Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


A solid rocket motor is test fired. Studies
such as this allow scientists to measure
the temperature changes to the sensitive
parts of the motor.
Reorganizing this equation by adding the change in internal energy of the sur-
roundings to both sides, we get
U
system
+ U
surroundings
= 0
Mathematically, this is a restatement of the first law of thermodynamics. If we
know how much energy the system loses, we automatically know that the sur-
roundings will gain that same amount. If we know how much energy the system
gains, we know that the surroundings must have lost that same amount. The total
energy change in the universe (system and surroundings) is zero. Energy is con-
served. The bottom line is that the law of conservation of energy is also the first law
of thermodynamics.
EXERCISE 5.3 The First Law of Thermodynamics
Calculate the change in the energy of a system if 51.8 J of work is done by the system
with an associated heat loss of 12.3 J.
First Thoughts
We must pay particular attention to the sign conventions for heat and work in this
problem. In this case, work is done by the system. Is this work positive or negative?
A useful system to keep in mind is you! That is, when you do work—by running,
dancing, or even moving your textbooks from one class to the next—you are using
energy. After the process of moving your body or your books, you have less energy
than you had before, so the work has a “−” sign. Similarly, the 51.8 J of work done
by the system means that its energy change, w
system
,is−51.8 J. The energy loss as
heat by the system (such as that accompanying your run as your body tries to stay
cool) also has a “−” sign, so q
system
=−12.3 J.
Solution
From the standpoint of the system,
U = q + w
U = −12.3 J + (−51.8 J) = −64.1 J
Further Insights
We want to reinforce the point that there is no such thing as heat or work within a
system. In other words, a system does not contain heat or work. Rather, heat and work
exist only as types of energy transfer between the system and the surroundings. Work
is done by or on a system to move it through a distance. A system can transfer 64.1 J
of energy as heat and work. It does not contain 64.1 J of heat and work.
PRACTICE 5.3
Calculate the change in the energy of a system if 84.7 J of work is done on the sys-
tem, with an associated loss of energy as heat of 39.9 J.
See Problems 11–14, 19, 20, and 89.
5.2 Keeping Track of Energy
In designing complex engineering equipment such as a space shuttle, an aircraft, or
a chemical plant, the scientist or engineer needs to know how hot the parts that are
exposed to exothermic chemical reactions will become. The scientist also needs to
know that the shuttle’s engine components, the parts within an aircraft engine, or
the containers that are used to hold the hot products of chemical manufacturing
processes won’t melt. This raises a whole series of questions that need answering,
such as“How can we quantify the amount of energy being produced?”“How much
178 Chapter 5 Energy

heat will a chemical reaction generate?” and “How quickly will the surrounding
materials heat up, and to what temperature?” Similar questions are raised in all
practical investigations of energy, such as studies into the amount of energy pro-
vided to us by food or the amount of energy needed to heat a building or to oper-
ate a train or an automobile.
The Units of Energy
We often talk about the chemist’s shorthand and the importance of having a
common set of units with which to communicate. We can start our examination
of units by doing a calculation of kinetic energy. Let’s suppose you are riding a bi-
cycle along a level road at 4.40 meters per second (about 10 miles per hour) and
that the total mass of both you and the bicycle is 85.0 kg. The kinetic energy of
forward motion possessed by this system (you plus the bicycle) would be
Kinetic energy =
1
2
mv
2
=
1
2
× 85.0 kg ×
4.40 m
s
2
= 823 kg·m
2
·s
−2
This calculation yields the units of energy (kg·m
2
·s
−2
) in terms of SI base units.
However, we typically refer to this collection of units as the
joule, and this is the
unit that we will use most often when we discuss energy exchanges in chemical
reactions.
1 joule (J) = 1 kg·m
2
·s
−2
The kinetic energy in our example is therefore 823 J.
From where did you and the bicycle get this energy? The immediate source was
the series of chemical reactions in your muscles that allowed you to pedal the bike
up to speed. These chemical reactions released energy that was extracted from
your food. The original source of that energy is the energy of sunlight that fell on
the plants that were used to make your food.
