Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

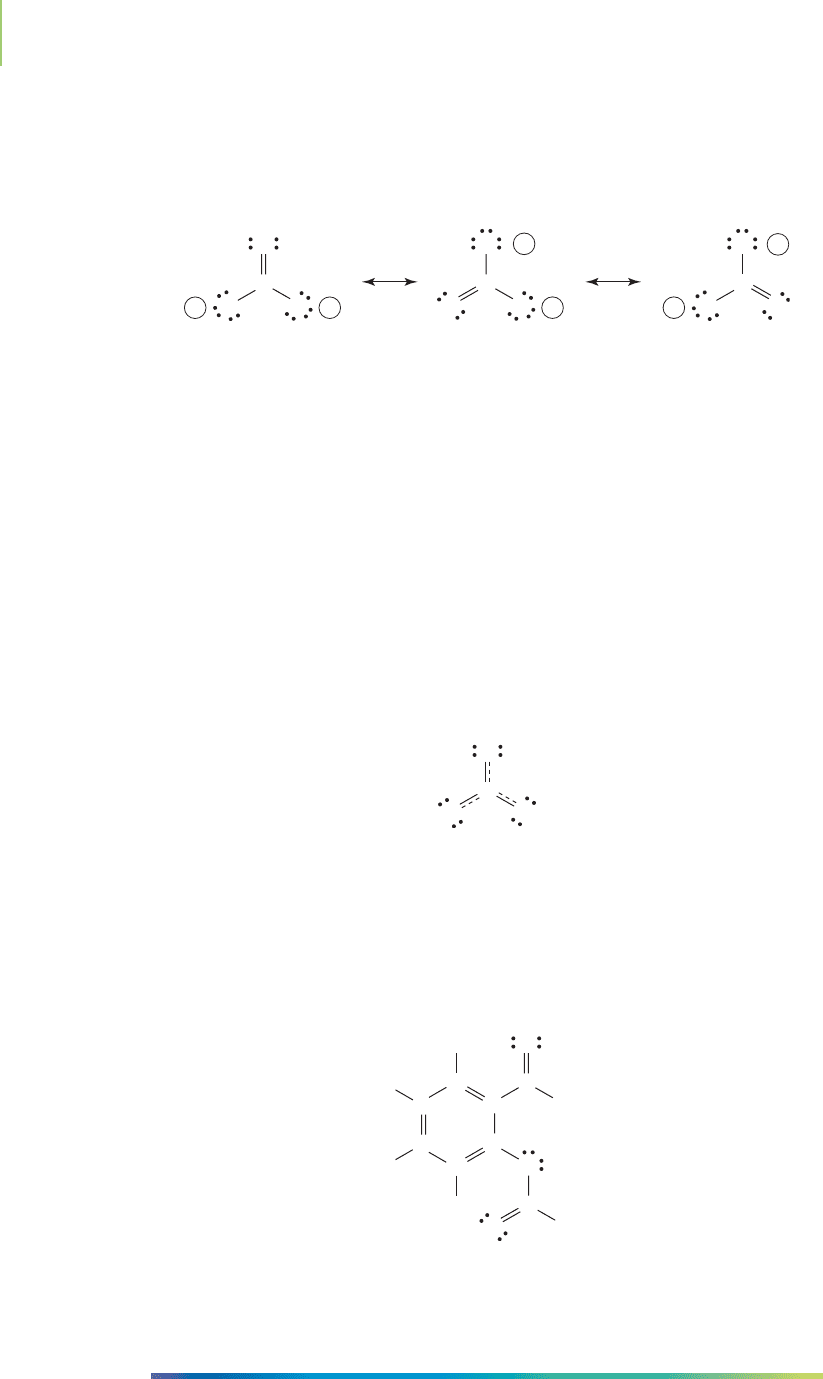
We could have shown the lone pair on one of the other atoms involved with
satisfying the octet rule for carbon. In fact, there are two other structures that also
seem to be satisfactory Lewis dot structures. Turn your textbook from “high
noon” to 4 o’clock (or “120 degrees”) to verify this fact.
Which one is correct?
Each one of the three structures is an equally good representation of the carbon-
ate ion. The only difference among these models is that the positions of the elec-
trons have changed. The relative positions of the atoms did not change.
A
resonance structure is a model of a molecule in which the positions of the
electrons have changed, but the positions of the atoms have remained fixed. All of
the resonance structures for a molecule are correct ways to draw the Lewis dot
structure. We show the relationship among these resonance structures by draw-
ing a double-headed arrow between them. However, we shouldn’t consider a single
resonance structure to be a discrete entity. Because the only difference is the loca-
tion of the electrons, the resonance structures must be considered together as the
model of the molecule. A combination of all of the resonance structures is the best
model. The resulting model is called the
resonance hybrid. The resonance hybrid
is an equal or unequal (based on experimental evidence) combination of all of
the resonance structures for a molecule. For the carbonate ion, the resonance hy-
brid looks like this:
Instead of full charges on two of the oxygen atoms, a partial charge (about
−0.67) exists on each of the oxygen atoms that adds up to the overall −2 charge
for the ion. Partial double bonds (1.33 times as much of a bond as a single bond)
are drawn to show how the three resonance structures combined to make one
resonance hybrid.
We now have a sufficiently complete model to draw the Lewis dot structure
for aspirin, or acetylsalicylic acid (C
9
H
8
O
4
).
We note that all the octets are filled and that the formal charges of all of the atoms
in the structure sum to 0.
