Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

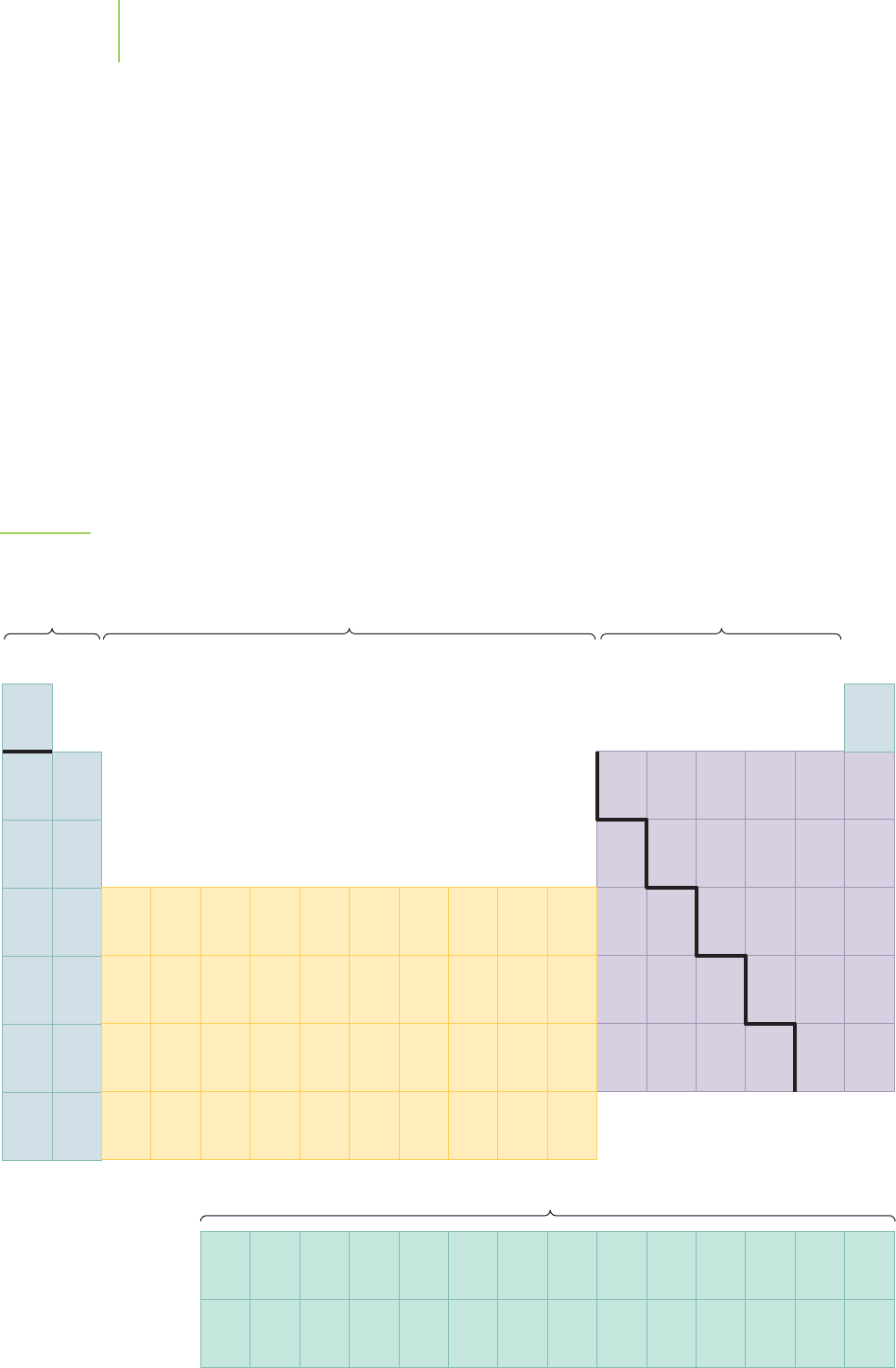
FIGURE 6.46
Periodic table with the electron configurations of all atoms in the gas phase. The “partial” electron configurations
are shown when the cores of the electron configuration can be deduced from the earlier periods.
248 Chapter 6 Quantum Chemistry: The Strange World of Atoms
1
H
1s
1
3
Li
2s
1
11
Na
3s
1
19
K
4s
1
37
Rb
5s
1
55
Cs
6s
1
87
Fr
7s
1
4
Be
2s
2
12
Mg
3s
2
20
Ca
4s
2
38
Sr
5s
2
56
Ba
6s
2
88
Ra
7s
2
21
Sc
4s
2
3d
1
39
Y
5s
2
4d
1
57
La
*
6s
2
5d
1
89
Ac
**
7s
2
6d
1
22
Ti
4s
2
3d
2
40
Zr
5s
2
4d
2
72
Hf
4f
14
6s
2
5d
2
104
Rf
7s
2
6d
2
23
V
4s
2
3d
3
41
Nb
5s
1
4d
4
73
Ta
6s
2
5d
3
105
Db
7s
2
6d
3
24
Cr
4s
1
3d
5
42
Mo
5s
1
4d
5
74
W
6s
2
5d
4
106
Sg
7s
2
6d
4
25
Mn
4s
2
3d
5
43
Tc
5s
1
4d
6
75
Re
6s
2
5d
5
107
Bh
7s
2
6d
5
26
Fe
4s
2
3d
6
44
Ru
5s
1
4d
7
76
Os
6s
2
5d
6
108
Hs
7s
2
6d
6
110
Ds
7s
2
6d
8
111
Rg
7s
1
6d
10
112
Uub
7s
2
6d
10
27
Co
4s
2
3d
7
45
Rh
5s
1
4d
8
77
Ir
6s
2
5d
7
109
Mt
7s
2
6d
7
28
Ni
4s
2
3d
8
46
Pd
4d
10
78
Pt
6s
1
5d
9
29
Cu
4s
1
3d
10
47
Ag
5s
1
4d
10
79
Au
6s
1
5d
10
31
Ga
4s
2
4p
1
49
In
5s
2
5p
1
81
Tl
6s
2
6p
1
5
B
2s
2
2p
1
13
Al
3s
2
3p
1
32
Ge
4s
2
4p
2
50
Sn
5s
2
5p
2
82
Pb
6s
2
6p
2
6
C
2s
2
2p
2
14
Si
3s
2
3p
2
33
As
4s
2
4p
3
51
Sb
5s
2
5p
3
83
Bi
6s
2
6p
3
7
N
2s
2
2p
3
15
P
3s
2
3p
3
34
Se
4s
2
4p
4
52
Te
5s
2
5p
4
84
Po
6s
2
6p
4
8
O
2s
2
2p
4
16
S
3s
2
3p
4
9
F
2s
2
2p
5
17
Cl
3s
2
3p
5
35
Br
4s
2
4p
5
53
I
5s
2
5p
5
85
At
6s
2
6p
5
10
Ne
2s
2
2p
6
18
Ar
3s
2
3p
6
36
Kr
4s
2
4p
6
54
Xe
5s
2
5p
6
86
Rn
6s
2
6p
6
2
He
1s
2
58
Ce
6s
2
4f
1
5d
1
90
Th
7s
2
5f
0
6d
2
59
Pr
6s
2
4f
3
5d
0
91
Pa
7s
2
5f
2
6d
1
60
Nd
6s
2
4f
4
5d
0
92
U
7s
2
5f
3
6d
1
61
Pm
6s
2
4f
5
5d
0
93
Np
7s
2
5f
4
6d
1
62
Sm
6s
2
4f
6
5d
0
94
Pu
7s
2
5f
6
6d
0
63
Eu
6s
2
4f
7
5d
0
95
Am
7s
2
5f
7
6d
0
64
Gd
6s
2
4f
7
5d
1
96
Cm
7s
2
5f
7
6d
1
65
Tb
6s
2
4f
9
5d
0
97
Bk
7s
2
5f
9
6d
0
66
Dy
6s
2
4f
10
5d
0
98
Cf
7s
2
5f
10
6d
0
67
Ho
6s
2
4f
11
5d
0
99
Es
7s
2
5f
11
6d
0
68
Er
6s
2
4f
12
5d
0
100
Fm
7s
2
5f
12
6d
0
69
Tm
6s
2
4f
13
5d
0
101
Md
7s
2
5f
13
6d
0
70
Yb
6s
2
4f
14
5d
0
102
No
7s
2
5f
14
6d
0
71
Lu
6s
2
4f
14
5d
1
103
Lr
7s
2
5f
14
6d
1
d-Transition elements
Period number, highest occupied electron level
Noble
gases
*
Lanthanides
**
Actinides
Representative
elements
IA
ns
1
Group
numbers
IIA
ns
2