An alternative unit used in energy exchange is related to our diet; many of us
use this unit as we “count calories” to assess the amount of energy available in the
food we eat. We do this by looking at the nutritional information on the food
package’s label, such as the one shown in Figure 5.13. That label gives us a mea-
sure of the energy content of food in units of
Calories (Cal)—note the capital C. In
a slightly confusing habit of nomenclature, the Calorie (with a capital C) is equal
5.2 Keeping Track of Energy 179
A bicycle and rider possess kinetic en-
ergy. The source of this kinetic energy is
from the food the bicyclist consumed.
Application
C
HEMICAL ENCOUNTERS:
Energy in Foods
FIGURE 5.13
Food labels contain information
about the chemical content of
the food and the amount of en-
ergy it supplies to the body. This
cereal supplies 180 Calories per
52-g serving.
Video Lesson: Energy, Calories,
and Nutrition

EXERCISE 5.4 The Power of Peanuts
Peanuts are a compact source of energy coming from proteins, fats, and carbohy-
drates found within. When these chemicals from one brand of peanuts are com-
bined with oxygen in the cells of the body, they release 625 Calories (note the capital
C) of usable energy per 1.00 × 10
2
g of peanuts. The following two questions
refer to the food energy in a 1.00-ounce (oz.) bag of peanuts. There are 28.4 g in
1.00 oz.
a. How many joules and how many kilojoules of energy are (ideally) available to
the body from the bag of peanuts?
b. A 100-watt (W) light bulb (1 W = 1 J/s) requires 100 joules to run for
1 second. How many hours would the light bulb run if the energy from the
peanuts were used to light the bulb?
180 Chapter 5 Energy
FIGURE 5.14
A food nutrition label from a box of
Zucaritas (Frosted Flakes) from Mexico.
Peanuts contain a lot of calories.
to a kilocalorie (kcal); that is, it is 1000 times larger than the calorie (cal)—note the
small c.
1 Cal = 1000 cal = 1 kcal
The values listed on most foods, even though you will often see the spelling calo-
ries on the label, are given in terms of the “big” Calorie, rather than the “little”
calorie. In any case, these alternative units for energy have entered everyday lan-
guage, in talk of “calorie-conscious lifestyles,” “high-calorie food,” “low-calorie
snacks,” and so on. How much is a calorie? One calorie (with a small c) equals
the amount of energy needed to raise the temperature of one gram of water by
one degree Celsius (for example, from 14.5°C to 15.5°C). The calorie is not an SI
unit, but it can readily be converted into joules:
1 cal = 4.184 J
The calorie is rather a small unit to use for measuring the energy available to
our bodies from the food we eat.A typical slice of bread, for example, can provide
our bodies with approximately 80,000 calories of usable energy. This equals
80 kilocalories, or 80 Calories.
1 calorie (cal) = 4.184 joules
1 kilocalorie = 4184 joules = 4.184 kilojoules = 1 Calorie
In many countries, food energy is stated in kilojoules, as in the box of Zucaritas
(Frosted Flakes) from Mexico that is shown in Figure 5.14.

5.3 Specific Heat Capacity and Heat Capacity 181
Solution
a. There are 4.184 kJ in 1 Cal (remember that the capital C signals kilocalories)
and 28.4 g of peanuts per bag. We can do our calculation as follows:
Kilojoules =
625 Cal
100 g
×
4.184 kJ
1Cal
×
28.4g
1 bag
= 743 kJ/bag
Joules =
743 kJ
bag
×
1000 J
1kJ
= 743,000 J/bag
b. We determined from part 1 that we have 743,000 J of energy available from the
peanuts. We can solve for time in hours knowing that there are 100 J/s, or
360,000 J/h.
Hours =
1s
100 J
×
1h
3600 s
× 743,000 J = 2.06 h ≈ 2h
PRACTICE 5.4
Apples supply us with approximately 30 Cal per 100 g, and an average apple weighs
about 200 g. How many apples would you need to eat to obtain the same amount of
energy as is supplied by the bag of peanuts?
See Problems 21–26, 90, and 91.
5.3 Specific Heat Capacity and Heat Capacity
A scientist working with the space shuttle engines is often concerned with the
heat generated during takeoff. The amount of heat given off by the burning
rocket fuel will need to be absorbed by something if the rocket is to remain intact.
The amount of insulation, and the type, will be of great importance. A chef faces
the same concerns when deciding on a method by which to pick up a hot pan.
Whether dealing with the space shuttle or the choice of metal cookware, the gen-
eral question is
How great will be the change in temperature when a specific mate-
rial absorbs or releases a certain amount of heat?
