
bonds is polarized away from the carbon atom. Electron-rich molecules such as
water (H
2
O), ammonia (NH
3
), and other compounds containing lone pairs can
react with the electron-starved carbon atoms in phosgene.
PRACTICE 8.7
Draw the Lewis dot structure for C
2
H
4
. for CH
3
N.
See Problems 39–48, 61–66, and 99.
Resonance Structures
Carbonate ion (CO
3
2−
) is a common polyatomic ion found in limestone, baking
powder, and baking soda. Addition of acid to the carbonate ion causes the for-
mation of carbonic acid (H
2
CO
3
), which decomposes rapidly into water (H
2
O)
and carbon dioxide (CO
2
). In baking, the carbon dioxide that is released causes
the bread to rise and makes its texture lighter.
Our first attempt at drawing the Lewis dot structure of the carbonate ion
results in the structure shown below. Carbonate has 24 electrons, 2 of them
responsible for the −2 charge, probably electrons from calcium (CaCO
3
), sodium
(Na
2
CO
3
), or whatever salt resulted in a cation that donated electrons to the
carbonate anion. The carbon atom in our structure still needs to share electrons
to satisfy the octet rule. Which atom is most likely involved in sharing electrons?
Using the formal charges on the atoms, we could reconfigure our electrons to
participate in a double bond with the carbon. At this point, the positive charge on
the carbon atom is gone, and all of the valences are filled (the octet rule is satis-
fied). The sum of the formal charges is equivalent to the charge on the carbonate
ion. This is a good Lewis dot structure for carbonate.
C
O
O
O
11
C
O
O
O
1
11
1
Cl ClC
O
F.C. (O) 6 2 4 0
F.C. (C) 4 4 0 0
F.C. (Cl) 7 1 6 0
Cl ClC
O
F.C. (O) 6 1 6 1
F.C. (C) 4 3 0 1
F.C. (Cl) 7 1 6 0
8.3 Covalent Bonding
327
Video Lesson: Resonance
Structures