IIIA
ns
2
np
1
IVA
ns
2
np
2
VA
ns
2
np
3
VIA
ns
2
np
4
VIIA
ns
2
np
5
VIIIA
ns
2
np
6
Representative elements
f-Transition elements
1
2
3
4
5
6
7
30
Zn
4s
2
3d
10
48
Cd
5s
2
4d
10
80
Hg
6s
2
5d
10
1
13 14 15 16 17
8 9 10 11 1234567
18
2
periodic table have the configuration [core]ns
1
, where [core] is the electron con-
figuration of the noble gas directly preceding the element in the periodic table.
The n is the row number that the element occupies in the periodic table, and n is
also the principal quantum number of the ns electron. It is this similarity in
ground-state electron configuration that gives all of the elements in this column
similar chemical properties, including metallic behavior, malleability, tendency to
form ions with a charge of +1, and high reactivity with water.
What happened to the 3d electrons? When we were talking about the effect of
adding more than one electron to the orbital energies of the hydrogen atom, we
made the point that interactions among the electrons removed the degeneracy
among the electrons in the n-shell orbitals, so that orbitals with increasing value
of l had increasing energy. The 3s is lower in energy than the 3p, which, in turn,
is lower than the 3d. The 3d orbital energy is so destabilized by the electron–
electron interactions that it actually rises in energy to be just a little higher in
energy than the 4s orbital for the atom in the gas phase. To form the lowest energy
electron configuration, the 4s subshell fills before the 3d subshell. The same prob-
lem is noted when we begin filling the f orbital subshell. The ground-state con-
figurations for the elements as gas-phase atoms are given in Figure 6.46.
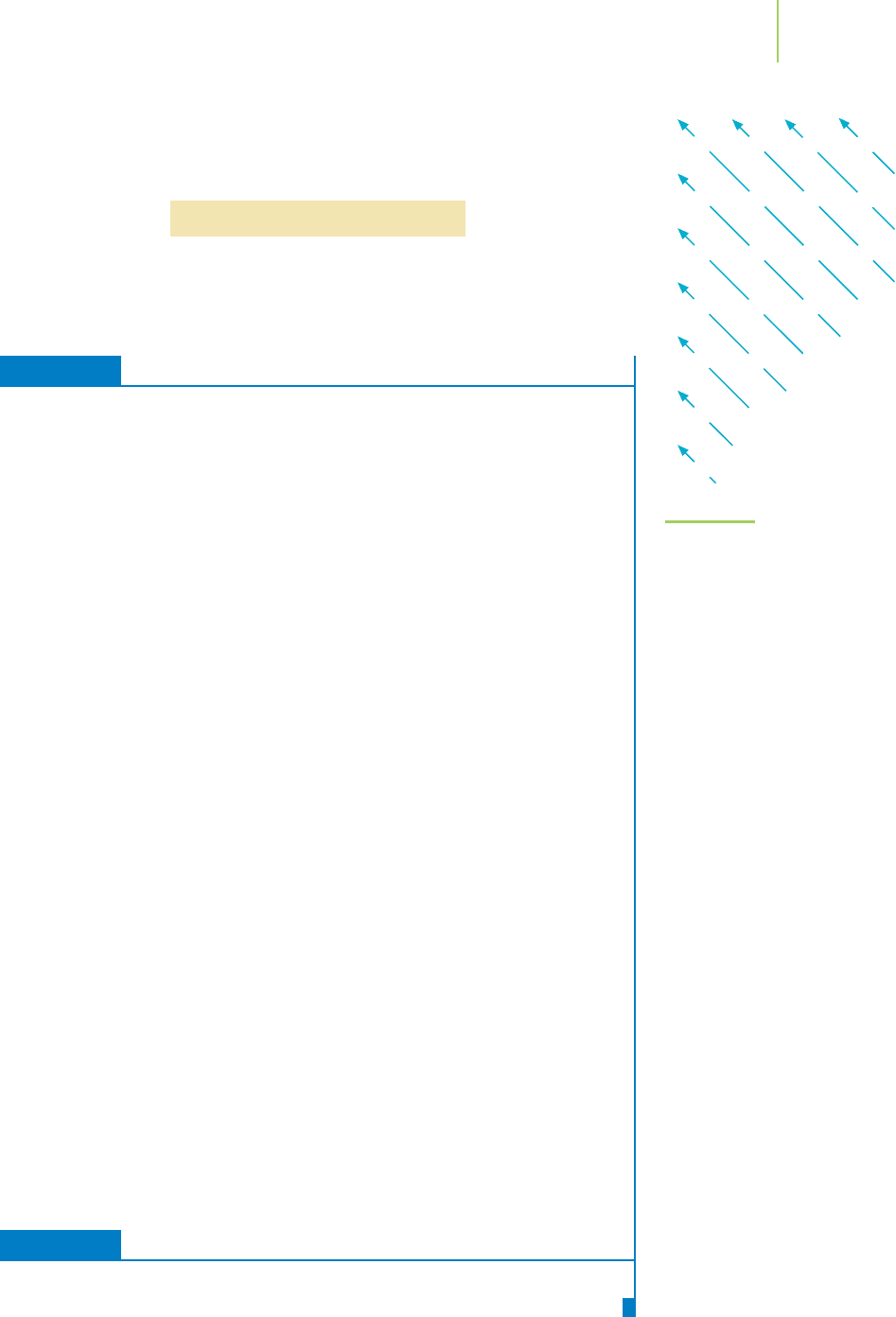
6.13 Electron Configurations and the Aufbau Principle 249
The order of filling is systematic and is illustrated by the diagram shown in Fig-
ure 6.47. For example, we can follow the arrows in the figure and add the
electrons to each subshell to obtain the ground-state electron configuration of
sulfur (16 electrons):
S: 1s
2
2s
2
2p
6
3s
2
3p
4
= [Ne]3s
2
3p
4
Using this information, we can obtain the electron configuration of iodine:
I: 1s
2
2s
2
2p
6
3s
2
3p
6
4s
2
3d
10
4p
6
5s
2
4d
10
5p
5
= [Kr]5s
2
4d
10
5p
5
EXERCISE 6.8 Practice with Electron Configurations
Write the ground-state electron configuration for vanadium and for tellurium.
