Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


268 Chapter 7 Periodic Properties of the Elements
Nonmetals possess characteristics that are generally quite different from those of
metals.
Nonmetals are generally
■
gases or dull, brittle solids at room temperature and pressure
■
poor conductors of electricity (with the exception of the form of carbon
known as graphite)
■
likely to form negatively charged ions when they react to form ionic com-
pounds
Despite being small in number, the nonmetals include some of the most abun-
dant elements found in living things, particularly the carbon, hydrogen, oxygen,
nitrogen, phosphorus, and sulfur atoms from which the chemicals of life are
largely made.
Between the metals and nonmetals in the periodic table we find elements
known as
metalloids,or semimetals. Specifically, boron, silicon, germanium, ar-
senic, antimony, tellurium, and astatine make up the list of elements we call met-
alloids. These elements share some of the characteristic properties of both the
metals and nonmetals, making it difficult to place them in either of these two
main categories. For example, silicon and germanium have properties of both
metals and nonmetals. Unlike the metals, these elements are not good conductors
of electricity, but unlike the nonmetals, they are not very poor conductors of elec-
tricity. Their ability to conduct electricity lies somewhere in between that of the
Selected Nonmetals, Their Sources in Nature,
and Some of Their Industrial Uses
Nonmetal Sources in Nature Industrial Uses
C Coal, graphite, diamonds Steel manufacture, pencil “lead”
Cl Salt (NaCl), briny water Water purification, manufacture
of dyes and explosives
N Air Fertilizers, gunpowder, low-
temperature reactions, inert
atmospheres
O Air Medical field, steel manufacture,
combustion
P Apatite (calcium hydroxy Fertilizer, chemical warfare agent,
phosphate) rat poison, steel manufacture
S Iron pyrite, galena, barite, pure Black powder, fertilizer, fireworks,
element rubber production
TABLE 7.4
52
Te
127.60
85
At
(210)
5
B
10.811
14
Si
28.086
32
Ge
72.64
33
As
74.922
51
Sb
121.760
The metalloids.
Video Lesson: General
Properties of Nonmetals
7.3 The Next Level of Structure—Groups in the Periodic Table 269
1
H
3
Li
11
Na
19
K
37
Rb
55
Cs
87
Fr
4
Be
12
Mg
20
Ca
38
Sr
56
Ba
88
Ra
21
Sc
39
Y
57
La*
89
Ac
†
22
Ti
40
Zr
72
Hf
104
Rf
23
V
41
Nb
73
Ta
105
Db
24
Cr
42
Mo
74
W
106
Sg
25
Mn
43
Tc
75
Re
107
Bh
26
Fe
44
Ru
76
Os
108
Hs
27
Co
45
Rh
77
Ir
109
Mt
110
Uun
111
Uuu
28
Ni
46
Pd
78
Pt
29
Cu
47
Ag
79
Au
3
IIIB
4
IVB
5
VB
6
VIB
7
VIIB
11
IB
12
IIB
31
Ga
49
In
81
Tl
5
B
13
Al
32
Ge
50
Sn
82
Pb
6
C
14
Si
33
As
51
Sb
83
Bi
7
N
15
P
34
Se
52
Te
84
Po
8
O
16
S
9
F
17
Cl