HERE’S WHAT WE KNOW SO FAR
■
The energy of an ionic bond is related to the charges on the ions and the dis-
tance separating the ions.
OH
CH
3
C
C
C
C
H
H
H
C
C
H
C
O
C
O
O
C
O
O
O
C
O
O
O
C
O
O
O
C
O
O
O
11
1
1 1
1
328 Chapter 8 Bonding Basics
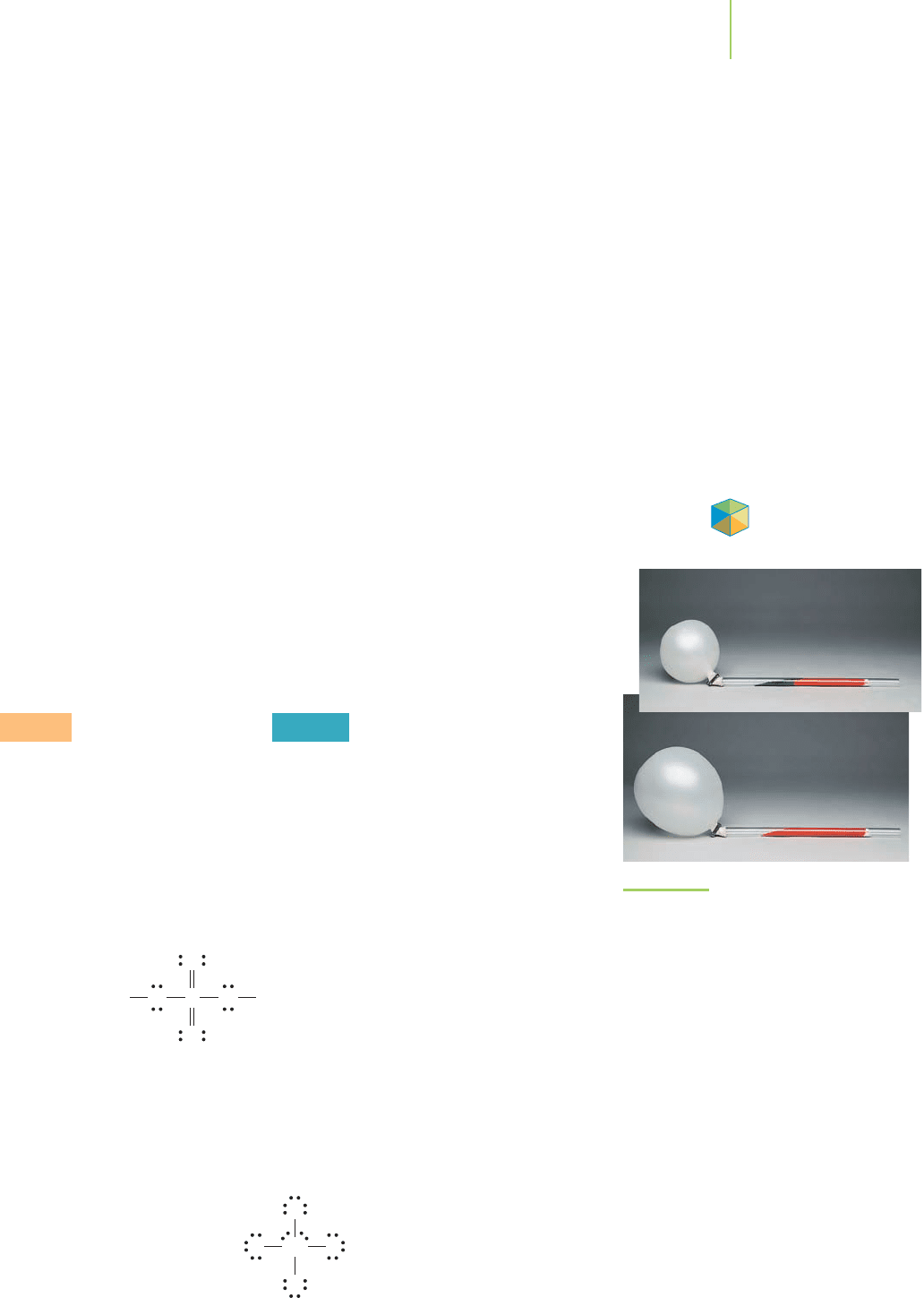
Application
■
Lewis dot structures can be used to draw a model of a compound. The octet
rule drives our prediction of the best model for a compound. The resulting
model represents the locations of electrons and atoms in a compound.
■
Electrons are shared in covalent bonds.
■
The atoms in a covalent bond are held at a distance that reflects the attraction
for the bonding electrons and the repulsion of the adjacent nuclei.
■
The Pauling electronegativity scale is the most widely used among several that
indicate the polarization of bonding electrons.
■
We can classify covalent bonds as polar and nonpolar, depending on the elec-
tronegativity difference between the atoms in the bond.
■
Formal charges help us to select the most likely structure among viable
alternatives.
■
Resonance structures exist in a molecule when electrons can shift positions
among different atoms while the positions of the atoms remain fixed.
Exceptions to the Octet Rule
In 1954, Robert Borkenstein, a captain in the Indiana State Police, developed a
Breathalyzer like that shown in Figure 8.17. A device similar to this was first used
by police to determine alcohol levels in drunk drivers. The Breathalyzer contains
potassium dichromate and sulfuric acid that converts exhaled ethanol
(CH
3
CH
2
OH) into acetic acid (CH
3
COOH). The conversion causes the chemical
reagent in the Breathalyzer to change color from yellow-orange to blue-green.