Every substance has a particular specific heat capacity (c), often shortened to
specific heat, which is defined as the amount of energy as heat needed to raise the
temperature of one gram of the substance by one degree Celsius (or one kelvin)
when the pressure is constant (see Table 5.2 for some representative examples of
specific heat). Substances with large values for their specific heat require more en-
ergy to raise their temperature than substances with small specific heat values.
For example, 1 g of water requires more than four times the energy to raise its
temperature 1.0°C than does aluminum.
The specific heat capacity of the water in your teapot at home is
4.184 J
g·
◦
C
. This means that it will require 4.184 joules (or 1 calorie) to raise the
temperature of 1 gram of the water by 1°C. Because the change in temperature in
degrees Celsius is equal to the change in temperature in kelvins, the specific heat
capacities can also be reported in units of
J
g·K
. Given the units of the specific heat
capacity, we can see how the heat required to raise the temperature of any com-
pound can be determined using the following equation:
q = m × c × T
where, q = heat c = specific heat capacity
m = mass T = change in temperature, in °C or K
Specific Heat Capacity
of Selected Materials
Specific Heat
Capacity
Substance J / g·°C
Water 4.184
Ethanol 2.460
Aluminum 0.902
Copper 0.385
Lead 0.128
Sulfur 0.706
Iron 0.449
Silver 0.235
TABLE 5.2
The piece of pie stays hot, while the
metal pie plate stays cool.
Video Lesson: CIA
Demonstration: Cool Fire
182 Chapter 5 Energy
This equation is useful in determining the amount of heat absorbed, or
released, during a temperature change for a substance. The amount of heat
needed to raise the temperature of any particular amount of a substance by
1°C is known as that substance’s
heat capacity (C), in units of
J
◦
C
. The heat
required to raise the temperature of this substance by a given amount is
q = C × T
J =
J
◦
C
×
◦
C
Let’s consider our teapot of water to help illustrate the use of these equations.
Assume that we determine the heat capacity of the entire quantity of water in
your teapot, when it is filled to the maximum, to be 6.27 kilojoules per
◦
C = (6.27 × 10
3
J/
◦
C)
.
We can use dimensional analysis to work out the mass of
water in the teapot, remembering that the specific heat capacity of water is
4.184 J/g·
◦
C
.
g water =
g·
◦
C
4.184 J
×
6.27 ×10
3
J
◦
C
= 1.50 ×10
3
g
We can also use this equation to determine the amount of heat required to
change the temperature of a particular substance. For instance, how much energy
as heat is required to raise the temperature of that teapot of water from a cold
5.00°C to the boiling point? (The change in temperature for the water will be
T = T
final
− T
initial
= 100.0°C − 5.00°C = 95.0°C.) Note that in finding our
answer, we will not determine the energy needed to vaporize the water. Instead,
we will calculate the heat required to arrive at a teapot full of 100.0°C water.
The required energy as heat is q = C ×T. Thus
6.27 ×10
3
J
◦
C
× 95.0
◦
C = 5.96 × 10
5
J = 596 kJ
1000 J = 1 kJ
Note how the dimensions work out to give you the proper units.