35
Br
53
I
85
At
10
Ne
18
Ar
36
Kr
54
Xe
86
Rn
2
He
58
Ce
90
Th
59
Pr
91
Pa
60
Nd
92
U
61
Pm
93
Np
62
Sm
94
Pu
63
Eu
95
Am
64
Gd
96
Cm
65
Tb
97
Bk
66
Dy
98
Cf
67
Ho
99
Es
68
Er
100
Fm
69
Tm
101
Md
70
Yb
102
No
71
Lu
103
Lr
1
IA
2
IIA
13
IIIA
14
IVA
15
VA
16
VIA
17
VIIA
18
VIIIA
*Lanthanide
series
†
Actinide
series
112
Uub
30
Zn
48
Cd
80
Hg
1
2
3
4
Period
5
6
7
9
VIIIB
810
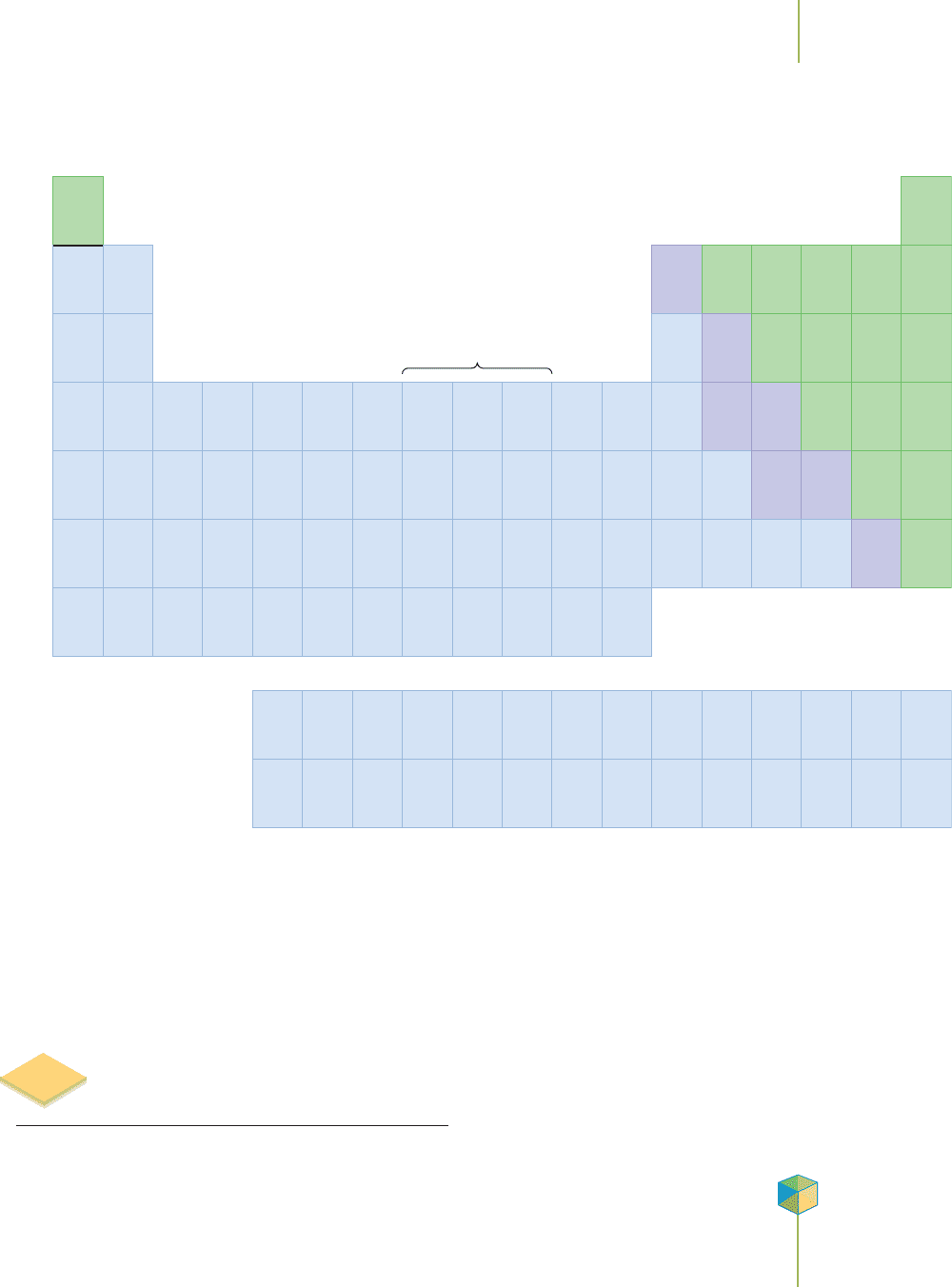
The nonmetals, shown in green, have properties that are opposite those of the metals, shown in blue.
metals and that of the nonmetals. We say that they are semiconductors, which
makes their use as materials in the computer-manufacturing industry ideal.
The subdivision into metals, nonmetals, and metalloids is the first level of
structure found within the periodic table. The next, and more fundamental, level
is the subdivision into the vertical columns called groups and the horizontal rows
called periods.
7.3 The Next Level of Structure—
Groups in the Periodic Table .
Elements within any of the vertical columns of the periodic table, which are
known as groups, share some significant chemical properties. There is a clear ex-
planation for the chemical similarities of elements within each group based on
each atom’s electron arrangements. For instance, elements in any of the
main
groups
, traditionally labeled IA through VIIIA, all have the same number of elec-
trons in their outer energy level, which corresponds to the highest principal
quantum number. The outer electrons—the
valence electrons—are very signifi-
cant in determining how an atom interacts with and reacts with other atoms. For
those elements known as transition elements, the definition of valence electrons
actually includes electrons in two energy levels.
Application
C
HEMICAL ENCOUNTERS:
Commercial Uses of
the Main-Group
Elements
3
Li
6.941
11
Na
22.990
19
K
39.098
37
Rb
85.468
55
Cs
132.905
87
Fr
(223)
270 Chapter 7 Periodic Properties of the Elements
Recent modifications to the periodic table have been made by the Interna-
tional Union of Pure and Applied Chemistry (IUPAC). One such modification is
the standardization of the numbers at the top of each of the columns of the peri-
odic table. In this system, the columns have been numbered from 1 to 18 from left
to right. Although this numbering system has eliminated confusion based on the
number of the column, vital information about the elements in the particular
groups of the periodic table has been lost. The authors prefer the use of the his-
torically common system of numbering the groups (IA, IIA, IB, etc.). You can
compare this time-honored system to the new IUPAC numbering system in the
periodic table on the inside front cover of this text.