The rest of the compounds in the reaction are colorless. After the test is per-
formed, the presence of the blue-green color indicates that the suspect has been
drinking. What is the Lewis dot structure for sulfuric acid?
2K
2
Cr
2
O
7
+ 8H
2
SO
4
+ 3CH
3
CH
2
OH n 2Cr
2
(SO
4
)
3
+ 2K
2
SO
4
+ 3CH
3
COOH + 11H
2
O
hh
Yellow-orange Blue-green
Drawing the structure, we see that the sulfur atom has the smallest formal charge
when there are six bonds from the sulfur to the adjacent atoms. Can this be
correct? There is still debate about the existence of two SPO bonds in sulfuric
acid (rather than single bonds to all atoms, which gives decidedly nonzero formal
charges—can you prove this?) but the structure does seem to fit our
understanding of Lewis dot structures and formal charges. Some exceptions to the
octet rule must be considered if we are to accept this Lewis dot structure model.
In 1962, Neil Bartlett, then at the University of British Columbia, reported the
first inert gas compound (XePtF
6
). This was quickly followed by reports of other
inert gas compounds. For example, researchers at the Argonne Laboratories pre-
pared xenon tetrafluoride (XeF
4
). In the Lewis dot structure of XeF
4
, the xenon
atom has twelve total electrons around it. What are the formal charges on the
atoms in this model?
FF
F
F
Xe
Formal charges
F.C. (S) 6 6 0 0
F.C. (O) 6 2 4 0
SHH
O
O
O O
8.3 Covalent Bonding 329
FIGURE 8.17
The Breathalyzer. This device can be
used to estimate how much alcohol a
person has consumed. Shown here is
a demonstration of the process that
occurs in the instrument.

How can sulfur and xenon have more than eight electrons around them in the
Lewis dot structure? Consider the orbitals that are available to valence electrons.
In the valence shell of oxygen, for example, there are 2s and 2p orbitals. In the
valence shell for sulfur, however, there are 3s,3p, and 3d orbitals. The 3s and 3p
orbitals contain electrons. A higher-energy unfilled set of orbitals (the 3d or-
bitals) can be used if needed to hold extra electrons. This means that the sulfur
atom in sulfuric acid (H
2
SO
4
) can have eight electrons in the 3s and 3p orbitals
and place the extra four electrons in the previously empty 3d orbitals. Xenon, in
XeF
4
, can also accommodate the extra electrons by placing them in the empty
5d orbitals. This is a general rule for atoms of the third row and higher of the pe-
riodic table. Atoms can have
expanded octets. Often this occurs when the valence
electron shell includes unfilled d orbitals.
Boron trifluoride (BF
3
) is an example of a compound in which the central
atom has fewer than eight electrons around it. What is the formal charge on the
boron atom in BF
3
?
Even though the formal charges on each atom indicate that the Lewis dot struc-
ture is satisfactory, the boron atom does not satisfy the octet rule. We’d predict
this molecule, then, to be quite reactive with molecules that are electron-rich. In
fact, boron trifluoride reacts quite violently with molecules containing a lone pair
of electrons (such as water and ammonia). The bond that forms is an example of
a
coordinate covalent bond. This bond is special because both electrons that make
the bond were donated from just one of the two atoms involved. However, after
the bond is made, it is indistinguishable from a normal covalent bond.
The coordinate covalent bond is fairly common, particularly in reactions in-
volving acids and bases. For instance, when we write the equation describing the
dissolution of gaseous HCl in water, we describe the formation of a coordinate
covalent bond.
HCl(g) + H
2
O(l) n H
+
(aq) + Cl
−
(aq) + H
2
O(l) n H
3
O
+
(aq) + Cl
−
(aq)
The hydronium ion (H
3
O
+
) contains a coordinate covalent bond between the
oxygen and one of the hydrogen atoms. As the strong hydrochloric acid dissolves
in the water, it ionizes into H
+
and Cl
−
. The hydrogen cation combines with a
pair of electrons from the oxygen of water, and a covalent bond results. In the
end, that bond is indistinguishable from the other OOH bonds in the hydro-
nium ion.
Superoxide (O
2
−
) is an extremely reactive anion that can cause severe damage
to living tissue. To protect itself, the body has evolved an enzyme, superoxide dis-
mutase, that quickly hunts down and destroys superoxide. What is the Lewis dot
structure for superoxide, which is more accurately called the dioxygen (−1) ion?
In drawing the structure, we note the presence of an odd number of total va-
lence electrons. Because bonding electrons and lone-pair electrons are pairs of
FF
F
B
FF
H
B
HHN
H
HHN
F
FF
F
B
330 Chapter 8 Bonding Basics
Video Lesson: Electronegativity,
Formal Charge, and Resonance
electrons, an odd number of total valence electrons in a Lewis dot structure im-
plies that there is a lone unpaired electron (called a
radical). The existence of a rad-
ical electron in a molecule often makes a molecule quite reactive. By placing a
radical electron in a Lewis dot structure, we generate an atom that doesn’t com-
plete an octet. Note the location of the formal charge in superoxide:
Energy of the Covalent Bond
Angina, a sudden pain in the chest, is a symptom of a heart that doesn’t have an
adequate flow of oxygenated blood to work properly. Treatments for this type of
heart disease require administration of drugs that dilate the coronary artery and
increase the flow of blood. Calcium channel blockers are a class of medicines used
to treat this disease. These compounds fit neatly into “pockets” within proteins
that control the flow of calcium ions into muscle cells. As the rate at which cal-
cium ion passes into the heart muscle is greatly reduced, the heart relaxes and its
arteries dilate. The result is a heart that has enough oxygen to function ade-
quately. One problem with calcium channel blockers is that the body
breaks specific bonds in these drugs and makes them into biologi-
cally inactive compounds (i.e., the drugs are rapidly metabolized).
