Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

338 Chapter 8 Bonding Basics
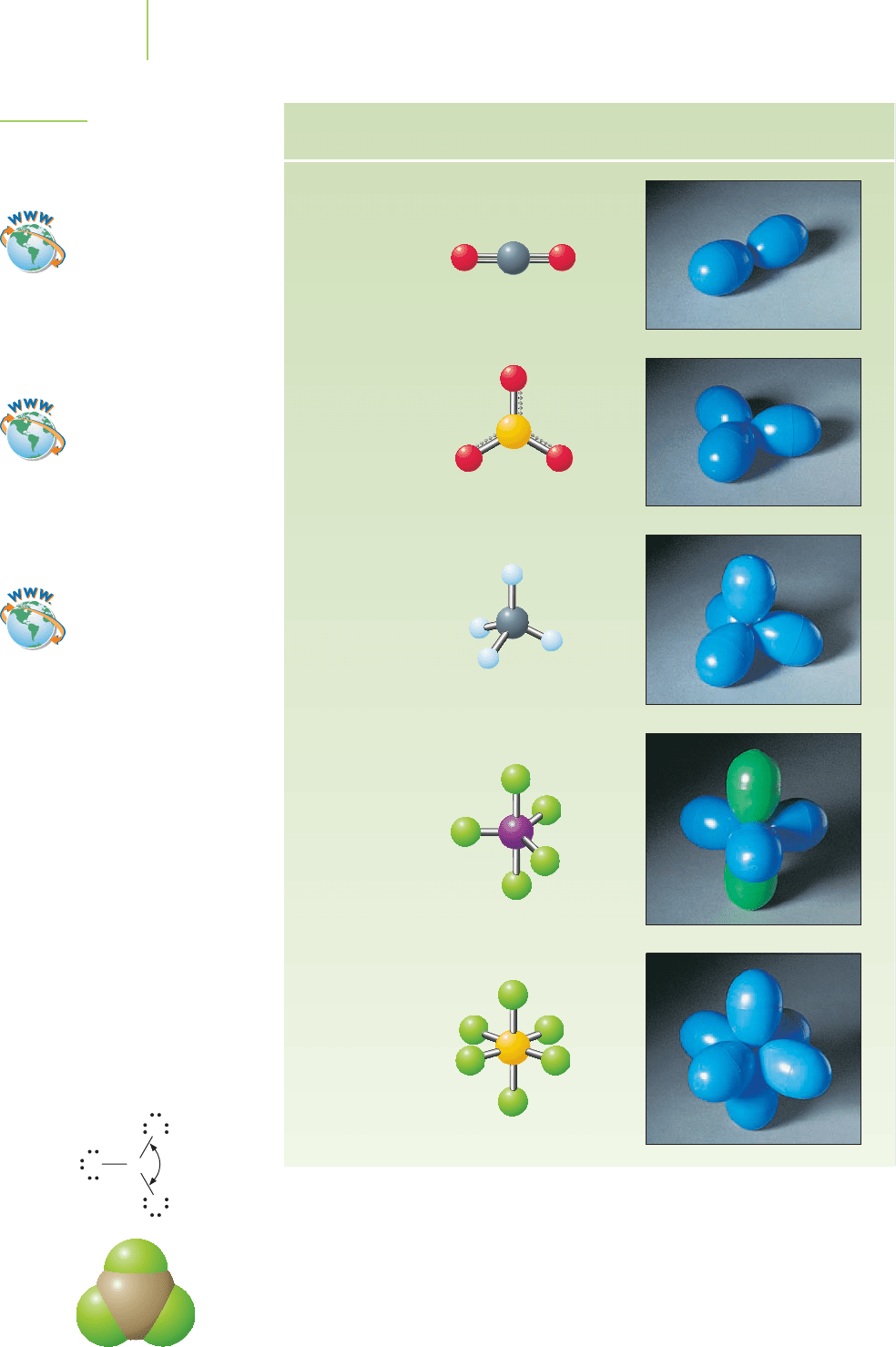
Molecular
geometry
Linear
Trigonal
planar
Tetrahedral
Example
CO
2
SO
3
CH
4
IF
5
SF
6
Trigonal
bipyramidal
Octahedral
FIGURE 8.21
Balloon analogy of the VSEPR theory.
Boron trifluoride (BF
3
), a compound used as a flux for soldering magnesium,
is shown below as a Lewis dot structure. This model shows that three electron
pairs (the three bonds) radiate from the central boron atom. The VSEPR model
dictates that the three electron pairs should occupy the corners of a triangle.
Therefore the electron-group geometry has trigonal planar geometry. The
F
F
F
B
120°
Visualization: VSEPR: Two
Electron Pair
Visualization: VSEPR: Three
Electron Pair
Visualization: VSEPR: Four
Electron Pair
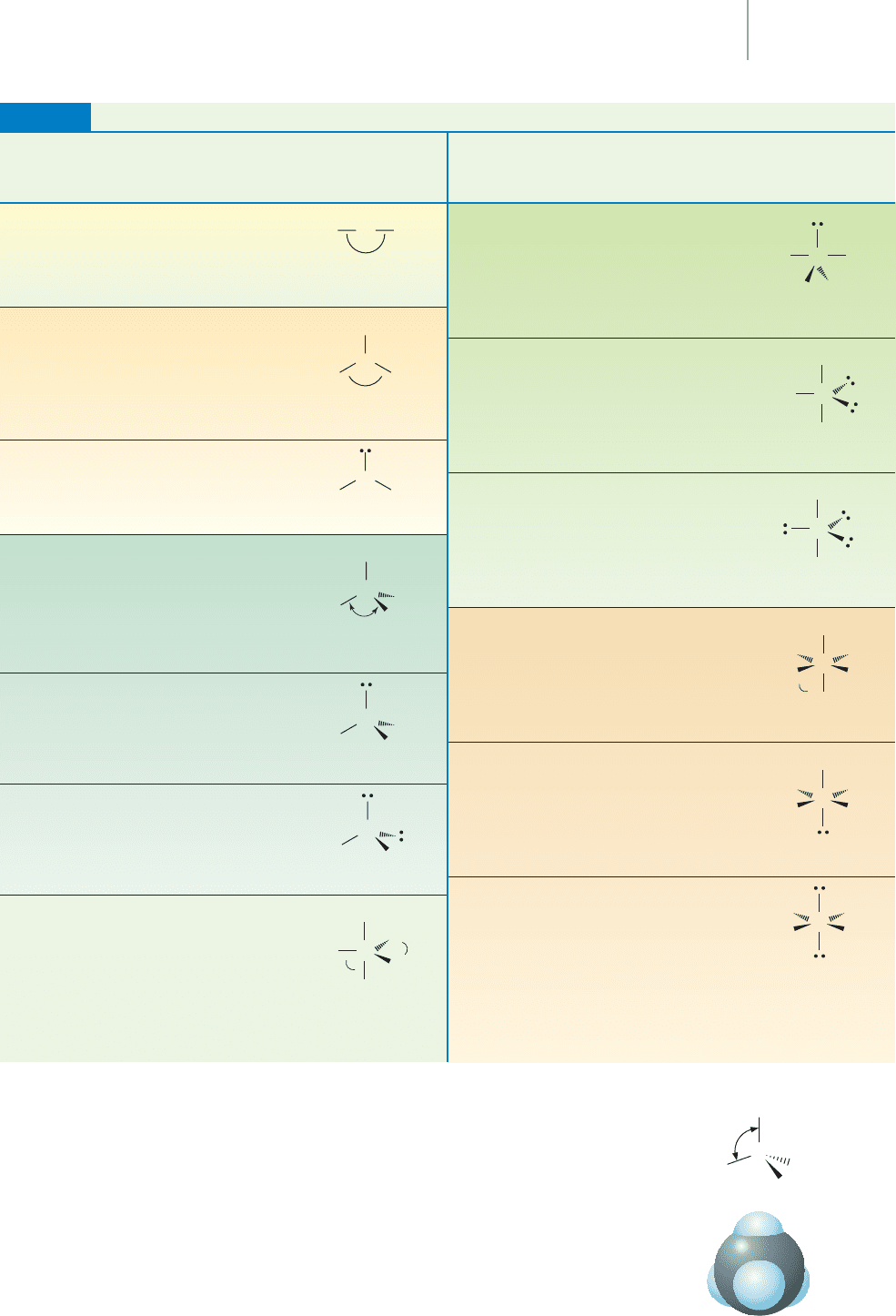
8.4 VSEPR—A Better Model 339
molecular geometry is also trigonal planar because there are no lone pairs on the
boron. Bond angles for FOBOF are 120°.