Group IA: Hydrogen and the Alkali Metals
The elements in Group IA, apart from hydrogen, are known as the alkali metals
because they are highly reactive elements that combine with water to form chem-
icals called alkalis. [Al qili is from the Arabic, describing the burning of the salt-
wort plant, which produces an ash that forms an alkaline (basic) solution. Alkali
metals form alkaline solutions when they react with water. We will discuss acids
and bases in Chapter 17.] The alkali metals all have one valence electron in an s
orbital, which they generally lose when reacting, to form ions with a +1 charge.
We don’t find the alkali metals as free elements in the environment because they
are so chemically active that their atoms have all reacted to form compounds or
dissolved ions. Sodium is not found in its elemental state but, rather, in the form
of sodium ions (Na
+
). Sodium ions are the most abundant positive ions in sea-
water, and they are also plentiful in the internal environment of our
blood and intercellular fluids.
Hydrogen shares some characteristics with the alkali metals,
particularly its tendency to react by forming a +1 ion (H
+
). In
other respects, however, it is quite different from the alkali metals.
It is not a metal; it can also form a negative ion, the “hydride” ion
(H
−
), when combining with other alkali metals; and two hydrogen
atoms can combine by covalent bonding to form the hydrogen
molecule (H
2
).
The alkali metals.
Group IIA: The Alkaline Earth Metals
The elements in Group IIA are known as the alkaline earth metals. These are also
reactive metals, although generally less reactive than the alkali metals. They have
two valence electrons within an s orbital, which they generally lose when reacting,
to form ions with a +2 charge. Compounds of these metals combined with
oxygen are reasonably common in the environment. Because these metal oxides
Some Commercial Uses of Group IA Elements
Hydrogen Fertilizers, plastics, pharmaceuticals, fuel, fuel cells
Lithium Glass making, television tubes, battery electrolytes, lithium–aluminum
alloys in the aerospace industry, greases
Sodium Nuclear reactors, reagent, salt, washing soda
Potassium Fertilizers, soaps and detergents, explosives, glass and water
purification
Rubidium Photoelectric cells
Cesium Special glass, radiation-monitoring equipment, atomic clock
Francium None (too rare)
TABLE 7.5
Video Lesson: Hydrogen, Alkali
Metals, and Alkaline Earth
Metals
Video Lesson: Hydrogen
Video Lesson: The Alkali Metals
Video Lesson: The Alkaline Earth
Metals

5
B
10.811
13
Al
26.982
31
Ga
69.723
49
In
114.818
81
Tl
204.383
Group IIIA.
4
Be
9.012
12
Mg
24.305
20
Ca
40.078
38
Sr
87.62
56
Ba
137.327
88
Ra
(226)
The alkaline earth
metals.
7.3 The Next Level of Structure—Groups in the Periodic Table
271
were so readily available in the Earth, and because they form
alkalis when dissolved in water, the elements in Group IIA were
originally given the name alkaline earth metals. For example, cal-
cium oxide (CaO) is an alkaline compound used in the manufac-
ture of cement and steel.
Group IIIA
The elements in Group IIIA are substantially less reactive metals than those in
either Group IIA or Group IA. They all have three valence electrons, two in an
s orbital and one in a p orbital. When they react to form ionic compounds, they
generally do so by losing the three outer electrons to form ions with a +3 charge.
Note that as the number of electrons that an atom loses to form an ion increases,
the reactivity of the element decreases. This is our first example of a trend within
the periodic table—a characteristic that varies in a logical and
regular manner as we move through the table. The most signifi-
cant Group IIIA element that we gather from the environment is
aluminum (23 million metric tons were produced worldwide in
2005, according to the International Aluminum Institute), found
in the ore known as bauxite, which is composed of aluminum
oxide (Al
2
O
3
) combined with varying amounts of water.
Some Commercial Uses of the Group IIIA Elements
Boron Glass making, soaps and detergents, nuclear
reactor control rods
Aluminum Packaging, especially soft drink containers, trans-
portation (lightweight components in motor
vehicles), window frames, aircraft parts, engines,
kegs, cooking oils, indigestion tablets
Gallium Semiconductors, microwave equipment
Indium Display devices, low-melting-point alloys and
solders, semiconductors, fire sprinkler systems
Thallium Rat poisons and hair removers (now banned)
TABLE 7.7
Some Commercial Uses of Group IIA Elements
Beryllium Telecommunications equipment, automotive electronics, computers,
undersea communications equipment, pipe products in the oil and gas
industry
Magnesium Automotive industry, bicycles, luggage
Calcium Metallurgic applications, lead and aluminum industries, nuclear
applications, cement, soil conditioner, water treatment
Strontium Cathode-ray tubes, automotive industry, special glass, fireworks, flares
Barium Given to patients with digestive disorders to highlight the bowels in
an X-ray, drilling fluid, oil for gas wells
Radium Cancer treatment, luminous paint for watches and clocks
TABLE 7.6
A conveyor belt at the Rio do Norte mining company in the
Amazon forest in Brazil piles up bauxite ore, a source of alu-
minum oxide. Mining bauxite can be very devastating to the
environment, but the Rio do Norte mining company has
been successful in reforesting the land that they’ve mined,
as evidenced by the green area in the middle of the photo.
