Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

Chemical Applications and Practices
41. Sodium and potassium are often found as ions in sports
drinks. Which is the larger element? Explain.
42. Helium and hydrogen have both been used in dirigibles (also
known as blimps or airships). Which of these two element’s
atoms is larger? Explain.
Section 7.6 Ionization Energies
Skill Review
43. For this calculation, use the first ionization values of sodium
and potassium found in the chapter. How many grams of
potassium could be ionized using the same energy that is re-
quired to ionize 1.00 g of sodium?
44. For this calculation, use the first ionization values of calcium
and potassium found in the chapter. How many grams of cal-
cium could be ionized using the same energy that is required
to ionize 5.00 g of potassium?
45. Within each of these two lists, rank the elements listed from
lowest to highest first ionization energy:
a. Al, Si, P b. Ne, Ar, Kr
Review your rankings. Would you make any changes if asked
to do the rankings by second ionization energy? If, so de-
scribe the reasons for any changes.
46. Within each of these two lists, rank the elements listed from
lowest to highest first ionization energy:
a. Li, Be, B b. K, Rb, Cs
Review your rankings. Would you make any changes if asked
to do the rankings by second ionization energy? If, so de-
scribe the reasons for any changes.
47. Based on the following ionization energy (I) information,
predict which element(s) are most likely metals and which
are nonmetals. Also, suggest the most likely periodic table
group for each of these “unknown” elements. (Note that
these values, all of which are in units of kilojoules per mole,
may not reflect actual ionization energies of known elements.)
disputed. If element 118 does exist, to which group in the
periodic table does it belong? Would its ionization energy be
expected to be higher or lower than that of its next neighbor
above it in the periodic table? Explain your answer.
50. Element 119 could be discovered in the near future. To which
group in the periodic table would it belong? Would its ion-
ization energy be expected to be higher or lower than that of
its neighbor directly above it in the periodic table? Explain
your answer.
51. The flow of potassium and sodium ions into and out of nerve
cells makes it possible for signals to be sent throughout our
bodies. The fact that you are reading right now is dependent
on this flow of ions.
a. What are the charges on the potassium and sodium ions,
respectively?
b. Which is the larger of the two ions, sodium or potassium?
c. Which of the two ions, sodium or potassium, is easier to
produce from their neutral atoms?
52. Calcium ions also have great importance in cells and move
into and out of other channels in cell membranes. What
would be the charge on a calcium ion? Would this ion be
larger or smaller than a potassium ion? Explain.
53. Explain why the first ionization energy of sodium is lower
than magnesium’s first ionization energy, but the second ion-
ization energy of sodium is higher than that of magnesium.
54. Examining the first ionization energies of the elements in
Period 4 of the periodic table reveals the general tendency for
an increase in ionization energy from left to right. However,
there is a slight drop in ionization energy from calcium to
gallium. Study the electron configuration of both of these el-
ements and offer an explanation for this apparent deviation
from the trend.
55. Many elements may be found in nature in a variety of oxida-
tion states. However, these statements express some limita-
tions. Explain the basis for each of these limitations.
a. Magnesium is never found naturally as the +3 ion.
b. Fluorine is never found naturally as the +1 ion.
c. Hydrogen is never found naturally in the +2 oxidation
state.
d. Aluminum tends to “prefer” the +3 ion naturally.
56. Explain the basis for each of these limitations on the oxida-
tion states in which certain elements appear.
a. Arsenic can often be found with a charge of +5 but never
with a charge of +6.
b. Titanium could be found with either a +2 or a +4 charge
but not with a +5 charge.
c. Potassium is never found naturally as a neutral element.
d. Tin can be found with a +2 or a +4 charge but not with
a +5 charge.
Section 7.7 Electron Affinity
Skill Review
57. Which of these would have the best chance of forming a
stable anion: S or Xe? Justify your choice.
58. Which of these would have the best chance of forming a
stable anion: Cl or Ar?
59. Which of these has the more exothermic value for electron
affinity: Cl or Ar?
298 Chapter 7 Periodic Properties of the Elements
I
1
(kJ/mol) I
2
(kJ/mol) I
3
(kJ/mol) I
4
(kJ/mol)
Element A 500 4600 5800 7460
Element B 1060 1900 2900 5000
I
1
(kJ/mol) I
2
(kJ/mol) I
3
(kJ/mol) I
4
(kJ/mol)
Element A 740 1440 7730 8670
Element B 2100 3230 4400 5500
48. On the basis of the following ionization energy (I) informa-
tion, predict which element(s) are most likely to be metals
and which to be nonmetals. Also, suggest the most likely pe-
riodic table group for each of these “unknown” elements.
(Note that these values, all of which are in units of kilojoules
per mole, may not reflect actual ionization energies of known
elements.)
Chemical Applications and Practices
49. Some claims have been made regarding the production (dis-
covery) of element 118. However, those claims have been
60. Which of these has the more exothermic value for electron
affinity: O or F?