Another useful quantity that relates the heat capacity of a substance is the
molar heat capacity, which is the heat capacity of one mole of the substance, in
J
mol·
◦
C
. Knowing the specific heat of water, we can calculate the molar heat
capacity of water using dimensional analysis:
4.184 J
g·
◦
C
×
18.0gH
2
O
mol H
2
O
=
75.3J
mol·
◦
C
Calorimetry
The thermochemist (a chemist who studies energy transfers in chemical reac-
tions) uses the information about specific heat capacity to determine the amount
of energy that is either gained or released by reactions. For example, the thermo-
chemist could provide information about the reaction that takes place in the
main engine of the space shuttle:
2H
2
(g) + O
2
(g) → 2H
2
O(g)
The scientist could provide the automobile designers with information about a
reaction that takes place in a gasoline engine:
2C
8
H
18
(l) + 25O
2
(g) → 16CO
2
(g) + 18H
2
O(g)
How does he or she accomplish this task? By performing these reactions under
carefully controlled conditions, and trapping and measuring the energy as heat
given off or absorbed, the thermochemist can obtain information about energy
transfers. This procedure is known as
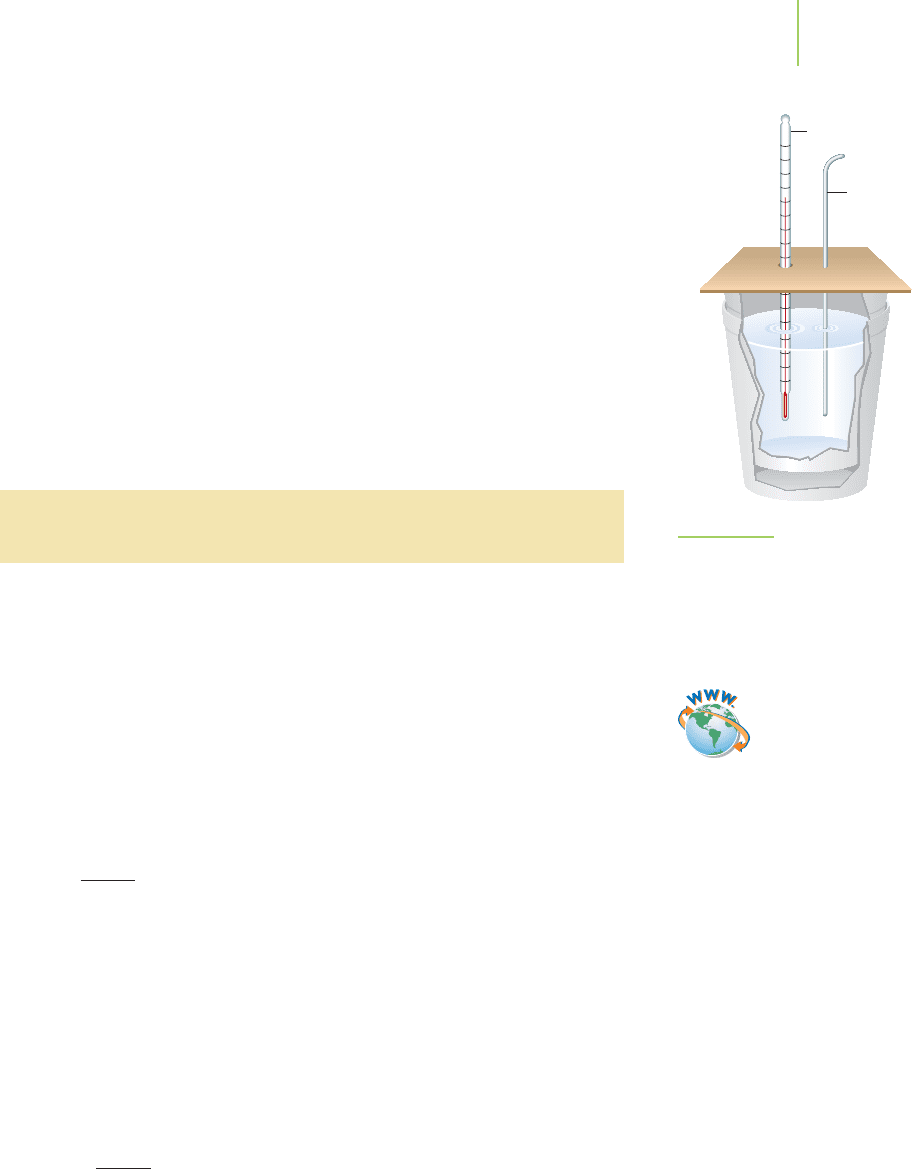
calorimetry, and the apparatus in which it is
performed is called a
calorimeter. To illustrate the key principles, we can construct
a calorimeter from two Styrofoam cups as shown in Figure 5.15. The cups act as
insulation to (ideally) prevent energy exchange between the contents of the inner
cup and the rest of the universe.
To understand the process, we can begin with a hot piece of iron (the system)
that we add to water (the surroundings), both within Styrofoam cups. Everything
outside the cups consititutes the rest of the universe. While crude looking, this
apparatus does a pretty good job of measuring the energy as heat transferred
from the system (the piece of iron, in this case) to the surroundings (the water).
