Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


Balmer’s Analysis and Atomic Structure
Balmer found that all of the wavelengths emitted from the hydrogen atom in the
visible portion of the spectrum could be fitted to an equation that relates the
wavelength (in nanometers) to an integer (n):
1
λ
= 1.0968 ×10
−2
1
2
2
−
1
n
2
nm
−1
The most significant thing about this equation is that it introduces to us a
quantum number, n, that can vary only in whole, positive increments. For example,
the equation predicts an infinite number of wavelengths in the spectrum gener-
ated by n = 3,4,5 ...all the way to infinity. If you don’t quite understand where
the quantum number comes from, you are in good company. Balmer did not un-
derstand it either, but he used the equation, complete with quantum numbers,
because it fits the data from the hydrogen atom. He had no theory or model that
predicted this fit and had no good reason to propose the quantum numbers—
apart from the fact that they worked.
Although in concept an infinite number of wavelengths can be emitted in the
Balmer spectrum, many wavelengths of light cannot be emitted. For example, the
longest wavelength of visible light emitted from the hydrogen atom (656.5 nm) is
found when n =3. The next longest wavelength in the Balmer emission spectrum
(486.1 nm) is at n = 4. No light is emitted with a wavelength between 486.1 nm
and 656.5 nm.
Although Balmer’s series is predominantly in the visible region of the electro-
magnetic spectrum, other hydrogen emission series exist that can all be fitted to
a similar form of an equation. This generalized equation was developed by the
Swedish physicist Johannes Rydberg in 1888 and is known as the Rydberg for-
mula. The main feature of the Balmer series occurs when we set n
f
=2. The other
quantum number is variable, and we’ll call it n
i
.
The Rydberg Formula:
1
λ
= 1.0968 ×10
−2
1
n
2
f
−
1
n
2
i
nm
−1
,
The Balmer Equation:
1
λ
= 1.0968 ×10
−2
1
2
2
−
1
n
2
nm
−1
,
Remember that Balmer didn’t attach a physical meaning to the numbers. He was
just looking at the data from the standpoint of a mathematician.
The physicist Theodore Lyman reported observing a series of wavelengths in
the ultraviolet and X-ray region. Each of those wavelengths could be calculated if
n
f
were held constant at 1 and if n
i
were varied from 2 to 3 to 4 to 5 to ∞. In addi-
tion, there is a series with n
f
=3, with quantum numbers n
i
=4,5,6,...∞, which
begins in the infrared region; another with n
f
= 4 and quantum numbers n
i
= 5,
6,7,...∞; another with n
f
= 5 and n
i
= 6,7,8,...∞; and so on, with n
f
increas-
ing in units of one up to infinity. Table 6.2 lists these first four series for the
hydrogen atom.
218 Chapter 6 Quantum Chemistry: The Strange World of Atoms
Spectral Series in the Hydrogen Atom
Spectral Series n
f
n
i
Wavelength Range
Lyman 1 2,3,4,... X-ray and UV
Balmer 2 3, 4, 5,... Visible
Ritz-Paschen 3 4, 5, 6,... Short-wave infrared
Brackett 4 5,6,7,... Long-wave infrared
TABLE 6.2
EXERCISE 6.3 Using the Balmer Equation
The longest wavelength for the Balmer series is found when n = 3:
1
λ
= 1.0968 ×10
−2
1
2
2
−
1
3
2
nm
−1
= 0.0015233 nm
−1
λ =
1
0.0015233 nm
−1
= 656.47 nm
What is the smallest energy of radiation for this series?
Solution
The smallest energy corresponds to the longest wavelength, because energy and
wavelength are inversely proportional. We just calculated the longest wavelength to
be 656.47 nm, and if we put this into SI units as 6.5647 × 10
−7
m, we can convert it
directly into the energy:
E
smallest
=
hc
λ
=
(6.626 ×10
−34
J·s) ×(3.00 × 10
8
m·s
−1
)
6.5647 ×10
−7
m
= 3.03 × 10
−19
J
PRACTICE 6.3
Calculate the wavelength of a photon corresponding to the collapse of an electron
from n = 4 to n = 3; from n =6 to n = 4; from n =6 to n = 5.
See Problems 25, 26, 30–34, and 111.
The equation describing the wavelengths of light found in the hydrogen emis-
sion spectrum has been
empirically derived, which means that it has been derived
from experiments and observations rather than from theory. In other words,
Balmer, Lyman, and other mathematicians and spectroscopists of the late
nineteenth century performed comprehensive measurements on the hydrogen
emission spectrum and fitted a mathematical expression to the data. Empirical fits
to data generally raise more questions than they answer, because scientists want to
know why the equation works.
Why, for example,is the hydrogen emission spectrum
quantized to give only certain wavelengths?
Figure 6.8 shows the visible region of
the emission spectra of several elements. Note that the wavelengths of light emit-
ted by each metal are specific to that element. For instance, if we focus an instru-
ment at one of those wavelengths and look specifically for that signal, we will
observe it only if that particular element is present in
the sample. In other words, if we tune our instrument
to one of the wavelengths in the copper spectrum, our
instrument will be able to detect if copper is present.
This process is quite useful to the environmental
chemist. By exciting atoms within a small sample of
river water, the chemist can detect the presence of
copper. In addition, the intensity of the light detected
by the instrument can be related to the quantity of
copper in the river water sample. It is currently possi-
ble to measure nearly two dozen elements in water at
the same time at the parts per billion level (ppb, micro-
grams, µg, of element per liter of solution) or parts per
trillion level (ppt, ng/L) by simultaneously focusing on
one discrete wavelength given off by each element.