61. Arrange the following atoms on the basis of electron affinity,
from least negative to most negative: Na, Mg, Al. Now
arrange them on the basis of their ability to form anions. Is
the order any different? If so, explain any changes you made
in the ordering.
62. Arrange the following list in order from least negative elec-
tron affinity value to most negative electron affinity value:
Br, Br
−
,K.
63. Because they all have a negative charge, the electrons in
atoms repel each other. Explain why the electron repulsion
in a fluorine atom is such a large factor in determining its
unusually small electron affinity value.
64. Explain why ionization energy is always a positive quantity,
whereas electron affinity may be positive or negative.
Chemical Applications and Practices
65. The first ionization energy of sodium is +495 kJ/mol. The
electron affinity of chlorine is −349 kJ/mol. When sodium
metal and chlorine gas are placed near each other, a violent
reaction takes place. After a sodium atom has lost an electron
and a chlorine atom has gained an electron, both are oppo-
sitely charged and have the stable configuration of noble
gases.
a. What is the total energy change that occurs in these two
processes? Is this exothermic or endothermic?
b. Two additional energy changes occur in this reaction: the
dissociation of a chlorine atom from Cl
2
(+158.8 kJ/mol)
and the energy released when the ions are combined
(−1030 kJ/mol). What can you determine about the spon-
taneous, violent reaction between sodium and chlorine
when all of the energy changes in this process are consid-
ered?
66. The first ionization energy of lithium is 5.392 eV. (1 eV =
96.485 kJ/mol). The electron affinity of fluorine is
−328 kJ/mol. When lithium metal and fluorine gas are
placed near each other, a reaction takes place. After the ion-
ization of lithium and the formation of anionic fluorine,
both are oppositely charged and have the stable configura-
tion of noble gases.
a. What is the total energy change that occurs in these two
processes? Is this exothermic or endothermic?
b. Two additional energy changes occur in this reaction: the
dissociation of a chlorine atom from F
2
(+158.8 kJ/mol),
and the energy released when the ions are combined
(−1030 kJ/mol). What can you determine about the
spontaneous, violent reaction between lithium and fluo-
rine when all of the energy changes in this process are
considered?
Section 7.8 Electronegativity
Skill Review
67. Arrange this list of atoms in a correct ranking from least elec-
tronegativity to highest electronegativity: Na, F, As, Li, S.
68. Arrange this list of atoms in a correct ranking from least elec-
tronegativity to highest electronegativity: K, P, O, Br, N.
69. Each of these situations depicts two atoms bonded to each
other. In each case, which atom is more likely to attract elec-
trons toward itself within the bond?
a. NOOb.SeOSc.BrOGe d. ClOO
70. Each of these situations depicts two atoms bonded to each
other. In each case, which atom is more likely to attract elec-
trons toward itself within the bond?
a. FOOb.POSc.HOCd.COS
Chemical Applications and Practices
71. Using the information in the chapter, determine the change
in electronegativity from Ga to Se. This change arises as the
number of protons is increased by three. The change in
proton number from Sc to Zn is nine. What is the change in
electronegativity from Sc to Zn? Based on the change in
number of protons, is this what you would expect? Why or
why not?
72. In an early section we noted that the trend in electron affin-
ity from fluorine to chlorine was a bit different than we
might expect. The trend in electronegativity is, however,
what we would expect; that is, it decreases from top to
bottom in the group. What aspect of the definition of elec-
tronegativity helps explain the difference between the two
trends?
Section 7.9 Reactivity
Skill Review
73. Arrange this list of metals in order from generally least reac-
tive to most reactive: Na Mg Rb
74. Arrange this list of nonmetals in order from generally least
reactive to most reactive: S Cl I
75. What is the relationship between first ionization energy and
reactivity in metals and in nonmetals?
76. What is the relationship between electron affinity and reac-
tivity in metals and in nonmetals?
Chemical Applications and Practices
77. The “coinage metals” are copper, silver, and gold.
a. Describe the location of these elements in the periodic
table.
b. Where are they located in the activity series of metals
used in the chapter?
c. Is the location of Group IB
consistent with periodic
reactivity and the
activity series?
Focus Your Learning
299
Coins made from copper,
silver, and gold. In the past,
your pocket might contain
coins made from these
three metals. Due to the
value of silver and gold, the
U.S. mint currently uses
other metals and mixtures
of metals known as alloys.
78. Aluminum metal is considered a fairly active metal. It cer-
tainly reacts with oxygen more vigorously than does iron.
The reaction of both metals with oxygen produces oxides
that have different characteristics. Contrast the properties of
the two oxides.
79. Nonmetals such as chlorine and oxygen are both considered
very reactive. However, they may also react with each other.
Using any available information, decide which of the two
would probably become more negative in the reaction? Ex-
plain the basis for your answer.
80. Until the 1960s the noble gases were considered chemically
inert, or totally unreactive. Eventually, however, some noble
gases were made to react. Among the first compounds of
noble gases, fluorine was typically a reactant. Explain why
fluorine was such a good candidate for this role of reactivity.
Section 7.10 The Elements and the Environment
Skill Review
81. What explanation can be given for the fact that iron is found
in much greater abundance in the Earth’s crust than in the
mantle?