For example, nifedipine, a common calcium channel blocker, persists
in the body for only 4–8 hours (Figure 8.18). Patients must continu-
ously take the drug to prevent heart damage due to lack of oxy-
genated blood.
Medicinal chemists are interested in designing drugs that are sim-
ilar in structure to nifedipine but resistant to metabolism. To do this,
the medicinal chemist must make a compound that fits into the same
pocket in the protein but contains bonds that do not break as easily
as nifedipine. The shape of the new drug, then, must be very similar
to the shape of nifedipine. The shape can be determined by building
a model of the compound.
How do scientists know which bonds are
susceptible to reactions within the body?
Because reactions break
O O
8.3 Covalent Bonding 331
Application
C
HEMICAL ENCOUNTERS:
Calcium Channel
Blockers
Outside of cell
Inside of cell
Cell
wall
Ca
2+
Ca
2+
Calcium
channel
Open Closed
H
N
N
O
O
O
O
O
+
–
O
FIGURE 8.18
Nifedipine, a calcium channel blocker
prescribed for some patients with heart
trouble. Carbon atoms are black, oxygen
atoms are red, nitrogen atoms are blue,
and hydrogen atoms are white.
The heart muscle can relax when the flow of
calcium ions to the heart is reduced. Calcium
channel blockers fit into the pockets of proteins
that control calcium intake, reducing the flow of
calcium to the heart.

bonds, the answer to this question is related to the strength of the different bonds
in a compound.
How strong are covalent bonds? Is there a relationship between the type of
bond and the strength of the bond? Is a multiple bond stronger than a single
bond? To answer these questions, we must rely on our ability to estimate the
amount of energy that it takes to dissociate a bond. The
enthalpy of bond dissoci-
ation
(
diss
H), or, simply, the bond dissociation energy, is the energy required to
break 1 mol of bonds in a gaseous species. The equation that describes bond dis-
sociation is shown below.
XOY(g) n X(g) +Y(g)
Bond breakage requires energy, so bond dissociation enthalpies are always en-
dothermic and the values for bond energy will always have a positive sign.
Table 8.6 lists the values for several important bonds. From the values in the table,
we note that single bonds require less energy to break than their corresponding
double or triple bonds. Compare the COC single bond (
diss
H = 347 kJ/mol),
aCPC double bond (
diss
H = 614 kJ/mol), and a CqC triple bond (
diss
H =
839 kJ/mol). Also note from the table that the length of the covalent bond shrinks
as we go from a single to a double to a triple bond for a given element or pair of
elements. For diatomic molecules, the bond dissociation energy can be measured
directly in the laboratory. The process is a little different for atoms that do not
form diatomic molecules. Other atoms close to the bond influence how electrons
are distributed in a bond and affect the energy it takes to break the bond. The data
in Table 8.6, then, are average bond dissociation energies.
The overall enthalpy change of a reaction (
rxn
H) can be estimated using
bond dissociation energies. Because the enthalpy change (a state function, inde-
pendent of path) in a reaction is the sum of all of the energy as heat added to the
system minus the sum of all of the energy as heat removed from the system at
constant pressure, the enthalpy change of a reaction can be determined by mea-
suring the enthalpies of bond breakage and bond formation. Breaking a bond
requires an addition of energy to the system. Forming a bond results in a release
of energy from a system. If we add up all of the enthalpies for the bonds that are
332 Chapter 8 Bonding Basics
Average Bond Dissociation Energies and Bond Lengths for Some Common Covalent Bonds
Energy Length Energy Length Energy Length
Bond (kJ/mol) (pm) Bond (kJ/mol) (pm) Bond (kJ/mol) (pm)
HOH 432 75 NOH 391 101 FOF 154 142
HOF 565 92 NOF 272 136 FOCl 253 165
HOCl 427 127 NOCl 200 175 FOBr 237 176
HOBr 363 141 NOBr 243 189 FOI 273 191
HOI 295 161 NON 160 145 ClOCl 239 199
COH 413 109 NPN 418 125 ClOBr 218 214
COF 485 135 NqN 941 110 ClOI 208 232
COCl 339 177 OOH 467 96 BrOBr 193 228
COBr 276 194 OOF 190 142 BrOI 175 247
COI 240 214 OOCl 203 172 IOI 149 267
COC 347 154 OOI 234 206 SOH 363 134
CPC 614 134 OOO 146 148 SOF 327 156
CqC 839 120 OPO 495 121 SOCl 253 207
CON 305 143 COO 358 143 SOBr 218 216
CP
N 615 138 CPO 745* 120 SOS 226 205
CqN 891 116 CqO 1072 113
*CPO bond energy in CO
2
is 799 kJ/mol.
TABLE 8.6
Video Lesson: Bond Properties
The enthalpy change of the reaction is calculated on the basis of this information.
The enthalpy change for the reaction is negative. When the reaction occurs, heat
is released.