First Thoughts
The structure of the periodic table gives us guidance for writing the ground-state
electron configuration of an element. The two key questions we ask are “In what
period is our element?” and “Is its highest partially filled energy orbital an s, p, d,or
f orbital?”
Solution
Vanadium, atomic number 23, is in Period 4 and is the third element in the 3d series
that begins with scandium, as shown in Figure 6.46. It will therefore have an argon
electron core with two 4s and three 3d electrons. Alternatively, you can use the tri-
angle in Figure 6.47 and fill the orbitals to an electron count of 23.
V (23 electrons): [Ar]4s
2
3d
3
= 1s
2
2s
2
2p
6
3s
2
3p
6
4s
2
3d
3
Tellurium, atomic number 52, is in Period 5 and Group VIA, in which p orbitals are
being filled. It will have a krypton electron core, with (reading across Group V in
Figure 6.46) two 5s electrons, ten 4d electrons, and six 5p electrons. Alternatively,
you can use Figure 6.47, as with vanadium, although counting electrons in this way
can get clerically messy.
Te (52 electrons): [Kr]5s
2
4d
10
5p
4
= 1s
2
2s
2
2p
6
3s
2
3p
6
4s
2
3d
10
4p
6
5s
2
4d
10
5p
4
Further Insights
Write the ground-state electron configuration of chromium.You will get [Ar]4s
2
3d
4
.
It is often said that one of the s electrons will move to the d orbital to give an ener-
getically more favorable (half–filled) 3d
5
configuration (that is, [Ar]4s
1
3d
5
). This re-
organization is noted when we’re talking about chromium atoms in the gas phase.
Molybdenum atoms ([Kr]5s
1
4d
5
) and copper atoms ([Ar]4s
1
3d
10
) are other exam-
ples where the reorganized electron configuration is observed in the gas phase.
It should be noted that these cases are specific to the gas-phase electron config-
uration, where interactions with other atoms cannot stabilize the predicted electron
configurations. Because these elements are not found in nature or generally used as
individual gas-phase atoms, we can use the predicted electron configurations in our
normal work.
PRACTICE 6.8
Write the ground-state electron configuration for gallium and strontium.
See Problems 99–108 and 118.
7s 7p
6s 6p
7d
6d
7f
5s 5p 5d 5f
4s 4p 4d 4f
3s 3p 3d
2s 2p
1s
6f
FIGURE 6.47
The order in which atomic orbitals are
filled. The order is determined by writing
the orbitals found in each energy level
in rows and then drawing diagonal lines
up and to the left. The order is 1s,2s,
2p,3s,3p,4s,3d,4p,5s,4d,5p,6s,4f,
5d,6p,....
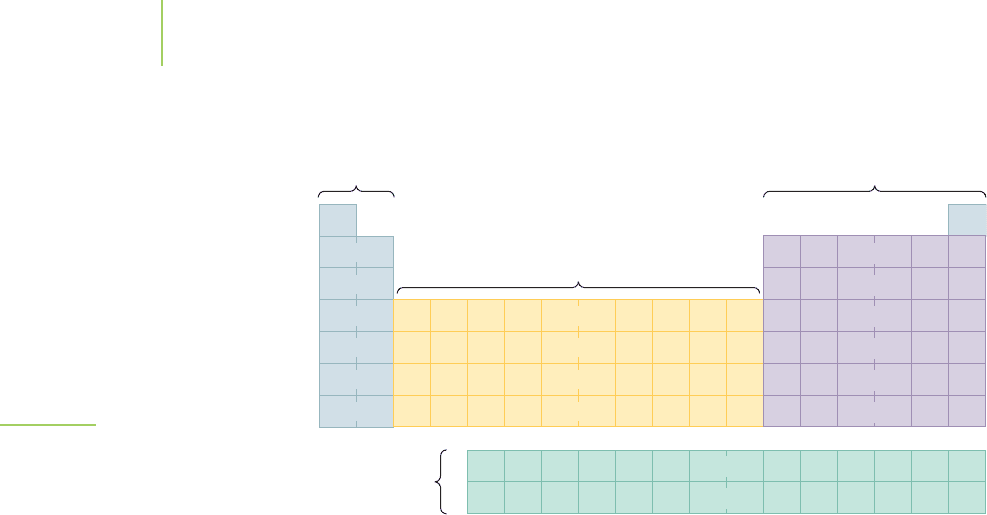
250 Chapter 6 Quantum Chemistry: The Strange World of Atoms
A Summary of the Ground-State Electron Configuration–Based Structure
of the Periodic Table
The periodic table can be divided into four regions based on the filling of orbitals
of the elements; see Figure 6.48.
■
Region 1: This region includes the alkali and alkaline earth metals, in which the
s orbitals are being filled. These Group IA and IIA elements, such as potassium
and calcium, are part of the main-group elements.
■
Region 2: This region includes the remainder of the main-group elements,
Groups IIIA through VIIIA, such as carbon, nitrogen, and chlorine, in which
the p orbitals are being filled.
■
Region 3: This region includes the transition elements, in which the d orbitals
are being filled. These include metals such as chromium, silver, and mercury.
■
Region 4: This includes the inner-transition elements, such as uranium and
plutonium, in which the f orbitals are being filled.
The structure of the periodic table is a very satisfying one for chemists be-
cause it gives rhyme and reason to the physical properties and chemical reactivity
of the elements. Although the periodic table was an outgrowth of Mendeleev’s
periodic law formulated in 1869, this early grouping of elements with similar
properties was done empirically, just by seeing how the material behaved. The
odd shape of the periodic table, however, simply begs for an explanation, and
quantum mechanics has given it! By solving the Schrödinger equation, we have
generated wave functions with unique sets of quantum numbers. By generating
the wave functions and quantum numbers, we have created a complete set of
electron orbitals, and by filling these orbitals according to the Aufbau principle,
we have generated the complete periodic table—its form, the properties of its
elements, everything!