Methane (CH
4
) is a molecule containing four electron pairs around the cen-
tral carbon atom. As a consequence, VSEPR predicts that the bonding pairs of
electrons should point to the vertices of a tetrahedron. Similarly, because of the
lack of lone-pair electrons on the carbon, the molecular geometry is tetrahedral
with HOCOH bond angles of 109.5°. A dashed wedge is used to illustrate that
the bond points behind the plane of the paper; the filled wedge protrudes in front
of the paper.
Shapes of the Electron-Group Geometries Predicted by VSEPR
Number Electron Number Number Electron Number
of Electron Group of Lone Molecular of Electron Group of Lone Molecular
Groups Geometry Pairs Geometry Groups Geometry Pairs Geometry
TABLE 8.7
5 Trigonal 1
bipyramidal
See-saw
5 Trigonal 2
bipyramidal
T-shaped
5 Trigonal 3
bipyramidal
Linear
6 Octahedral 0
Octahedral
6 Octahedral 1
Square
pyramidal
6 Octahedral 2
Square planar
A
G
G
G
G
A
G
G
G
G
G
A
90
G
G
G
G
G
G
A
G
G
A
G
G
G
A
G
G
GG
2 Linear 0
Linear
3 Trigonal planar 0
Trigonal planar
3 Trigonal planar 1
Bent/angular
4 Tetrahedral 0
Tetrahedral
4 Tetrahedral 1
Trigonal pyramidal
4 Tetrahedral 2
Bent/angular
5 Trigonal 0
bipyramidal
Trigonal
bipyramidal
A
G
G
G
G
G
90
120
A
G
G
A
G
G
G
A
G
109.5
G
G
G
A
GG
A
G
120
GG
A
180
GG
C
H
109.5°
H
H
H
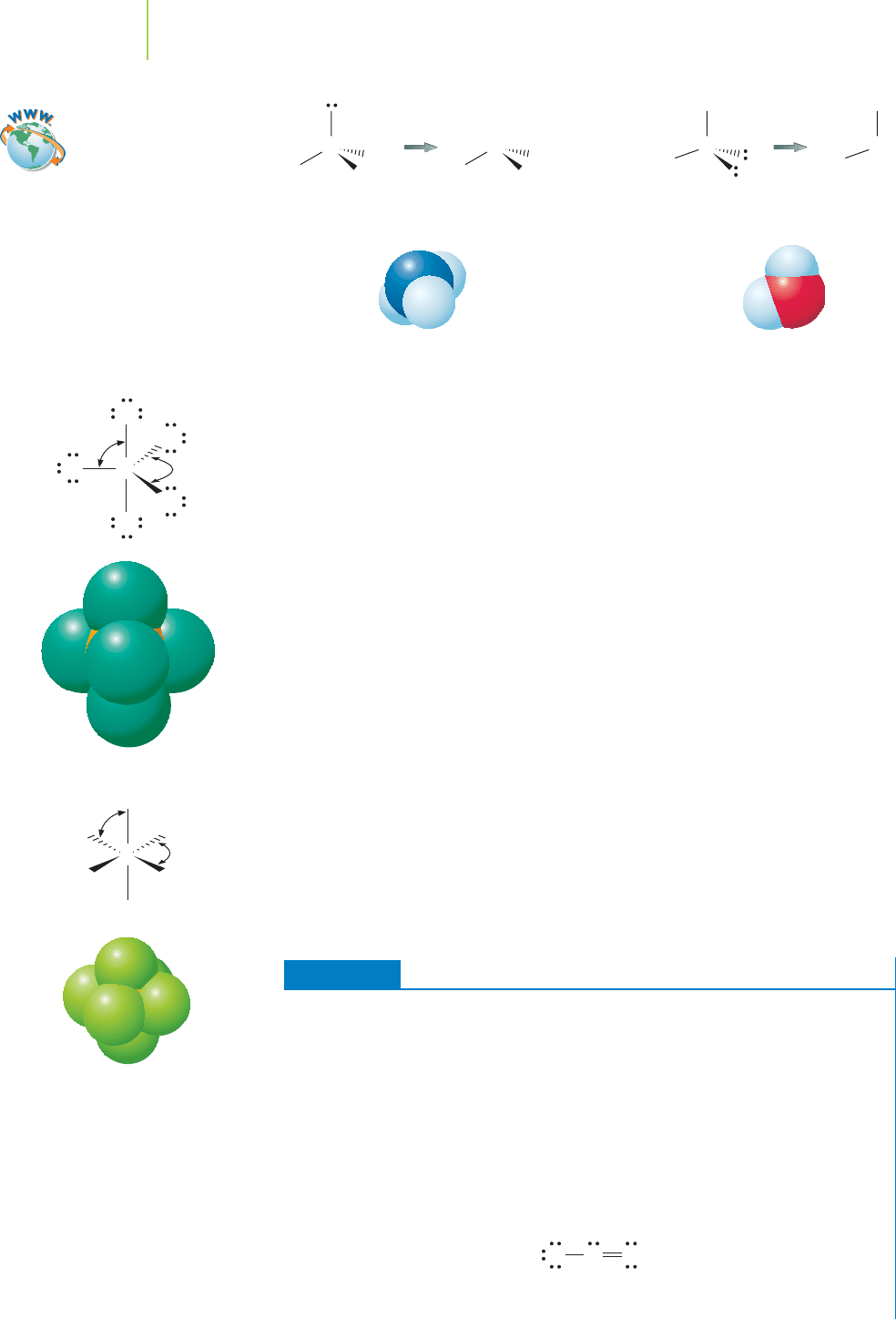
Ammonia (NH
3
), a compound used extensively as a fertilizer in farming, pos-
sesses three
bonding electron pairs and one lone pair. VSEPR models predict a
tetrahedral electron-group geometry. Because one of the electron groups is a lone
pair, the molecular geometry of ammonia is predicted to be trigonal pyramidal.
