Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.

a. If the value of H for the reaction is −453 kJ, what is the
value of H for the reverse of the reaction?
b. What is the value of H for this reaction if 10.0 g of
phosphoric acid is produced?
c. Is this reaction endothermic or exothermic?
d. If 1.50 g of P
4
O
10
(s) and 2.50 mL of water were mixed,
how many grams of phosphoric acid would result?
e. What is the enthalpy change for the process outlined in
part d?
f. If 10.0 g of P
4
O
10
were mixed with 1.00 kg of water at
25.0°C, what would be the final temperature of the water?
101. Sodium hydroxide pellets can be used to unclog a drain.
a. What is the enthalpy change when 15.0 g sodium
hydroxide is added to 1.00 L water?
b. What is the molarity of this solution? (Assume that the
volume change is negligible.)
102. A student wishes to prove the existence of silver ions in a
particular solution that was supposedly made by dissolving
silver nitrate in water.
a. Explain how this could be determined using a solution of
sodium sulfide, and write a reaction that illustrate this
method.
b. What is the molar enthalpy change for the reaction of a
solution of silver nitrate and sodium sulfide?
103. A 0.10 lb sample of sodium metal is added to 1.00 gallon
water.
a. Assuming no change occurs in the volume of the sample,
what would be the concentration of the resulting solution
of sodium hydroxide?
b. What is the enthalpy change for this process?
Thinking Beyond the Calculation
104. Phosphoric acid is used in many soft drinks to add tartness.
This acid can be prepared through the following reaction:
P
4
O
10
(s) + 6H
2
O(l) → 4H
3
PO
4
(aq)
208 Chapter 5 Energy
22.7°C
152.06 g =
Mass of
calorimeter
Water
added
Metal block
at 96.3°C
234.95 g =
Mass of
calorimeter
and water
257.88 g =
Mass of
calorimeter,
water, and
metal block
24.1°C
Phosphoric acid
96. A student’s coffee cup calorimeter, including the water it
contains, has been calibrated in a manner similar to that de-
scribed in Problem 46. The heat capacity was found to be
55.5
J
◦
C
. If a 65.8-g sample of an unknown metal, at 100.0°C,
was placed in the calorimeter initially at 25°C, and an equi-
librium temperature of 29.1°C was reached, what is the
specific heat of the metal?
97. The foods we eat provide fuel to keep us alive. Burning a
0.500-g sample of vegetable oil provides enough heat to
raise the temperature of a calorimeter by 2.5 K. Assuming
the heat capacity of the calorimeter to be 7.5
kJ
K
, determine
the heat of combustion for 1 g of the oil.
98. A student performs the experiment shown graphically here.
What is the specific heat of the block of metal used in the ex-
periment? (Assume that the heat capacity of the empty
calorimeter is 7.5
J
◦
C
.)
99. The reaction of the gases ethane (C
2
H
6
) and oxygen gives
gaseous carbon dioxide and water vapor.
a. Write the balanced chemical reaction for this combustion.
b. What is the enthalpy change for this process?
c. If 250.0 grams of ethane is consumed in the reaction, how
many grams of carbon dioxide would be produced?
d. Why should precautions be taken to avoid performing
the reaction outlined in part c in an enclosed space?
100. Under certain conditions, the reaction of chlorine gas with
metallic iron can produce iron(III) chloride.
a. Write the balanced chemical reaction for this reaction.
b. If 8.44 grams of iron(III) chloride are produced, how
many grams of iron were required?
c. What is the enthalpy change required to produce 8.44
grams of iron(III) chloride? (
f
H(FeCl
3
) =−399 kJ/mol;
S(FeCl
3
) = 142 J/kmol;
f
G =−344 kJ/mol.)
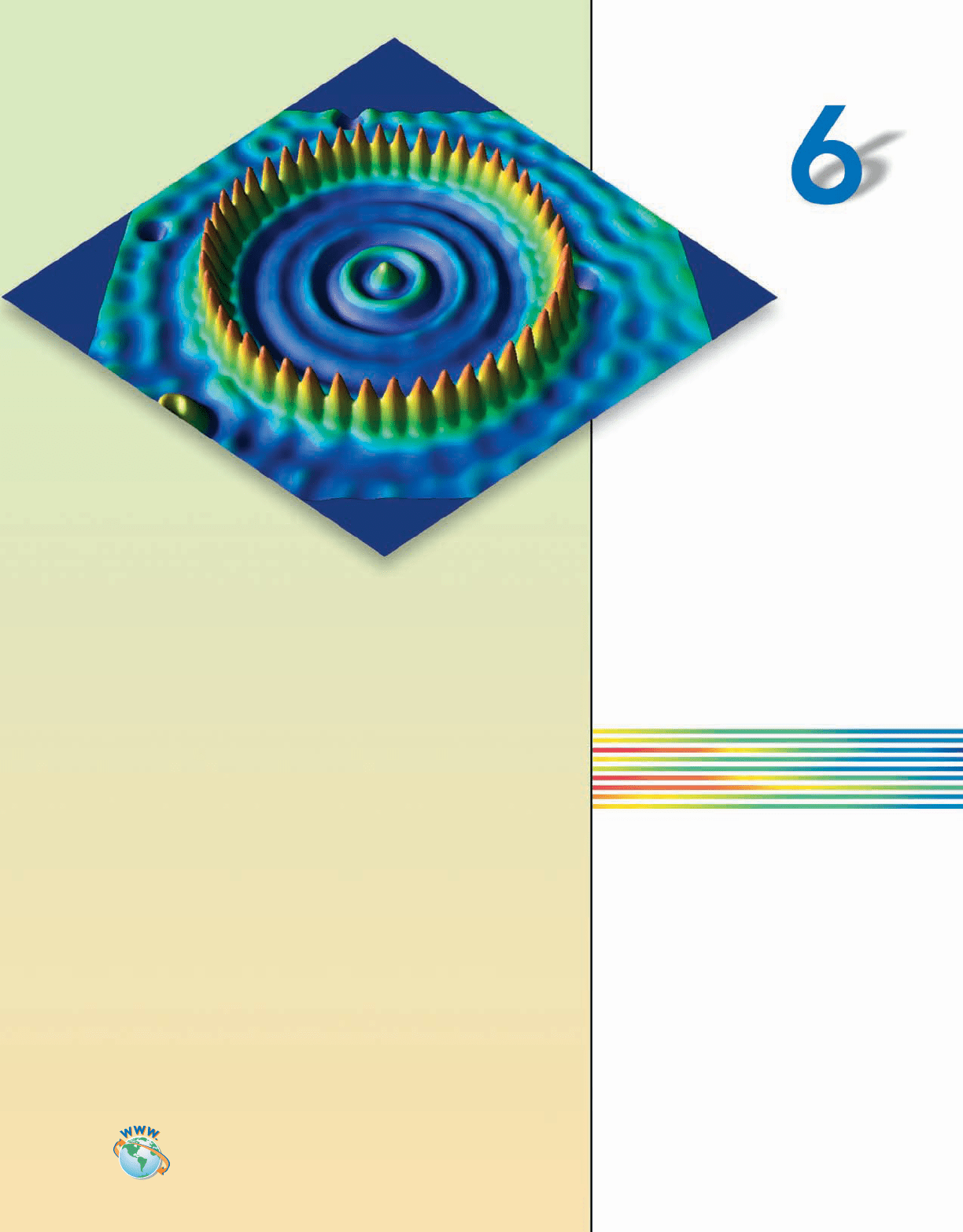
209
Scanning tunneling microscope (STM)
image showing iron atoms adsorbed on
a copper surface forming a “quantum
corral” at a very low temperature (4 K).
The image shows the contour of elec-
tron density. The corral is about 14.3 nm
in diameter.
209
Contents and Selected Applications