1 mol CPO bonds × 745 kJ/mol =+745 kJ
2 mol COCl bonds × 339 kJ/mol =+678 kJ
2 mol OOH bonds × 467 kJ/mol =+934 kJ
Total bonds broken =+2357 kJ
2 mol HOCl bonds × (−427 kJ/mol) =− 854 kJ
2 mol CPO bonds × (−799 kJ/mol) =−1598 kJ
Total bonds formed =−2452 kJ
Total energy absorbed =+2357 kJ
Total energy released =−2452 kJ
Net energy change (H) =− 95 kJ
8.3 Covalent Bonding 333
broken and subtract all the enthalpies for the bonds that are formed, we can ar-
rive at the enthalpy change of the reaction.
rxn
H = (
diss
H)
breaking bonds
− (
diss
H)
making bonds
To use this equation, we have to know which bonds are broken and which are
formed in a reaction. This is where Lewis dot structure models come in. By ex-
amining the Lewis dot structures of the products and reactants, we can determine
which bonds are broken and which are formed. Then we can tally up the energy
required to break all of the bonds in the reactants, and all of the energy released
when the bonds in the products form. The difference between the two is the en-
thalpy change for the reaction. As we go through this process, we must keep in
mind that using bond enthalpies to calculate the approximate enthalpy change in
a reaction is strictly a mathematical tool; chemical reactions do not actually occur
by breaking bonds into individual atoms and then having the atoms combine in
different configurations. The actual chemistry is much more subtle and is con-
tinuous, consisting of a series of energy changes along the way as bonds are
broken and formed. We will discuss the subtleties of reaction mechanisms in
some detail in Chapter 15.
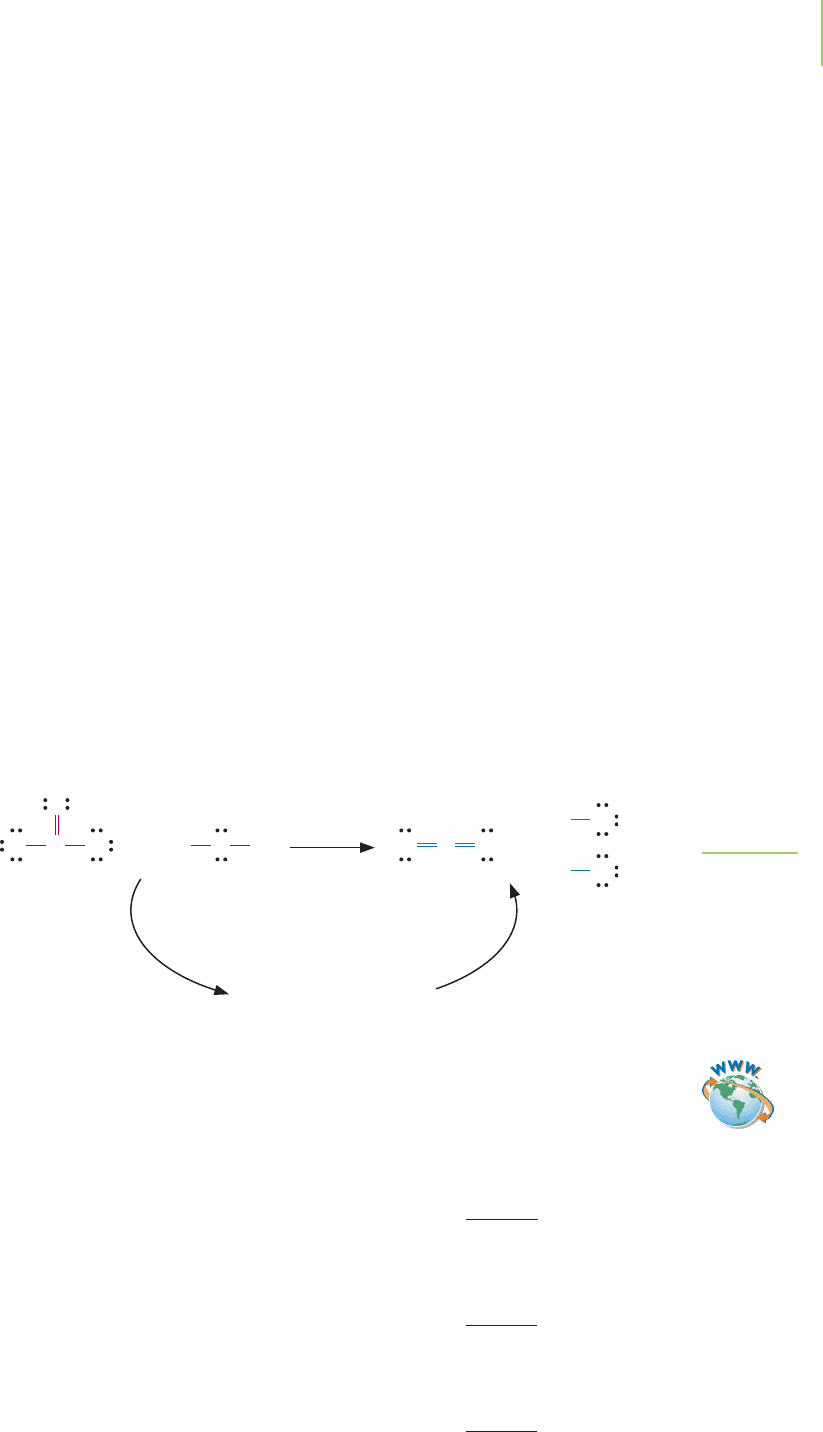
The reaction of water and phosgene illustrates how we use this handy tool in
the chemist’s toolbox to determine approximate bond enthalpy changes during
reactions:
H
2
O(l) + COCl
2
(g) n CO
2
(g) + 2HCl(aq)
We must draw the reaction and the Lewis dot structure for each compound. In
this reaction we break one CPO bond, two COCl bonds, and two OOH bonds,
and form two CPO bonds and two HOCl bonds (Figure 8.19).