82. What substance that is common in the Earth’s crust accounts
for most of the silicon and oxygen (the two highest-ranking
elements in the crust?)
83. a. What is the third most abundant substance, by volume, in
the Earth’s atmosphere?
b. How many particles of that substance would be found in
one mole of dry air?
84. a. What is the fourth most abundant substance, by volume,
in the Earth’s atmosphere?
b. How many molecules of that substance would be found
in one mole of dry air?
Chemical Applications and Practices
85. Table 7.15 lists the ranked abundance of elements in the
Earth’s crust by mass. Which element(s) in that list are most
likely to be found in an uncombined state? (Uncombined,in
this case, means “not combined with other elements.”)
86. In Table 7.15, titanium is listed as more abundant, by mass,
than hydrogen. Convert the mass to moles and number of
atoms. If the ranking were to be redone by number of atoms,
would hydrogen still be below titanium? Prove your answer.
Comprehensive Problems
87. For each of these, provide a brief summary of his contribu-
tion to organizing the properties of elements into a useful
arrangement.
a. Johan Dobereiner
b. John Newlands
c. Dmitri Mendeleev
88. Indium (element 49) is an important element, but it is
not commonly used in textbook examples. Use the Internet
or another text reference to determine a chemical and a
physical property that indium has in common with gallium,
element 31.
89. a. What is the most common form of steel used in the
world?
b. Based on composition, what is the chief difference
between this most common type of steel and other types
of steel?
90. Based on the characteristics listed, which type of element
(metal, nonmetal, or metalloid) is being described?
a. At room temperature the sample is a dull, brittle solid.
The element is most likely to assume a negative charge
in ionic compounds.
b. At room temperature the sample is a solid that is able to
conduct electricity.
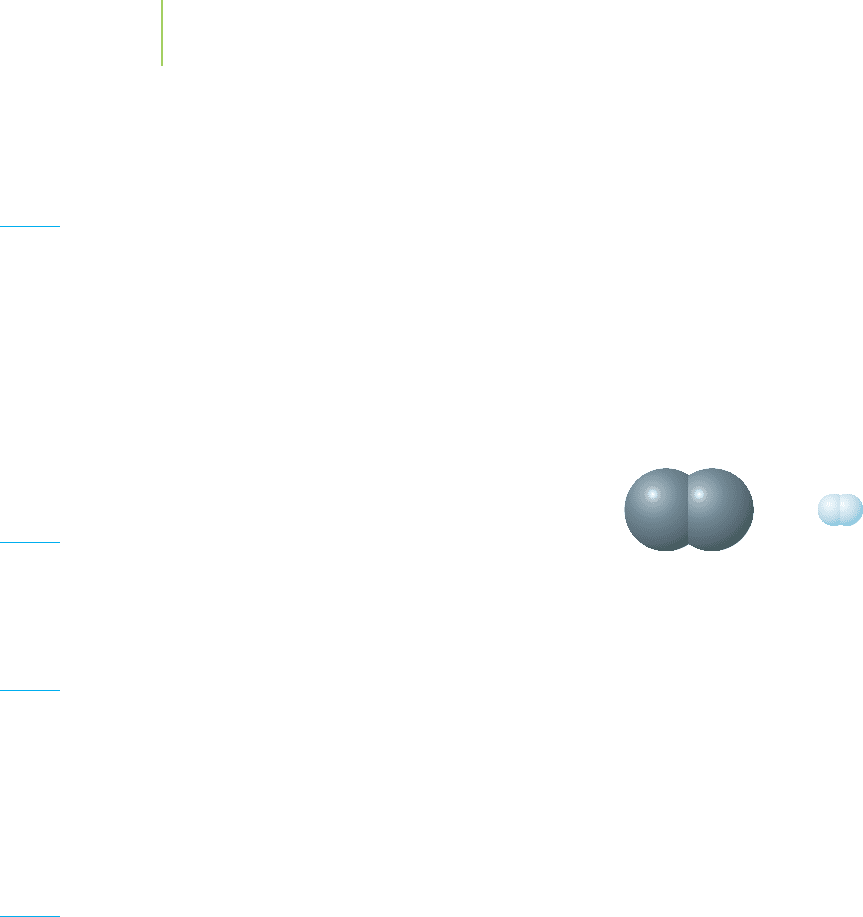
91. Given the information depicted in the illustration below,
what would you predict for the bond length between a
carbon and a hydrogen atom?
92. Contrast the meaning of the term valence electrons in the
context of calcium and in the context of chromium.
93. a. Using the first 18 elements on the periodic table, prepare
a graph with number of valence electrons on the y axis
and group number on the x axis. Is this a periodic func-
tion? Explain why or why not.
b. Prepare another graph of the same elements, using the
most common oxidation number on the y axis and the
group number on the x axis. Is this a periodic function?
Explain why or why not.
94. The definition of the atomic radius of an atom is basically
straightforward; it is one-half the distance between the
nuclei of a molecule made of two identical atoms.
a. What problems would arise if we were to define the
radius as one-half the diameter of an atom?
b. How does metallic radius differ from covalent radius?