Their efforts challenge the notion that the Amazon forest
must be destroyed to tap its riches and offer an opportunity
to learn how to repair degraded forests.
Video Lesson: Aluminum
Video Lesson: CIA
Demonstration: The Reaction
between Al and Br
2
6
C
12.011
14
Si
28.086
32
Ge
72.64
50
Sn
118.710
82
Pb
207.2
Group IVA.
272 Chapter 7 Periodic Properties of the Elements
Group IVA
The elements in Group IVA, with four valence electrons, two in an s orbital and
two in individual p orbitals, occupy the “center ground” of the main-group ele-
ments, and they include elements of central importance to living things and the
fabricated materials with which modern society survives. Carbon, at the top of
the group, can be regarded as the basic elemental “building block”of life, because
the chemicals of life are largely based on chemical chains and rings of carbon
atoms with various other atoms attached. Carbon does not generally form ions.
Instead, it forms covalent bonds (we will discuss these in Chapter 8), in which
electrons are more or less shared between two atoms. Group IVA also includes sil-
icon, the basis of the “silicon chips” of the computer industry, and the semicon-
ductor germanium, also used in the manufacture of computer chips.
Group VA
The elements in Group VA have five valence electrons, two sharing an s orbital
and three occupying individual p orbitals. When they form ions, these elements
generally gain three electrons to form ions with a −3 charge. The Group VA ele-
ments also readily participate in covalent bonding. Nitrogen, for example, makes
up 80% of the volume of the Earth’s atmosphere in the form of N
2
molecules.
Nitrogen is also a crucial component of many covalently bonded compounds
required for life, such as DNA, proteins, and many vitamins and hormones. Phos-
phorus is also crucial for life, being one of the atoms found in DNA, for example,
and being part of the compounds found in fertilizers that are used to grow the
food we consume.
Some Commercial Uses of the Group IVA Elements
Carbon Commercial and military aircraft, fibers, thermoplastic
matrix materials, petrochemicals, clothing, dyes,
fertilizers, fuels, pharmaceuticals
Silicon Aluminum alloys, silicones, silicon chips used in
computers, semiprecious stones
Germanium Semiconductors, transistors,
catalysts (substances that
greatly increase the rate of a reaction without being con-
sumed) in polymer production, glass for infrared devices
Tin Coatings for other metals, bronze, soft solder, pewter,
special paint used on boats to prevent barnacles
Lead Storage batteries, cable covering, radiation shielding, pipes,
pewter, pottery, additive in gasoline, lead crystal glass
TABLE 7.8
7
N
14.007
15
P
30.974
33
As
74.922
51
Sb
121.760
83
Bi
208.980
Group VA.
Some Commercial Uses of the Group VA Elements
Nitrogen Fertilizers, plastics, explosives, dyes
Phosphorus Fertilizers, matches, detergents, coating to prevent
corrosion
Arsenic Pesticides, wood preservatives, semiconductors,
special glass
Antimony Flame retardants, pigments, lubricants, ammunition,
used to harden other metals
Bismuth Industrial and laboratory chemicals, pharmaceuticals,
cosmetics, replacement for lead in steel alloys and alu-
minum in ceramics, high-temperature superconductors,
indigestion tablets
TABLE 7.9
Video Lesson: General
Properties of Carbon
Video Lesson: Silicon
Video Lesson: Nitrogen
Video Lesson: Phosphorus

7.3 The Next Level of Structure—Groups in the Periodic Table 273
Group VIA: The Chalcogens
The elements in Group VIA are known as the chalcogens, a name derived from
the Greek khalkos (“copper”), because the copper ores contain the elements in
this group. All of the elements in Group VIA have six valence electrons, two in an
s orbital and four distributed within three p orbitals. When they form ions, the
Group VIA elements gain two electrons to form ions with a −2 charge.
In looking for Group VIA elements of significance to ourselves and the envi-
ronment, we immediately turn to oxygen. Oxygen gas, in the form of O
2
mole-
cules, makes up about 21% of the atmosphere and is the gas we must breathe in
order to stay alive. We need it because it combines with hemoglobin, as we noted
in the chapter opening, and reacts with food molecules to release the energy that
powers all life. We have also met, in Chapter 2, the relatively rare form of oxygen
known as ozone (O
3
) that is a vital part of our environment high in the atmos-
phere, where the ozone layer absorbs harmful UV rays, but is a troublesome
environmental pollutant at ground level.