Table 6.3 gives several of the elements, the emis-
sion wavelengths typically used for detection, and the
minimum concentration of the element that can be
6.3 Atomic Emission and Absorption Spectroscopy, Chemical Analysis and the Quantum Number 219
Application
C
HEMICAL ENCOUNTERS:
Simultaneous
Determination of
Elements in Water
Simultaneous Determination of Elements in Water
Wavelength Detection Limit
Element (nm) (ppb = µg/L)
Al 396.152 1.5
As 188.980 3.5
Ba 455.403 0.04
Ca 315.887 1.5
Cd 214.439 0.3
Cr 267.716 0.5
Cu 324.754 0.3
Mo 202.032 0.8
Pb 220.353 3.0
Zn 213.857 0.3
TABLE 6.3
Source: Fast Analysis of Water Samples Comparing Axially and Radially Viewed
CCD Simultaneous ICP-AES, Varian, Inc., http://www.varianinc.com/image/
vimage/docs/products/spectr/icpoes/atworks/ icpes028.pdf (accessed
February 2006).
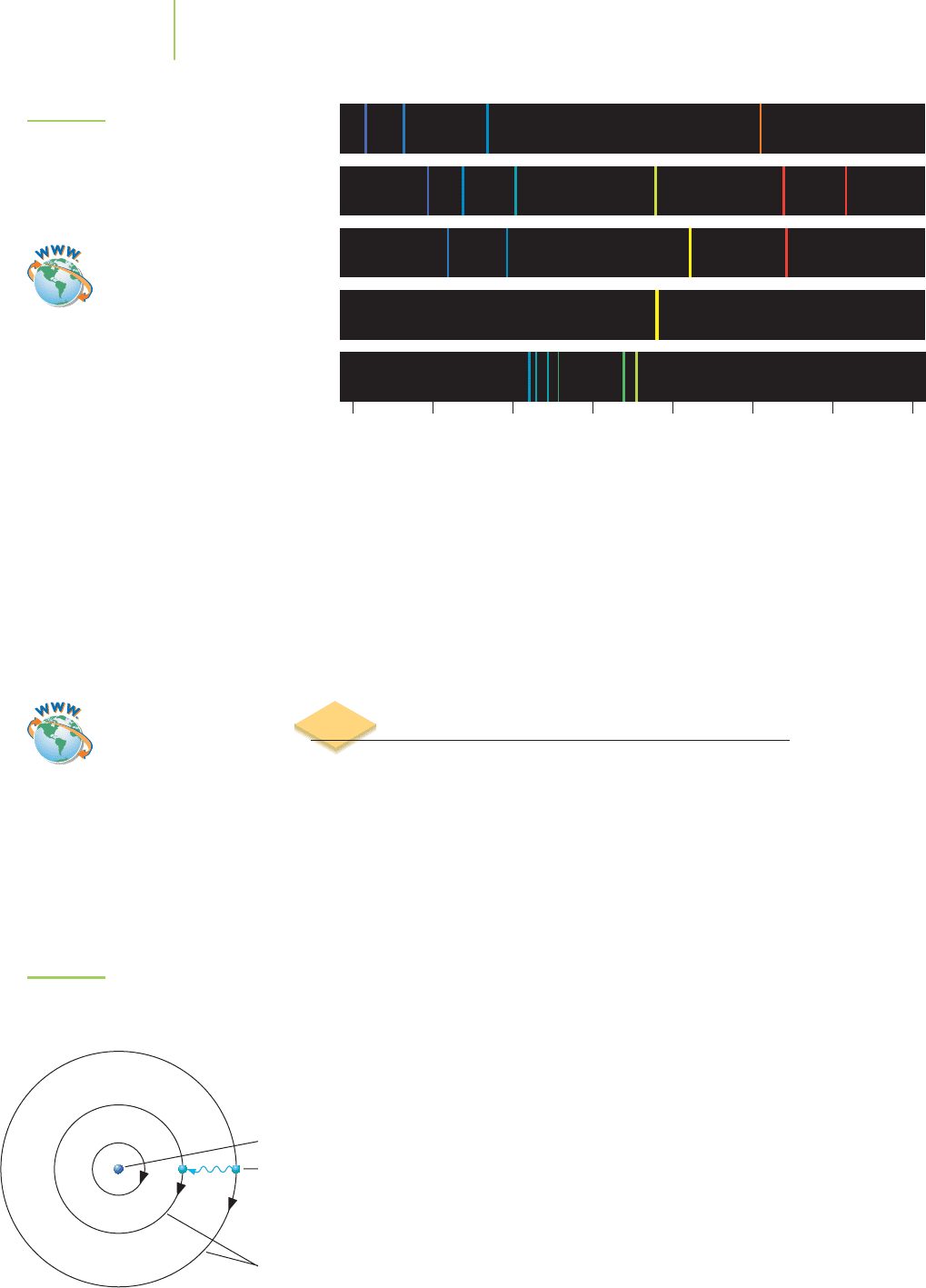
220 Chapter 6 Quantum Chemistry: The Strange World of Atoms
He
Li
Na
Cu
Wavelength (nm)
H
400 450 500 550 600 650 700 750
FIGURE 6.8
The emission spectra of different metals.