95. Explain why, when comparing the radius of two atoms, it
would be important not to base the comparison solely on
which atom has the greater number of protons.
96. a. This reaction depicts an atom being ionized. To which
side of the reaction (left or right) should you show the
energy term for the reaction?
X → X
+
+ e
−
b. Select the correct response for each of these situations:
The smaller the value for ionization energy, the (less,
more) easily ionization will occur. The smaller the value
for ionization energy, the (less, more) chemically reactive
a metal will be.
97. Seldom do we get to describe a chemistry situation as
“always” happening without exception. However, this state-
ment is always true: Successive ionization energies in an
atom are always higher than previous values. What underly-
ing factors make this statement true?
98. Electron shielding or electron screening provides a descriptive
way to explain how the attraction for an electron by the nu-
cleus can vary in different electron arrangements. However,
H–H bond
75 pm
C–C bond
154 pm
300 Chapter 7 Periodic Properties of the Elements

electron screening can have a slightly different description
when applied to elements in the same row on the periodic
table than when applied to elements in the same group.
Contrast the type of screening, and its effectiveness, when
the term is applied to a period and when it is applied to a
group.
99. Arsenic and selenium are next to each other in Period 4 of
the periodic table. In general the trend, from left to right on
the table, is in favor of an increase in ionization energy.
However, when you compare the values for As and Se, you
note that the ionization drops slightly instead of increasing.
What is the basis for this situation?
100. Radioactive fallout from nuclear testing can contain signif-
icant amounts of unstable strontium. This can be particu-
larly harmful to young children, who have rapidly growing
bone structures. What common charge would strontium
ions have? What element is an important component in
bone development? Why is exposure to radioactive stron-
tium such a grave danger to young children?
101. a. The electron affinity of chlorine is approximately
−350 kJ/mol. Does this indicate an exothermic or an en-
dothermic reaction?
b. Write out the reaction, including placing the energy term
in the equation, that depicts the “electron affinity reac-
tion” for the process described in part a.
102. When summarizing the periodic trends in ionization
energy, atomic radius, electronegativity, and (to some
extent) electron affinity, we can typically indicate the trend
with a one-directional arrow to show the trend increasing
or decreasing from left to right in a row, or from top to
bottom in a group. Explain why the trends for increasing
reactivity start from the center of the periodic table and
move outward in both directions.
103. In the chapter, we note that tool steel contains 4.38% by
mass of chromium and 0.864% by mass of carbon.
a. What is the electron configuration of chromium?
b. If a steelmaker wishes to make 1.0 kg of tool steel, which
element (carbon or chromium) would require a larger
number of moles?
c. How many atoms of chromium would be present in an
88.7 g sample of tool steel?
104. The halogens are so-named because they react with metals
to make salts.
a. How many valence electrons do each of the atoms in this
group contain?
b. Write the balanced reaction that occurs between sodium
metal and iodine crystals.
105. According to Table 7.13, the human body contains the same
mass percent of silicon and potassium. Which occurs in the
body in a greater number of moles?
106. If the periodic table was arranged, in order, from smallest
atom (based on atomic radius) to largest atom
a. What atom would be listed first?
b. What atom would be listed last?
c. Would the noble gases still be aligned in a column?
d. How many helium atoms would be needed to create a
line of atoms 1.0 inches long (assuming that the atoms
are just touching and placed in a straight line)?
107. In the chapter, we mention that francium-210 can be made
from gold-197 by bombarding the gold with oxygen-18.
a. How many protons, neutrons, and electrons are found in
one atom of francium-210?
b. How many grams of francium-210 could be made from
2.50 g gold-197?
c. Which has a larger atomic radius—francium-210 or
gold-197? (Assume specific isotopes do not differ in their
atomic radius.)
Thinking Beyond the Calculation
108. An aqueous solution of sodium bicarbonate (NaHCO
3
)
reacts with aqueous hydrochloric acid to produce carbonic
acid (H
2
CO
3
) and sodium chloride (NaCl).
a. Write a balanced equation illustrating this reaction.
b. Indicate the group and period for each of the elements
involved in the reaction.
c. Indicate those elements in the reaction that can be char-
acterized as metals.
d. Carbonic acid decomposes in solution to produce carbon
dioxide and water. Write the balanced equation for this
reaction.
e. If 10.0 mg of sodium bicarbonate is added to 275 mL of
water and reacted completely with a stoichiometric
amount of HCl, what concentration (in ppm) of sodium
chloride will result? (Assume no volume change.)
f. Using the information from part e, determine what the
concentration (in ppm) of sodium ions will be.
g. A pastry chef may wish to perform a reaction similar to
this using sodium bicarbonate and acid. Why would the
chef wish to perform this reaction?
Focus Your Learning
301
Reaction of sodium bicarbonate and HCl.

Bonding
Basics
Bottles in the medicine chest. The active
ingredients in these medications are a
mix of molecules and ionic compounds,
two classes of compounds that differ in
the way their atoms are bound together.