Group VIA also contains sulfur, another element that is both vital for life and
associated with harmful pollution. We need the sulfur that is a part of proteins
and some vitamins and other important biochemicals, as well as fertilizers and
industrial chemicals such as sulfuric acid (H
2
SO
4
), but oxides of sulfur such as
sulfur dioxide (SO
2
) are pollutants that can cause “acid rain.”
8
O
15.999
16
S
32.065
34
Se
78.96
52
Te
127.60
84
Po
(209)
The chalcogens.
Application
Some Commercial Uses of the Group VIA Elements
Oxygen Steel making, metal cutting, chemical industry
Sulfur Lead–acid storage batteries, fertilizers, water treatment,
petroleum refining, drying agents
Selenium Photoreceptors, glass colorant, pigments, metallurgic
and biological applications, photoelectric cells, photo-
copiers, semiconductors
Tellurium Additive in steel and other metal alloys, catalyst in syn-
thetic rubber production
Polonium Source of alpha radiation, heat source in space vehicles
TABLE 7.10
Sulfur can be found near volcanoes as
yellow rocks.
Video Lesson: Oxygen
Video Lesson: Sulfur

2
He
4.003
10
Ne
20.180
18
Ar
39.948
36
Kr
83.798
54
Xe
131.293
86
Rn
(222)
The noble gases.
274 Chapter 7 Periodic Properties of the Elements
Group VIIA: The Halogens
The elements in Group VIIA are known as the halogens (from the Greek hals +
gen, meaning “salt makers”) because they are reactive nonmetals that can com-
bine with metal ions to form the chemicals we call salts. All of the elements in
Group VIIA have seven valence electrons, two in an s orbital and five distributed
among three p orbitals. When they form ions, as happens when they become part
of salts, they gain one electron per atom to form ions with a −1 charge. The most
common example of a salt is sodium chloride (NaCl), which we know as
common table salt, and we will meet many other examples throughout this book.
Chloride ions (Cl
−
) are abundant in seawater and in the blood and intercellular
fluids of the body. Tiny amounts of fluoride ions (F
−
) are important in making
our teeth resistant to decay.
Group VIIIA: The Noble Gases
The elements of Group VIIIA are all largely unreactive gases, and the group is col-
lectively known as the
noble gases, in the sense of aloof nobility—being distin-
guished from the other, much more reactive elements. The noble gases, which are
also known as the
inert gases, are present in very small amounts in the environ-
ment, but through their remarkable stability, they reveal to us one of the most sig-
nificant secrets of chemistry. All the noble gases apart from helium have eight
valence electrons distributed between a full s orbital and three full p orbitals. The
9
F
18.998
17
Cl
35.453
35
Br
79.904
53
I
126.904
85
At
(210)
The halogens.
Some Commercial Uses of the Group VIIA Elements
Fluorine Welding metals, frosting glass, used in the nuclear
power industry, insulating gas for high-power electric-
ity transformers, as an additive to municipal water
supplies
Chlorine Chemical warfare, bleach, PVC plastic, water
purification
Bromine Flame retardants, pharmaceutical intermediates,
swimming pool disinfectants, industrial water
treatment, air conditioning absorption systems, pre-
cious metal leaching, fuels, additives, insecticides,
photography
Iodine Conductive polymers, fuel cells, dyes, photography,
pharmaceuticals, industrial catalyst
Astatine Nuclear reactors
TABLE 7.11
Some Commercial Uses of the Group VIIIA Elements
Helium Used by divers to dilute the oxygen they breathe, bal-
loons, low-temperature research
Neon Filling discharge tubes, ornamental lighting
Argon Filling discharge tubes, provide an inert atmosphere for
high-temperature metallurgic processes, light bulbs
Krypton High-quality light bulbs
Xenon Research purposes
Radon Gives off alpha particle radiation
TABLE 7.12
Video Lesson: Halogens
Video Lesson: Aqueous Halogen
Compounds
Video Lesson: Properties
of Noble Gases
fact that atoms of these elements do not naturally react either with themselves or
with any other elements (although some compounds of xenon, krypton, and
radon have been made under extreme conditions) indicates that they have an ex-
ceptionally stable valence electron arrangement. This arrangement is known as a
stable
octet because it contains eight valence electrons. Helium, with only two va-
lence electrons, also has an exceptionally stable electron arrangement because it
has a completely full energy level. It is the “odd one out” among the noble gases
in that, with only two electrons, it does not have a stable octet but nevertheless
shares the remarkable stability of the other inert gases. When we examine chem-
ical bonding in more detail in Chapter 8, we will find that many chemical reac-
tions can be understood in terms of the participating atoms acquiring electron
arrangements that are similar to the stable valence electron arrangements of the
noble gases.
Although they are very rare, we have found various good uses for the noble
gases of the environment. Helium, for example, is well known as the “lighter than
air” gas within party balloons, weather balloons, and airships that allows them to
rise upward. Neon has found fame as the gas within “neon” lights. In light bulbs,
argon, mixtures of argon and nitrogen, or, in newer “halogen” bulbs, krypton and
xenon are used as inert gases to prevent the tungsten filament from oxidizing, and
xenon has similar uses in flash photography.