6.1 Introducing Quantum Chemistry
6.2 Electromagnetic Radiation
6.3 Atomic Emission and Absorption Spectroscopy, Chemical
Analysis and the Quantum Number
Chemical Encounters: Simultaneous Determination of Elements in Water
6.4 The Bohr Model of Atomic Structure
Chemical Encounters: The Nature and Applications of Lasers
6.5 Wave–Particle Duality
6.6 Why Treating Things as “Waves” Allows Us to Quantize Their
Behavior
6.7 The Heisenberg Uncertainty Principle
6.8 More About the Photon—the de Broglie and Heisenberg
Discussions
6.9 The Mathematical Language of Quantum Chemistry
6.10 Atomic Orbitals
Chemical Encounters: The Scanning Tunneling Microscope
6.11 Electron Spin and the Pauli Exclusion Principle
Chemical Encounters: Nuclear Spin and Magnetic Resonance Imaging
6.12 Orbitals and Energy Levels in Multielectron Atoms
6.13 Electron Configurations and the Aufbau Principle
Quantum
Chemistry:
The Strange
World
of Atoms
Go to college.hmco.com/pic/kelterMEE for online learning resources.

210
Funny things happen when you shrink the
scale of observation down below that which you
can see with your eye, even when it is aided by the
most powerful optical microscope. Things that once ap-
peared to have a single value, a single space on the lab bench,
or a single speed at which they move through space now get
smeared out over time and space so that you can discuss only the prob-
ability of where they might be at a given time or how fast they might be trav-
eling when you finally catch up with them. Nothing at this level seems absolute
anymore.
The field of chemistry that models the behavior of atoms and molecules at the
atomic level is called
quantum chemistry, and the physical description of this model is
called
quantum mechanics. Because our everyday
experiences do not easily prepare us for what
we observe in experiments done on the
atomic level, the whole idea of quantum me-
chanics might at first appear strange or even
incomprehensible. However, the rules, or pos-
tulates, of quantum theory are elegant and
precise, of great utility, and readily under-
standable if we put aside our “macroscopic”
expectations.
Why is it useful for us to know about
quantum chemistry?
Quantum chemistry is important because
we use it to predict chemical reactivity and
other kinds of chemical properties. For exam-
ple, we need quantum chemistry to answer
questions such as, How is the energy of sun-
light captured by plants? and Why do leaves and flowers have distinctive colors?
There is so much richness in our macroworld that is truly revealed only by looking
deep within, at the nanoworld that underlies it.
The colors of these flowers, and the fact that sunlight helps them grow, can
be explained in terms of the basic interactions of light with matter. These
interactions are governed by quantum chemistry.
The smallest unit of
water is H
2
O.
6.1 Introducing Quantum Chemistry
A liter of water can be divided in half to give two half-liters of water. Each of those
half-liters can itself be divided into halves (two quarter-liters), and so on. But
the process cannot go on indefinitely because we will eventually be left with a
single water molecule. We could break this molecule down into two hydrogen
atoms and an oxygen atom, but the pieces would no longer be water. The H
2
O
molecule is the smallest unit, or quantum, of water. Energy can be quantized, too.
The quantum of light is called a
photon, the smallest possible amount of light
energy there can be. In general, a
quantum (plural quanta) is a single indivisible
unit of something. Many familiar things also come in quanta, including eggs,
cats, and chemistry books. Yet it is the quantization of light energy into photons
that will play a key part in our understanding of atomic and molecular
structure—and in all the chemistry we can explain and predict as a result. Light
energy is a type of electromagnetic radiation, so let us begin by looking at it from
that point of view.