Similarly, the Lewis dot structure model of water indicates that there are two
bonding pairs and two lone pairs on the central oxygen atom. The VSEPR model
dictates that the electron groups should be arranged in a tetrahedral shape. How-
ever, the molecular geometry, determined by considering that the lone pairs are
invisible, is bent, or angular. We’ll discuss the subtleties of bond angles later in
this section.
For molecules that contain an expanded octet, there may be five or six elec-
tron groups about the central atom. Consider the structure of PCl
5
. This com-
pound is used as a catalyst in the manufacture of acetylcellulose (the plastic film
on which motion pictures are printed). The Lewis dot structure of phosphorus
pentachloride shows five electron groups attached to the central atom.VSEPR in-
dicates that the molecule has a trigonal bipyramidal electron-group geometry,
and the molecular geometry is also trigonal bipyramidal. This model has some
interesting features. In particular, there are two distinct positions for chlorine
atoms within the trigonal bipyramid. Two locations, with bond angles of 90°
from one chlorine to the next nearest neighbor, occupy the
axial positions (up and
down), and three locations occupy the
equatorial positions (outward) with bond
angles of 120° between them. Molecules that contain six electron groups occupy
the octahedral electron configuration. Each of the positions in the octahedron is
the same. All atoms have 90° bond angles to the next closest neighbor. An exam-
ple of this shape is found in SF
6
.
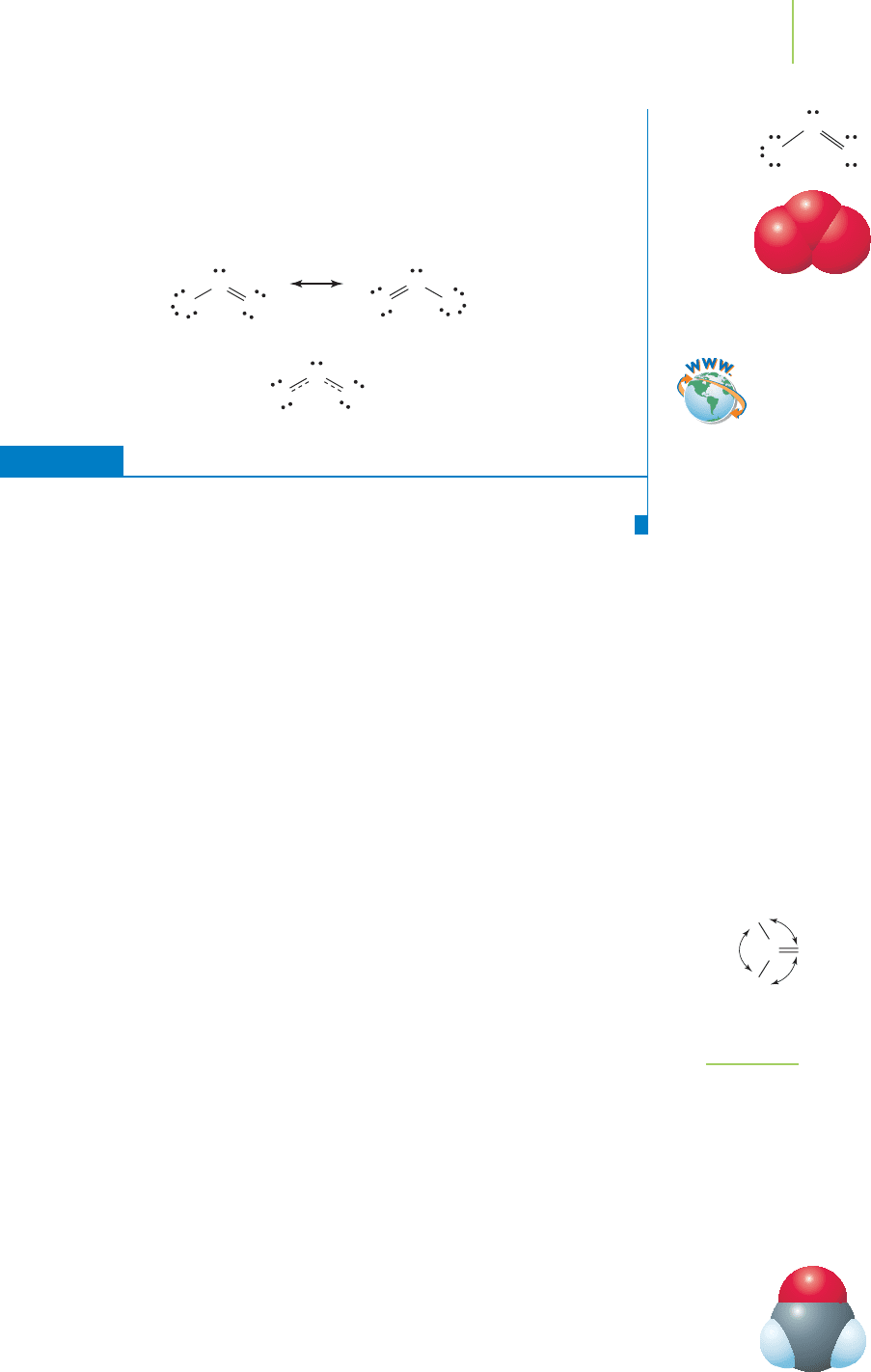
EXERCISE 8.9 Ozone and VSEPR
Ozone is an important molecule in our atmosphere because it protects us from the
harsh ultraviolet rays of the sun. What is the shape of ozone (O
3
)?
First Thoughts
Although a Lewis dot structure might provide the answer, we should apply the
VSEPR model to assist us in developing the best model possible at this point in our
discussion.
Solution
The Lewis dot structure model shows a single bond and a double bond with a lone
pair on the central oxygen atom. Each of these is an electron group.
With three electron groups, ozone has a trigonal planar electron-group geometry.
The VSEPR model indicates that the molecular geometry is bent.
O OO
340 Chapter 8 Bonding Basics
Cl
Cl
Cl
P
90°
120°
Cl
Cl
S
F
F
F
F
F
F
90°
90°
O
H
H
N
H
H
H
N
H
H
H
Electron-group
geometry
Electron-group
geometry
Molecular
geometry
Molecular
geometry
WaterAmmonia
O
H
H
Video Lesson: Molecular Shapes
for Steric Numbers 2–4
Video Lesson: Molecular Shapes
for Steric Numbers 5 and 6
8.4 VSEPR—A Better Model 341
Further Insights
We should further develop our model of ozone to show resonance structures. Be-
cause it is known that chlorofluorocarbons (CFCs) react with ozone, it might be
useful to consider the reactivity of ozone. Knowing the strengths of the polar cova-
lent bonds could help us determine the reactivity.