Cl C
C
++Cl
Cl
Cl
HCl
H
H
H
Cl
HO
O
O
H
COCl
2
+ H
2
OCO
2
+ 2HCl
OCO
O
→
FIGURE 8.19
Reaction of phosgene with water. Energy
is added to the system to break all of the
bonds in the reactants. Energy is released
when the bonds in the products are
formed.
Video Lesson: Using Bond
Dissociation Energies

334 Chapter 8 Bonding Basics
The difference between the energy absorbed and the energy released =net energy
change
(H)= − 95 kJ
.
EXERCISE 8.8 Calculating the Enthalpy of Combustion
Calculation of the enthalpy of combustion can be useful in determining whether
a compound could make a good fuel. Calculate the enthalpy of combustion for
methane (found in natural gas) using bond dissociation enthalpies.
CH
4
(g) + 2O
2
(g) n CO
2
(g) + 2H
2
O(g)
First Thoughts
This problem requires that we first draw the Lewis dot structures of all of the com-
pounds in the reaction. After correctly drawing the structures, we can determine the
type of bond that breaks or forms.
What sort of answer do we expect? We know
that the combustion of methane is highly exothermic, supplying heat to countless
homes as natural gas is consumed in heaters.
Solution
4 mol COH bonds × 413 kJ/mol =+1652 kJ
2 mol O
2
bonds × 495 kJ/mol =+990 kJ
Total bonds broken =+2642 kJ
2 mol CPO bonds × (−799 kJ/mol) =−1598 kJ
4 mol OOH bonds × (−467 kJ/mol) =−1868 kJ
Total bonds formed =−3466 kJ
Total energy absorbed =+2642 kJ
Total energy released =−3466 kJ
Net energy change (H) =−824 kJ
The reaction is exothermic. Heat is given off during the combustion of methane, so
our answer makes sense.
Further Insight
Calculations such as this can be used to find a more efficient alternative fuel source.
For example, toluene (C
7
H
8
, a major component of gasoline) releases 3933 kJ/mol,
or 43 kJ/g, during its combustion. Based on this information, methane, releasing
824 kJ/mol, or about 52 kJ/g, gives off nearly 20% more energy as heat than does
toluene on a gram-to-gram basis. The best alternative on a gram-to-gram basis may
well be hydrogen, for which the energy released is about 280 kJ/mol, or 140 kJ/g!
This is one of several reasons why hydrogen is being developed as an alternative to
fossil fuels.
PRACTICE 8.8
Calculate the enthalpy change for the reaction of methanol and hydrogen bromide.
CH
3
OH(aq) + HBr(aq) n CH
3
Br(aq) + H
2
O(l)
See Problems 72–74.
8.4 VSEPR—A Better Model 335
HERE’S WHAT WE KNOW SO FAR
■
Atoms can exceed the octet rule if they have available d orbitals in their valence
shell.
■
The enthalpy of a reaction can be determined from the energy absorbed dur-
ing bond breakage or released during bond formation.
■
We can combine the net changes in energy from bond-breaking and bond for-
mation because bond enthalpy is a state function.
■
Between two particular atoms, breaking multiple bonds requires more energy
than breaking single bonds.
8.4 VSEPR—A Better Model
Knowing the strength of bonds in molecules such as nifedipine is useful for de-
termining information about the biological processes that break down the mole-
cule and render it useless as a pharmaceutical. However, the three-dimensional
structure of molecules is even more important in determining the biological
activity of the compound.
For instance, the opium poppy has long been used for its ability to alleviate
pain. The ancient Sumerians in 3400
B.C. enjoyed its use so much that they
referred to it as the “joy plant.” It wasn’t until 1827 that the active ingredient,