*
**
*
**
2s
1s 1s
3s
4s
5s
6s
7s
2p
3p
4p
5p
6p
7p
3d
4d
5d
4f
5f
6d
Main-group
elements
s-block
Main-group
elements
p-block
Transition elements
d-block
Inner transition
elements
f-block
FIGURE 6.48
The periodic table can be divided into
regions (known as blocks) that illustrate
the type of atomic orbital that is being
filled.
Key Words 251
The Bottom Line
■
We can interconvert among the wavelength, fre-
quency, and energy of electromagnetic radiation.
(Section 6.2)
■
Atoms and molecules absorb and emit electromag-
netic radiation to gain and lose energy, but only cer-
tain wavelengths of radiation can be absorbed and
emitted. (Section 6.3)
■
The wavelengths of electromagnetic radiation ab-
sorbed and emitted by an atom are characteristic of
that atom and can be used to probe the atomic
structure. (Section 6.3)
■
One of the first models of atomic structure, the Bohr
model, quantized the energies and spatial locations
of the electrons to explain the hydrogen emission
spectrum. Although the model was not correct in
other details, quantum energy and orbit locations
were breakthrough concepts and are key concepts in
the modern picture of the atom. (Section 6.4)
■
Electronic transitions between the quantized energy
levels are responsible for atomic absorption and emis-
sion spectra. The energies are given by
E = E
f
− E
i
,
where the subscripts i and f stand for initial and final
states. (Section 6.4)
■
The energy of electromagnetic radiation absorbed or
emitted in an electronic transition must exactly match
the energy of the transition. (Section 6.4)
■
At the atomic scale, all things show both wave and
particle behavior. This concept is known as wave–
particle duality. (Section 6.5)
■
The wave and particle natures of quantum objects,
such as electrons and photons, are linked in the de
Broglie equation: λ = h/p. (Section 6.6)
■
The Heisenberg uncertainty principle,
x
p ≥ h/4π,
places limits on how precisely we can simultaneously
measure position and momentum. (Sections 6.7
and 6.8)
■
All quantum systems, like the hydrogen atom, can be
completely described by the Schrödinger equation:
ˆ
H
n
= E
n
n
. (Section 6.9)
■
The probability distribution function
n
∗
n
tells us
where to find electrons in the atom, and the shape
described by this function is called the atomic or-
bital. (Section 6.9)
■
The orbitals are associated with the probability of
finding the electron within a certain region of space.
(Section 6.9)
■
Each electron wave function has a set of four quan-
tum numbers associated with it. They are n, the
principal quantum number; l, the angular momen-
tum quantum number; m
l
, the magnetic quantum
number; and m
s
, the electron spin quantum number.
(Section 6.10)
■
The principal quantum number n indicates the elec-
tron shell that an electron is in, and n, l, and m
l
de-
termine the shape and orientation of the orbital
in three-dimensional space. (Section 6.10)
■
Only two possible electron spin states exist:
m
s
=+
1
/
2
and m
s
=−
1
/
2
. (Section 6.10)
■
The Pauli exclusion principle states that in a given
atom, no two electrons can have the same set of
the four quantum numbers − n, l, m
l
, and m
s
.
(Section 6.11)
■
We can envisage multielectron atoms being assem-
bled by placing electrons into the orbitals one by
one, starting with the lowest energy level, according
to the Aufbau principle. The complete listing of oc-
cupied orbitals is called the ground-state electron
configuration. (Section 6.13)
■
Hund’s rule for ground-state electron configurations
states that when orbitals of equal energy are available,
the lowest energy configuration for an atom is the
one with the maximum number of unpaired elec-
trons with parallel spins. (Section 6.13)
Key Words
absorption spectroscopy The measurement of how atoms
and molecules absorb electromagnetic radiation as a
function of the wavelength or frequency of the radia-
tion. (p. 216)
amplitude The distance between the highest point and
the midpoint (zero) of a wave. (p. 212)
angstrom A unit of distance equal to 10
−10
m. It is sym-
bolized by Å. (p. 211)
angular As a function of the angles that describe the
orientation of the radius in spherical polar coordi-
nates. (p. 234)
angular momentum quantum number The quantum
number, l, that describes the shape of the orbital;
l can be any whole-number value from zero to n −1.
(p. 235)

252 Chapter 6 Quantum Chemistry: The Strange World of Atoms
Aufbau principle As protons are added to the nucleus, in-
creasing the atomic number of the atom, electrons
are added successively to the next highest energy or-
bital. The method of filling hydrogen atomic orbitals
to get the ground-state electron configuration of a
multielectron atom by adding the electrons one at a
time until all are accommodated; from the German
phase for “building up.” (p. 245)
classical mechanics The physical description of macro-
scopic behavior based on Newton’s equations of
motion. (p. 225)
core electrons Electrons in the configuration of an atom
that are not in the highest principal energy shell.
(p. 247)
d orbital An orbital with quantum number l = 2.
(p. 240)
degenerate orbitals Orbitals that have equal energies.
(p. 236)
electromagnetic spectrum The entire range of radiation
as a function of wavelength, frequency, or energy.
(p. 211)
electron configuration The complete list of filled orbitals
in an atom. (p. 245)
electron shielding The ability of electrons in lower
energy orbitals to decrease the nuclear charge felt by
electrons in higher energy orbitals. (p. 244)
electron spin quantum number The quantum number, m
s
,
that describes the spin of an electron. Also known as
the spin angular momentum quantum number.
(p. 236)
electron subshell The energy level occupied by electrons
that share the same values for both n and l.(p. 235)
electronic transition A change in atomic or molecular
energy level made by an electron bound in an atom
or molecule. (p. 221)
emission spectroscopy The measurement of how atoms
and molecules give off electromagnetic radiation as a
function of the wavelength or frequency of the radia-
tion. (p. 216)
emission spectrum A plot of the intensity of radiation as
a function of wavelength or frequency in an emission
experiment. (p. 217)
empirically derived Derived from experiments and ob-
servations rather than from theory. (p. 219)
energy levels The allowed orbits that electrons may
occupy in an atom. (p. 221)
excited state Any higher energy state than the ground
state characterized by the existence of an electron in
an orbital that violates Hund’s Rule and/or the
Aufbau principle. (p. 221)
f orbital An orbital with quantum number l = 3.
(p. 241)
frequency A descriptor of electromagnetic radiation.
Defined as the number of waves that pass a given
point per second, in units of 1/s (s
−1
). (p. 211)
ground state The lowest energy state of an atom.
(p. 221)
Hamiltonian operator A mathematical function that is
used in the Schrödinger equation. (p. 232)
Heisenberg uncertainty principle There is an ultimate un-
certainty in the position and the momentum of a
particle. Reducing the uncertainty in one increases
the uncertainty in the other. (p. 230)
Hund’s rule When orbitals of equal energy are available,
the lowest energy configuration for an atom has the
maximum number of unpaired electrons with paral-
lel spins. (p. 247)
laser An acronym for “light amplification by stimulated
emission of radiation.” (p. 224)
magnetic quantum number The quantum number, m
l
,
that describes the orientation of the orbital; m
l
can be
any whole-number value from −l to +l. Also known
as the orbital angular momentum quantum number.