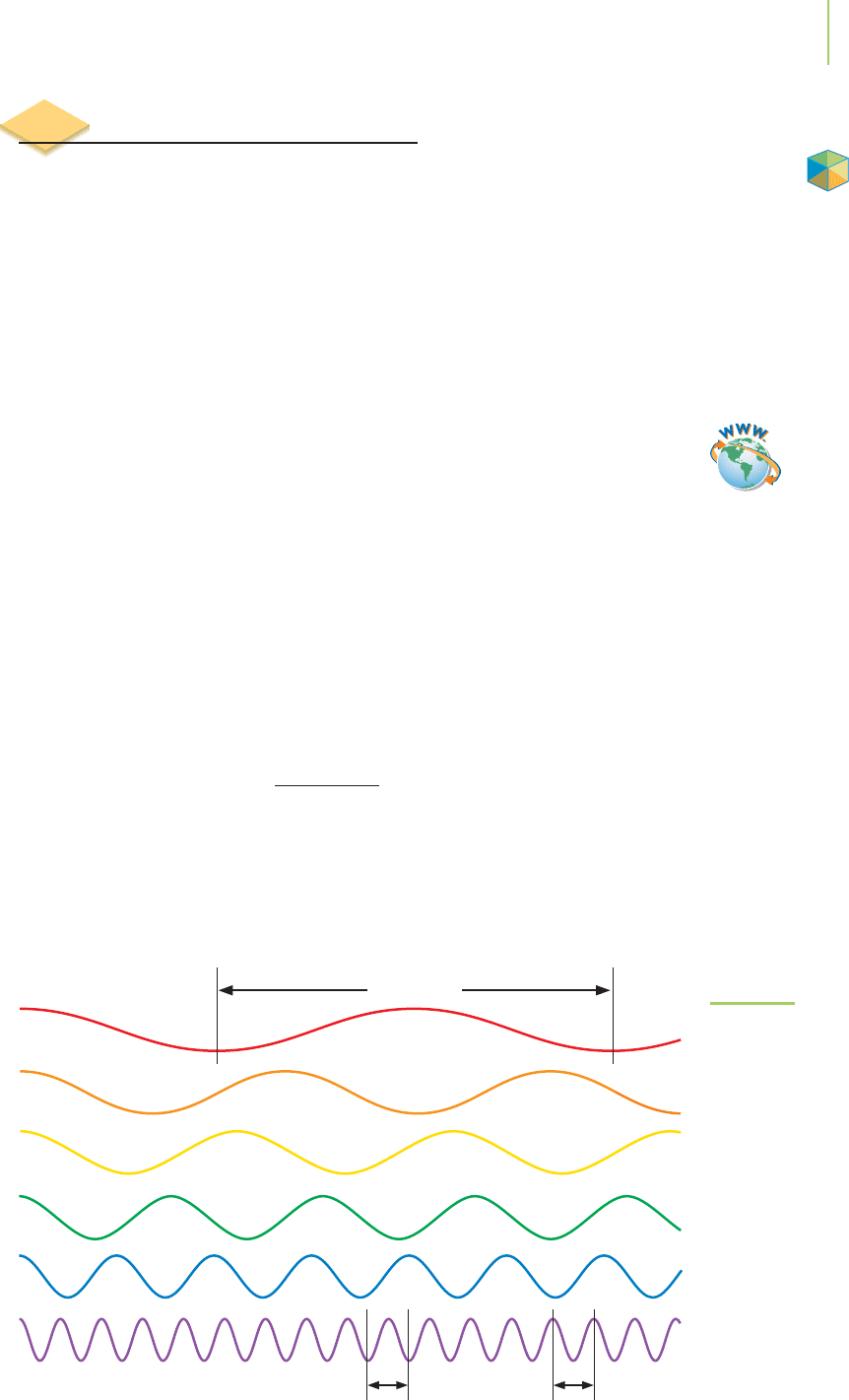
6.2 Electromagnetic Radiation 211
6.2 Electromagnetic Radiation
When you open your eyes in the morning, information carried by light, a type of
electromagnetic radiation, floods into your brain. Light lets you see where you
are, notice what sort of day it is, and perhaps locate the button to turn on the tele-
vision news. The television signal arrives from the television station—or, in more
recent times, from a communication satellite—in the form of electromagnetic ra-
diation, as does most of the other information we need to live our lives. Studying
electromagnetic radiation from stars enables scientists to figure out the structure
of the universe. Studying the interaction of atoms with electromagnetic radiation
led to the modern view of atomic structure, the subject of this chapter.
Electromagnetic radiation, such as visible light, can be described as electro-
magnetic waves that transmit energy through space. Each wave is just a part of
the
electromagnetic spectrum, the entire range of electromagnetic radiation that is
possible. These waves have a wavelength, λ (the Greek letter lambda), frequency,
ν (the Greek letter nu), and speed (see Figure 6.1). The
wavelength is defined
as the distance from the top of one crest of the wave to the top of the next crest. In the
past, wavelengths were measured using a unit known as the
angstrom (Å),where
1Å = 10
−10
m. The use of this unit of length has been supplanted by units in the
SI system, such as nanometers. The
frequency of electromagnetic radiation is
defined as the number of waves that pass a given point per second. So, the frequency
is reported in units of 1/s (s
−1
), known as a hertz (Hz).
The speed of light in a vacuum is given the symbol c and is a fundamental
physical constant equal to 299,792,458 meters per second (this is usually rounded
to 3.00 × 10
8
m
.
s
−1
). How fast is the speed of light? We can get a handle on this
by considering that the Sun is, on average, 93 million miles, or 1.5 ×10
11
m, from
the Earth. Sunlight takes a little more than 8 minutes to reach our planet.