Each wavelength emitted is unique to
that element, which makes it possible to
analyze the element’s concentration at
very low concentrations.
reliably detected (“detection limit”). Note that the majority of these wavelengths
are in the ultraviolet region of the electromagnetic spectrum. Atomic emission,
absorption, and related spectroscopy techniques are among the most important
in chemistry for the analysis of elements in all manner of samples from brass to
blood. It is through atomic spectra that we learned core ideas about the funda-
mental structure of the atom.
6.4 The Bohr Model of Atomic Structure
In Chapter 2, we saw how the model of the atom evolved from the indivisible
spheres proposed by Dalton all the way to the “nuclear atom” view that emerged
from Rutherford’s discovery of the nucleus. We also discussed the way in which
scientific models develop and noted that useful information is gleaned even from
models that eventually turn out to be incorrect or only partially correct. Like the
earlier models, the Bohr model we are about to consider provides an incomplete
and oversimplified view of how atoms are really constructed. But it is useful to
examine this model and to appreciate how it solved certain puzzles of atomic
emission and contributed to scientists’ development of the quantum picture of
the atom. The work is so important that it earned Niels Bohr the Nobel Prize in
physics in 1922.
The simplest atom is that of hydrogen with a single electron and a nucleus of
a single proton. According to the Bohr model of the hydrogen atom, the electron
is found some distance from the nucleus, whirling around it in a circular orbit, as
shown in Figure 6.9. The electron can be in any one of an infinite number of pos-
sible orbits around the nucleus, each orbit at a particular distance, or radius, from
the nucleus. Not just any radius will do, however. The orbits are spatially
quantized. Much like the layers of an onion, they are confined to specific
locations, in spheres of ever-increasing radii, r, expressed in nanometers,
according to the equation:
r = 0.052917 n
2
nm
where n, our quantum number for the Bohr model, can be 1, 2, 3, 4,...,∞.
Electrons in orbits close to the nucleus travel much more rapidly than
electrons in the outside orbits. This means that the kinetic energy of an
FIGURE 6.9
Schematic of the Bohr model of
the hydrogen atom.
n = 3
n = 2
Electron
Nucleus
Electron
orbits
n = 1
Video Lesson: CIA
Demonstration: Flame Colors
Video Lesson: The Bohr Model
electron in the orbits close to the nucleus is greater than the kinetic energy of those
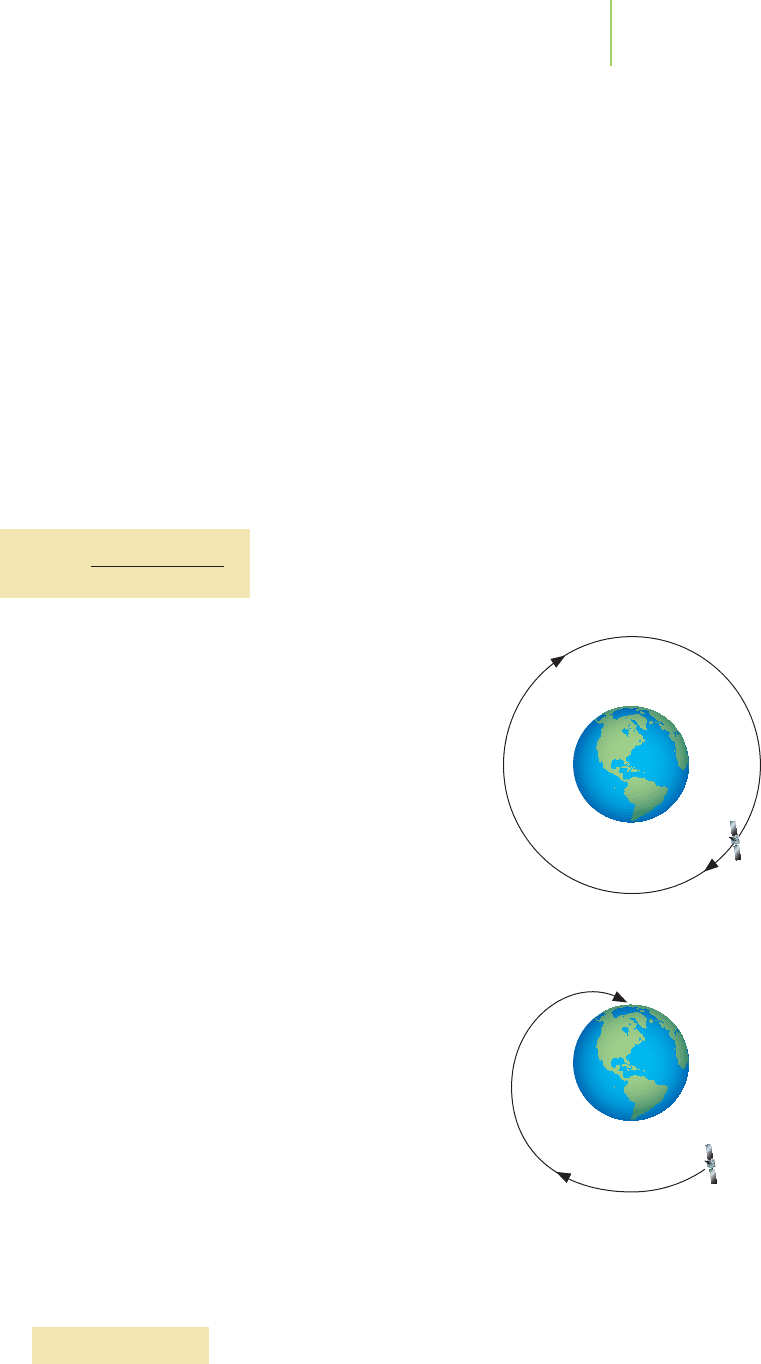
in the outer orbits. This is true for satellites that orbit the earth just as it is for elec-
trons. The space shuttle circles the planet Earth many times during a voyage in a
low Earth orbit of between 185 and 650 km; it must travel at a very high speed,
about 27,900 km/h, in order to maintain its orbit.On the other hand, geosynchro-
nous satellites, such as weather and communications satellites, are 35,800 km
above the Earth’s surface and travel at a speed of only 11,300 km/h. They appear to
remain stationary over a specific place because their movement just matches the
rotation of our planet.