morphine, was isolated from the poppy and produced commercially. The anal-
gesic effects of morphine are one of the reasons why it continues to be used today
to treat postoperative pain. However, morphine has serious side effects, which in-
clude respiratory depression, constipation, muscle rigidity, physical dependence,
and a high potential for abuse. The medicinal chemist, the pharmacognocist, and
the biochemist have been working together to try to prepare compounds that
have beneficial properties similar to those of morphine but lack its harmful side
effects. To develop a compound with a similar mode of action, they must deter-
mine not only the shape of the morphine molecule but also the shape of the
active site on the receptor (the biological molecule to which morphine binds in
the human body). Recent efforts have determined that the shape of the active
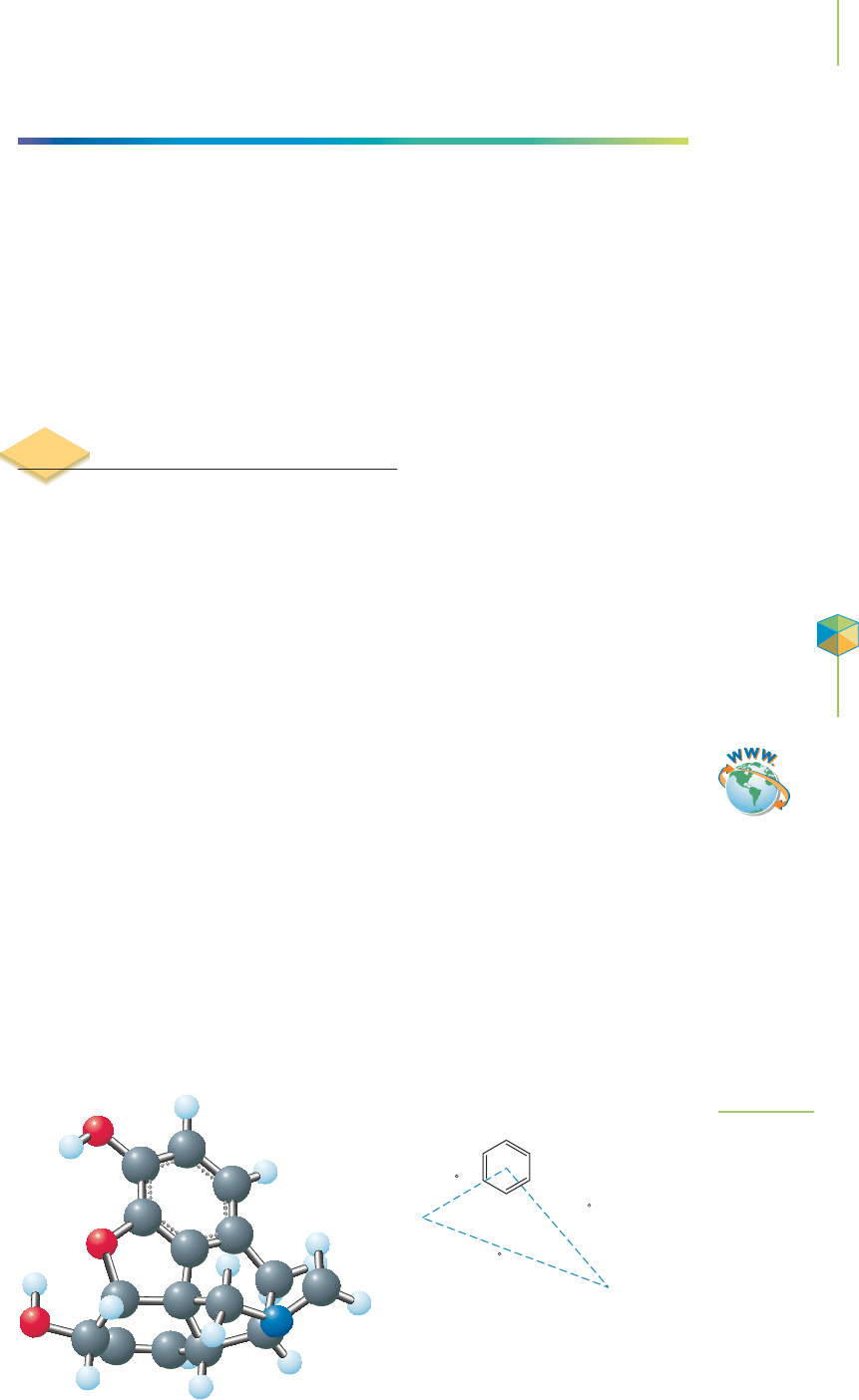
site must accommodate a structure with particular dimensions, as shown in Fig-
ure 8.20. Because there are so many different probable locations for the active site,
computers were used to complete most of the work mapping the shape of the
active site on the receptor.
Application
C
HEMICAL
ENCOUNTERS:
Focus on Morphine
(A)
(B)
(C)
N
4.5 A
7.6 A
Hydrophobi
c
region
6.7 A
+
FIGURE 8.20
The structure of morphine and the opioid
receptor binding requirements.
Tutorial: VSEPR Theory

Lewis dot structures would not be very helpful in determining the shape of
the morphine molecule or the active site in a case like this.
What are the limita-
tions of the Lewis dot structure models?
Does a Lewis dot structure tell us anything
about the three-dimensional shape of a molecule? The Lewis dot structure might
lead us to believe that all molecules are flat, planar structures. But people, gerbils,
roses, and guitars all occupy three dimensions, and all of these things are com-
posed of molecules, so it makes sense that molecules occupy three-dimensional
space. Even so, the idea of a “three-dimensional molecule” was fervently debated
when it was first introduced. Many chemists could not believe that molecules
would be anything other than flat.
The orientation of atoms in a molecule plays a major role in determining its
properties. For instance, the Lewis dot structure of water (H
2
O) can be drawn in
two different ways. In one, it is a linear molecule, in the other, it is bent. Which is
the correct way to draw water? If water were a linear molecule, it would have
completely different properties from those we observe. The consequences of this
slight change would have drastic effects in the real world. An ocean of linear water
molecules would dissolve oxygen quite well and probably kill most of the life in
336 Chapter 8 Bonding Basics
In the 1800s, Jacobus Henricus van’t Hoff
(Dutch physical chemist, 1852–1911)
and Achille Le Bel (French chemist, 1847–1930) inde-
pendently came to the conclusion that molecules must
exist as three-dimensional structures. Despite the fact
that established thought on the structure of molecules
asserted that they were flat, these researchers advanced
their theory for public and professional scrutiny. Using
careful theoretical considerations of well-studied mole-
cules (such as tartaric acid), van’t Hoff concluded that
molecules had to occupy a three-dimensional structure.
It was a very compelling argument for some. For others,
it was heresy.