302
Contents and Selected Applications
8.1 Modeling Bonds
8.2 Ionic Bonding
Chemical Encounters: The Uses and Behavior of Sodium Chloride
Chemical Encounters: Focus on Zeolites
Chemical Encounters: Fluoridated Water and Tooth Decay
8.3 Covalent Bonding
Chemical Encounters: Tetraethyl Lead in Gasoline
Chemical Encounters: Calcium Channel Blockers
8.4 VSEPR—A Better Model
Chemical Encounters: Focus on Morphine
8.5 Properties of Ionic and Molecular Compounds
Go to college.hmco.com/pic/kelterMEE for online learning resources.
Americans spend more than $250 billion per
year on prescription and over-the-counter (OTC)
medicines, about one-half of the world’s total. Our
medicine cabinets are fairly well stocked. If you and your
family are typical consumers, you may have headache pills,
muscle relaxers, antacids, cough syrup, and a couple of old bottles
of antibiotics. A quick check of the active ingredients reveals that these
bottles contain such compounds as ibuprofen, magnesium salicylate, sodium
bicarbonate, and pseudoephedrin. One of the more common ingredients in a
pain-relieving OTC medicine is aspirin (a molecular compound). Sodium bicarbon-
ate (an ionic compound) is typically used as an antacid. These two compounds
work in different ways to relieve common maladies, and they are fundamentally
different in their chemical makeup. They do not have the same numbers and types
of atoms, although this alone isn’t enough to explain why these compounds differ
radically in many properties, including melting point, boiling point, solubility in
water, and chemical reactivity. The main difference lies in their designation as mole-
cular compounds or ionic compounds, and this designation is made on the basis of
the way in which the atoms are bound together.
Biochemists, medicinal chemists, and
pharmacognocists (who study the properties
of drugs, especially focusing on those from natural sources) are familiar with molec-
ular and ionic compounds. One of the fundamental questions asked by these scien-
tists is
How do the bonds within a compound, the shape of the compound, and the physical
properties of the compound contribute to its ability to treat a disease or common malady?
In this chapter, we will answer this question and others as we explore how atoms
are held together, and how this bonding determines the shapes and properties of
compounds.
8.1 Modeling Bonds
We will begin our discussion by looking at the ways in which researchers
view the compounds with which they work. Although the formula of a po-
tential drug can be useful for determining the number and types of atoms it
contains, it doesn’t say much about the molecular shape. Aspirin—acetylsal-
icylic acid—which we discussed in Chapter 3, has the formula C
9
H
8
O
4
.The
formula alone provides no information about how aspirin structurally inter-
acts with the body to alleviate a headache. To find out more, we must look
deeper into the atom—into the role that the electron plays in determining
shapes. Our first key idea is that the different arrangements of the electrons in a
compound help determine the shape and the properties of that compound.
Three Kinds of Bonds
In their search for marketable pharmaceuticals, biochemists, medicinal
chemists, and pharmacognocists use a wide range of
molecular models, three-
dimensional depictions of the structure of molecules, as tools to examine the
shape and properties of compounds. To be ideal for the marketplace, a com-
pound should
■
effectively treat a particular disease, malady, or ailment
■
have no serious side effects
■
be inexpensive to mass-produce
Acetylsalicylic acid
C
9
H
8
O
4
303
The molecular models that researchers use in their search for marketable drugs
serve as representations of the
chemical bonds, the forces that hold atoms together,
within a compound. Chemical bonds arise between atoms when some of the out-
ermost electrons on the bonding atoms interact. In some cases, the electrons tend
to congregate on one of the atoms of the bond. In other cases, the electrons are
shared more or less equally between the atoms. A vital point to keep in mind is
that there are no absolutes. There are many degrees of electron sharing, from more
or less complete ownership by one atom to about equal sharing by both atoms,
and every possibility on the scale of sharing can occur. Still, we classically think
of three types of chemical bonds: the covalent bond, the ionic bond, and the
metallic bond.
Sodium chloride, the stuff we sprinkle on our French-fries, is an example of a
compound with an ionic bond. The
ionic bond lies at one end of the spectrum of
chemical bonds, where the atoms are held together by the force of attraction of
opposite charges. We say that one or more electrons have been removed from one
atom (remember, there are no absolutes!) and congregate on the other atom in
the compound.
Aspirin is an example of a compound in which the atoms are held together by
covalent bonds. The
covalent bond lies at the other end of the spectrum of chem-
ical bonds, where electrons are shared between the atoms. The positively charged
nuclei on either end of the bond attract the negatively charged electrons. It is this
force that holds the atoms together.
The aluminum atoms in a soda can are held together with a metallic bond.
The
metallic bond is a special type of bond in which metal cations are spaced
throughout a sea of mobile electrons. We’ll learn more about this bonding
pattern in Chapter 13.