PRACTICE 8.9
Indicate the VSEPR model and bonding angles for HNO
3
,CCl
4
, and NH
3
.
See Problems 75–80, 85, 86, and 88.
Advanced Thoughts on the VSEPR Model
VSEPR theory illustrates how electron pairs repel each other because they occupy
space and have similar charge. Does the degree of repulsion depend on the type
of electron pair that we’re examining? To put it another way, is there a difference
in the size and shape of different electron pairs? Lone pairs of electrons are big
compared to bonding pairs of electrons. Gillespie illustrated this fact by noting
the repulsions in terms of the size of the electron pair. The result is that the dif-
ferent types of electron pairs can be ordered in terms of the three-dimensional
space they require:
Lone pairs triple bonds double bonds single bonds
Note that the space required in the VSEPR model is not the same as the bond
lengths, in which a triple bond between, for example, two carbon atoms is shorter
than a double bond, which, in turn, is shorter than a single bond between the
atoms.
Why do we need to discuss the space that bonds and lone pairs require? Let’s
consider the physical shape of a molecule of ammonia. According to the VSEPR
rules, ammonia (NH
3
) has a tetrahedral electron geometry. The lone pair on the
nitrogen, however, is much more repulsive than the bonding pairs of electrons. In
response to the repulsions and three-dimensional space requirements, the bond-
ing pairs move closer together. The result is that the HONOH bond angles in
ammonia, at 107°, are smaller than those of the true tetrahedron. The same rea-
soning holds for the experimentally measured bond angle in water (104.5°). The
angle is severely pushed by the presence of two lone pairs on the central oxygen
atom.
Gillespie noted that multiple bonds require more space than single bonds.
Let’s examine the structure of formaldehyde (H
2
CO) as an example. The Lewis
dot structure shown in Figure 8.22 predicts three electron groups (one double
bond and two single bonds) surrounding the central carbon atom.
VSEPR correctly predicts a trigonal planar electron-group geometry. Experi-
mentally, though, the bond angles in formaldehyde are not 120°. Because the
double bond is larger than the single bond, we predict the HOCOH bond angle
to be less than 120°. Experimental evidence suggests that the actual angle is 116°.
Similarly, the HOCOO bond angle should be larger than 120°. Experimentally,
it has been measured at 122°.
O
O
O
O
OO
O
O
O
OO
O
Ozone
O
3
C
H
H
Formaldehyde
O
122
116
122
FIGURE 8.22
Multiple bonds affect bond
angles in formaldehyde (CH
2
O).
Video Lesson: Predicting
Molecular Characteristics Using
VSEPR Theory
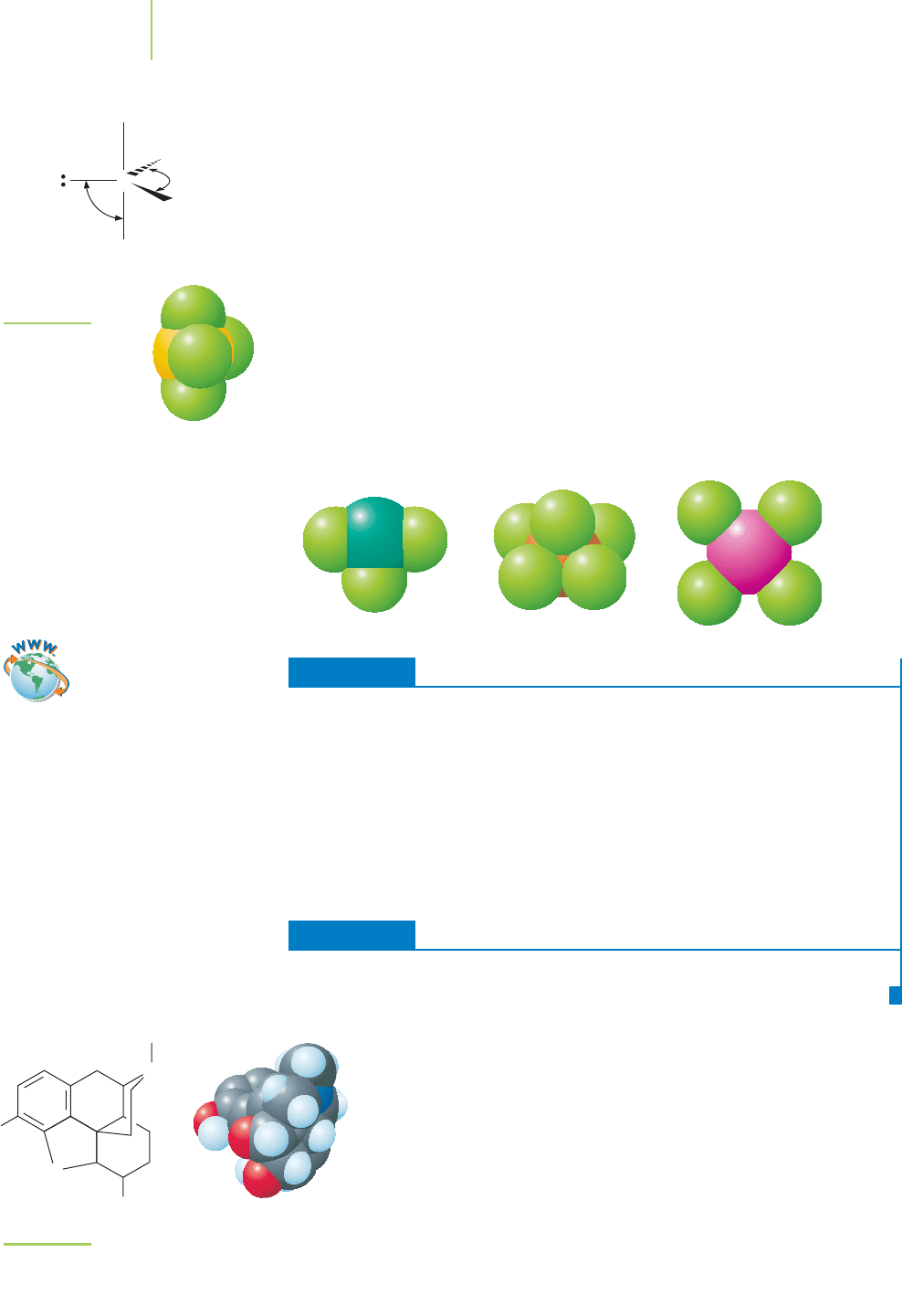
Using this information, we can determine the molecular geometry for sulfur
tetrafluoride (SF
4
) with 34 valence electrons. The molecule is typically used in the
laboratory to make other fluorine-containing compounds. The VSEPR model
predicts the trigonal bipyramidal electron-group geometry shown in Figure 8.23.