EXERCISE 7.3 Group Fun
How many electrons are found in the valence shell of each of these elements?
a. Be b. As c. I d. In
Solution
a. Two, both in the 2s orbital
b. Five, two in the 4s orbital and three in the 4p orbitals
c. Seven, two in the 5s orbital and five in the 5p orbitals
d. Three, two in the 5s orbital and one in the 5p orbitals
PRACTICE 7.3
Indicate which element is described by each of these phrases.
a. Two valence electrons, both in the 6s orbital
b. One valence electron, in the 3s orbital
c. Six valence electrons, two of them in the 2s orbital
d. Four valence electrons, and the element is a nonmetal
See Problems 23 and 24.
The Transition Elements
In the middle of the periodic table we find elements known as the transition ele-
ments
, with the inner transition elements usually shown as a separate block, as in
Figure 7.1. The transition elements are arranged in short groups, historically la-
beled IB through VIIIB in the case of the main transition elements, and currently
unlabeled for the inner transition elements.
The elements in the first period of the inner transition elements are known as
the
lanthanides, and the second period forms the actinides. These inner transition
elements are shown separately, primarily for the visual ease of having the periodic
table fit into a convenient space. A more logical version of the periodic table is
shown in Figure 7.5, with the inner transition elements fitted where they belong
Uses of the
noble gases.
7.3 The Next Level of Structure—Groups in the Periodic Table
275
Video Lesson: Transition Metals
and Nonmetals
Video Lesson: Properties of
Transition Metals
Video Lesson: CIA
Demonstration: Copper
One-Pot Reactions
in terms of their atomic numbers. The inner transition elements make up the
f-block, and they have some specific chemical characteristics due to electrons in
f orbitals acting as valence electrons in addition to those in the outer s orbital. We
most often think of the inner transition elements such as uranium and pluto-