1.5 ×10
11
m ×
1s
3.00 ×10
8
m
= 500 s (= 8.3 min)
We can also use the speed of electromagnetic radiation to tell us about dis-
tances on smaller scales. New global positioning system (GPS) devices receive
signals from at least three of the more than two-dozen GPS satellites in orbit
20,200 km (12,500 mi) above the Earth’s surface. At that distance, it takes about
Long wave
(low frequency) Wavelength
Wavelen
g
th Wavelen
g
th
Short wave
(high frequency)
FIGURE 6.1
Sine waves of similar amplitude, with
wavelength and frequency illustrated.
Application
Video Lesson: The Wave Nature
of Light

0.07 s for signals from each satellite to reach the receiver. Each satellite’s signal will
take a slightly different amount of time than the others to reach the running
watch or car receiver, because each is a little farther from or closer to you, de-
pending on your location. The differences in time of receipt of the signal—on the
order of milliseconds—from just three of these satellites can be used to locate
your position within a meter or two in three dimensions (Figure 6.2).
Wavelength, frequency, and speed are interrelated by the equation
ν =
c
λ
Note the inverse relationship between frequency, ν, and wavelength, λ. As one in-
creases,the other decreases.The illustrationof thewavesin Figure6.1also indicates
the
amplitude of a wave, which is the distance between its highest point and zero.
EXERCISE 6.1 Conversion Between Frequency and Wavelength
What frequency of light has a wavelength of 585 nm, which appears orange to
human eyes?
Solution
Because the speed of light is expressed in meters per second, it is useful to convert
the wavelength, 585 nm, to meters:
585 nm ×
1 ×10
−9
m
1nm
= 5.85 × 10
−7
m
We can use the relationship among the speed of light, wavelength, and frequency to
solve for frequency:
ν =
c
λ
3.00 ×10
8
m·s
−1
5.85 ×10
−7
m
= 5.13 ×10
14
s
−1
We can also do the equivalent calculation by dimensional analysis:
3.00 ×10
8
m
s
×
1
5.85 ×10
−7
m
= 5.13 × 10
14
s
−1
PRACTICE 6.1
What is the wavelength of light that has a frequency of 7.45 × 10
14
Hz? Should the
wavelength be shorter or longer than that in the exercise?
See Problems 5a, 6a, and 11–14.
212 Chapter 6 Quantum Chemistry: The Strange World of Atoms
(latitude, longitude)
FIGURE 6.2
A GPS watch and how it works. The watch
receives signals from at least three satellites.
On the basis of the time it takes each signal
to reach the watch, the distance from each
satellite is calculated. The exact position of
the watch is then triangulated from the three
distances.
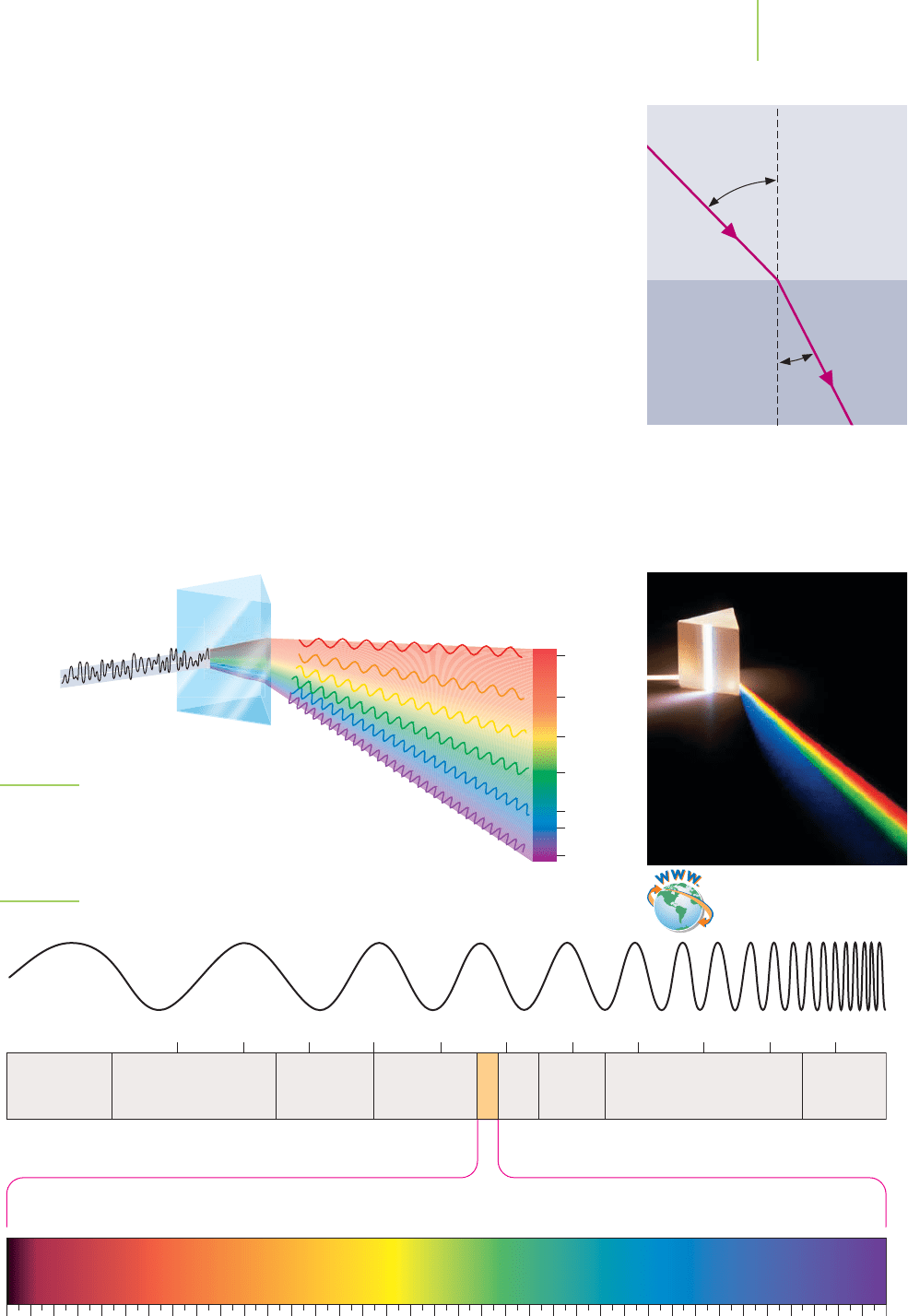
Even though we mention that light is a wave, no matter is actually “waving” as the
light travels through space. When electromagnetic radiation travels through
matter, such as water, air, or a pane of glass, the electrons and nuclei of the matter
tend to follow the oscillatory motion of the electromagnetic radiation, slowing
the wave a little. The end result is that electromagnetic radiation travels a bit
more slowly through matter than in a vacuum.