However, because one of the electron groups is a lone pair, the molecular geom-
etry is not a trigonal bipyramid. Which one of the positions, axial or equatorial,
should be occupied by the lone pair of electrons? Because the lone pair requires
more space than the bonding pairs of electrons when we are using the VSEPR
model, the lone pair will occupy the position with the fewest repulsions. In the
trigonal bipyramid, the most unhindered site is one of the equatorial positions.
In fact, lone pairs in trigonal bypyramids will always occupy the equatorial posi-
tions. The space-eating lone pairs then push against the other electron pairs and
distort them from their ideal bond angles. The result for SF
4
is referred to as a see-
saw structure. The same logic can be applied to the construction of ClF
3
(T-shaped molecular geometry), BrF
5
(square pyramidal molecular geometry),
and XeF
4
(square planar molecular geometry).
342 Chapter 8 Bonding Basics
F
F
F
120°
90°
S
F
FIGURE 8.23
Sulfur tetrafluoride is
a molecule with see-
saw geometry.
The molecular geometries of CIF
3
(T-shaped), BrF
5
(square pyramidal), and
XeF
4
(square planar) are affected by the
presence of lone pairs around the central
atom in each molecule.
EXERCISE 8.10 A Closer Look at Ozone
When we apply the VSEPR rule to ozone, our initial picture is that it is a bent mol-
ecule with an OOOOO bond angle of 120°. Given that different electron groups
have different degrees of repulsions, what do you predict to be the actual bond angle
in ozone?
Solution
The lone pair on the central oxygen occupies more space than the bonding pairs, so
the bond angle becomes smaller between the adjacent oxygen atoms. The experi-
mentally measured angle (OOOOO) is 116.8°.
PRACTICE 8.10
Which molecule has larger bond angles, SO
2
or H
2
O?
See Problems 81–84 and 87.
Let’s use what we now know to examine the three-dimensional
structure of morphine. The formula reveals little: C
17
H
21
NO
3
.As
we noted at the start of this section, the Lewis dot structure, just
like the formula, fails to illustrate the three-dimensional structure
of morphine. The structure contains trigonal planar and tetrahe-
dral carbon atoms arranged to make a scaffold that holds the OH
groups pointed at one end of the molecule. The nitrogen resides at
the opposite end of the molecule as shown in Figure 8.24. The scaf-
fold holds these groups at specific distances, allowing morphine to
fit into the opioid receptor quite well. We can get a handle on the
structure of large molecules such as morphine by analyzing the
329670_la_08_24
Kelter/Mosher: The Nature of Change 1/e
Houghton Mifflin Company — 100%
N
CH
3
HO
OH
O
FIGURE 8.24
The VSEPR model allows us to understand and
interpret the three-dimensional structure of
morphine, as described in the text.
Visualization: VSEPR: Iodine
Pentafluoride
8.5 Properties of Ionic and Molecular Compounds 343
three-dimensional structure at each carbon atom. By examining each atom in the
structure and determining the geometry about it, we can build the overall shape of a
molecule.
Medicinal chemists need to be able to determine the shapes of the molecules
they construct in order to assess the fit with an appropriate receptor in the body.
This application stems from the simple rule that “structure follows function.”
That is, the three-dimensional arrangement of atoms within a molecule is just as im-
portant to the function (and chemical properties) of a molecule as are the identities
of the atoms that make up the molecule. If the geometry of one of the bonds in
morphine were not aligned just right for the opioid receptor, morphine wouldn’t
interact well as an analgesic agent.
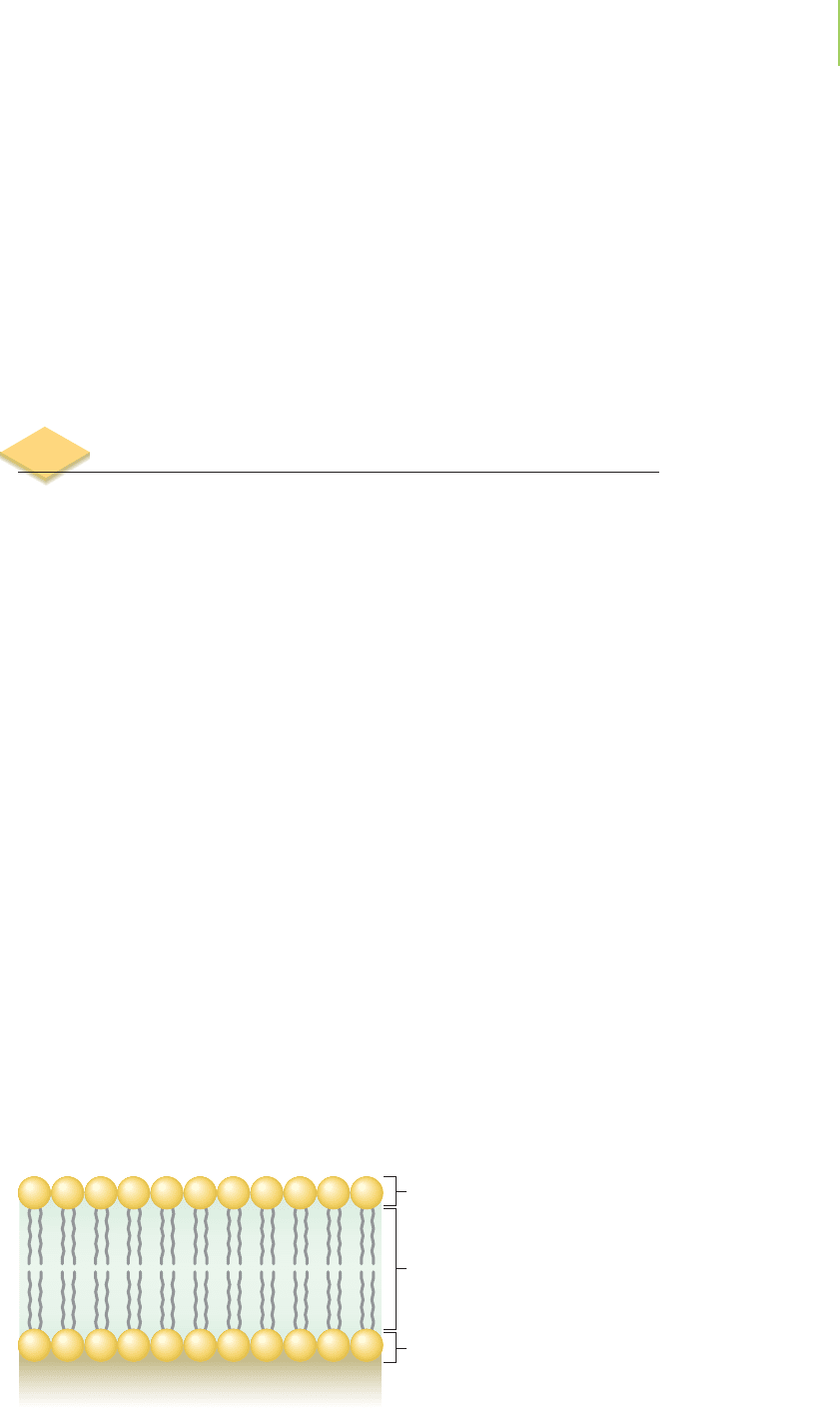
8.5 Properties of Ionic and Molecular Compounds
The cells within our body are held together by membranes that not only define
the limits of the cell but also regulate the passage of materials into and out of the
cell. Because most targets that interact with drugs are located inside the cell, any
newly developed pharmaceutical agent’s activity is related to the drug’s ability to
cross the cell membrane. A cross section of the cell membrane exposes three dis-
tinct regions with which a drug must be able to have favorable interactions in
order to pass across the membrane. The first and last regions of the cell mem-
brane will interact well only with compounds that have an overall polarization of
electrons toward one end of the molecule. The middle portion of the membrane
interacts well with molecules that lack this overall polarization, and this differ-
ence makes the cell membrane difficult to cross. Any new drug, then, must be
carefully designed if its target lies inside the cell. How do medicinal chemists
know whether their new drug will be able to enter cells? In this section, we will
discuss how the polarization of electrons can be used to determine this ability
and some of the other properties associated with molecules.