(p. 235)
molecular orbitals Electron orbitals that are appropriate
for describing bonding between atoms in a molecule.
(p. 244)
node A point in the wave function where the amplitude
is always zero. (p. 233)
orbital The volume of space to which the electron is re-
stricted when it is in a bound atomic or molecular
energy level. (p. 233)
p orbital An orbital with quantum number l = 1.
(p. 239)
Pauli exclusion principle In a given atom, no two elec-
trons can have the same set of the four quantum
numbers − n, l, m
l
, and m
s
.(p. 242)
photon A single unit of electromagnetic radiation.
(p. 210)
Planck’s constant A fundamental constant equal to
6.62608 × 10
−34
J
.
s. (p. 215)
principal quantum number The quantum number, n, that
describes the energy level of the orbital; n can be any
whole-number value from 1 to infinity. (p. 234)
principal shell The energy level that is occupied by elec-
trons with the same value for the principal quantum
number (n). (p. 234)
quantum A single unit of matter or energy. (p. 210)
quantum chemistry The study of chemistry on the atomic
and molecular scale. (p. 210)
quantum mechanics The physical laws governing energy
and matter at the atomic scale. (p. 210)
quantum number A number used to arrive at the solu-
tion of an acceptable wave function that describes the
properties of a specific orbital. (p. 218)
radial Along the radius in spherical polar coordinates.
(p. 234)
rest mass The mass a particle has when it is stationary.
(p. 231)
s
orbital An orbital with quantum number l =0.
(p. 238)
Schrödinger equation The mathematical expression that
relates the wave function to the energy in a quantum
system. (p. 232)
Focus Your Learning 253
Focus Your Learning
The answers to the odd-numbered problems and some selected
problems appear at the back of the book, as represented by the
blue numbering.
Section 6.1 Introducing Quantum Chemistry
Skill Review
1. At the submicroscopic level we typically discuss the probabil-
ity of events and objects rather than their location and
individual speeds. Describe one event or object that is best
explained using probability. Is this event considered “macro”
or “micro”?
2. Use the analogy of flipping a coin to explain the term
probability.
Section 6.2 Electromagnetic Radiation
Skill Review
3. As you walk through the produce section of a supermarket,
you will see various colors of vegetables. Say the grocer
decided to organize the vegetables in order of increasing fre-
quencies of their reflected light—a novel marketing concept.
Indicate in what sequence these vegetables would appear: a
red tomato, a green zucchini squash, a purple eggplant, and
a yellow spaghetti squash.
4. The three colors of a traffic light are red, yellow, and green.
The green light is placed at the bottom, with yellow in the
center and red on top. Are these colors in order, from bottom
to top, by frequency or by wavelength? Justify your answer.
5. a. What is the frequency of an X ray with a wavelength of
1.5 × 10
−2
nm?
b. What is the energy, in joules, associated with a photon of
this frequency?
c. What would be the energy of a mole of such photons?
6. a. What is the frequency of visible light with a 400-nm
wavelength?
b. What is the energy, in joules, associated with a photon of
this frequency?
c. What would be the energy associated with a mole of these
photons?
7. In what region on the electromagnetic radiation spectrum
would a wavelength of 2.5 × 10
4
nm be placed? What would
be the frequency and energy of this radiation?
8. In what region on the electromagnetic radiation spectrum
would a wavelength of 5.8 × 10
2
nm be placed? What would
be the frequency and energy of this radiation?
9. Calculate your height in nanometers, meters, and light-years.
Which do you find the most convenient unit for this
application?
10. Calculate the length of a 12-in ruler in nanometers, meters,
and lightyears. Which do you find the most convenient unit
for this application?
11. Cell phones operate at frequencies of 824 to 894 MHz. What
range of wavelengths is this? To which part of the electro-
magnetic spectrum does this correspond?
12. Helium–neon (HeNe) lasers are both cheap and common.
They are even sold as attachments to novelty key chains. If
the helium–neon mixture lases at 0.632 µm, what is its fre-
quency? To which part of the electromagnetic spectrum does
this correspond?
13. Radio stations broadcast with frequencies given in mega-
hertz, where 1 MHz = 10
6
s
−1
. This means that when a sta-
tion advertises itself as Radio WXYZ located at 98.3 on your
dial, it is broadcasting at 98.3 MHz. What wavelength does
Radio WXYZ use in its broadcast?
14. A wireless Internet connection broadcasts its signal in the
1200-MHz range. To what wavelength does this correspond?
15. Chlorophyll, the green pigment found in plants, absorbs visible
radiation best in the red region (at about 675 nm) and in the
blue-violet region (at about 440 nm). What are the energies of
the photons collected by plants at these two wavelengths?
16. What are the energies of photons emitted from a 100-MHz
magnetic resonance imager?
17. How much energy resides in 1 mol of photons whose wave-
length is 440 nm? . . . 675 nm?
18. How much energy resides in 2 mol of photons with a fre-
quency of 1.5 × 10
14
Hz? . . . in 0.75 mol?
spectroscopy
The measurement of how atoms and mol-
ecules interact with electromagnetic radiation as a
function of the wavelength or frequency of the radia-
tion. (p. 216)
standing wave The constructive interference of two or
more waves that results in the presence of nodes in
fixed locations. (p. 226)
valence electrons The electrons in an atom, ion, or mol-
ecule that are in the highest principal energy shell.
(p. 247)
wave function The mathematical equation describing a
system, such as an electron, atom, or molecule, that
contains all physical information that can be ob-
tained for the system by quantum mechanics.
(p. 232)
wave–particle duality The quantum mechanical theorem
that states that all things have both wave and particle
natures simultaneously and that both of these natures
can be observed on the atomic scale. (p. 227)
wavelength A descriptor of electromagnetic radiation.
Defined as the distance from the top of one crest to
the top of the next crest of the electromagnetic wave.
(p. 211)
254 Chapter 6 Quantum Chemistry: The Strange World of Atoms
Chemical Applications and Practices
19. a. The process of photosynthesis is quite complex. However,
one of the main considerations is that plant pigments
absorb visible light to power reactions that convert carbon
dioxide and water into food and produce oxygen. If a plant
absorbs blue light that has a wavelength of 565 nm, what is
the energy per photon that is being absorbed?
b. A typical ratio between photons absorbed and oxygen
molecules produced is 8:1. How much energy is required
to produce one molecule of oxygen in this manner?