Within the perfect Styrofoam calorimeter, the energy as heat lost by the system
equals that gained by the surroundings. Mathematically, this is represented by
q
system
=−q
surroundings
m
system
× c
system
× T
system
=−m
surround
× c
surround
× T
surround
After all of the energy as heat has been transferred, the final temperatures of the
system (iron) and that of the surroundings (water) within the calorimeter are iden-
tical. For example, let’s determine the change in temperature of a water bath
when a 155-g piece of iron at 95.0°C is added to 1.000 kg of water at 25.0°C. We
keep in mind that the energy as heat lost by the system and that gained by the sur-
roundings are equal and opposite in magnitude; q
system
=−q
surroundings
. Here are
the data.
The Block of Iron
m
system
= 155 g of iron
c
system
=
0.449 J
g·
◦
C
(from Table 5.2)
T
system
= ? We do not know the final temperature of the system, but we
know that it will be less than 95.0°C, because the surrounding water is
cooler than the piece of iron. Let’s call the change in temperature, T
system
,
“T
f
− 95.0°C.” The change in temperature is “−” because the final
temperature is less than the initial temperature.
The Water
m
surround
= 1.000 × 10
3
g of water
c
surround
=
4.184 J
g·
◦
C
T
surround
= ? We do not know the final temperature of the surroundings,
but we know that it will be greater than 25.0°C, because the piece of iron
(the system) is hotter than the water (surroundings,) and will therefore
transfer energy as heat into the water. Let’s call the change in temperature,
T
surround
,“T
f
− 25.0°C.” The change in temperature is “+” because the
final temperature is greater than the initial temperature.
5.3 Specific Heat Capacity and Heat Capacity 183
Stirrer
Thermometer
FIGURE 5.15
A Styrofoam calorimeter. The inner cup of
the calorimeter contains both the system
and the surroundings. The outer cup pro-
vides additional insulation to keep the
surroundings within the set-up.
Video Lesson: Constant Pressure
Calorimetry
184 Chapter 5 Energy
We can now set the equations for the energy exchanges as heat between the system
and the surroundings equal to each other (but opposite in sign!) and solve:
m
system
× c
system
× T
system
=−m
surround
× c
surround
× T
surround
155 g ×
0.449 J
g·
◦
C
× (T
f
− 95.0°C) =−1.000 × 10
3
g ×
4.184 J
g·
◦
C
× (T
f
− 25.0°C)
69.595 J
◦
C
× (T
f
− 95.0°C) =
−4184 J
◦
C
× (T
f
− 25.0°C)
69.595T
f
− 6611.525 =−4184T
f
+ 104600
4253.595T
f
= 111211.525
T
f
= 26.145 = 26.1°C
The final temperature of the water (and the piece of iron) will be
26.1°C.
However, our “perfect” Styrofoam calorimeter isn’t perfect. It