nium in terms of their nuclear rather than their chemical properties.
The transition elements of the d-block have electrons in d orbitals that can
count as valence electrons, in addition to the electrons in the s orbital of their
highest energy level. This situation of having valence electrons in two energy
276 Chapter 7 Periodic Properties of the Elements
IA
IIA IIIA IVA
VA
VIA VIIA
VIII
A
6p
5d
4s
5s
6s
7s
2s
3s
1s
4f
5f
Period
4
5
6
7
2
3
1
6d
4d
3d
5p
4p
3p
2p
1s
FIGURE 7.5
Periodic table with the lanthanides and actinides where they belong in terms of electron arrangement.
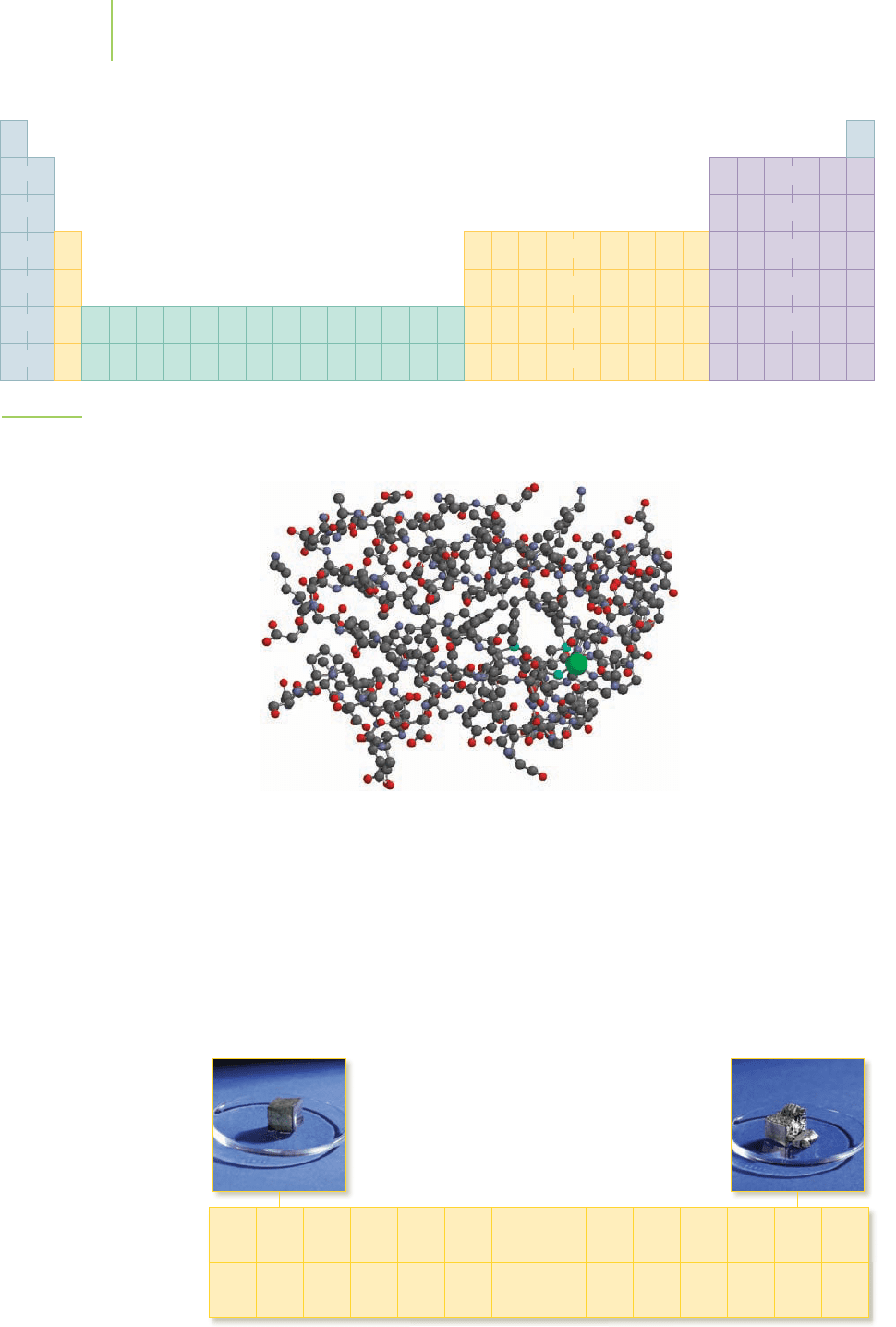
Transition metals, such as the copper
atom (green), are an essential part of
some enzymes. This three-dimensional
model is spinach plastocyanin, an
enzyme involved in energy production
in some plants. The hydrogen atoms
have been omitted from the model to
make it easier to see the other atoms.
58
Ce
140.116
90
Th
232.038
59
Pr
140.908
91
Pa
231.036
60
Nd
144.24
92
U
238.029
61
Pm
(145)
93
Np
(237)
62
Sm
150.36
94
Pu
(244)
63
Eu
151.964
95
Am
(243)
64
Gd
157.25
96
Cm
(247)
65
Tb
158.925
97
Bk
(247)
66
Dy
162.500
98
Cf
(251)
67
Ho
164.930
99
Es
(252)
68
Er
167.259
100
Fm
(257)
69
Tm
168.934
101
Md
(258)
70
Yb
173.04
102
No
(259)
71
Lu
174.967
103
Lr
(262)
The f-block elements.
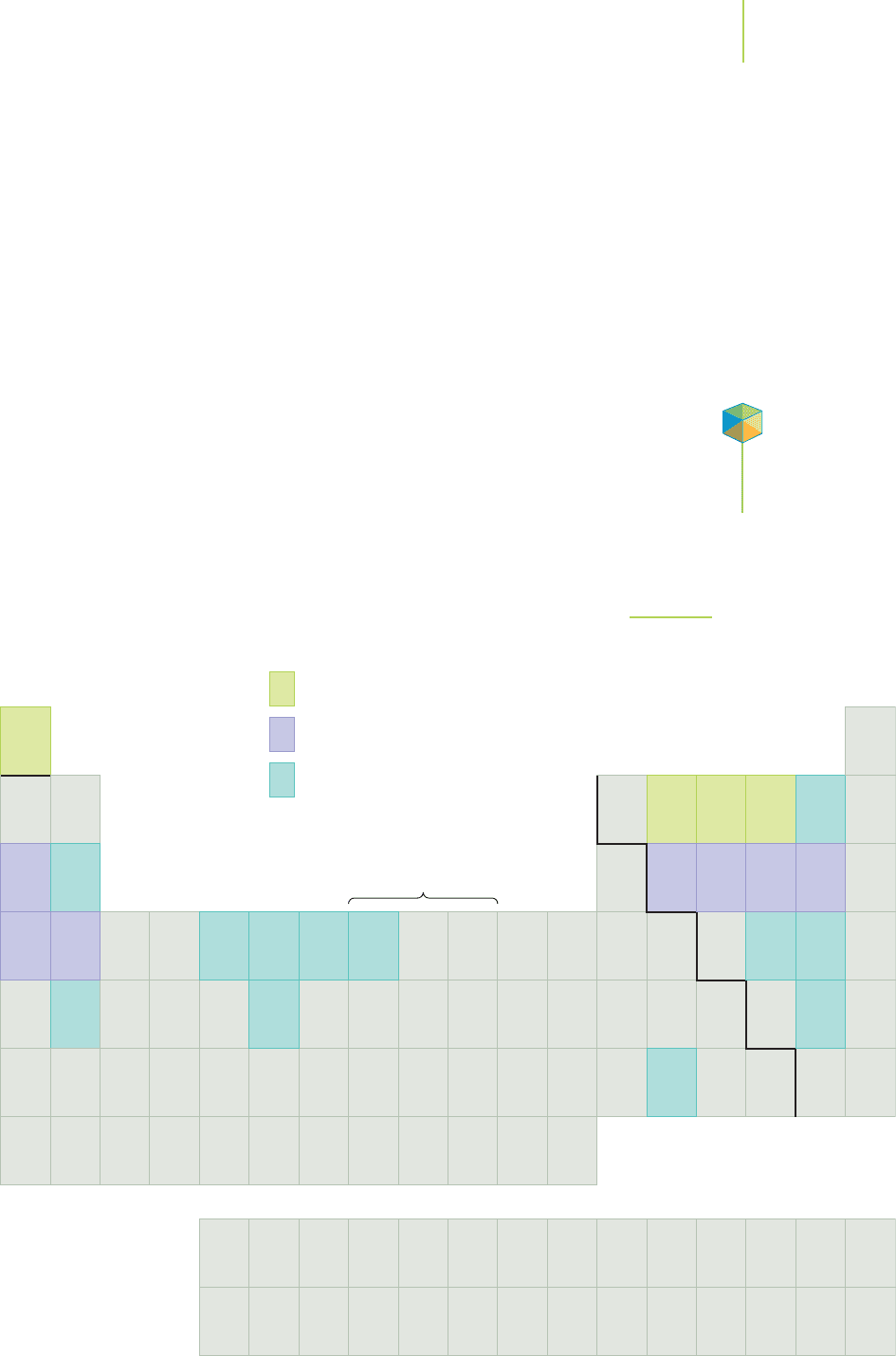
7.3 The Next Level of Structure—Groups in the Periodic Table 277
levels arises because the energies of the electrons involved are very close. It results
in some interesting and important chemical characteristics, such as the ability to
readily form ions with different values of charge.
The special chemistry of the transition elements, such as iron, cobalt, copper,
and zinc, makes many of them vital for life. Biochemists, for example, have
become quite used to the idea that when a difficult feat of chemistry is achieved
within a living thing, it is very often catalyzed by an enzyme (a biological sub-