Lewis Dot Symbols
The first step in the construction of the molecular model of a compound, such as
aspirin, sodium chloride, or aluminum, is drawing an atom itself. We recognize
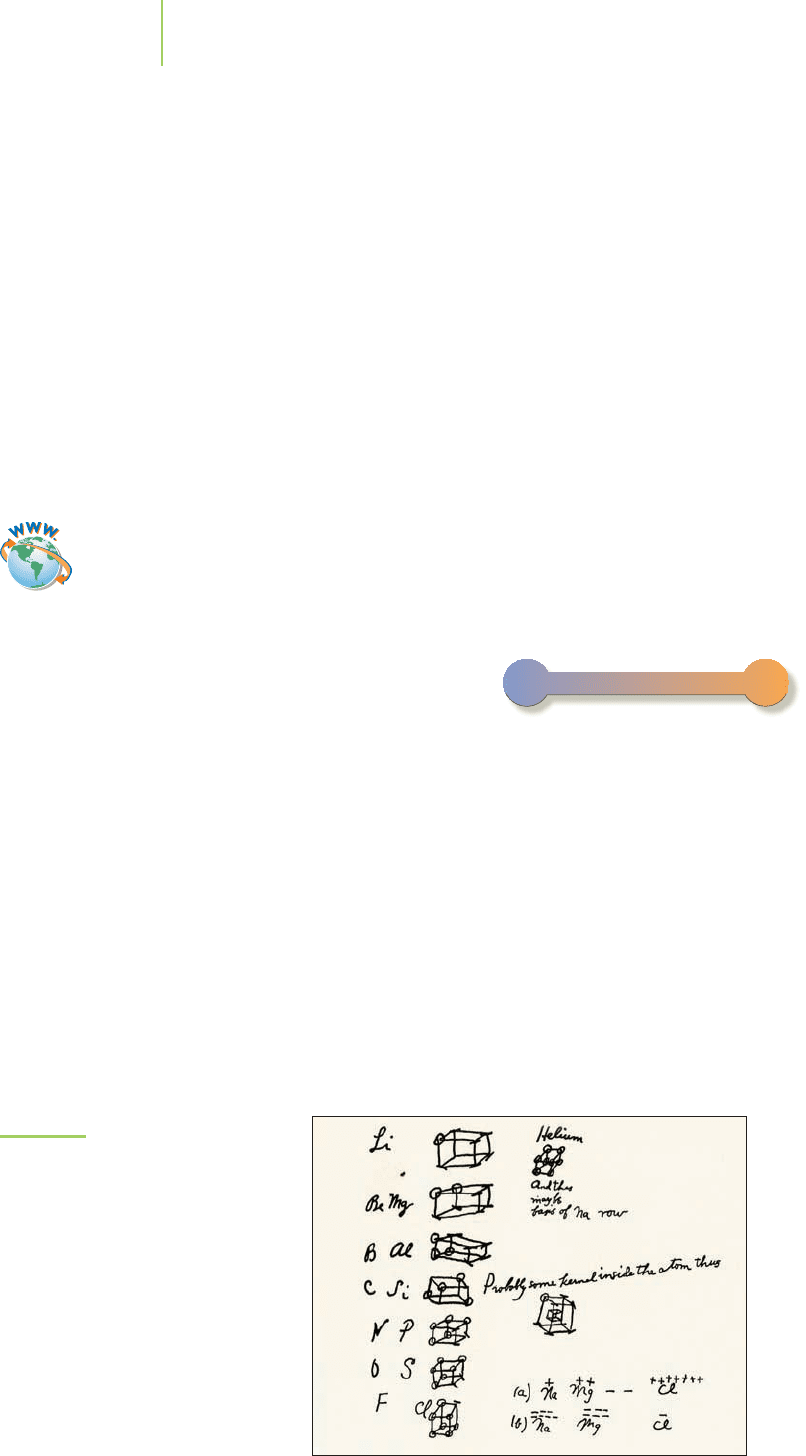
that the “business part” of the atom is its set of valence electrons. In 1916,
G. N. Lewis (Figure 8.1) developed a useful shorthand representation employing
Covalent and ionic bonds lie at opposite ends of the bonding spectrum. Real
bonds between different elements are neither purely ionic nor purely covalent.
Purely
covalent
Purely
ionic
“Real” bonds
304 Chapter 8 Bonding Basics
FIGURE 8.1
The American chemist G. N. Lewis
(1875–1946) developed a model of
atomic bonding that is still used today.
These notes, written by Lewis in 1902,
illustrate his thinking on how electrons
were arranged around an atom. The
Lewis dot symbols that we use today are
slightly modified from this original work.