Adolph Wilhelm Hermann Kolbe (1818–1884),
one of the prominent German chemists of the time,
vehemently discounted the theory of three-dimensional
molecules. His flat models seemed to provide the best
explanation for the properties that he observed. He took
such an aggressive stance on this issue that he attempted
to discredit van’t Hoff and his colleagues. Without exper-
imenting to determine whether the theory was correct,
he published letters in prominent chemistry journals
just to tarnish the new theory. In one of his published
articles, Kolbe wrote as follows:
I have recently published an article giving as one of the
reasons for the contemporary decline of chemical research
in Germany the lack of well-rounded as well as thorough
chemical education. Many of our chemistry professors
labor with this problem to the great disadvantage of our
science. As a consequence of this, there is an overgrowth
of the weed of the seemingly learned and ingenious but in
reality trivial and stupefying natural philosophy. This nat-
Issues and Controversies
Flat molecules and ethics in science
ural philosophy, which had been put aside by exact sci-
ence, is at present being dragged out by pseudoscientists
from the den which harbors such failings of the human
mind, and is dressed up in modern fashion like a freshly
rouged prostitute whom one tries to smuggle into good
society where she does not belong.
A J. H. van’t Hoff of the Veterinary School in Utrecht
has, as it seems, no taste for exact chemical investigation.
He has thought it more convenient to mount Pegasus
(apparently on loan from the Veterinary School) and to
proclaim in his “La chimie dans l’espace” how, during his
bold flight to the top of the chemical Mount Parnassus,
which he ascended in his daring flight, the atoms appeared
to him to have grouped themselves throughout the
Universe.
Journal für praktische Chemie, 1877
The responsibility of scientists to maintain objectiv-
ity in the methodical practice of science carries over into
the public realm as well. To practice science with less
than complete objectivity results in professional ridicule
and increased public skepticism. Tens of thousands of
chemistry-related articles are published worldwide each
year. Highly regarded journals use the system of prepub-
lication “peer review,” in which knowledgeable scientists
evaluate the validity of the research recounted by the
authors of articles. While not perfect, it is the best system
we know of to keep the scientific process as honest as
possible. The best indication that chemistry is over-
whelmingly done by ethical workers is that there are so
many advances in our discipline every year, and these
advances are based on communicating meaningful re-
sults of chemical research.

8.4 VSEPR—A Better Model 337
the sea. Even the beading of water as it runs down a window would not be possi-
ble if water were a linear molecule. Water is a bent molecule. Can we prove this
with a model—one that will pass the test of properly predicting the shapes of mol-
ecules with which we are unfamiliar? As we continue this discussion, please keep in
mind that models are, by their nature, simplifications that help us predict chemical
behavior. Because they simplify often subtle chemistry, they do not always properly
predict what happens. Yet we use models because the best ones give us insight and
some degree of predictive power. Such is the case with the VSEPR model, which
we describe next.
Description of VSEPR
Ronald J. Gillespie (1924–) and Sir Ronald Nyholm (1917–1971) introduced the
valence shell electron-pair repulsion model (VSEPR; pronounced “VES–per”) in
1957 to facilitate the construction of three-dimensional molecular structures.
This model is based on the repulsion between pairs of valence electrons (which
have similar charges). The key assumption in the
VSEPR model is that bonding
pairs and lone pairs of electrons move away from each other and orient them-
selves in three-dimensional space to give minimum repulsions (lowest energy
configurations.) To assist in visualizing this process, imagine pairs of electrons as
balloons tied together (Figure 8.21). Two balloons tied together orient in such a
way that they are opposite each other. Three balloons tied together orient them-
selves in a triangular shape, and four balloons push themselves to occupy the
corners of a tetrahedron. What happens if you distort the shapes by pushing the
balloons together and then letting go? The balloons push away again to return,
ideally, to the original shape.
VSEPR models determine the shape of a molecule on the basis of the number
of electron groups about the central atom. The resulting model of the molecule
represents the positions of the electron groups in three dimensions (the
electron-
group geometry
). Electron groups include lone pairs and bonding electrons; a
single bond, a double bond, or a triple bond counts as a single electron group.
However, when we determine the experimental shape of a molecule, we actually
find the positions of the different nuclei (the
molecular geometry). The actual
shape of the molecule can be different from the electron-group geometry, but
that doesn’t mean that the molecular geometry is unattainable from the electron-
group geometry. In fact, we can use the VSEPR model’s electron-group geometry
to determine the molecular geometry if we consider the geometry of the atoms to
contain “invisible” lone pairs. The result is the molecular geometry. Let’s consider
some examples of the VSEPR model to illustrate this process. Table 8.7 shows the
shapes of each of the electron-group geometries that we will discuss.
Examples of Three-Dimensional Structures
Using the VSEPR Model
Beryllium chloride (BeCl
2
) is a toxic white solid primarily used to make beryl-
lium metal. The Lewis dot structure of this compound indicates to us that the
beryllium atom is surrounded by two bonding pairs of electrons. By analogy, we
can consider the central atom in BeCl
2
by tying two balloons together. The bal-
loons push apart to occupy opposite orientations, so VSEPR predicts the elec-
tron-group geometry to be linear with a ClOBeOCl bond angle of 180°. The
molecular geometry is also linear because there are no lone-pair electrons around
the central beryllium atom.
This type of geometry is also predicted for carbon dioxide (CO
2
). The Lewis
dot structure of CO
2
indicates that there are only two electron groups in the form
of double bonds that extend from the central carbon atom. Carbon dioxide is a
linear molecule.
Cl Be
180°
Cl
OC
180°
O
Visualization: VSEPR
Video Lesson: Valence Shell
Electron-Pair Repulsion Theory