The increase in kinetic energy of the negatively charged electron closest to the
nucleus is offset by a greater decrease in the potential energy due to its position
relative to the positive nucleus. In other words, because opposite charges attract,
the closer the electron and the nucleus are to each other, the lower the potential
energy of the electron. The key outcome is that electrons closer to the nucleus have
lower energy than those farther away.
We call the allowed orbits
energy levels, because the electron in a given orbit
will have a constant total energy according to this equation:
E
n
=−
2.1786 ×10
−18
J
n
2
What is especially interesting about this equation is that the quantum number n
is the same quantum number we discovered in the equation for the radius. Note
that this equation calculates the energy of a given orbit as a negative number. This
is because a completely free electron (corresponding to n = ∞) is assigned an energy
of zero. When electrons become bound within atoms, their energy falls, and be-
cause it is falling from zero, it must become negative. This means that as an elec-
tron falls from higher orbits to lower ones, its energy has increasingly larger
negative values relative to zero, the value for the free electron. The lower values of
the quantum number have more negative energies than the higher values. The
most negative value for energy occurs when n = 1. At this orbit (n = 1), the elec-
tron is as strongly bound to the atom as is possible, and it is in its lowest energy
state in the atom. Unless energy is supplied to the hydrogen atom, its electron will
tend to be in the most strongly bound level, closest to the nucleus. As we noted
above, for the hydrogen atom, this is the smallest quantum number, n = 1. Any
atom that contains all its electrons in their lowest possible energy levels is said to
be in the
ground state, like the ground floor of a building.
Larger quantum numbers, n = 2,3,4,...,∞, represent progressively higher
atomic energy levels, just as the second, third, fourth,...floors of a building rep-
resent progressively higher gravitational energies. When electrons are moved
from the ground state into these higher energy levels, we say that they occupy
excited states. Atoms and molecules in excited states generally tend to relax back
down to their ground states after a short period of time.
Energy must be supplied to the hydrogen atom to promote its electron from
the ground state (n = 1) to any other state (n > 1). Conversely, the atom must
lose energy when the electron falls from any state to a lower energy state. These
electronic transitions, as they are called, can be explained very well by the quan-
tum picture of the Bohr atom. The electron must gain or lose the exact amount
of energy that separates two energy levels (two orbits) in order to jump between
the levels. The change in energy is expressed mathematically as
E = E
f
− E
i
where E
f
is the energy of the final state of the electron, E
i
is the energy of the initial
state of the electron, and
E is the difference in energy between these two levels.
6.4 The Bohr Model of Atomic Structure 221
In 1922 Niels Bohr (1885–1962) was
awarded the Nobel Prize in physics for his
work on the structure of the atom and his
explanation of how atoms emit light.
Speed just right
Speed too slow
The speed of a satellite is related to its
distance from the planet it circles.