If we allow an electromagnetic wave to strike a more dense transparent mate-
rial at an angle, the radiation will change direction as it enters. We use this effect
in lenses made for eyeglasses, cameras, or telescopes. The angle through which a
ray of light or other electromagnetic ray bends depends on its frequency; higher-
frequency (shorter-wavelength) rays are bent more than lower-frequency ones.
This is why a glass prism, shown in Figure 6.3, is used to separate the frequencies
of light to generate a spectrum, familiar to us as the colors of a rainbow.
The visible light spectrum of Figure 6.3 represents only a small fraction of the
entire electromagnetic spectral range, shown in Figure 6.4. Our eyes do not re-
spond to anything outside the visible range, but our technology can detect and
use the invisible frequencies. For example, very long wavelengths are used for
radio transmission and are called radio waves. The shortest radio waves, at tenths
of meters, are called microwaves and are used in cellular phone communications,
radar systems, and microwave ovens.
6.2 Electromagnetic Radiation 213
Less dense
More dense
r
i
The direction in which light travels is
bent as the electromagnetic radiation
moves from a substance of given density
to another.
Orange
Red
Yellow
Green
Blue
Indigo
Violet
Spectrum
Sunlight
Prism
FIGURE 6.3
The glass prism separates out the frequencies of light from
the electromagnetic spectrum by wavelength.
Visible spectrum
400450500550600650750 700
10
20
10
19
10
18
10
17
10
16
10
15
10
14
10
13
10
12
10
11
10
10
Frequency (s
–1
)
Wavelength (nm)
Gamma
rays
Near
infrared
Far
infrared
TV FM AM
Radio waves
X-raysMicrowaves
Visible
Radar
Far
ultra-
violet
Near
ultra-
violet
FIGURE 6.4
Electromagnetic spectrum as a function of energy.
Visualization: Refraction of
White Light

214 Chapter 6 Quantum Chemistry: The Strange World of Atoms
33.0
33.4
36.0
37.0
37.6
38.0
38.6
39.5
40.0
40.5
41.0
42.5
43.5
45.5
46.9
47.0
47.2
48.2
50.2
50.4
51.4
52.6
54.25
Gigahertz
FIGURE 6.5
The radiofrequency band is packed with assigned frequencies. How will we add more as new technologies become available?
1. McClain, Dylan Loeb, “Directing Traffic in the Radio Spectrum’s
Crowded Neighborhood,” New York Times, February 24, 2000 p. D7.
2. International Telecommunications Union, http://www.itu.int/
(accessed October 2005).
When we think of traffic jams, images of
cars stuck in a sea of smog and blaring
horns come to mind. Yet with the furious surge of tech-
nological innovation in consumer products such as cel-
lular phones and other two-way mobile communications
devices, a new electromagnetic traffic jam has been pro-
duced. The frequencies of the radio wave part of the
electromagnetic spectrum are becoming uncomfortably
cluttered, and the assignment of the frequencies in this
range of about 3 kilohertz (3000 s
−1
) to 300 gigahertz
(3.00 × 10
11
s
−1
) is becoming what one newspaper re-
porter called “a high-wire act achieved through techno-
logical advances and the careful management of the
radio spectrum.”
1
The International Telecommunications Union
(ITU), headquartered in Geneva, Switzerland, is respon-
sible for developing standards and procedures related to
the assignment of radio frequencies “based on the prin-
ciple of equal rights of all countries, large or small, to
equitable access to these bands.”
2
In the United States, there is a dual system of admin-
istration. Federal use is administered by the National
Telecommunications and Information Administration
(NTIA). All other uses are managed by the Federal Com-
munications Commission (FCC). About 93.1% of the
total spectrum has shared use; only 5.5% is exclusively
allocated for private use and 1.4% for government use.
Figure 6.5 shows the division of the radio spectrum
into 450 bands, many of which have specific allocations.
To keep up with the extraordinary demand for new radio
frequency assignments, and to resolve the occasionally
complex issues that accompany these assignments, the
ITU began to meet every
2 years starting in 1993
instead of the prior
meeting schedule
of every 20 years. In the
United States, one of the
most complex issues is
the allocation of frequen-
cies in response to ever
more requests. How can
we make it technologi-
cally possible to stuff
more users into a finite
wavelength region by
reducing the necessary
“bandwidth” (how wide
the frequency band needs
to be for the transmis-
sion) for each user? Is it
possible to create com-
munications devices that
use alternative frequencies? The personal cellular phone
was just a dream 30 years ago. Digital audio was not even
on the horizon. What advances might we anticipate in
the next 20 years that will squeeze the frequency range
even more?