Bond Dipoles
The polarization of electrons in a bond is commonly referred to as a bond dipole.
A difference in the electronegativity between the atoms on either end of the bond
can be used to illustrate this polarization. Earlier in this chapter, we noted that
polarization of the bonding electrons gave rise to a polar covalent bond. If the po-
larization were large enough, an ionic bond would result. In order to incorporate
this information into the structure of a compound, we typically write delta plus
(δ+) and delta minus (δ−) on the atoms in the bond. Alternatively, the bond
Interacts with polarized compounds
Outside of cell
Inside of cell
Interacts with unpolarized compound
s
Interacts with polarized compounds
Cross section of a cell membrane.

344 Chapter 8 Bonding Basics
Dipole Moments of Some Common Binary Compounds
Dipole Dipole
Compound Moment (D)* Compound Moment (D)*
H
2
0.00 ClF 0.88
HF 1.91 BrF 1.29
HCl 1.07 NO 0.16
HBr 0.79 CO 0.13
HI 0.38 O
2
0.00
*D stands for debye.
TABLE 8.8
dipole can be represented by drawing an arrow over the bond pointing to the
more electronegative atom. For example, H
2
lacks a bond dipole because of the
lack of polarization of the electrons in the bond. Hydrogen fluoride (HF), how-
ever, contains a strongly polarized bond and can be drawn with the bond dipole
to illustrate this.
We can also draw an electron density distribution diagram of HF that shows the
dipole in a more visually appealing and detailed way.
Dipole Moment
The net polarization of electrons in a molecule is known as the dipole moment.For
a molecule, this is a function of the orientation and the magnitude of each of the
individual bond dipoles. Mathematically, the dipole moment (
) is defined as
=Q × d
in which the charge at either end of the dipole (Q) times the distance between the
charges (d) yields the dipole moment in Coulomb
.
meters (C
.
m). In honor of
Peter Debye (1884–1966), who won the 1936 Nobel Prize in chemistry for his
work on molecular structures, we usually refer to dipole moments in terms of the
number of debyes (D):
3.34 × 10
−30
C
.
m = 1 D (debye)
Bond dipoles can be observed experimentally in binary molecules as the di-
pole moment. When the molecule is placed in an electric field, the unequal dis-
tribution of charge in the molecule (δ+ on one end of the bond and δ− on the
other end) causes the molecule to orient itself in such a way as to maximize elec-
trostatic attractions. In this way, the molecule behaves similarly to a small bar
magnet. The resulting orientation can be measured and the degree of polariza-
tion calculated. The trends observed in dipole moments of some common binary
molecules (Table 8.8) seem to follow our expectations for the polarization of the
bonds. As we would predict, hydrogen fluoride has the greatest dipole moment of
all the hydrogen halides (HF, HCl, HBr, HI) because the electronegativity differ-
ence in this series is greatest for HF.
For molecules with more than two atoms, however, an individual bond dipole
is harder to measure. Instead, the dipole moment that is measured is related to
the sum of all of the bond dipoles in the molecule. For example, hydrogen
cyanide (HCN) contains two bond dipoles. Both bond dipoles point in the same
NC
H
FH
The electron density distribution diagram
of HF shows the positive (in blue) and
negative (in red) ends of the bond dipole.
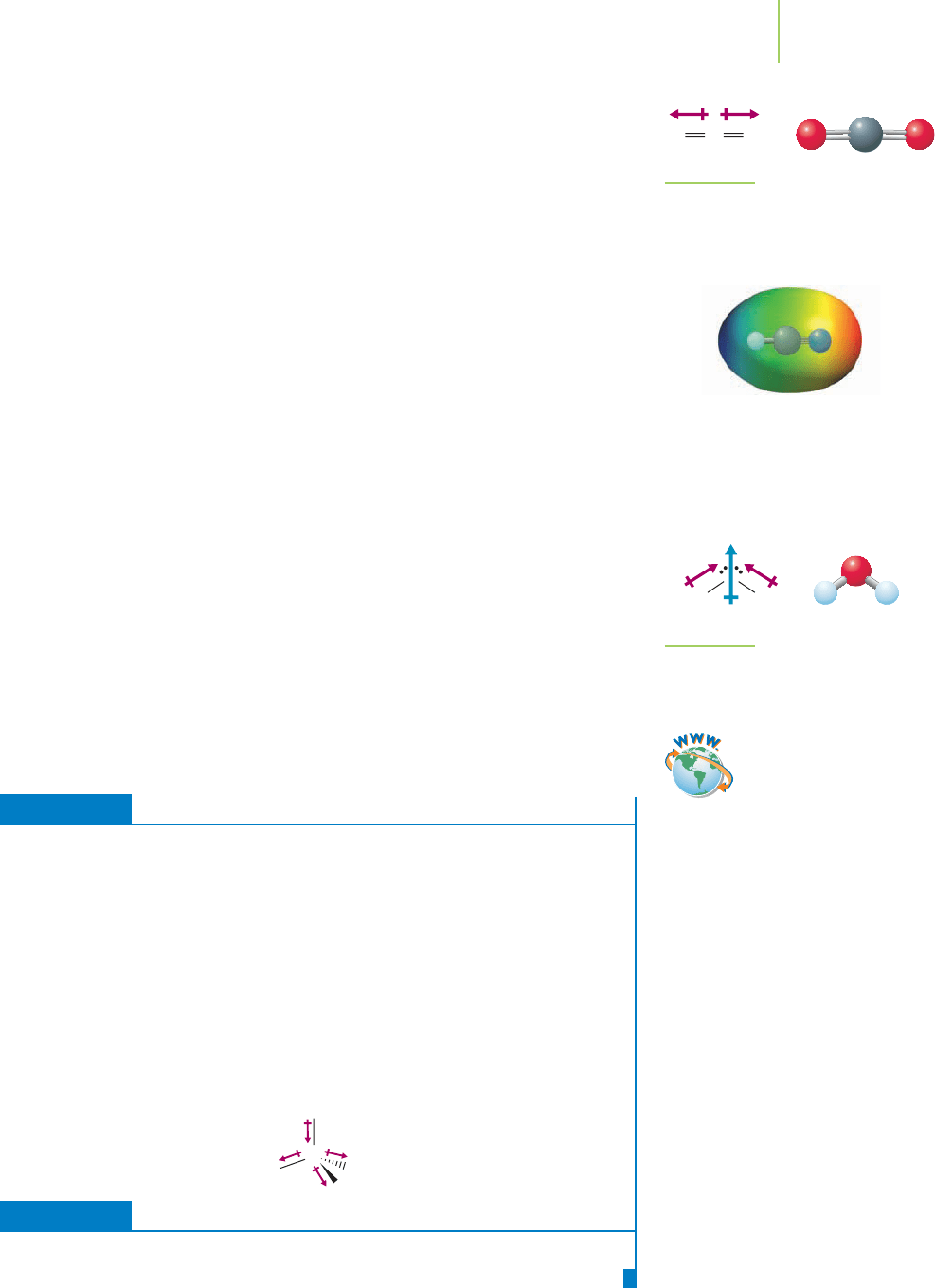
8.5 Properties of Ionic and Molecular Compounds 345
direction, giving rise to an overall polarization of the electrons in the molecule,
and a molecule that aligns itself in an electric field and has a dipole moment of
about 3.0 D. Carbon dioxide also has two bond dipoles, shown in Figure 8.25.