still allows some energy as work to be transferred from the sys-
tem to the surroundings. In addition, the calorimeter itself can
participate in the transfer of energy as heat, distorting the calcu-
lations. We can address these problems and eliminate small er-
rors in our measurements if we arrange things so that no work is
done by the system or on the system (w = 0). In cases like this,
the heat for any reaction (q) will be equal to the total change in
the energy of the system, because the equation U = q + w will
simplify to U = q. We can achieve this using a technique called
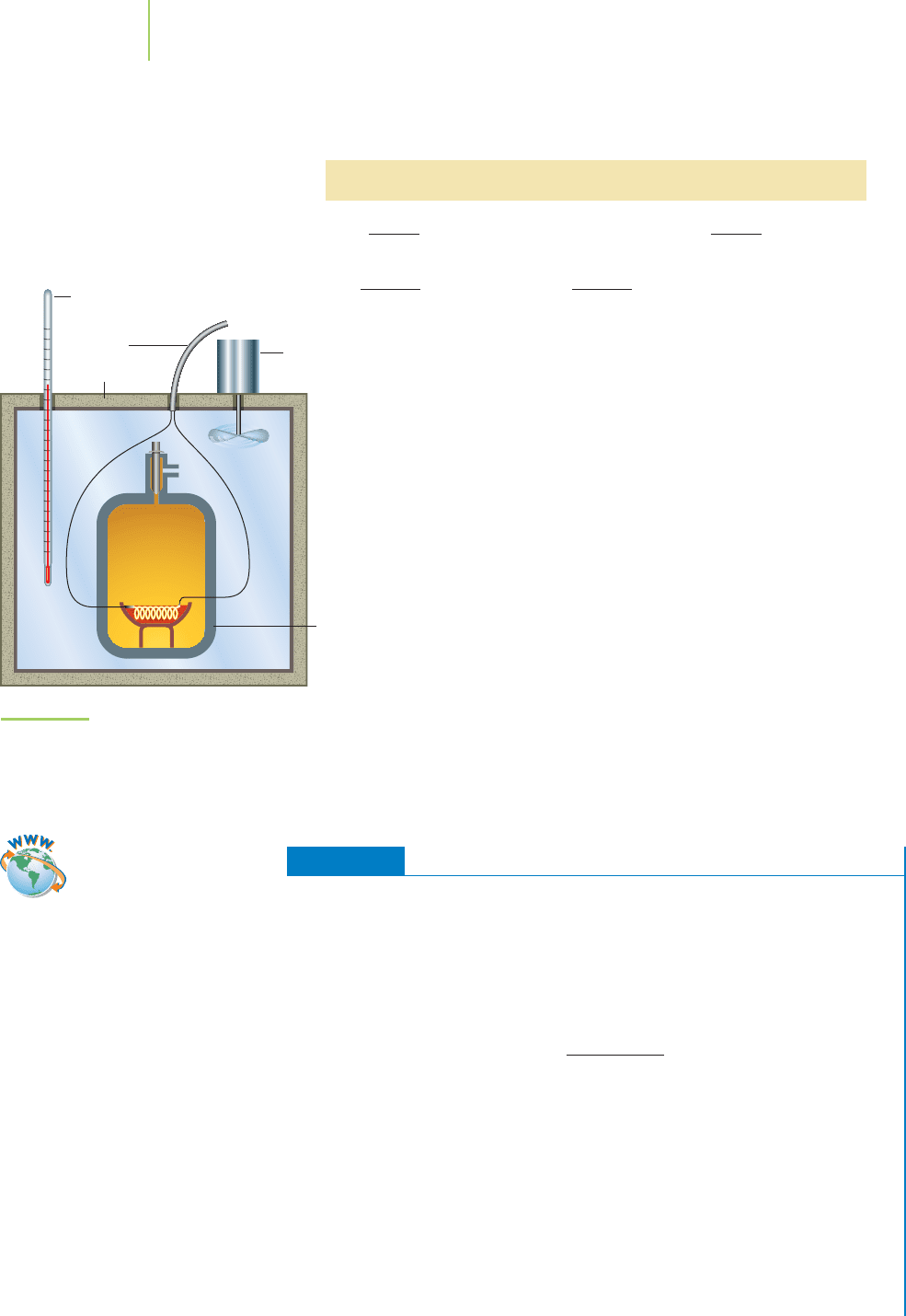
constant-volume calorimetry, in which the reacting system is
sealed within a steel chamber of fixed volume, called a
bomb
calorimeter
, shown in Figure 5.16.When a combustion reaction is
ignited inside a bomb calorimeter, heat is transferred between
the reaction and the surrounding water and calorimeter cham-
ber. We can then calculate the heat released by the reaction.
EXERCISE 5.5 Calculations with a Calorimeter
Each bomb calorimeter is different, but its own heat capacity can be determined ex-
perimentally using a substance that releases a known amount of energy. Once cali-
brated in this way, the calorimeter can be used to determine the heat output of other
chemicals.
a. Glucose (C
6
H
12
O
6
), also known as “blood sugar,” is the main sugar that serves
to transport chemical energy through the blood and distribute it to the body’s
cells. Glucose is known to release
2.80 ×10
3
kJ
mol
when combined with excess
oxygen at 298 K (25°C). A sample of glucose weighing 5.00 g was burned with
excess oxygen in a bomb calorimeter.The temperature of the calorimeter rose
by 2.40°C. Calculate the heat capacity of the calorimeter in joules per degree
Celsius.
b. Propane gas has the formula C
3
H
8
and can be used as a source of heat for
cooking and domestic heating. For storage purposes, it is liquefied and stored
in canisters, often under the name of liquefied petroleum gas (LPG). A 4.409-g
sample of propane was burned with excess oxygen in the bomb calorimeter
calibrated in part 1. The temperature of the calorimeter increased by 6.85°C.
Calculate how much energy as heat is released per mole of propane burned
under these conditions.
Thermometer
Wire for electric
ignition
Stirrer
Steel
“bomb
”
Reactants
Insulation
Water
FIGURE 5.16
A bomb calorimeter. The combustion reaction under study
is conducted within the steel bomb submerged in the
water bath. The heat given off by the reaction is measured
by the change in the temperature of the water bath.