Issues and Controversies
Putting the Squeeze on Radio Frequencies
Cell phones, video phones, and
text messaging are just some of
the modern conveniences that
are squeezing the available
bandwidth.

6.2 Electromagnetic Radiation 215
How do we use the different regions of the electromag-
netic spectrum? Infrared radiation (IR), spans about 10
−6
to
10
−3
m in wavelength and can be detected with special
“heat-sensing” scopes. IR rays are associated with the vibra-
tions of molecules, so you can feel the warmth they generate
in your skin.
The Sun’s ultraviolet radiation (UV) at approximately
10
−7
m can give you a tan, or a burn, depending on the wave-
length and how long you sit in the sun or under a UV lamp.
The short wavelength of X-ray radiation, at about 10
−10
m, is approximately the
same size as atomic distances. This allows X-rays to be used to detect the spatial
arrangements of atoms. Gamma rays have the shortest waves in the electromag-
netic spectrum and are also the most energetic. They are a by-product of many
nuclear reactions and are very damaging to most materials, including those that
make up living things. Table 6.1 gives selected commercial (that is, related to
commerce) uses for radiation from various regions of the electromagnetic
spectrum.
The shorter the wavelength, or the higher the frequency, of the electromag-
netic radiation, the greater the energy associated with it. This relationship
between wavelength, or frequency, and the energy of a single photon was deter-
mined in 1900 by Albert Einstein (1879−1955) by extending the work of Max
Planck (1858−1947).
E = hν =
hc
λ
In this equation, E is the energy carried by each photon of the electromagnetic ray,
and h is
Planck’s constant, equal to 6.6260693 × 10
−34
J
.
s. This equation enables
us to calculate the energy of a single quantum of electromagnetic energy. As we’d
predict, the energy each photon carries is very small.
EXERCISE 6.2 The Energy of Electromagnetic Radiation
Compare the energy of a photon of visible radiation at 605 nm, the red light that we
detect with our eyes, and that of the X-ray radiation at 2.00 × 10
−10
m that we use
in medical diagnosis.
First Thoughts
Which has greater energy, a photon of red light or an X-ray? Our own experience
tells us that the calculation should result in a higher energy value for the X-ray.
Some Commercial Uses of Electromagnetic Radiation
Region Example of Use
Radio wave Communication
Microwave Cooking and reheating foods
Infrared Heating, drying, and cameras for finding thermal “hot spots” in products
Visible Detection of low concentrations of elements in steel, milk, water, etc.
Ultraviolet Germicide for killing bacteria in foods, in water, and on surfaces
X-ray Skeletal imaging, inspecting luggage at airports
Gamma ray Finding defects in pipe welds and castings,
inspecting containers
TABLE 6.1
An infrared picture of a home can show where most of the heat
is lost. Note that most of the heat loss in this home, shown as
bright yellow colors, occurs through its windows.
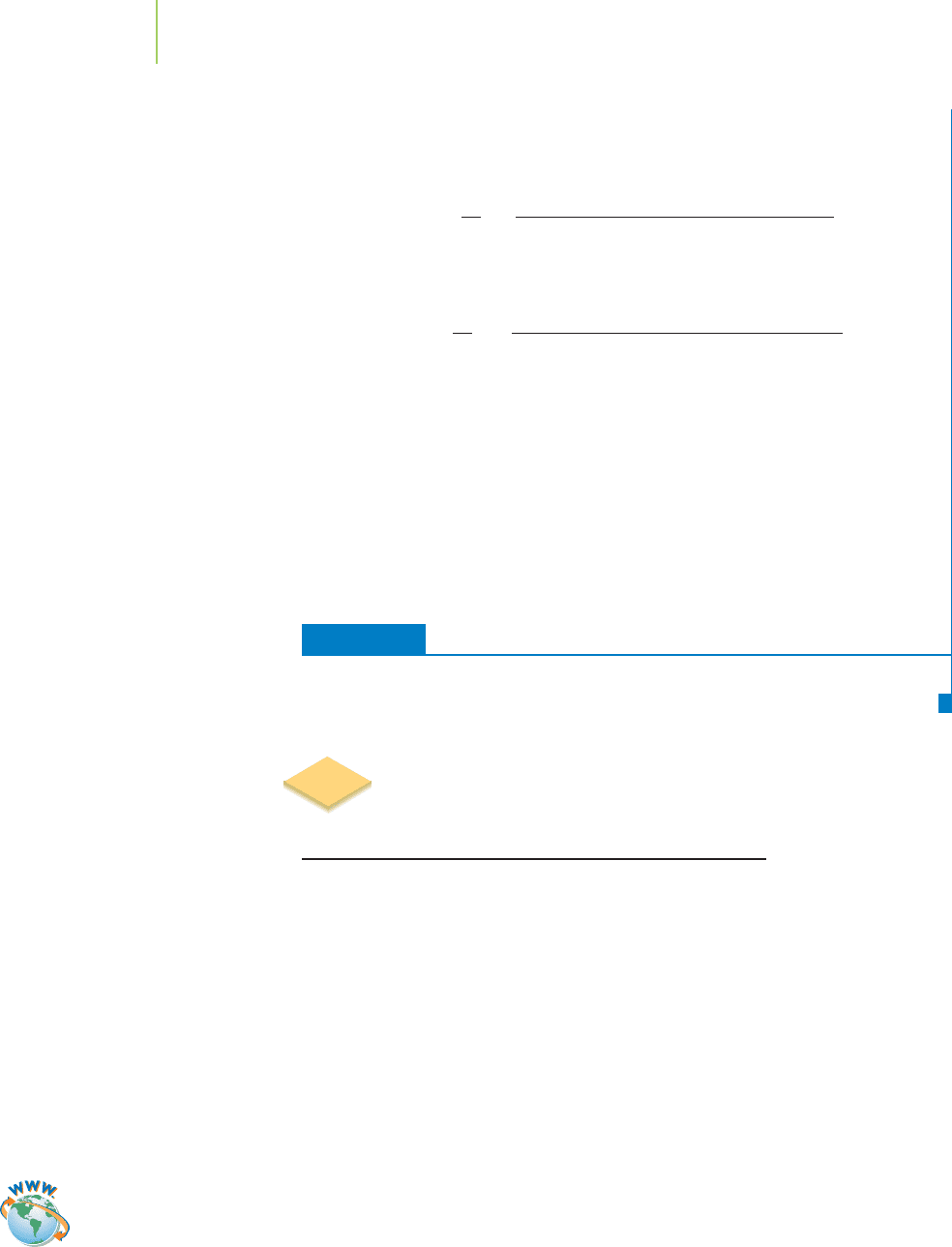
Solution