However, they point opposite to each other. It is as though you and an equally
strong friend are involved in a tug of war. Your pull cancels out your friend’s pull,
and the flag in the middle doesn’t move. The molecule lacks the ability to align it-
self in an electric field, and the dipole moment of CO
2
is 0.00 D.
The dipole moment is a measure of the
polarity of a molecule. In some cases,
the bond dipoles cancel each other out, as in CO
2
. The result is a nonpolar mole-
cule
. In other cases, the bond dipoles don’t cancel out, as in HCN. The net result
is a
polar molecule. At the start of Section 8.4, we noted that the shape of a mole-
cule is very important in determining its properties. In particular, we mentioned
that some of water’s important properties result from its shape. Is water a polar
or nonpolar molecule? Water has a tetrahedral electron-group geometry and,
because of the existence of two lone pairs on the oxygen atom, has a bent molec-
ular geometry. The bond dipoles of water point toward the oxygen, but because
the molecule is bent, the net dipole moment is not zero. In fact, water has a dipole
moment (1.85 D) and is a polar molecule (Figure 8.26).
The dipole moment can be used by the medicinal chemist, who can measure
the “fingerprint” of a molecule with an infrared (IR) spectrophotometer. How
does this instrument work? The IR spectrophotometer records the energy associ-
ated with the vibrations of a molecule, as long as the vibration produces a change
in the dipole moment. The resulting information can be used to classify com-
pounds in terms of the types of bonds they contain, and it also reveals the iden-
tity of compounds through a comparison to known samples. Other professionals
have recently applied IR spectroscopy to determine the quality of motor oil, to
find the amount of carbon monoxide in automobile exhaust, and to identify
different polyester fibers from crime scenes.
EXERCISE 8.11 Does CHCl
3
Have a Dipole Moment?
Does CHCl
3
(chloroform, a compound formerly used as the major component in
cough syrups) have a dipole moment? Give the Lewis dot structure for chloroform,
and show bond dipoles and the dipole moment for the molecule, if any exists.
Solution
The Lewis dot structure model of CHCl
3
indicates that the central carbon atom
contains four electron pairs. Because they are all bonding pairs of electrons, chloro-
form has a tetrahedral geometry. By placing individual bond dipoles on the mole-
cule, we note that there is a net dipole moment (1.04 D) to the molecule. We would
therefore predict that chloroform is a polar molecule.
The electron density distribution
diagram of HCN shows that this is a
polar molecule.
O
H H
FIGURE 8.26
The bond dipole in water. Note the large
dipole moment (blue arrow) that arises
from the sum of the two bond dipoles.
C
H
Cl
C
l
Cl
PRACTICE 8.11
Predict the bond dipoles and overall dipole moment for N
2
and NH
3
.
See Problems 95 and 96.
CO
O
FIGURE 8.25
Carbon dioxide contains two bond
dipoles, but overall the molecule lacks a
dipole moment.
Visualization: Polar Molecules
Polar Versus Nonpolar
As we mentioned before, we can use the dipole moment of a molecule to illus-
trate its overall polarity. In a polar molecule, a dipole moment exists. In a nonpo-
lar molecule, no dipole moment exists. However, it isn’t really that cut and dried.
Molecules reside on a scale of polarities from the purely nonpolar to the highly
polar. Water, with its two polar covalent bonds and resulting dipole moment, is
a polar molecule. However, it isn’t the most polar molecule. Why do we need to
know about polarity? The polarity of a molecule can be a useful predictor of the
solubility of the molecule. In other words, “like dissolves like.” Polar molecules
generally dissolve in polar solvents. Nonpolar molecules generally dissolve in
nonpolar solvents. And polar molecules typically do not dissolve in nonpolar
solvents. However, like all rules, this one has exceptions.
One exception is that not all molecules of the same polarity dissolve in each
other. For example, water and chloroform (HCCl
3
) do not mix together, in spite
of the fact that they are both polar.
Why is this so? In short, solubility is affected by
more factors than relative molecular polarities. The structure of a molecule is
very much a part of the equation, but the rules of solubility are best determined
by considering the forces of interaction between dissolving molecules. See Chap-
ter 12 for more information about polarity, structure, and solubility.