20. Ozone (O
3
) is important in our upper atmosphere because it
aids in filtering out harmful ultraviolet rays. Ultraviolet rays
may be classified as either UV-A, UV-B, or UV-C. The UV-B
rays cause the most problems for earth-based organisms. For
example, higher incidences of “jumping genes” that cause
mutations may be related to exposure to UV-B.
a. What is the energy in 1 mol of UV-B photons that have a
wavelength of 312 nm?
b. What is the energy of 1 mol of photons with a wavelength
of 600 nm? To what type of radiation does this correspond?
21. UV-B radiation is responsible for “sunburn” in humans. A
helpful advancement in technology is the personal UV detec-
tor. This small device uses a photoelectric response. An ex-
ample is a gallium-based device to convert absorbance into
an electrical signal. If the device gave a maximum reading at
290 nm, what energy is being absorbed? To what frequency
does this correspond?
22. a. Some snakes have the ability to detect infrared radiation
(IR). Are they detecting energy that is higher or lower in
energy than human eyes can see?
b. What is the source of typical IR wavelengths?
c. A television remote control may use IR with a frequency
of 1 × 10
13
cycles per second. To what energy does this
correspond?
23. One way to gain information about the origin and functions
of stars within the universe is to study the origin and distrib-
ution of the hundreds of gamma ray sources in the sky. If one
such source were producing high-energy gamma rays of
1.6 × 10
−8
J, what wavelength would astronomers have
detected? What is the frequency of this radiation?
24. If an astronomer recorded energy from a distant star at 3.6 ×
10
−10
J, what wavelength would he have detected? What is the
frequency of this radiation?
Section 6.3 Atomic Emission and Absorption
Spectroscopy, Chemical Analysis and the Quantum
Number
Skill Review
25. When bombarded with high-energy electrons, copper metal
gives off radiation with a wavelength of 1.54 Å. What is the
frequency of this radiation? To which range of the electro-
magnetic spectrum does this correspond?
26. Sodium arc lamps, which are used as automobile headlights
and street lights, are colored by the sodium doublet: electro-
magnetic radiation produced by excited sodium atoms found
at 5895 Å and 5904 Å. What color are these lights?
27. Much of the radiation striking the earth from the sun has a
wavelength of approximately 500 nm. Express this wave-
length in meters, angstroms, centimeters, and inches.
28. Express 280-nm ultraviolet radiation in meters, angstroms,
centimeters, and inches.
29. According to the Balmer equation, the wavelength of emitted
light from hydrogen can be calculated from the whole-
number values of n. The difference between n = 5 and n = 4
is only 1. The difference between n = 2 and n = 1 is also
only 1. Why don’t the two conditions produce the same
wavelength of emitted light in hydrogen?
30. The Balmer equation can be used to calculate the wavelength
of light emitted from excited hydrogen. To what initial value
of n in hydrogen would an emitted wavelength of 5547 Å cor-
respond? Explain why this value of n is never noted for
hydrogen.
31. The first two wavelengths of the Balmer series of the hydro-
gen emission spectrum are 6562.1 Å, 4860.8 Å. What are the
next three values in this series? What are the frequencies and
energies of the emission lines?
32. What is the highest frequency of the Balmer series of the
hydrogen emission spectrum? What is the lowest frequency
of this series?
Chemical Applications and Practices
33. The presence of cadmium in drinking water is undesirable
because exposure to large amounts has been associated with
weakening of bones and joints. The wavelength of electromag-
netic radiation strongly absorbed by cadmium is 214.439 nm.
a. What is the frequency of that light?
b. In what range of the electromagnetic spectrum would you
classify this frequency?
c. Which other element has an absorbance wavelength
closest to (and therefore possibly difficult to distinguish
from) cadmium? Consult Table 6.3 for additional
information.
34. An adaptation of the Balmer equation makes it possible to
calculate other emitted wavelengths from excited hydrogen.
For example, if the final value for n is 3, then emitted light is
in the infrared area of the spectrum. These line spectra are
known as the Paschen series. Calculate the wavelength emit-
ted when an electron in hydrogen drops from the fourth
Bohr level to the third.
Section 6.4 The Bohr Model of Atomic Structure
Skill Review
35. Calculate the energy of an electron in each of these Bohr
energy levels:
a. n = 1b.n = 3c.n = 5d.n = 7
36. Calculate the energy of an electron in each of these Bohr
energy levels:
a. n = 2b.n = 4c.n = 6d.n = 8
37. Calculate the energy a photon released from a hydrogen atom
in each of these transitions:
a. n = 4 to n = 1c.n = 5 to n = 4
b. n = 3 to n = 1d.n = 7 to n = 2
38. Calculate the energy a photon released from a hydrogen atom
in each of these transitions:
a. n = 2 to n = 1c.n = 4 to n = 3
b. n = 4 to n = 2d.n = 8 to n = 2
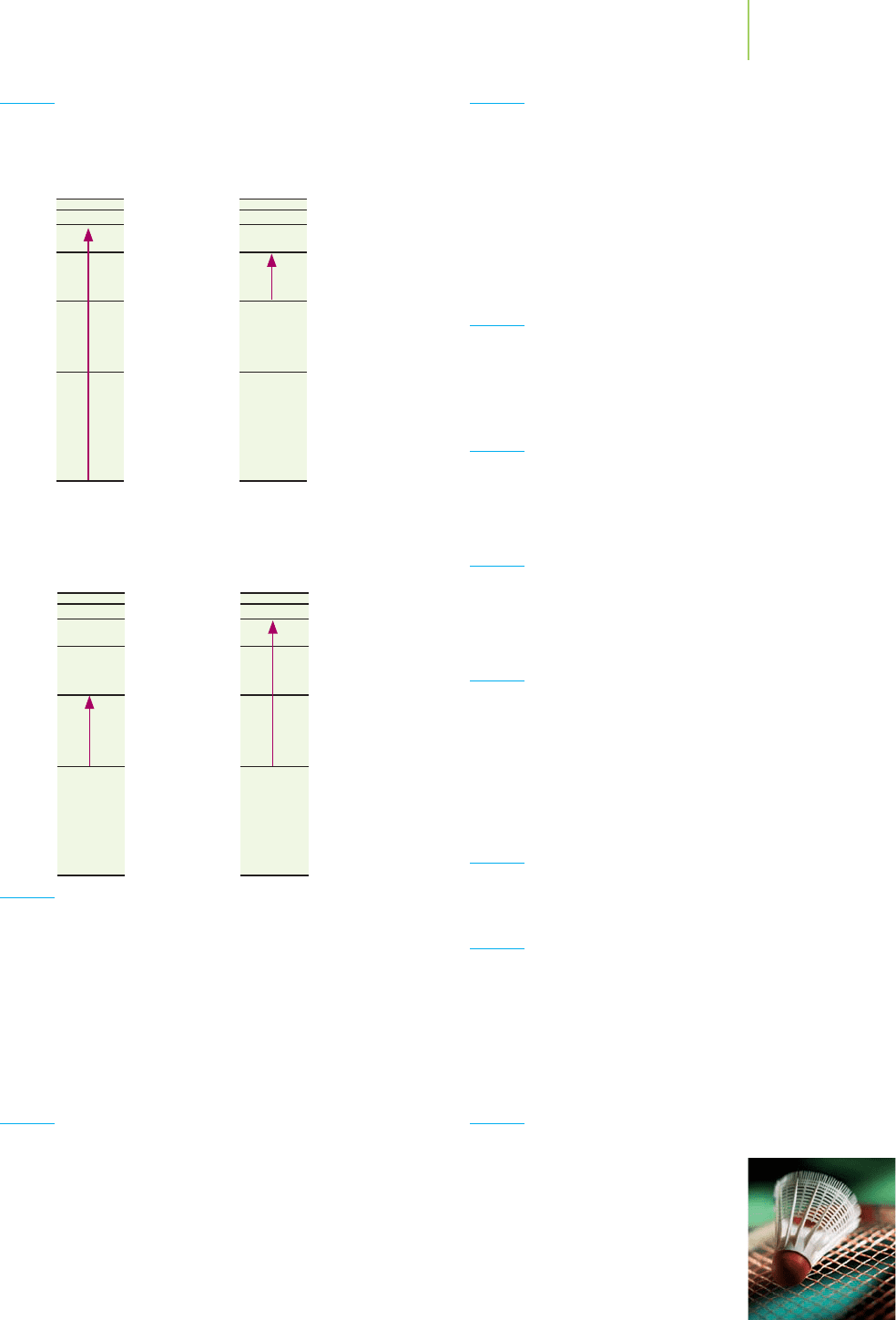
n = 6
n = 5
n = ∞
n = 4
n = 3
n = 2
n = 1
n = 6
n = 5
n = ∞
n = 4
n = 3
n = 2
n = 1
n = 6
n = 5
n = ∞