Video Lesson: Bomb Calorimetry
(Constant Volume)
c. If the energy released from 1 mole of propane determined in part 2 were used
to heat 90.0 kg (9.00 × 10
4
g) of water originally at 30.0°C, what would be
the final temperature of the water? The specific heat capacity of water is
4.184 J
g·
◦
C
. (Remember to convert the energy released per mole of propane
from kilojoules to joules!)
Solution
a. We first need to calculate the number of moles of glucose (C
6
H
12
O
6
) in the
5.00 g used. The molar mass of glucose is 180.0 g/mol. We calculate the
number of moles used, and then use this to calculate the energy released per
degree Celsius, as follows:
Moles C
6
H
12
O
6
= 5.00 g C
6
H
12
O
6
×
1 mol C
6
H
12
O
6
180.0gC
6
H
12
O
6
= 0.0278 mol
Heat capacity of calorimeter =
2.80 ×10
3
kJ
1 mol
× 0.0278 mol ×
1
2.40
◦
C
=
32.4kJ
◦
C
b. The molar mass of propane (C
3
H
8
) is 44.09 g/mol. Knowing this enables us to
calculate the number of moles of propane burned and, therefore, the amount
of energy released. We’ll need to use the heat capacity of the calorimeter to
correct for the effect of the calorimeter:
Moles C
3
H
8
= 4.409 g C
3
H
8
×
1 mol C
3
H
8
44.09 g C
3
H
8
= 0.1000 mol C
3
H
8
Energy change per mole C
3
H
8
=
32.4kJ
◦
C
× 6.85
◦
C ×
1
0.1000 mol C
3
H
8
=
−2.22 ×10
3
kJ C
3
H
8
mol C
3
H
8
We add a negative sign to the value because energy is released from this
reaction; that is, the reaction is exothermic.
c. Remember that q = c × m × T. This rearranges to
T =
q
c × m
T =
2.22 ×10
6
JC
3
H
8
4.184 J
g·
◦
C
× (9.00 ×10
4
g)
= 5.90
◦
C
The temperature of the water will rise to nearly 36°C in a total of 90.0 L
(about 24 gal) of water!
PRACTICE 5.5
A sample of benzoic acid (C
6
H
5
COOH) weighing 2.442 g was reacted with excess
O
2
in a bomb calorimeter. The temperature rose from 26.34°C to 39.20°C. The heat
capacity of the calorimeter was
5.02 kJ
◦
C
. Calculate the energy released in
kilojoules per mole as heat for the reaction.
See Problems 27, 28, 31, 32, 37–46, 51, 52, 95, 96, and 98.
5.3 Specific Heat Capacity and Heat Capacity 185
186 Chapter 5 Energy
HERE’S WHAT WE KNOW SO FAR
■
Changes in the internal energy of a system are determined by the sum of the
work and the energy change as heat for the process. The signs on these terms
are vital, because they define whether energy is transferred to the system or to
the surroundings.
■
The heat capacity of a substance reflects its ability to absorb heat. A large heat
capacity indicates that the addition of a large amount of energy is required in
order to change the temperature of the substance.
■
A calorimeter can be used to measure the amount of heat transfer between the
system and the surroundings. We may use the equation m
system
× c
system
×
T
system
=−m
surround
× c
surround
× T
surround
to determine the specific situa-
tion of the heat transfer.
5.4 Enthalpy
Many of the reactions we do in the laboratory do not occur under constant-
volume conditions. Any gases that are generated are often free to expand outward
into the atmosphere. These reactions occur under constant-pressure conditions,
because the release of gas or any other expansion of volume will occur until the
pressure of the products of the reaction becomes equal to the atmospheric
pressure.
Under constant-pressure conditions, we cannot use the simple relationship
U = q but instead have to work with the slightly more complex U = q + w to
take into account any work done by the system or done on the system. When a
reaction occurs under constant-pressure conditions, the only type of work the
system will be able to do on the surroundings is called “pressure–volume work”
(see Section 5.1), such as the work done by the system when a released gas is al-
lowed to expand. Under these conditions of constant pressure, the work done by
the system is equal to −P × V (pressure multiplied by the change in volume),
so the equation U = q + w can be written as
U = q
p
− PV
where q
p
is the heat of reaction at constant pressure. We can rearrange that equa-
tion to get
q
p
= U + PV
We introduce a new term, enthalpy, which is symbolized by H. Enthalpy is
measured in the units of energy (joules) and is defined as the sum of the internal
energy and the pressure–volume product of a system:
H = U + PV
A change in enthalpy ( H) can be defined as H = U + (PV) and is equal
to q
p
.