We use Planck’s constant and the speed of light to obtain the energies of each
wavelength:
E =
hc
λ
=
(6.626 ×10
−34
J·s) ×(3.00 ×10
8
m·s
−1
)
6.05 ×10
−7
m
= 3.29 ×10
−19
J for visible light
E =
hc
λ
=
(6.626 × 10
−34
J·s) × (3.00 × 10
8
m·s
−1
)
2.00 ×10
−10
m
= 9.94 × 10
−16
J for X-rays
Further Insights
The energy of the X-ray is 3000 times greater than that of the visible light. Does our
answer make sense?
Yes, it does, because our experience and our discussion up to
this point agree that X-rays are much more powerful than visible radiation. This
also explains why X-rays can do a lot more damage to living tissue than visible light.
Used with caution, however, they can penetrate the body and help physicians diag-
nose broken bones and tumors. Their damaging powers can also be used to destroy
cancerous tissue.
PRACTICE 6.2
What is the energy associated with microwaves of wavelength 8.00 mm?
See Problems 5b, 5c, 6b, 6c, and 15–24.
6.3 Atomic Emission and Absorption
Spectroscopy, Chemical Analysis
and the Quantum Number
Electromagnetic radiation can be used to transfer energy to and from atoms and
molecules. Energy emitted from or absorbed by an atom or molecule is often
found as a collection of discrete wavelengths that depend on the structure of the
atom or molecule. Using the technique known as
spectroscopy, chemists can
identify and quantify elements in all types of samples—from food and water to
brass and stainless steel. Spectroscopy, the study of how substances interact with
electromagnetic radiation as a function of wavelength or frequency, helps us deter-
mine the molecular composition of both synthetic and natural drugs, as well as
the kinds of organic molecules present in interstellar space or a sample of blood.
Figure 6.6 shows the solar spectrum, the range of visible light that is emitted
from the Sun. Note in the figure the discrete dark lines. These are an all-
important feature in atomic spectroscopy because, much like each person’s
unique fingerprint in a crime scene analysis, each element leaves its unique fin-
gerprint that we can use to identify it unequivocally via
emission spectroscopy
(studying radiation emitted vs. wavelength) or absorption spectroscopy (studying
radiation absorbed vs. wavelength).
Spectra have been obtained from the Sun since the beginning of the nine-
teenth century, and elemental analysis via emission spectroscopy has been done
since the late 1850s. For example, Kirchhoff and Bunsen, who published their
discovery of the elements cesium (in 1860) and rubidium (in 1861), did so by
looking at the emission spectra of these elements in a flame. However, they did
not understand the relationship of the wavelengths of light that they observed to
the atomic structure of the elements. Unraveling this connection was left to
216 Chapter 6 Quantum Chemistry: The Strange World of Atoms
Video Lesson: Absorption and
Emission
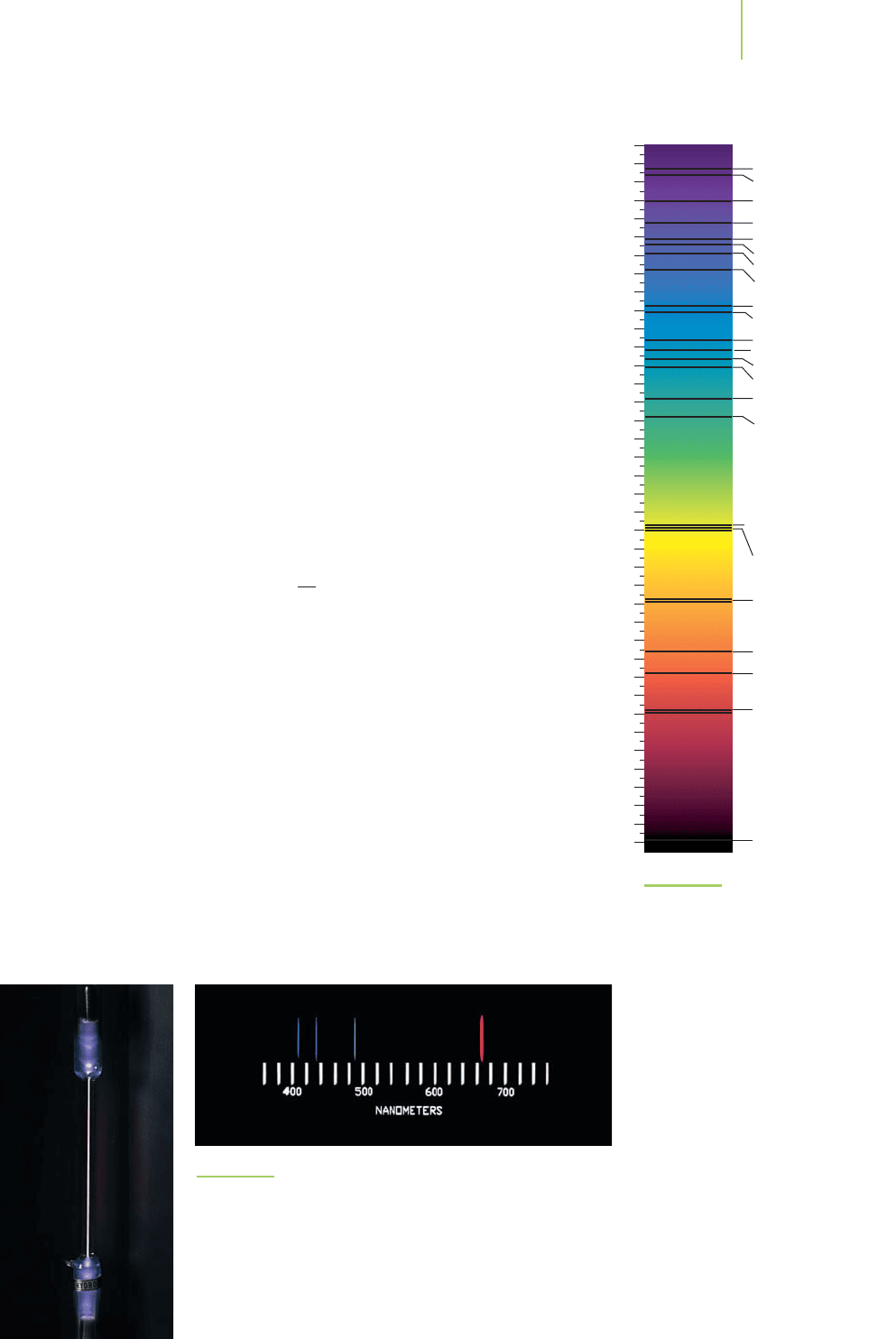
Johann J. Balmer, a mathematician who numerically analyzed in detail
the hydrogen spectrum obtained by the physicist Anders Ångstrom
and others, and who published his results in 1885. The next part of our