Slower-moving objects must have
higher orbits or they will crash back
to the surface.

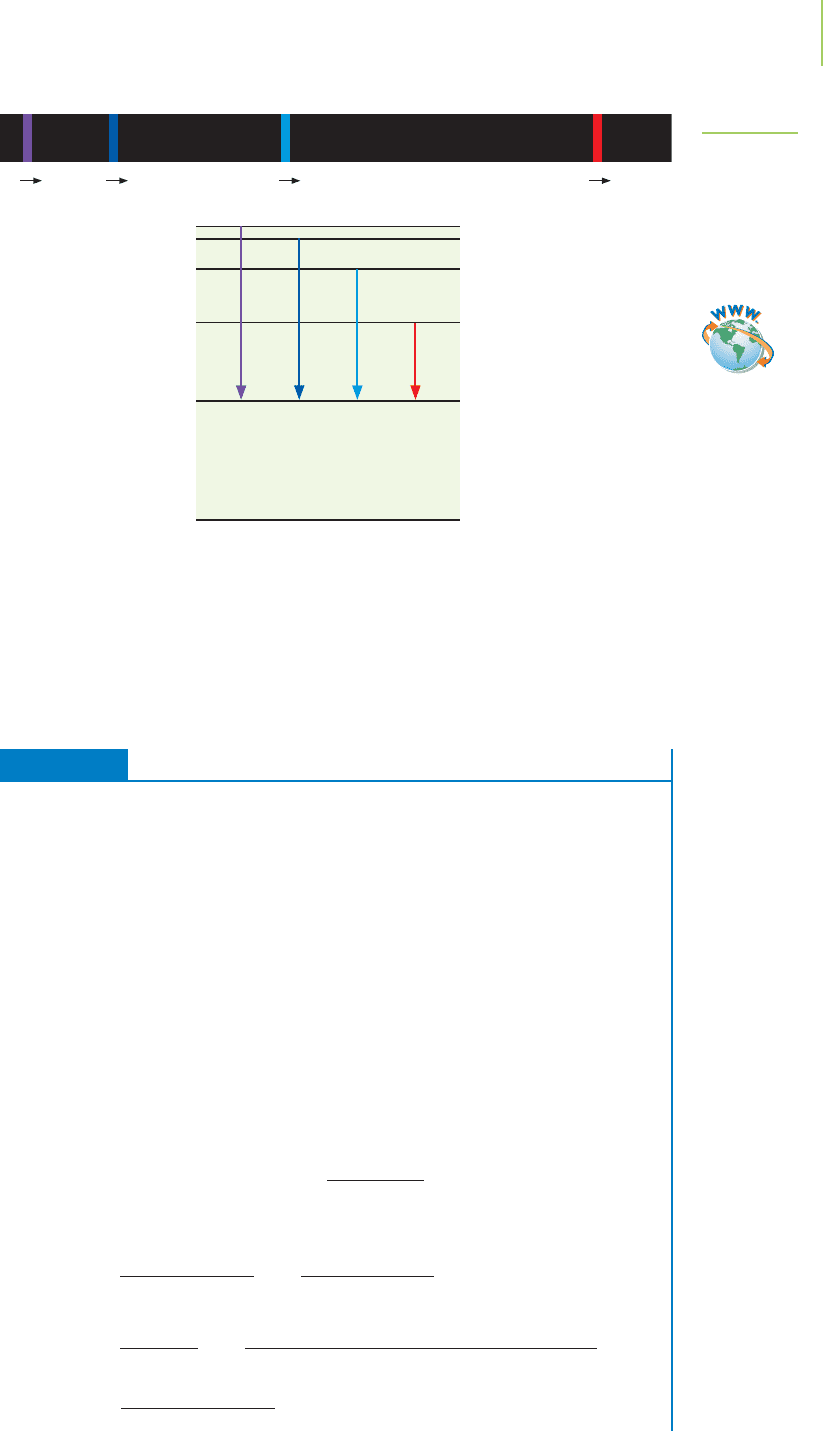
The change in energy made during an electronic transition can be either pos-
itive or negative as long as the energy is conserved. If
E is positive, energy is sup-
plied to the atom to make the electron jump from a lower to a higher state. If
E
is negative, energy is released from the atom because the electron falls from a
higher to a lower energy level, as shown in Figure 6.10.
One way to supply or release the required energy is for the atom to absorb or
emit electromagnetic radiation. This is where the hydrogen emission spectrum
proves useful. If we write the equation for hydrogen emission in terms of energy
instead of wavelength, we can show that the emission lines are the result of elec-
tronic transitions from the higher energy levels (or states) of the excited hydrogen
atom to lower energy states. We call the quantum number of the initial energy
level, n
i
and that of final energy level n
f
:
E =−2.1786 × 10
−18
J
1
n
2
f
−
1
n
2
i
Note that the energy change is a discrete value. In other words, the energy is quan-
tized. For the electron to move from one energy level to a lower energy level, it
must lose this discrete amount of energy. Knowing that the energy is related to
the frequency indicates that h
ν
, the electromagnetic energy, is equal to the energy
lost in the transition,
E:
hν =
hc
λ
= E
This relationship (∆E = h
ν
), although somewhat similar to that proposed by
Einstein, was proposed by Niels Bohr in an effort to correlate the energy emitted
from a hydrogen atom during an electronic transition with the frequency of ob-
served light. The relationship is referred to as the Bohr frequency condition.
If we combine the last two equations to calculate the wavelength of radiation
predicted for the hydrogen emission spectrum and convert from meters to
nanometers (1 m = 10
9
nm), we get
1
λ
= 1.097 ×10
7
1
n
2
f
−
1
n
2
i
m
−1
= 1.097 ×10
−2
1
n
2
f
−
1
n
2
i
nm
−1
222 Chapter 6 Quantum Chemistry: The Strange World of Atoms
4th level—high energy
Ground level—low energy
e
–
e
–
e
–
Nucleus
Photon
e
–
e
–
e
–
Nucleus
Photon
FIGURE 6.10
Energy changes that occur in an atom. An increase in energy results in promotion of an electron to a
higher energy level. A release of energy is observed when the electron returns to its original state.
6.4 The Bohr Model of Atomic Structure 223
This is the equation that Rydberg, Balmer, and others found by analyzing the
hydrogen emission spectrum and empirically fitting an equation to the results.
When n
f
=1, we get the Lyman series. When n
f
=2, we get the Balmer series. We
can continue this process until we generate all of the energies of electromagnetic
radiation observed to be emitted from the hydrogen atom. These electronic tran-
sitions in the visible region are depicted schematically in Figure 6.11.
EXERCISE 6.4 Energy-to-Frequency Conversion in the Hydrogen Atom
A hydrogen atom in one of its excited states has an energy of −1.5129 × 10
−20
J.
What frequency of radiation is emitted when the atom relaxes down to its ground
state (n = 1)?
First Thoughts
An important idea here is that an atom moving from its excited state to its ground
state releases energy (
E is −), typically as light, whereas an atom that goes from the
ground state to an excited state absorbs energy (
E is +).