Visualization: Covalent Bonding
Tutorial: Covalent and Ionic
Bonding
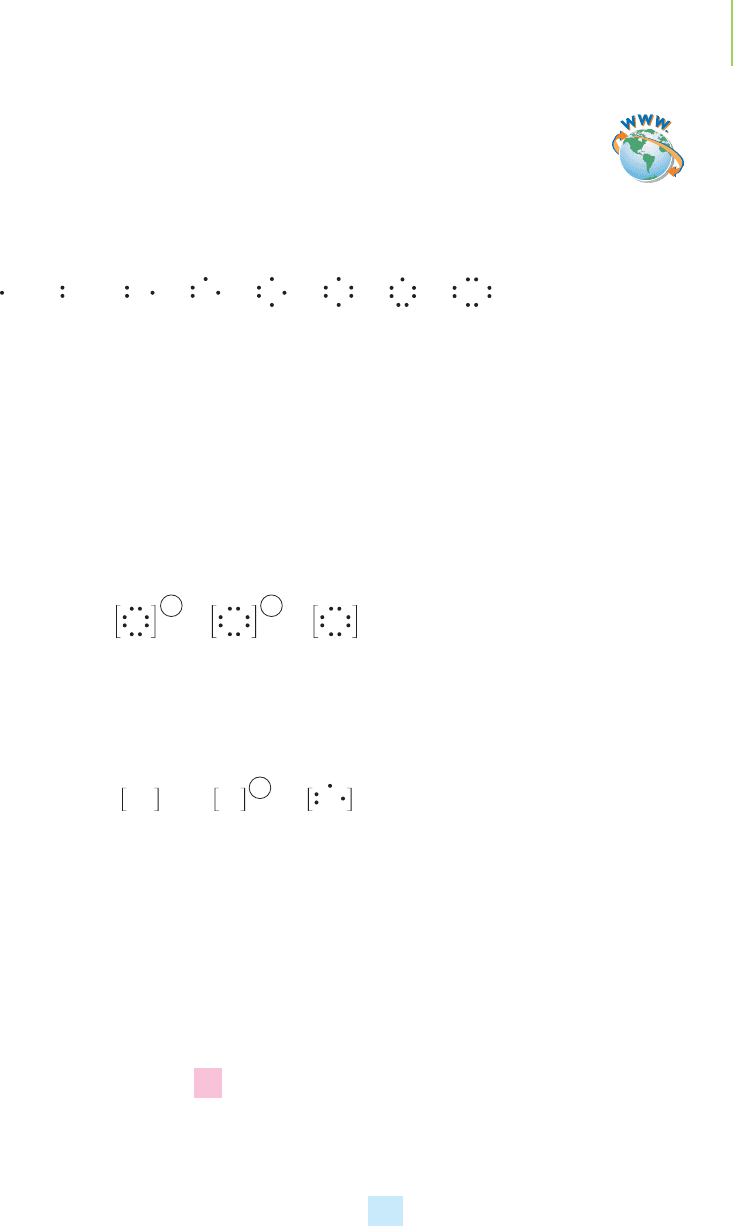
Lewis dot symbols. Typically, the first two dots, representing valence electrons in
the s orbital, are placed one at a time, as a pair, to the side of the element symbol.
The next three dots, representing valence electrons in the p orbitals, are placed
individually on the other sides of the element symbol. The remaining electrons
are placed alongside of the first three dots to indicate pairs of electrons in the
p orbitals. The Lewis dot symbols for the elements of Period 2 are shown below.
Lewis dot symbols can be used to represent ions as well. Because an ion is just
an atom (or a group of atoms) with a different number of electrons than the un-
charged species, we can draw its Lewis dot symbol. For instance, the fluoride ion
is a fluorine atom plus an electron (F
−
). To distinguish ions from Lewis dot sym-
bols of atoms, we place brackets around the drawing, and we place the charge on
the ion outside the brackets. Note in the diagram below that the nitrogen anion
has three extra electrons and the sulfur anion has two extra electrons. All three
have eight valence electrons and the same electron configuration as the period’s
noble gas (Ar for S and Ne for F and N). This idea is important in
Lewis dot
structures
, and we’ll revisit it later in this chapter.
Lewis dot symbols can also be drawn for cations. In a monatomic cation, elec-
trons have been removed from the atom (or group of atoms,) resulting in a pos-
itive charge. When we write the Lewis dot symbols, we indicate the number of
valence electrons and the resulting positive charge.
Electron Configuration of Ions
We determined the electron configuration of the elements in Chapter 6 using the
Aufbau principle. Electron configurations can also be used to describe which
atomic orbitals in ions contain electrons. For example, the electron configuration
of the sodium ion contains one electron less than that of the sodium atom. This
ion, which is necessary for proper contraction of heart tissue and electrolyte
balance inside and outside of the body’s cells, has an electron configuration that
lacks the valence electron from the sodium atom.
Na: 1s
2
2s
2
2p
6
3s
1
Na
+
:1s
2
2s
2
2p
6
In anions such as the fluoride ion (F
−
, found in toothpaste and added to most
U.S. municipal water supplies to help prevent tooth decay), the electron configu-
ration shows the addition of another electron to the valence shell:
F: 1s
2
2s
2
2p
5
F
−
:1s
2
2s
2
2p
6
We also know from our discussion of ionization energy that valence electrons are
the most accessible of the electrons in an atom. The addition of an electron to an
atom to make an anion occurs in the valence shell. Similarly, cations can be made
by removing valence electrons.
How do the electron configurations of Na
+
and F
−
compare with each other and with the noble gas nearest to them in atomic number
on the periodic table?