n = 4
n = 3
n = 2
n = 1
n = 6
n = 5
n = ∞
n = 4
n = 3
n = 2
n = 1
Focus Your Learning
255
39. Calculate the wavelength of a photon that would cause these
transitions:
a. n = 1 to n = 2c.n = 2 to n = 4
b. n = 3 to n = 5d.n = 1 to n = 6
e. f.
40. Calculate the frequency of a photon that would cause these
transitions:
a. n = 8 to n = 10 c. n = 4 to n = 8
b. n = 3 to n = 6d.n = 4 to n = 5
e. f.
41. What is the shortest wavelength that can be emitted by the
hydrogen atom in the Brackett spectral series? What is the
longest wavelength in this series? Consult Table 6.2 for assis-
tance with this problem.
42. According to the Bohr model of the hydrogen atom, what is
the closest an electron can get to the nucleus? What is the far-
thest it can get from the nucleus? (If the latter answer seems
absurd, it might amuse you to know that it is the same value
predicted by present-day quantum chemistry.)
Chemical Applications and Practices
43. The emission spectrum being given out by a mixture of gases,
possibly including hydrogen, includes an emission line with
an energy of 4.84 × 10
−19
J per photon. Is this emission pos-
sible for hydrogen? (To make this problem more manageable,
consider transitions within the first eight energy levels only.)
44. The emission spectrum for a specific sample of gas includes
an emission line with an energy of 7.38 ×10
−19
J per photon.
Is this emission possible for hydrogen? (To make this prob-
lem more manageable, consider transitions within the first
eight energy levels only.)
45. To ionize hydrogen is to remove its single electron. We con-
sider a free electron to have, with respect to an electron in
hydrogen, zero energy. In other words, the initial state is n = 1
and the final state is n = ∞. Calculate the energy needed to
ionize an electron from the ground state.
46. Calculate the energy released when an electron is added to a
hydrogen nucleus. Assume the transition is n = ∞ to n = 1.
Section 6.5 Wave–Particle Duality
Skill Review
47. In a classical sense, compare matter and waves in terms of
permissible energy values, spatial positions, and momentum.
48. How did the quantum view change the classical distinction
between matter and waves? What is the quantum view called?
Chemical Applications and Practices
49. What is the momentum of an electron that is moving at 68%
of the speed of light? (Use 9.1 × 10
−31
kg as the mass of the
electron.)
50. What is the speed of an electron that has a momentum of
9.7 × 10
−23
kg ·m · s
−1
?
51. A freight train locomotive weighs about 415,000 lb and has a
top speed of about 100.0 mi/h. What is its momentum?
52. The average speed of an electron in the ground state of
the hydrogen atom is 2.19 × 10
6
m/s. What is the average
momentum of an electron in this state?
53. What is the momentum of a photon with a wavelength of
540 nm?
54. What is the momentum of a photon with a frequency of
1.0 × 10
14
Hz?
Section 6.6 Why Treating Things as “Waves”
Allows Us to Quantize Their Behavior
Skill Review
55. List two particle-like properties of electrons.
56. List two wave-like properties of electrons.
Chemical Applications and Practices
57. The average radius in the second Bohr orbit (n =2) is 2.116 ×
10
−10
m. Using the equation 2πr = nλ, calculate the wave-
length for the standing electron wave.
58. The approximate wavelength for an electron in hydrogen’s
first energy level has been calculated to be 3.3 × 10
−10
m.
What is the ratio of this wavelength to the diameter of ten
hydrogen atoms (7.4 × 10
−11
m)? What might be some rea-
sons why the diameter isn’t closer in size to the wavelength?
59. In badminton, the object being struck by the racket is called
a shuttlecock. Although the shut-
tlecock may be made of various
materials, it mass must be close
to 5.00 g. What is the wavelength
of a served shuttlecock that is
moving at 78 mi/h? Without
doing exact calculations, indicate
whether the wavelength of a soft-
ball moving at the same speed
would be larger or smaller.
A shuttlecock.
256 Chapter 6 Quantum Chemistry: The Strange World of Atoms
60. If a proton, mass = 1.67 × 10
−27
kg, were moving as fast as
the electron in the ground state of hydrogen (2.1 × 10
6
m/s),
what would be the wavelength?
Section 6.7 The Heisenberg Uncertainty Principle
Skill Review
61. In the uncertainty relationship, what do the symbols p and x
represent? Explain the importance of noting that ∆p and ∆x
multiplied must be equal to or greater than a constant.
62. Taking a photograph of a moving object—for example, a
person sprinting—will cause some blurring of the actual
person’s position when the photograph is developed. There-
fore, the more blur, the better you represent movement. If the
entire scene were re-shot using a faster shutter speed, what
information would you gain and what would you lose in the
photo?
Chemical Applications and Practices
63. Using the mass of an electron as 9.11 ×10
−31
kg and velocity
as 2.1 ×10
6
m/s, what would you calculate as the uncertainty
in the position,
x
, for the electron if the uncertainty in the
velocity were 5.0%? What would be the answer to this if
the uncertainty in the velocity were 10.0%? How does this
uncertainty compare to the radius of the hydrogen atom
(3.7 × 10
−11
m)?