Solution
The energy of the photon emitted must exactly match the energy lost by the hydro-
gen atom as the electron moves from a higher energy level to its ground state:
E
photon
=−E
electron
hν =−
(
E
f
− E
i
)
ν =
−
(
E
f
− E
i
)
h
The final state energy is given by
E
f
=−
2.1786 ×10
−18
J
n
2
=−
2.1786 ×10
−18
J
1
2
=−2.1786 ×10
−18
J
ν =−
(E
f
− E
i
)
h
=−
(−2.1786 ×10
−18
J) − (−1.5129 × 10
−20
J)
6.626 ×10
−34
J·s
ν =−
(−2.163 ×10
−18
J)
6.626 ×10
−34
J·s
= 3.265 × 10
15
s
−1
6 2 5 2 4 2 3 2
n = 6
n = 5
n = 4
n = 3
n = 2
n = 1
FIGURE 6.11
The visible electronic transitions of the
hydrogen emission spectrum. The energy
of the radiation observed must exactly
match the difference between energy of
the excited state and that of the ground
state (most energetically stable state).
Visualization: The Line Spectrum
of Hydrogen
224 Chapter 6 Quantum Chemistry: The Strange World of Atoms
Lasers and Their Applications
Lasing Medium Wavelength Application
TABLE 6.4
Diode
Helium/neon (HeNe)
Argon and krypton
CO
2
HeCd
Solid state
Nd:YAG
Ruby
ArK excimer
635 nm (red)
670 nm (deep red)
780 nm, 800 nm, 900 nm,
1550 nm (Infrared)
632.5 nm (red)
457.9 nm (violet-blue)
488 nm (blue)
514 (green)
646 (red)
1060 nm (mid-IR)
442 nm (violet blue)
325 nm (UV)
1064 nm (IR)
694 nm (red)
193 nm (UV)
CD players, DVDs, laser printers,
laser pointers, high-speed fiber
optics, dental surgery
Alignment, barcode scanners, laser
pointers, light shows, blood cell counting
Forensic medicine, high-performance printing,
general and ophthalmic surgery, holography,
light shows
Industrial metal cutting, welding, surgery
Nondestructive testing and spectroscopy
Materials processing (drilling, cutting,
welding), target designation, surgery,
hair removal
Laser eye surgery
Further Insights
Our discussion is focusing on the hydrogen atom. Helium, with its two electrons,
was discovered in 1868 by the British astronomer Norman Lockyer and, in separate
observations of the emission spectrum from the Sun, Pierre Janssen of France. The
solar spectrum showed lines never before seen, indicating a previously unknown
element. Lockyer named the element helium after the Greek word for Sun, Helios.
The discovery was confirmed in 1895 by the Scottish chemist William Ramsey, who
saw the same spectrum when looking at gases coming from the heating of the
uranium-containing mineral cleveite. Ramsey is known for discovering all of the
noble gases except radon.
PRACTICE 6.4
What wavelength of radiation needs to be supplied to a hydrogen atom in its ground
state to raise it to its excited state that has an energy of −1.5129 × 10
−20
J?
See Problems 35–40 and 43–46.
We make use of the emission of light from excited atoms everyday. For exam-
ple, when electric current is passed through a tube containing a small amount
of neon gas, it causes the neon atoms to become excited. As they return to the
ground state, they emit light.
What is this contraption?
When the tube is bent to spell words, we refer to the contraption as a neon
sign. Another example of light emission from excited atoms is found in
lasers.
Lasers (the acronym stands for “light amplification by stimulated emission of
radiation”) provide a powerful light source because they involve the simultaneous
collapse of a large population of excited atoms to the ground state.
The light emitted by each excited atom has the same phase (the peaks and
troughs of the light waves line up), giving rise to a powerful emission of light.
Table 6.4 sheds some light on common lasers and their uses.
224 Chapter 6 Quantum Chemistry: The Strange World of Atoms
Application
C
HEMICAL
ENCOUNTERS:
The Nature and
Applications of
Lasers
A laser pointer,
one example of
the many uses
of lasers.
A neon sign emits light when an electric
current is passed through the tube.

6.5 Wave–Particle Duality 225
HERE’S WHAT WE KNOW SO FAR
■
Electromagnetic radiation is characterized by wavelength, frequency, and
energy. These values can be interconverted.
■
Electromagnetic radiation is quantized into photons, the smallest unit of
radiation.
■
An atom can gain or lose discrete units of energy when an electron jumps
between the orbits allowed by the Bohr model.
■
Each line in the hydrogen emission spectrum is created by the light released
when an electron falls between two of the allowed orbits in the Bohr model.
■
Each time an electron falls between two levels, one photon of light is released,
carrying exactly the amount of energy equal to the difference between the two
energy levels.
■
The frequencies (and therefore wavelengths) of light emitted are determined by
the energy levels available for electrons to fall between. In other words,the light
coming out of the atom is a consequence of the atom’s electronic structure.
6.5 Wave–Particle Duality
We can dig deeper into the explanations that enable us to make sense of atoms if
we look at some apparent contradictions between the way the world appears on
the basis of everyday experience and the way it behaves at the level of individual
atoms. These two views of the world are known as the classical mechanical and
quantum mechanical views. In
classical mechanics we have particles and we have
waves, each with their own distinct characteristics. The particles do not act like
waves, and the waves do not act like particles. This is the kind of behavior we see
on a macroscopic level, as shown in Figure 6.12. In quantum mechanics, we can
still talk about particle and wave behavior, but the distinction between particles
and waves becomes fuzzier, although it is still of great importance, as we shall see.