discussion shows how we can get a hydrogen spectrum and how
Balmer’s conclusions advanced the understanding of atomic structure.
Obtaining the Hydrogen Spectrum
The hydrogen emission spectrum can be obtained using a quartz tube
with a metal electrode at each end. If the tube is then filled with hydro-
gen gas (H
2
) and a very high voltage is applied across the electrodes, the
tube glows as shown in Figure 6.7. In this apparatus, electrons jump
from the negative electrode to the positive electrode, transferring
energy to the hydrogen molecules in the tube. Enough energy is trans-
ferred to make some of the molecules dissociate into energetically ex-
cited hydrogen atoms (
H
∗
). The extra energy can be released if the
atoms emit light, represented by the expression
H
∗
→ H + hν
where
ν
is the frequency of the light emitted by the excited hydrogen
(
H
∗
) and h is Planck’s constant. If you wished, you could write the
energy term as hc/λ:
H
∗
→ H +
hc
λ
In this case, you would talk instead about the wavelength of light
emitted.
After they lose energy by emitting radiation, the hydrogen atoms
(H) eventually recombine to form H
2
, the stable form of hydrogen
under normal conditions.
H + H
→
H
2
Each of the recombined molecules is then able to absorb more energy
from the electrical discharge to dissociate into two more excited atoms,
and the cycle begins again. This makes the tube glow continuously as
electrical energy is converted into electromagnetic energy.
If we pass the light emitted by the excited hydrogen atoms through a
prism, we can separate the light into individual wavelengths to get the
hydrogen
emission spectrum, as shown in Figure 6.7. Note that unlike the
Sun’s emission, only specific, discrete wavelengths of light are emitted in
the hydrogen emission spectrum.
6.3 Atomic Emission and Absorption Spectroscopy, Chemical Analysis and the Quantum Number 217
750
700
650
600
550
500
450
400
Wavelengths
(nm)
Absorption
lines
393 nm Calcium
397 nm Calcium
410 nm Hydrogen
423 nm Calcium
431 nm Iron, Calcium
434 nm Hydrogen
438 nm Iron
447 nm Helium
471 nm Helium
492 nm Helium
587 nm Helium
667 nm Helium
501 nm Helium
467 nm Iron
486 nm Hydrogen
496 nm Iron
517 nm
518 nm
527 nm Iron
656 nm Hydrogen
687–688 nm Oxygen (O
2
)
759–762 nm Oxygen (O
2
)
628– 629 nm Oxygen (O
2
)
Magnesium
Sodium
589 nm
590 nm
FIGURE 6.6
The spectrum of light from the Sun
includes dark bands at certain frequen-
cies, caused by particular elements in
the Sun absorbing light at these fre-
quencies. This phenomenon can be used
to identify which elements are present
in the sun. The existence of helium was
postulated by this method.
FIGURE 6.7
The hydrogen emission spectrum. An electric current is passed
through a tube of hydrogen gas, which causes some of the mole-
cules to dissociate to form excited hydrogen atoms (H*). The H*
atoms lose the energy by emitting radiation, which is separated
by wavelength with a glass prism. The intensity of the emitted
radiation can then be observed directly with your eye or can
be captured on film with electronic detectors such as a photo-
multiplier tube, or a change-coupled device.