They have the same electron configuration as neon,
1s
2
2s
2
2p
6
. We say that the electron configuration of Na
+
is isoelectronic with (has
the same electron configuration as) F
−
and Ne.
Na
Al
N
3
N F
S
32
NeF
ONCBBeLi
8.1 Modeling Bonds 305
Lewis dot symbols of elements 3 to 10.
Lewis dot symbols of some
monatomic anions.
Lewis dot symbols of cations.
Video Lesson: Valence Electrons
and Chemical Bonding
The position of the electrons as either valence or core electrons on an atom
can help us understand the structure of a molecule such as aspirin. Knowing
where the electrons on the atom reside and how those electrons behave is of ut-
most importance in developing our three-dimensional model of a molecule. As
we’ll see shortly, the number of valence electrons on the atom is also important to
building our best possible model.
Octet Rule
In any chemical reaction, one of the driving forces is the ability of each atom to
reach a stable electron configuration. Electrons shuffle around until each atom
has its most energetically stable arrangement of electrons. As in F
−
and Na
+
, the
most stable electron configuration of the main-group elements is isoelectronic
with a noble gas. We refer to this as the
octet rule because the stable arrangement
for all of the noble gases beyond helium has eight valence electrons. For the ele-
ments H and He and the ions Li
+
and Be
2+
, the rule is also known as the duet rule
because of the need for only two electrons to fill the valence shell of the first row
elements. In other words, main-group atoms typically react by changing their num-
ber of electrons in such a way as to acquire the more stable electron configuration of
a noble gas. A full valence shell around an atom is a good situation for an atom,
because it then has the same electron configuration as a noble gas. We’ll use this
rule a lot as we put together atoms to make ionic compounds and molecules.
EXERCISE 8.1 Writing Lewis Dot Symbols
Write the electron configuration, and the Lewis dot symbol, for both P
3−
and Al
3+
.
With which element are they isoelectronic?
Solution
The P
3−
anion has three electrons more than the phosphorus atom. Therefore, the
electron configuration is 1s
2
2s
2
2p
6
3s
2
3p
6
. This is the same electron configuration as
argon. The Lewis dot symbol shows an octet of electrons.
The Al
3+
cation is missing three electrons, compared to the neutral atom. Its
electron configuration is 1s
2
2s
2
2p
6
, which is isoelectronic with neon. The Lewis dot
symbol also illustrates an octet of electrons.
PRACTICE 8.1
Write the electron configuration, write the Lewis dot symbol, and determine which
atoms are isoelectronic with S
2−
,F
−
,Mg
2+
, and Br
−
.
See Problems 1–4 and 9–14.
8.2 Ionic Bonding
We noted before that sodium chloride is an ionic compound. In ancient times, it
was a highly sought-after seasoning used in cooking and pickling. According to
the United States Geological Survey (USGS), 210 million metric tons of sodium
chloride were harvested from salt water or mined from deposits in the ground in
2005 (see Figure 8.2). In the United States, most of the salt that is harvested is
used to manufacture chlorine gas and sodium hydroxide. About 37% of the total
is used to de-ice highways. Only 3% is used in the food industry. Even so, the
average American consumes more than 1.5 kg of salt each year!
The human body requires only about 500 mg of sodium per day, yet the aver-
age American ingests between 2300 and 6900 mg each day. This high level of
306 Chapter 8 Bonding Basics
Application
C
HEMICAL
ENCOUNTERS:
The Uses and
Behavior of
Sodium Chloride

8.2 Ionic Bonding 307
sodium consumption can contribute to hypertension,
sleep apnea, and other disorders.
Sodium chloride (NaCl) is a compound held to-
gether by ionic bonds. As we saw in Chapter 4, sodium
chloride, like all other Group IA salts, dissociates
essentially completely into its ions when added to
water. It is a strong electrolyte and is a good example of
a typical ionic compound. Other examples of impor-
tant ionic compounds are shown in Table 8.1 and Fig-
ure 8.3. Note that some ionic compounds contain ionic
and covalent bonds. For example, calcium carbonate
contains an ionic bond between the calcium ion and
the carbonate ion. The carbon and oxygen atoms in the
carbonate ion are covalently bonded to each other. What makes sodium chloride
a “typical” ionic compound? The answer lies in the nature of its bonding and
properties, which we now explore.
FIGURE 8.2
Harvested sodium chloride is stored in
piles near the purification plant.
Important Ionic Compounds
Compound Formula Selected Use
Calcium carbonate CaCO
3
Limestone, chalk
Calcium chloride CaCl
2
Sidewalk salt
Iron(III) oxide Fe
2
O
3
Pigment
Magnesium hydroxide Mg(OH)
2
Milk of magnesia
Sodium carbonate Na
2
CO
3
Glass, soaps, and detergents
Sodium bicarbonate NaHCO
3
Baking soda
Sodium chloride NaCl Production of chlorine and sodium
hydroxide, seasoning, saline
solutions
Sodium fluoride NaF Toothpaste
TABLE 8.1
FIGURE 8.3
Important ionic compounds.