According to classical mechanics, particles have exact locations, and their posi-
tions can be defined precisely in space. Classically, a particle can move at any speed
(below the speed of light), provided that it is supplied with the necessary energy.
Also, if a particle is moving, it carries momentum (p), which is defined as mass
multiplied by velocity (v):
p = m ×v
A 100-ton coal car moving at 30 mi/h has considerably more momentum than a
1-ton compact car moving at the same speed. A coal car traveling at 30 mi/h has
more momentum than another coal car that is crawling
along the tracks. Both mass and velocity contribute to
momentum.
One of the main descriptors a particle has is mass. In
addition, because all particles can carry momentum, they
must have mass. This means that we can use momentum to
test whether something is behaving like a classical particle
or not.
The Bohr model of the hydrogen atom defies the classi-
cal logic of the behavior of particles. Although electrons
have momentum and therefore carry mass, they are quan-
tized in the Bohr model both in terms of where they can be
and in terms of how much energy they can have. The quan-
tized nature of space and energy is not the kind of behavior
we expect from particles on a macroscopic level. For example,
FIGURE 6.12
A person is very different from a wave. In
the quantum realm the nature of people
(particles) and waves become intertwined
in strange but very significant ways.
The lighter of two cars has much less momentum, even if the
vehicles are traveling at the same speed. Consequently, it usually
suffers the greater amount of damage in a collision.

226 Chapter 6 Quantum Chemistry: The Strange World of Atoms
FIGURE 6.13
A tuned violin string plays a note when it vibrates
at a specific frequency, when a standing wave is set up
between the end points of the string. A different note
can be played when the player’s finger is used to vary
the length of the string. But only certain lengths—
certain positions of the player’s finger—will allow a
proper note to be played when a standing wave of
a different frequency occurs. The notes are a set of
quantized frequencies.
when we think of a moving race car, we envision it as capable of possessing any
velocity or mass within a continuous range. This is not true for the quantum me-
chanical view of the atom.
However, quantized energy states and locations have long been known in
everyday life. For example, musicians who play stringed instruments quantize the
energy of the vibration of the strings by shortening or lengthening the string
being played. This changes the frequency of the string’s vibration by setting the
wavelength of the vibration, as shown in Figure 6.13. A wave vibrating between
two fixed endpoints is called a
standing wave.
The crucial word here is wave. All of the cases that were known to create quan-
tized states prior to the Bohr atom were systems that could be described by
“waves,” oscillating forms of energy that had a specific wavelength and frequency.
To clarify the distinction between the ideas of particles and waves, we can first list
the characteristic classical properties of particles as follows:
In the Classical Mechanical View of the World:
■
Particles have a continuous range of available energies and positions (such as
a person going out for a walk).
■
Particles have a single value of energy and position measured at any one time
(such as a person seated in a chair).
■
Particles carry momentum (have mass, such as the mass of the person).
■
Particles are quantized (exist as individual particles, such as the person himself
or herself).
We can now build up a similar list of criteria for the classical mechanical view of
waves. In the view of classical mechanics, waves are neither composed of single
units nor located at exactly one place in space as are individual particles. As we
can see in our picture of the violin string in Figure 6.13, the wave exists every-
where between the bridge and the player’s finger. The amplitude changes between
these two sites, but the wave exists along the whole vibrating part of the violin
string. It is smeared out over a pretty large volume of space.
Waves can also be quantized by confining them to a specific region in space,