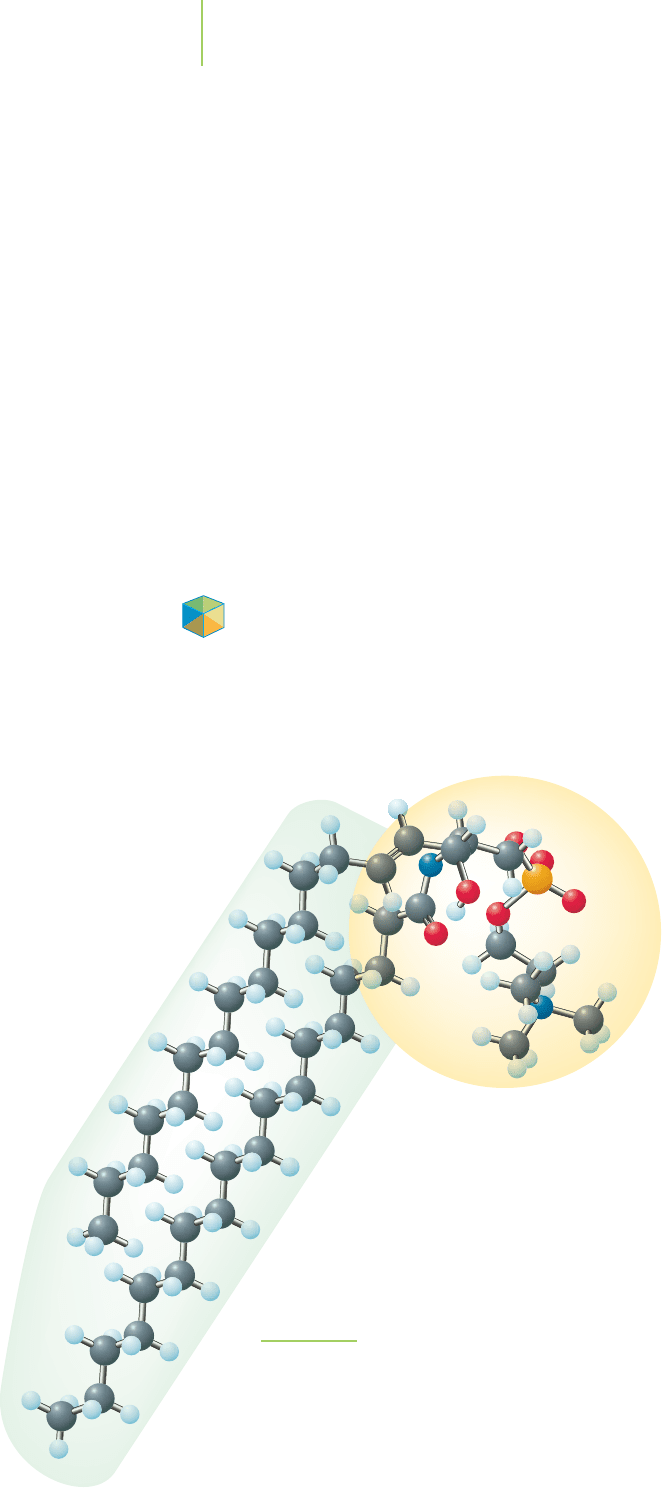
One of the major components in cell membranes is sphingomyelin (Fig-
ure 8.27). This molecule contains large bond dipoles at one end and very small
bond dipoles at the other. Because the molecule is so long, one end of the mole-
cule is polar and the other end is nonpolar. A cell membrane is made up of two
layers of sphingomyelins with the nonpolar ends pointing toward each other. A
medicinal chemist interested in developing a new drug must consider the impli-
cations of this fact. As we discussed earlier in this section,
a new drug that must penetrate a cell to cause a bi-
ological response must cross the outer polar re-
gion of the cell membrane, pass through the
large nonpolar region of the membrane,
and then cross the inner polar region of
the membrane. In other words, a drug
that targets a location inside a cell must
be able to dissolve in polar and nonpo-
lar solvents. It must have some polar
and some nonpolar character.
Just as the construction engineer
must model a building in such a way as
to take into account all expected mishaps,
we must try to address all of the observed
properties when we construct models of mole-
cules. The models discussed in this chapter ad-
dress the shape, dipole moment, and polarity of a com-
pound, but they do not adequately explain bond lengths, bond strengths, and the
reactivity of molecules. In Chapter 9, we will examine models that do a more
accurate job of explaining some of the properties of molecules.
346 Chapter 8 Bonding Basics
Application
Head
Tail
FIGURE 8.27
Sphingomyelin, one of the many molecules that
makes up the structure of the cell membrane. Note
that the tail of the molecule is nonpolar and the
head is polar.
Key Words 347
The Bottom Line
■
Models are an important tool that chemists use to
help them determine the properties of molecules.
(Section 8.1)
■
Estimation of the properties of a molecule on the
basis of the structure of that molecule is only as
good as the model of that molecule. (Section 8.1)
■
Bonding can range across the full spectrum
between equal sharing and complete transfer of
electrons. (Section 8.1)
■
The three main types of bonds are ionic, polar
covalent, and metallic. (Section 8.1)
■
An anion is always bigger than the atom from
which it is derived. A cation is always smaller than
the atom from which it is derived. (Section 8.2)
■
The lattice enthalpy is an important expression of
the energetic stability of a salt. (Section 8.2)
■
We can use bond enthalpy calculations to deter-
mine the approximate energy change involved in a
reaction. (Section 8.3)
■
Lewis dot structures are useful in constructing a
simple model showing the location of atoms within
a molecule. (Section 8.3)
■
Resonance hybrids offer an overall picture of a
molecule. Individual resonance structures do not
adequately describe a molecule. (Section 8.3)
■
VSEPR theory describes the shapes of molecules
better than Lewis dot structures. The model drawn
using VSEPR provides a three-dimensional picture
of the molecule. (Section 8.4)
■
The polarity of a molecule is related to the overall
forces of the individual bond dipoles in the mole-
cule and to the molecule’s three-dimensional shape.
(Section 8.5)
Key Words
axial position The position of a group when it is
aligned along the z axis of a molecule. (p. 340)
boiler scale The deposit of calcium carbonate (or cal-
cium sulfate) on the inside of water pipes. (p. 309)
bond dipole The polarization of electrons in a bond
that results in a separation of partial charges in the
bond. (p. 343)
bond dissociation energy The energy required to break
1 mol of bonds in a gaseous species. Also known as
the enthalpy of bond dissociation. (p. 332)
bonding electron pairs Pairs of electrons involved in
a covalent bond. Also known as bonding pairs.
(p. 340)
bonding pairs Pairs of electrons involved in a covalent
bond. Also known as bonding electron pairs.
(p. 322)
bond length The average distance between the nuclei of
bonded atoms. (p. 319)
Born–Haber cycle A diagrammatic representation of the
formation of an ionic crystalline solid using Hess’s
law. The cycle reveals the lattice enthalpy, which is
difficult to obtain by direct measurement. (p. 313)
chemical bonds A sharing of electrons between two
adjacent atoms. This sharing can be complete, par-
tial, or ionic in nature. (p. 304)
coordinate covalent bond A covalent bond that results
from the donation of two electrons from one of the
two atoms involved in the bond. The resulting bond
is indistinguishable from other covalent bonds.
(p. 330)
Coulomb’s law The force between two particles is pro-
portional to the product of the charges (Q) on each
particle divided by the square of their distance of
separation (d). (p. 314)
covalent bond A sharing of electrons between two adja-
cent atoms. This sharing can be complete or partial.
(p. 304)
crystalline lattice A highly ordered, three-dimensional
arrangement of atoms, ions, or molecules into a
solid. (p. 308)
dipole moment The polarization of electrons in a mole-
cule that results in a net unequal distribution of
charges throughout the molecule. (p. 344)
double bond A covalent bond consisting of two indi-
vidual bonding pairs of electrons. (p. 326)
duet rule The exception to the octet rule involving the
atoms H and He. A full valence shell for the atoms H
and He. (p. 306)
electron-group geometry The positions of the groups of
electrons (lone pairs and bonding pairs) around a
central atom in three dimensions. (p. 337)
electronegativity The ability of an atom in a molecule
to attract shared electrons to itself. (p. 319)
enthalpy of bond dissociation The enthalpy change
related to breaking 1 mol of bonds in a gaseous
species. Also known as the bond dissociation energy.
(p. 332)