stance that significantly increases the rate of a process; see Chapter 22) that in-
cludes a transition metal ion at the “active site,” where the chemical reaction
occurs. Transition elements are also widely used as catalysts in the chemical in-
dustry and, in the automotive industry, as rhodium- and platinum-based cat-
alytic converters in cars.
The Elements of Life
Figure 7.6 shows the periodic table with all of the elements that make up the
human body highlighted in color and their relative abundances indicated, and
Table 7.13 lists the relative abundance of these elements within us. Note the loca-
tion of these elements in the periodic table. Can you suggest any generalizations
about their locations? Can you offer any explanations for your suggestions?
The two most clear-cut generalizations are that the human body is largely
constituted of elements only from the upper right-hand portion of the periodic
table and that the noble gases are not part of the chemistry of life.
1
H
1
H
3
Li
11
Na
11
Na
19
K
37
Rb
55
Cs
87
Fr
4
Be
12
Mg
20
Ca
38
Sr
56
Ba
88
Ra
21
Sc
39
Y
57
La*
89
Ac
†
22
Ti
40
Zr
72
Hf
104
Rf
23
V
41
Nb
73
Ta
105
Db
24
Cr
24
Cr
42
Mo
74
W
106
Sg
25
Mn
43
Tc
75
Re
107
Bh
26
Fe
44
Ru
76
Os
108
Hs
27
Co
45
Rh
77
Ir
109
Mt
110
Uun
111
Uuu
28
Ni
46
Pd
78
Pt
29
Cu
47
Ag
79
Au
3
IIIB
4
IVB
5
VB
6
VIB
7
VIIB
11
IB
12
IIB
31
Ga
49
In
81
Tl
5
B
13
Al
32
Ge
50
Sn
82
Pb
6
C
14
Si
33
As
51
Sb
83
Bi
7
N
15
P
34
Se
52
Te
84
Po
8
O
16
S
9
F
17
Cl
35
Br
53
I
85
At
10
Ne
18
Ar
36
Kr
54
Xe
86
Rn
2
He
58
Ce
90
Th
59
Pr
91
Pa
60
Nd
92
U
61
Pm
93
Np
62
Sm
94
Pu
63
Eu
95
Am
64
Gd
96
Cm
65
Tb
97
Bk
66
Dy
98
Cf
67
Ho
99
Es
68
Er
100
Fm
69
Tm
101
Md
70
Yb
102
No
71
Lu
103
Lr
1
IA
2
IIA
13
IIIA
14
IVA
15
VA
16
VIA
17
VIIA
18
VIIIA
Principle components
Major minerals
Trace minerals
*Lanthanides
†
Actinides
112
Uub
30
Zn
48
Cd
80
Hg
1
2
3
4
Period
5
6
7
9
VIIIB
810
Application
C
HEMICAL
ENCOUNTERS:
The Elements
of Life
FIGURE 7.6
The elements that make up you.