64. Which of these diagrams of an atom most agrees with the
Heisenberg uncertainty principle? Explain your answer, and
indicate why the other two choices do not conform to the
Heisenberg uncertainty principle.
a. b. c.
Section 6.8 More About the Photon—the de Broglie
and Heisenberg Discussions
Skill Review
65. What is the momentum of an X-ray that has a wavelength of
6.5 × 10
−10
m?
66. How does the momentum of the X-ray in Problem 65 com-
pare to the momentum of a photon of green light that has a
wavelength of 560 nm?
67. What is the momentum of a mole of X-rays with wavelength
6.5 × 10
−10
m?
68. What is the momentum of a mole of photons with wave-
length 560 nm?
Chemical Applications and Practices
69. What is the momentum of a 190-lb chemistry professor
walking through a chemistry lab at 3.0 mi/h?
70. In order for you to see the strolling professor of Problem 69,
light photons would have to bounce off the professor and
enter your eyes. If some of that light had a wavelength of
565 nm, what would be the momentum of the photon?
Section 6.9 The Mathematical Language of Quantum
Chemistry
Skill Review
71. Acceptance of the Schrödinger equation literally knocked the
electrons out of Bohr’s orbit concept. However, the term
orbital was maintained as a connection to the quantization of
energy states proposed in Bohr’s model. Explain the meaning
of an electron orbital.
72. a. What is meant by the term node when it is applied to
waves?
b. What is the relationship among nodes, wavelength, and
energy in the context of standing waves?
Chemical Applications and Practices
73. In both the Bohr model of electronic structure and the
Schrödinger model, electrons appear to change energy levels
by a method that does not show the electron making a grad-
ual transition. Using the wave model, explain why a stable
standing wave is not possible between two adjacent energy
levels. Does this explain why there is no gradual transition
between states?
74. a. What is a photon?
b. In what way does a photon resemble particles, and in what
way does it resemble light?
Section 6.10 Atomic Orbitals
Skill Review
75. The sequence in each line that follows represents values for
the quantum numbers for an electron in a hydrogen atom.
Select any sequence(s) that are not possible and explain the
problem(s).
nl m
l
a. 3 −10
b. 2 +2 +1
c. 3 +2 +3
d. 1 +1 +1
e. 4 +3 −2
76. The sequence in each line that follows represents values for
the quantum numbers for an electron in a hydrogen atom.
Select any sequence(s) that are not possible and explain the
problem(s).
nl m
l
a. 3 +10
b. 2 +2 +1
c. 3 0 −3
d. 5 +40
e. 2 +3 −2
Electron
Nucleus
Diffuse cloud of electrons

Focus Your Learning 257
77. When an electron is in the fifth energy level, how many sub-
levels are possible? How many orbitals are possible?
78. When an electron is in the fourth energy level, how many
sublevels are possible? How many orbitals are possible?
79. When l = 3, how many degenerate orbitals are possible?
80. When l = 6, how many degenerate orbitals are possible?
81. How many sets of quantum numbers are possible when
n = 5?
82. How many sets of quantum numbers are possible when
n = 3?
83. We often use the phrase the shape of an orbital. Indicate what
restrictions apply when the phrase is used, and explain why
the phrase is a bit of an abstraction.
84. Name two distinguishing features that the three p orbitals in
the same level have in common. What property allows them
to be identified separately?
85. Define and give an example of both a radial node and a
planar node.
86. In which energy level would the f orbitals make their first
appearance? (Use a quantum mechanical proof for your
answer.)
Chemical Applications and Practices
87. a. The explanation for scanning tunneling microscopic pic-
tures relies on the concept presented in the quantum me-
chanical atomic model. When the tip of the STM probe
nears an atom, what actually touches?
b. What is meant by quantum tunneling?
88. The “photograph” that is produced by a scanning tunneling
microscope is not a photograph in the traditional sense. In
what way does the image differ from a traditional photo-
graph?
Section 6.11 Electron Spin and the Pauli Exclusion
Principle
Skill Review
89. The four quantum numbers representing an electron may be
shown as: (3, 1, 0, ). What is the value of the fourth quan-
tum number? What is the name given to the fourth quantum
number?
90. If the four quantum numbers for an electron were (3, 2, 1,
−
1
/
2
) what would be the four quantum numbers for an elec-
tron in the same orbital as the first electron?
Chemical Applications and Practices
91. The magnetic properties of elements are related to the
number of unpaired electrons in the atoms. Of the following,
which would have unpaired electrons?
CCaONeZn
92. The magnetic properties of elements are related to the
number of unpaired electrons in the atoms. Of the following,
which would have unpaired electrons?
NSNaHeSc
93. If the Pauli exclusion principle were not used, show how the
configuration of the electrons in oxygen would appear to
allow no unpaired electrons.
→
94. Among the elements from 21 to 30, which would have the
highest number of unpaired electrons?
Section 6.12 Orbitals and Energy Levels in Multielectron
Atoms
Skill Review
95. Name three things that will be the same in multielec-
tron atoms’ orbital descriptions, and name one critical
difference.
96. How many radial and how many nonradial nodes, respec-
tively, will be possible for each of these electron orbitals?
What will be the letter designation for each of the repre-
sented orbitals?
a. n = 3; l = 2b.n = 3; l = 0c.n = 4; l = 3
Chemical Applications and Practices
97. Would you expect it to be easier to remove the outermost
electron from He or He
+
? Explain the basis of your answer.
98. Would an electron in the 3p sublevel experience more nu-
clear pull than an electron in the 3d sublevel? (Assume that
the electron is in the same atom and that the atom’s sub-
levels up to 3s are filled.)
Section 6.13
Electron Configurations and the Aufbau Principle
Skill Review
99. a. What is the written notation for the ground state of the
nitrogen atom?
b. Nitrogen commonly forms a −3 ion. What is the electron
configuration of the ion?
100. In the ground state of manganese, there are five electrons
that would occupy the 3d sublevel. Show the way these elec-
trons could be configured to follow Hund’s rule and a way
that would violate Hund’s rule.
101. Report, for each of the following, which element is being
represented.
a. [Ne]3s
2
3p
2
b. [Ne]3s
2
3p
5
c. [Ar]4s
1
d. [Kr]5s
2
102. Report, for each of the following, which element is being
represented.
a. [Ne]3s
2
b. [He]2s
2
2p
5
c. [He]2s
1
d. [Ar]4s
2
103. Write the electron configuration for element 21. Explain
why the correct configuration shows the 4s sublevel filling
with electrons before the 3d sublevel.
104. Write out the ground-state electron configuration for ele-
ment 19.
Chemical Applications and Practices
105. Which, if any, of the following contain unpaired electrons
in their ground state?
KCaFeZnNe
106. Which, if any, of the following contain unpaired electrons?
K
+
Ca
2+
Fe
3+
Zn
2+
Ne
+
107. Evidence indicates that copper has no unpaired electrons
in the 3d sublevel. What would have to be the ground-state
electron configuration of copper to make this possible?