Moreover, if the pressure of the system is constant, PV can change only as a
consequence of changes in volume, so (PV ) is equal to PV. Under these
constant-pressure conditions, the definition of the change in enthalpy becomes
exactly the same as the definition for the heat of reaction at constant pressure,
namely q
p
:
At constant pressure: q
p
= U + PV
And H = U + PV
So H = q
p
Video Lesson: Heats of
Reaction: Enthalpy

Heats of reaction measured under constant-pressure conditions are known as
changes in enthalpy. It is important to remember that this unfamiliar term really
just refers to the familiar idea of energy exchange as heat for a reaction that pro-
ceeds under constant-pressure conditions.
In situations where only very small amounts of work are done by a system or
on a system, the value of w is very small compared to q
p
. In these situations, the
easily measured heat of reaction (q
p
), which also equals the enthalpy change
(H), is approximately equal to the total energy change of the system U
system
.
This is useful when studying the chemistry of living things. For instance, most
biochemical reactions occur in body fluids in which there are negligible changes
in volume and, therefore, negligible contribution to the energy change of the sys-
tem from work. However, if a chemical reaction produces a gas, then the work com-
ponent is not necessarily negligible because the change in volume becomes large.
The most interesting and useful value to us when we are studying energy and
chemistry is the total energy change (U) of the chemicals in the system as they
react. This is not always easy to measure directly, so one of the most significant
facts about enthalpy change (H) values is that they provide a readily measured
approximation to the U values in which we are really interested.
Standard Enthalpies of Reaction
Comparing changes in enthalpies for reactions is a tricky business. Not only do
the enthalpies need to be measured at the same temperature, but to be meaning-
ful, they also require the same conditions. Comparisons are often made with
heats of reaction obtained when all of the reactants and products are in their
standard states, as illustrated in Figure 5.17. Then the enthalpy of the reaction be-
comes known as a
standard enthalpy of reaction (
rxn
H°). What is the standard
state of a reactant or product? The most commonly used standard states for ther-
modynamic work are as follows:
■
For a pure solid, liquid, or gas, the standard state is the state of the substance
at a pressure of exactly 1 atmosphere (1 atm), which equals 101,325 Pa.
(IUPAC has adopted 100,000 Pa, known as 1 bar, as the standard pressure, but
1 atm is still in widespread use.)
■
For any substance in solution, the standard state is at a concentration of
exactly 1 molar.*
We indicate that a thermodynamic value has been determined
under standard conditions by using the degree sign (°), so a stan-
dard enthalpy of reaction would be indicated as
rxn
H°.
Any substance subjected to these standard conditions is said to
be in its standard state. For example, water is present all around us
in three main forms: as the liquid water that runs from our taps, as
the water vapor in the air, and as the solid water such as the ice in
our freezers. The standard state of water, however, is the liquid form
in which pure water appears at 1 atm of pressure. Most of the water
around us is not “pure water” because it has other chemicals dis-
solved in it. To be in its standard state, a substance must be pure.
The
reference form of an element is the most stable form of the
element at standard conditions. For the element oxygen at 1 atm
and 25°C, the reference form is O
2
, rather than the less stable
allotrope O
3
.
5.4 Enthalpy 187
In reactions that involve gases, H does
not equal U because the reaction does
work on the surroundings. Explosions are
an extreme example of reactions that
generate gases and do work on the sur-
roundings.
*Standard states for thermodynamic properties are often tabulated at 25°C. However,
the definition for a substance in its standard state does not require the temperature to be
25°C. For example, one could calculate the enthalpy of reaction at 350°C, although to
do so, one would need access to thermodynamic values tabulated at this temperature.
Chlorine
Cl
2
(g)
Ethanol
C
2
H
6
O(l)
Bromine
Br
2
(g, l)
Sodium chloride
NaCl(s)
Glucose
C
6
H
12
O
6
(s)
FIGURE 5.17
Examples of some compounds in their standard states.