as we do when we tie the two ends of the violin string down with the bridge and
our finger. In order for the amplitude of the wave to go to zero at the ends, the
number of wavelengths that must fit between the two ends must be some half-
integral number of the wavelength:
Length of string = nλ
/2
In this example, the length of the string, l, sets the possible wavelengths that the
vibration can have to λ = 2l, l,(2/3)l,(1/2)l,(2/n)l,...,0.This is illustrated in
Figure 6.14, where we can see the first few allowed standing waves. Note that
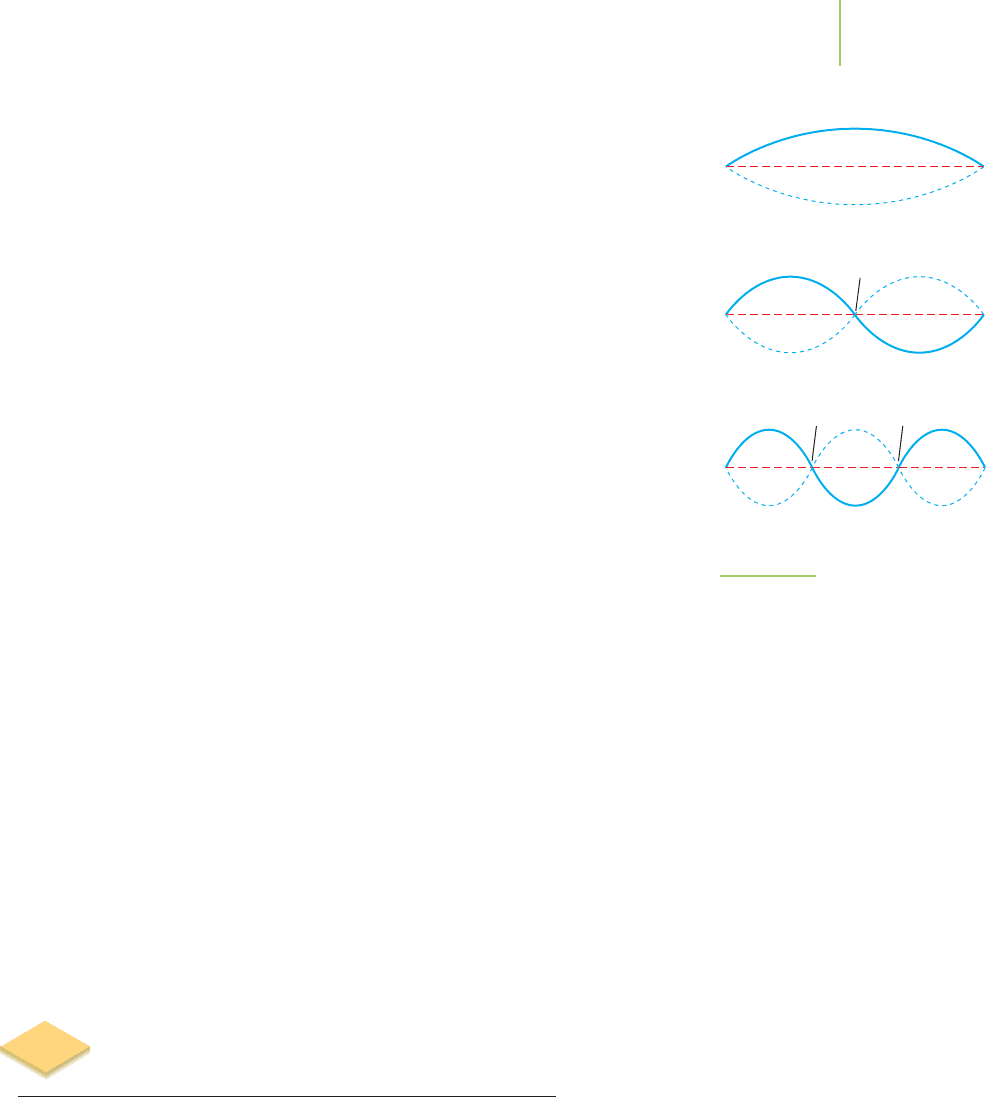
6.6 Why Treating Things as “Waves” Allows Us to Quantize Their Behavior 227
whereas 2l (n = 1) and l (n = 2) are permissible values, no wavelengths between
these two values are allowed.
We can now list the characteristic classical mechanical properties of waves:
In the Classical Mechanical View of the World:
■
Waves have specific energy values (such as the specific notes on a violin
string).
■
Waves occupy a range of spatial positions all at the same time (such as the
positions of the vibrating violin string).
■
Waves do not carry momentum (have no mass; the sound waves from the
violin don’t have a mass).
■
Waves have a continuous range of amplitudes (smallest, single wave units;
such as the loudness of each note from the violin).
In our everyday (macroscopic) world, the characteristics of particles and
waves are separate. But here is a truly strange thing: If we investigate matter and
energy on a quantum level, where dimensions are on the order of atomic spacing,
things we once regarded as particles, such as electrons, can also behave like waves,
and things that were traditionally thought of as waves, such as light, can also behave
like particles. The electrons will still behave like particles, and the electromagnetic
radiation will still retain the properties of waves. But the distinction between par-
ticles and waves is no longer absolute. All quantum “things”—electrons, protons,
photons of electromagnetic radiation, whatever—have the properties of both waves
and particles simultaneously.
This schizophrenic behavior is called the
wave–particle duality, which pro-
poses that at very small dimensions, things behave like both waves and particles,
regardless of how they behave on the macroscopic level. The earliest experiments
performed on the electron investigated particle behavior by measuring the
electron mass (the Millikan oil drop experiment, Chapter 2) and position (J. J.
Thomson and the cathode ray tube, Chapter 2). However, we can show that the
energy quantization observed in the hydrogen emission spectrum or predicted by
the Bohr model results from the wave nature of the electron. The electron be-
haves as both a particle and a wave. It has mass, carries momentum, and is found
as a discrete single unit, which are properties expected of a classical particle, but
when contained in an atom or molecule it is spatially and energetically quantized,
which are properties expected of a classical wave.
6.6 Why Treating Things as “Waves”
Allows Us to Quantize Their Behavior
To see why the wave nature of the electron allows its energy levels to be quantized,
look at Figure 6.15, where we have drawn a “cross section” of several possible
orbits for the Bohr model. The Bohr model is three-dimensional, with spherical
orbits, but that would be hard to show schematically, so we will show a circular
cross section of the orbit.
Much like the standing wave vibrating on the violin string, only certain wave-
lengths will fit on a circle. If the radius of the circle is r, we can quantize the
allowed wavelengths, λ, to be integral multiples of the circumference, which you
might recall from your high school geometry class has a length of 2
πr:
Circumference = 2πr = nλ
In other words, we can fit n waves of wavelength λ around the circumference of
the circle, as shown in Figure 6.16.
1 half-wavelength
Node
2 half-wavelengths
3 half-wavelengths
Node Node
FIGURE 6.14
Overtones of a violin string. In addition
to the half-wavelength standing wave,
the violin also produces integral multi-
ples of the half-wavelength. These
vibrations are more energetic than the
half-wavelength vibration and are not
deliberately played by a violinist.
